Abstract
This is a case of a 4-cm left extrathoracic subclavian artery aneurysm (SCAA) in a 58-year-old man with an aortic root and abdominal aortic aneurysm. The patient had features suggestive of genetic arteriopathy, including vertebral artery tortuosity, pectus excavatum, tall stature, and scoliosis. The SCAA was successfully repaired with an inline prosthetic graft and anastomotic pledgets via a supraclavicular approach. Genetic testing revealed an FBN1 pathogenic variant consistent with Marfan syndrome. Repair is satisfactory 2 years later. Patients with SCAA should include consideration of genetic arteriopathy. Open repair of the extrathoracic SCAA in Marfan syndrome is recommended.
Keywords: Marfan syndrome, Subclavian artery aneurysm, Vertebral tortuosity, Heritable thoracic aortic disease, Genetic arteriopathies
Subclavian artery aneurysms (SCAA) are rare and there is a paucity of literature regarding surgical repair technique. We present a case of a 4-cm left extrathoracic SCAA in a patient with an aortic root and abdominal aortic aneurysm to describe the surgical repair technique and the workup undertaken for this diagnosis. The patient was ultimately diagnosed with Marfan syndrome (MFS). Consent was obtained to publish this case.
Case report
This is a case of a 58-year-old man who was referred for an enlarging extrathoracic 4-cm left SCAA without mural thrombus or calcification and extending into the axillary artery. The initial diagnosis was made during workup for an aortic root aneurysm for which he underwent a valve-sparing root and ascending aortic replacement. The left SCAA at initial diagnosis measured 2.9 cm (Fig 1). The patient did not smoke or have a history of hypertension or hyperlipidemia. There was no history of trauma to the left upper extremity. Additional pathology included a 1.5-cm right subclavian artery, a 3.1-cm infrarenal abdominal aortic aneurysm, and ectatic bilateral common iliac arteries. Family history was negative for aneurysms or dissections; however, there was a history of sudden death (maternal grandfather).
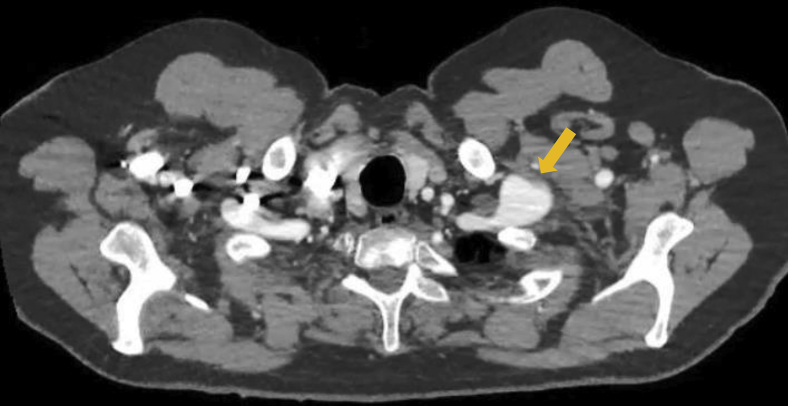
Fig 1.
Axial image of computed tomography angiogram showing the left subclavian artery aneurysm (SCAA).
On examination, the patient is tall (6’4”) with down-slanting palpebral fissures, striking iridodenesis bilaterally (history of lens replacement), a narrow slightly high arched palate, and a surgically absent uvula. He has a pulsatile aneurysm at the base of the neck in the left supraclavicular space, mild pectus excavatum, mild scoliosis, and hypermobility of small joints, but no arachnodactyly. There were no signs or symptoms of thoracic outlet compression. The left SCAA enlarged over the subsequent 2 years of follow-up since discovery and also demonstrated significant bilateral vertebral artery tortuosity (Fig 2).
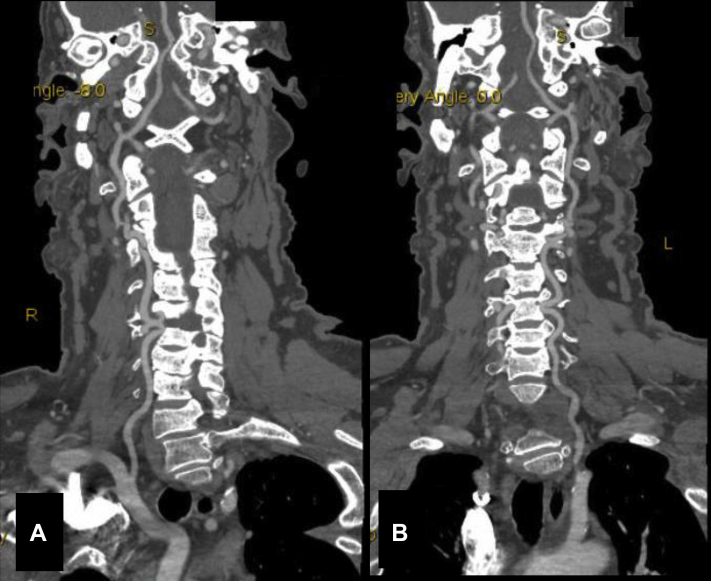
Fig 2.
Maximum intensity projection of computed tomography angiogram showing the right (A) and left (B) vertebral arteries tortuosity.
Surgical technique
Given the constellation of findings suggestive of a heritable etiology, we offered an open surgical repair. We discouraged an endovascular approach owing to the highly mobile area would place any stents at risk for fracture (Fig 3). Imaging also confirmed that this was not a poststenotic dilation aneurysm related to thoracic outlet compression; thus, a bypass without first rib resection was chosen as the optimal repair option.
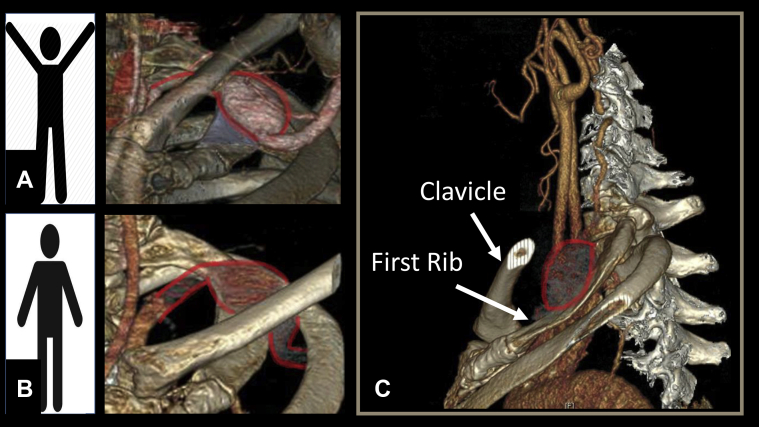
Fig 3.
Three-dimensional reconstruction imaging demonstrating the left subclavian artery position with the arms up (A) and down (B), and the relationship to the clavicle and the first rib (C).
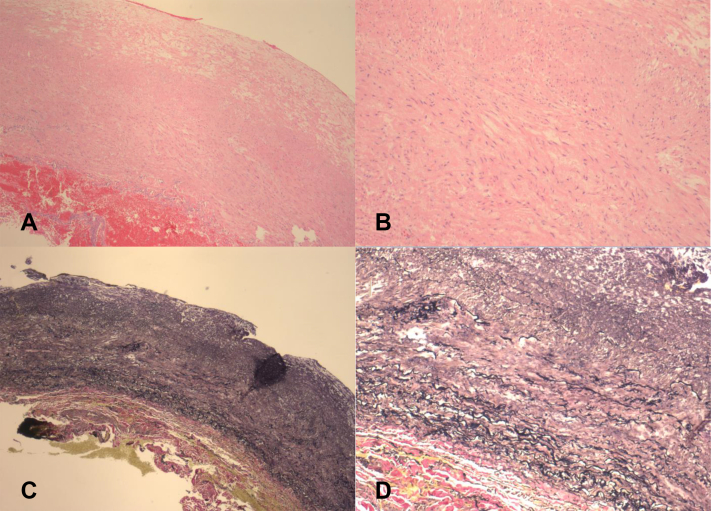
The repair was performed under general anesthesia.1 The patient was placed in the supine position with a shoulder roll and the neck extended and turned to the right. A transverse supraclavicular incision two finger breadths above the clavicle and lateral to the sternocleidomastoid muscle was made. The incision was then carried through the platysma and subplatysmal flaps raised. The scalene fat pad was mobilized beginning at the lateral edge of the internal jugular vein. The phrenic nerve overlaying an attenuated anterior scalene muscle was identified and preserved. The SCAA occupied most of the space. The anterior scalene muscle attachment to the first rib was circumferentially mobilized and the tendon sharply divided under direct vision. This maneuver exposed the SCAA. Gentle traction allowed exposure of the proximal inflow and distal outflow (Fig 4, A, B). Gentle clamping with vascular clamps of the arterial outflow and inflow was performed after systemic heparinization. The artery was friable without significant atherosclerotic calcification or signs of inflammation or dissection (Fig 4, C). An 8-mm Dacron interposition bypass (end-to-end anastomosis) between the mid subclavian artery and proximal most aspect of the axillary artery using 5-0 Prolene sutures with pledgets circumferentially (Fig 4, D). After restoration of flow, heparin reversal, and hemostasis confirmed, the sac was then closed over the bypass graft. Pathology showed an arterial wall with medial myxoid degeneration and elastin fragmentation (Fig 5).
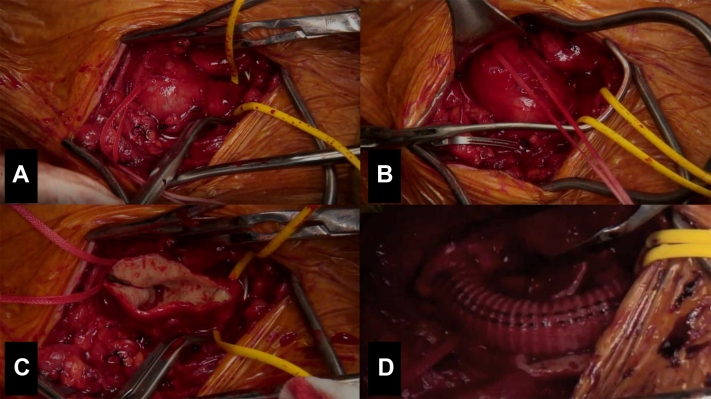
Fig 4.
Intraoperative photos of the left subclavian artery aneurysm (SCAA) with the vessel loop surrounding the subclavian artery (A), umbilical tape surrounding the proximal axillary artery distal to the aneurysm (B), open aneurysm sac (C), and interposition 8-mm Dacron graft (D).
Fig 5.
Pathology imaging of the left subclavian artery aneurysm (SCAA) wall showing medial myxoid degeneration and elastin fragmentation on Hematoxylin and eosin stain (H&E) (A and B) and Verhoeff-Van Gieson stain (VVG) (C and D) at original magnifications of ×4 and ×10, respectively.
The patient returned to work 1 month later. After insurance related delays, genetic testing revealed a heterozygous FBN1 pathogenic variant (c.7865G>C, p.Cys2622Ser), consistent with MFS. Additional genetic counseling was provided. The 2-year follow-up shows a patent bypass with no stenosis or pseudoaneurysm and an unchanged abdominal aorta.
Discussion
True SCAA are rare, representing less than 1% of arterial aneurysms.2 The etiology is variable and includes atherosclerotic aneurysms, trauma, post stenotic dilation in thoracic outlet syndrome, and genetic arteriopathies such as MFS, Loeys Dietz syndrome, and vascular Ehlers-Danlos syndrome.3, 4, 5, 6, 7, 8 Our case demonstrates the workup that was undertaken upon diagnosis of an extrathoracic SCAA owing to MFS to differentiate it from the other etiologies and how this dictated the surgical decision making. Workup includes attention to features of genetic arteriopathies, such as musculoskeletal abnormalities (scoliosis, pectus abnormalities), other concurrent aneurysms/dissections, arterial tortuosity, and family history of aneurysms, dissections, and sudden death.9, 10, 11 Although genetic testing was delayed owing to insurance preauthorization, the heighted index of suspicion for MFS or Loeys Dietz syndrome as the etiology and ruling out thoracic outlet compression as part of the etiology allowed us to plan our approach and repair accordingly.
Although rare, SCAA carry the risk of thrombosis, distal embolization, compression of adjacent structures, and rupture.4 The location of the SCAA as intrathoracic or extrathoracic dictates the surgical approach, with open surgical repair of extrathoracic SCAA as the gold standard. In this case, owing to the suspicion for a genetic arteriopathy and the fragile texture of the artery, circumferential pledgets were used to buttress and provide additional support for the suture line (felt strips can also be used). It is worth noting that although the tissue quality was fragile, requiring gentle manipulation, traction, and clamp application, the consistency was that of what we expected in a patient with a history of successful root repair and ascending aortic replacement. Although endovascular stent grafts are an attractive minimally invasive repair option,12, 13, 14 this approach is not suited for extrathoracic SCAAs in the thoracic outlet region owing to the high mobility in this area and the high likelihood of failure owing to compression and fracture.13,14 As such, we recommend endovascular stent grafts (such as Viabhan endoprosthesis) for patients who are high-risk surgical candidates or in circumstances of rupture in which the stent grafts provide rapid control of hemorrhage.15 In rare circumstances of mycotic SCAA, coil embolization maybe appropriate.16
Our case demonstrates the importance of genetic testing to establish the diagnosis. The results allow us to offer cascade testing to his first-degree relatives as well as plan for annual surveillance follow-up of the right SCA dilation and the small abdominal aortic aneurysm.
Conclusions
Patients with SCAA should include consideration of genetic arteriopathy. Open repair of extrathoracic SCAA in MFS is recommended.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Ur R., Shalhub S., Banning S., Manteghi L.K., Revels J., Hamlat C.A. Open surgical repair of an extrathoracic distal left subclavian artery aneurysm. 2019. www.jvascsurg.org/article/S0741-5214(19)30957-7/fulltext Available at:
- 2.Mohan I.V., Stephen M.S. Peripheral arterial aneurysms: open or endovascular surgery? Prog Cardiovasc Dis. 2013;56:36–56. doi: 10.1016/j.pcad.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Yetman A.T., Roosevelt G.E., Veit N., Everitt M.D. Distal aortic and peripheral arterial aneurysms in patients with Marfan syndrome. J Am Coll Cardiol. 2011;58:2544–2545. doi: 10.1016/j.jacc.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Vierhout B.P., Zeebregts C.J., van den Dungen J.J., Reijnen M.M. Changing profiles of diagnostic and treatment options in subclavian artery aneurysms. Eur J Vasc Endovasc Surg. 2010;40:27–34. doi: 10.1016/j.ejvs.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Davidovic L.B., Zlatanovic P., Ducic S., Koncar I., Cvetic V., Kuzmanovic I. Single center experience in the management of a case series of subclavian artery aneurysms. Asian J Surg. 2020;43:139–147. doi: 10.1016/j.asjsur.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Shalhub S., Black J.H., 3rd, Cecchi A.C., Xu Z., Griswold B.F., Safi H.J. Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes. J Vasc Surg. 2014;60:160–169. doi: 10.1016/j.jvs.2014.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morisaki K., Kobayashi M., Miyachi H., Maekawa T., Tamai H., Takahashi N. Subclavian artery aneurysm in Marfan syndrome. Ann Vasc Surg. 2012;26:731.e1–731.e4. doi: 10.1016/j.avsg.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu R.J., Lue J., Ehlert B.A., Grimm J.C., Hicks C.W., Black J.H., 3rd Surgical management of peripheral vascular manifestations of Loeys-Dietz syndrome. Ann Vasc Surg. 2017;38:10–16. doi: 10.1016/j.avsg.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Musunuru K., Hershberger R.E., Day S.M., Klinedinst N.J., Landstrom A.P., Parikh V.N. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2020;13:e000067. doi: 10.1161/HCG.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 10.Morris S.A., Orbach D.B., Geva T., Singh M.N., Gauvreau K., Lacro R.V. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124:388–396. doi: 10.1161/CIRCULATIONAHA.110.990549. [DOI] [PubMed] [Google Scholar]
- 11.Meester J.A.N., Verstraeten A., Schepers D., Alaerts M., Van Laer L., Loeys B.L. Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann Cardiothorac Surg. 2017;6:582–594. doi: 10.21037/acs.2017.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maskanakis A., Patelis N., Moris D., Tsilimigras D.I., Schizas D., Diakomi M. Stenting of subclavian artery true and false aneurysms: a systematic review. Ann Vasc Surg. 2018;47:291–304. doi: 10.1016/j.avsg.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Beregi J.P., Prat A., Willoteaux S., Vasseur M.A., Boularand V., Desmoucelle F. Covered stents in the treatment of peripheral arterial aneurysms: procedural results and midterm follow-up. Cardiovasc Intervent Radiol. 1999;22:13–19. doi: 10.1007/s002709900322. [DOI] [PubMed] [Google Scholar]
- 14.Zehm S., Chemelli A., Jaschke W., Fraedrich G., Rantner B. Long-term outcome after surgical and endovascular management of true and false subclavian artery aneurysms. Vascular. 2014;22:161–166. doi: 10.1177/1708538113479514. [DOI] [PubMed] [Google Scholar]
- 15.Marjanovic I., Tomic A., Maric N., Pecarski D., Sarac M., Paunovic D. Endovascular treatment of the subclavian artery aneurysm in high-risk patient - a single-center experience. Vojnosanit Pregl. 2016;73:941–944. doi: 10.2298/VSP150420091M. [DOI] [PubMed] [Google Scholar]
- 16.Kische S., Ince H., Peuster M. Coil occlusion of a subclavian mycotic aneurysm. Catheter Cardiovasc Interv. 2010;75:1116–1120. doi: 10.1002/ccd.22389. [DOI] [PubMed] [Google Scholar]