Abstract
Background:
Men who have sex with men (MSM) are disproportionately burdened by gonorrhea and face high rates of extragenital (rectal and pharyngeal) infection, which is mostly asymptomatic and often missed by urogenital-only screening. Extragenital screening likely remains below Centers for Disease Control and Prevention-recommended levels. Because increasing screening coverage is often resource-intensive, we assessed whether improved extragenital screening among men already presenting at clinics could lead to substantial reductions in prevalence and incidence.
Methods:
We calibrated an agent-based model of site- and race-specific gonorrhea infection in MSM to explicitly model multisite infection within an individual and transmission via anal, orogenital, and ororectal sex. Compared with current screening levels, we assessed the impact of increasing screening at (1) both extragenital sites, (2) only the rectal site, and (3) only the pharyngeal site among men already being urogenitally screened.
Results:
All scenarios reduced prevalence and incidence, with improved screening at both extragenital sites having the largest effect across outcomes. Extragenitally screening 100% of men being urogenitally screened reduced site-specific prevalence by an average of 42% (black MSM) and 50% (white MSM), with these values dropping by approximately 10% and 20% for each race group when targeting only the rectum and only the pharynx, respectively. However, increasing only rectal screening was more efficient in terms of the number of screens needed to avert an infection as this avoided duplicative screens due to rectum/pharynx multisite infection.
Conclusions:
Improved extragenital screening substantially reduced site-specific gonorrhea prevalence and incidence, with strategies aimed at increasing rectal screening proving the most efficient.
Gonorrhea has transformed from a manageable challenge to an urgent public health threat in recent years with increasing rates of reported diagnoses since 2009 and growing antimicrobial resistance.1,2 The burden is particularly acute for men who have sex with men (MSM)—estimated diagnoses in MSM increased 375.5% (1368.6–6508.0 diagnoses per 100,000 MSM) from 2010 to 2018.1 Although improved screening is likely a key driver of this increase, real changes in incidence may also be contributing.1,3
Gonorrhea control is complicated by its infection characteristics—it can infect distinct anatomical sites (the urethra, rectum, and pharynx) within the same person (multisite infection) without spreading between sites, allowing transmission via different sex acts.4 Gonorrhea is understood to transmit via insertive and receptive anal5–10 and orogenital sex,2,8,10,11 and some studies hypothesize that it may also spread via ororectal sex11–13 and kissing.2,11–15 Additionally, symptom likelihood varies by site. Male urogenital infections are mostly symptomatic, whereas extragenital infections (rectal and pharyngeal) are mostly asymptomatic,16,17 meaning that most symptomatically tested infections are urogenital. However, extragenital-only infections are common. One study evaluating National HIV Behavioral Surveillance data from a community venue-based sample of sexually active MSM in 5 U.S. cities found a 4.5% and 4.6% prevalence of rectal and pharyngeal gonorrhea, respectively.18 Another analysis of STD Surveillance Network (SSuN) data from patients attending 42 US sexually transmitted infection (STI) clinics found a 10.2% and 7.9% prevalence of rectal and pharyngeal gonorrhea, respectively, among MSM tested at those sites. It also found that approximately 50% of total diagnosed infections in MSM were extragenital and that, among men triple-site tested, 70% of extragenital infections would have been missed with urogenital-only testing.19 The role of pharyngeal infection also remains unclear, with some hypothesizing that it could be an asymptomatic reservoir of untreated infection.2,8,13 Overall, it is important to better discern how extragenital gonorrhea factors into infection dynamics.
The Centers for Disease Control and Prevention (CDC)’s current gonorrhea screening guidance for MSM is to screen each anatomical site of reported exposure at least once annually, regardless of condom use and to screen higher-risk MSM every 3 to 6 months.4 Asymptomatic site-specific screening data are very limited, making it challenging to distinguish symptomatic testing from asymptomatic screening. Despite these gaps, site-specific screening is broadly believed to remain below current recommended levels, particularly for extragenital sites.19–25 Until May 2019, no nucleic acid amplification tests were Food and Drug Administration-cleared for extragenital specimens, which may have limited their use.23,26 Additional explanations for failures to screen include lack of provider awareness, provider time limitations, provider discomfort with sexual history taking and genital examinations, and patient reluctance.27
The recent emergence of strains resistant to the remaining first-line antibiotics is also a cause for concern.28 Gonorrhea isolates from MSM have historically shown more resistance than those from men who have sex with women, and the MSM community could be particularly affected by the latest resistant strains.29 The pharynx may also be an important source of resistance given its low antibiotic penetration, colonization with other Neisseria species, and incidental exposure to other antibiotics while asymptomatically infected.2 Although pharyngeal infection is common,18,19 relatively few epidemiological studies or models incorporate it.11 We found 6 mathematical models that include pharyngeal gonorrhea—4 simulate non–US MSM populations,8,10,30,31s and 2 capture US MSM populations using community health clinic data.32s, 33s
Despite limited site-specific screening data, there is a need to understand the possible impact of expending resources to improve adherence to CDC screening recommendations in light of increasing diagnosis rates and antibiotic resistance concerns. The high positivity of extragenital sites suggests that they can act as transmission reservoirs. Furthermore, increasing screening coverage is difficult and resource-intensive. Therefore, we examined whether targeting increased extragenital screening for patients already presenting for regular screening services could lead to substantial reductions in prevalence and incidence at all anatomical sites. We used an agent-based modeling framework because it allowed us to explicitly model site-specific gonorrhea infection within a given individual and transmission via different acts within a sexual network.
MATERIALS AND METHODS
Structure
We modeled gonorrhea and HIV infection dynamics in a sexual network of 18- to 38-year-old non-Hispanic black MSM (BMSM) and white MSM (WMSM) by extending the EpiModelHIV modeling platform.34s EpiModelHIV provides a framework for agent-based models of HIV and has been extensively described in prior studies.9,35s,36s The sexual network in our model was comprised of main, casual, and 1-time partnerships.
We extended EpiModelHIV to incorporate pharyngeal infection and screening (Fig. 1), as well as transmission to and from the pharynx via orogenital and ororectal sex. Sex acts stochastically occurred according to weekly rates that varied based on the partnership type. Calibrated transmission probabilities were 55% urogenital to rectal, 20% rectal to urogenital, 38% urogenital to pharyngeal, and 1% pharyngeal to urogenital. Bidirectional probabilities for ororectal sex were predetermined at 5%.8,10–13 Symptom likelihood varied, with urogenital infection set to be 82% symptomatic; rectal, 8%; and pharyngeal, 0%.5,8,16,36s–38s There was background site-specific natural recovery. We predetermined untreated urogenital and rectal infections to clear after 245 days on average,36s and calibrated pharyngeal infections to clear after 182 days on average. Infections could be diagnosed through symptom-driven testing or asymptomatic screening, which varied by site. For this analysis, we modified screening for asymptomatic infection to include pharyngeal infection (Fig. 1). Recommended treatment did not vary across sites, and we assumed that treatment for 1 detected site-specific infection cleared any other infected sites within the same person.4
Figure 1.
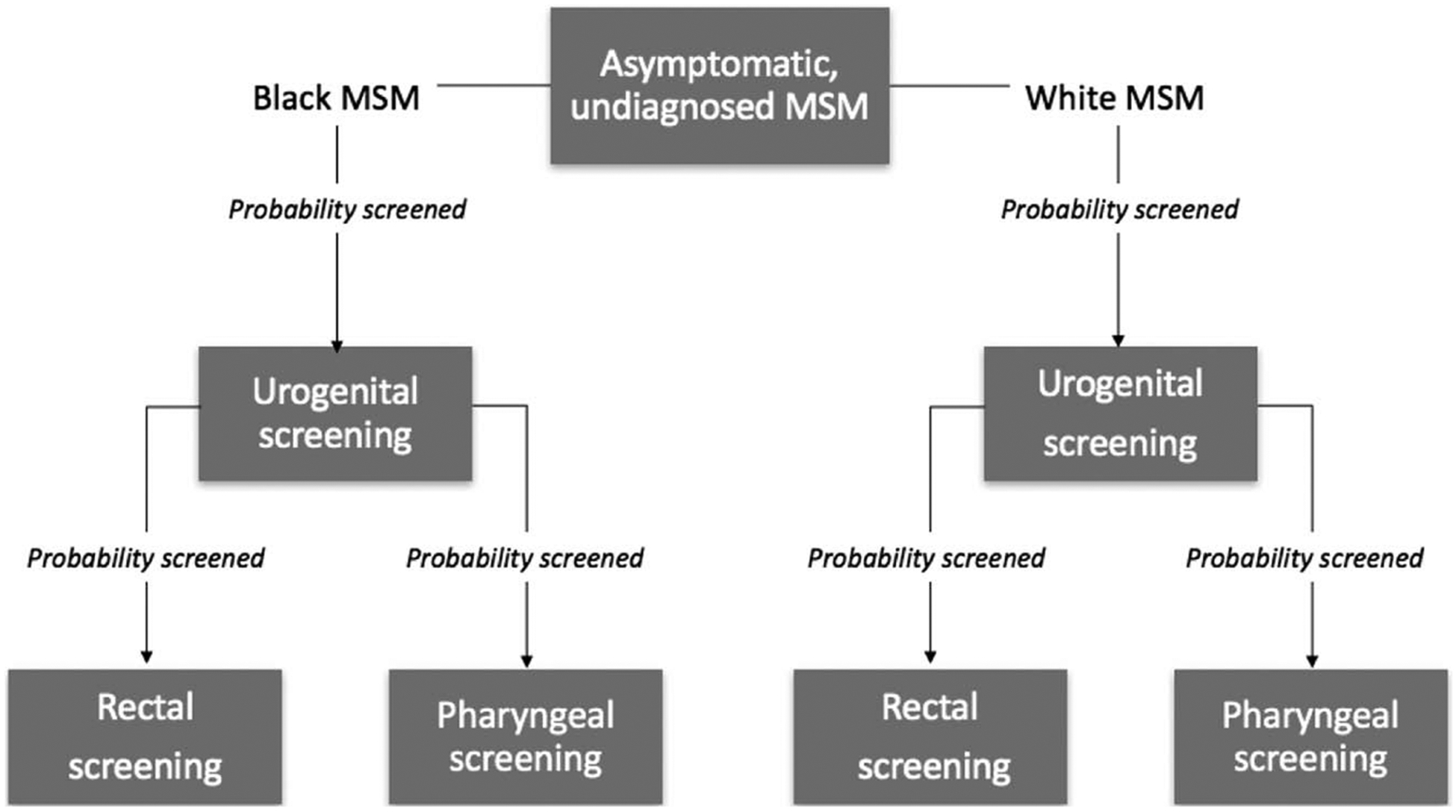
Screening pathway for asymptomatic, infected or uninfected, undiagnosed, BMSM and WMSM. Each week, there was a probability that a given man was urogenitally screened. If this occurred, there were independent probabilities that they were also screened at each extragenital site. No extragenital screening occurred without an accompanying urogenital screen.
We used a race-stratified model to reflect the substantial disparities observed for gonorrhea.38s–40s We differed parameters where data were available, including for network structure (e.g., degree distributions, mean partnership durations, age mixing, age-specific mortality), sexual behaviors (e.g., sex act rates, sex role versatility, condom use, condom failure), and the HIV testing and care cascade (e.g., testing frequency, treatment initiation, status disclosure, circumcision status). These parameters are detailed in other articles,9,36s and in the Appendix, http://links.lww.com/OLQ/A503. Due to a lack of race- and site-specific data, we did not stratify gonorrhea screening by race.
As in EpiModelHIV, we included HIV in our model, with prevalent urogenital and rectal gonorrhea infection increasing HIVacquisition risk. We did not model an increased risk of gonorrhea acquisition given an HIV infection. In this analysis, HIV diagnosis status did not change the probability of gonorrhea screening due to a lack of reliable data to inform the estimates.
Data
The EpiModelHIV platform derives most of its sexual partnership and behavioral parameters from two 2011–2014 Atlanta HIV and STI network studies of non-monogamous and sexually active MSM.36s, 39s, 40s These studies only collected data on anal sex partnerships. We assumed that including orogenital and ororectal sex would not change the proportion of men in main partnerships (ie, men in main partnerships were not exclusively engaging in orogenital and/or ororectal sex), but that we would see an increase in the proportion of men in casual and one-time partnerships only involving orogenital and/or ororectal sex. We used data on the relative frequency of different sex act types from a national survey of 24,787 MSM that recorded self-reported sexual behaviors during men’s most recent male-partnered sexual event.41s
Data on current national site-specific screening levels were limited—the CDC’s SSuN did not collect population-level site-specific data until relatively recently and most literature estimates came from potentially unrepresentative STI clinic reporting. We assigned a weekly urogenital asymptomatic screening probability of 0.978%, extrapolating the value from another model’s national estimate of the annual MSM screening rate.42s Among men screened at the urethra, we set a 37.5% probability of also being screened at the rectum and an independent 37.5% probability of also being screened at the pharynx after reviewing the available data.19,20,24 43s, 44s
Calibration
The model was calibrated to (1) race- and site-specific gonorrhea prevalence and incidence and (2) race-specific HIV prevalence. We derived all calibration targets from the Atlanta studies,36s excluding those for pharyngeal gonorrhea. Pharyngeal targets were based on a prospective cohort of MSM in California.38s We did not have targets for pharyngeal incidence and prevalence in BMSM or urogenital prevalence in WMSM. The model was calibrated using approximate Bayesian computation with sequential Monte Carlo.45s We selected parameters to be included in the calibration based on their measurement uncertainty, importance to model behavior, and relevance to our research questions. In early model calibrations, we estimated site-specific screening values, but these values did not substantially deviate from our point estimates. To reach our data targets, we selected other parameters to estimate via calibration that had a larger impact on model behavior, and used priors informed by a literature review where data on specific parameters were scarce.2,8–11 Calibrated parameters included race-specific anal and orogenital sex act rate scalars, race-specific HIV and STI condom failure probabilities, untreated pharyngeal gonorrhea duration, and anal and orogenital sex transmission probabilities. See Appendix for details on all methods and data.
Scenarios
We ran the calibrated model for 60 years (the burn-in period) to reach equilibrium in a population of 10,000 MSM, after which we introduced the different screening strategies (each simulated 128 times) and ran the model for another 10 years. During the burn-in period, site-specific screening remained constant at estimated current levels.
We evaluated the population-level impact of varying the extragenital screening probability from a baseline of 37.5% to 60%, 80%, and 100% (referred to as screening levels) among urogenitally-screened men. The urogenital screening probability was held constant. The comparison was the calibrated model with site-specific screening at estimated current levels. We examined 3 scenarios targeting increased screening at (1) both extragenital sites, (2) only the rectal site, and (3) only the pharyngeal site. These were applied to the entire population. Under each scenario, the extragenital sites remained independent.
Outcomes
Because gonorrhea can infect multiple anatomical sites within the same individual, we calculated outcomes at the level of the infection and the case. Infection-based outcomes counted site-specific infections separately, with each man able to have up to 3 concurrent infections at the urethra, rectum, and/or pharynx. Case-based outcomes measured the overall infection status of an individual, counting multisite infections within the same man once. We calculated infection and case prevalence and incidence per 100 person-years, the proportion of baseline infections averted (PIA) and cases averted (PCA), and the number of screens needed to avert 1 infection (NTAI) and 1 case (NTAC). We measured outcomes at the simulation level with the exception of the NTAI and NTAC. For these, we used bootstrapping to draw 100 samples and, within each sample, calculated the ratio of the mean difference in screens to the mean difference in infections or cases. We generated the 95% confidence interval for the mean ratios across the samples. Outcomes were calculated from all 10 years of each scenario with the exception of prevalence and incidence, which were measured from the last year.
RESULTS
Calibrated Baseline Model
In the calibrated baseline model, the dominant transmission pathway was rectum to urethra (37.11% of weekly transmissions) followed by urethra to pharynx (32.96%), and urethra to rectum (20.39%) (Fig. 2A). There were 73.25% of the weekly prevalent infections that were single site, 26.47% for dual-site, and 0.34% for triple site (Fig. 2B). There were 77.83% for extragenital-only.
Figure 2.
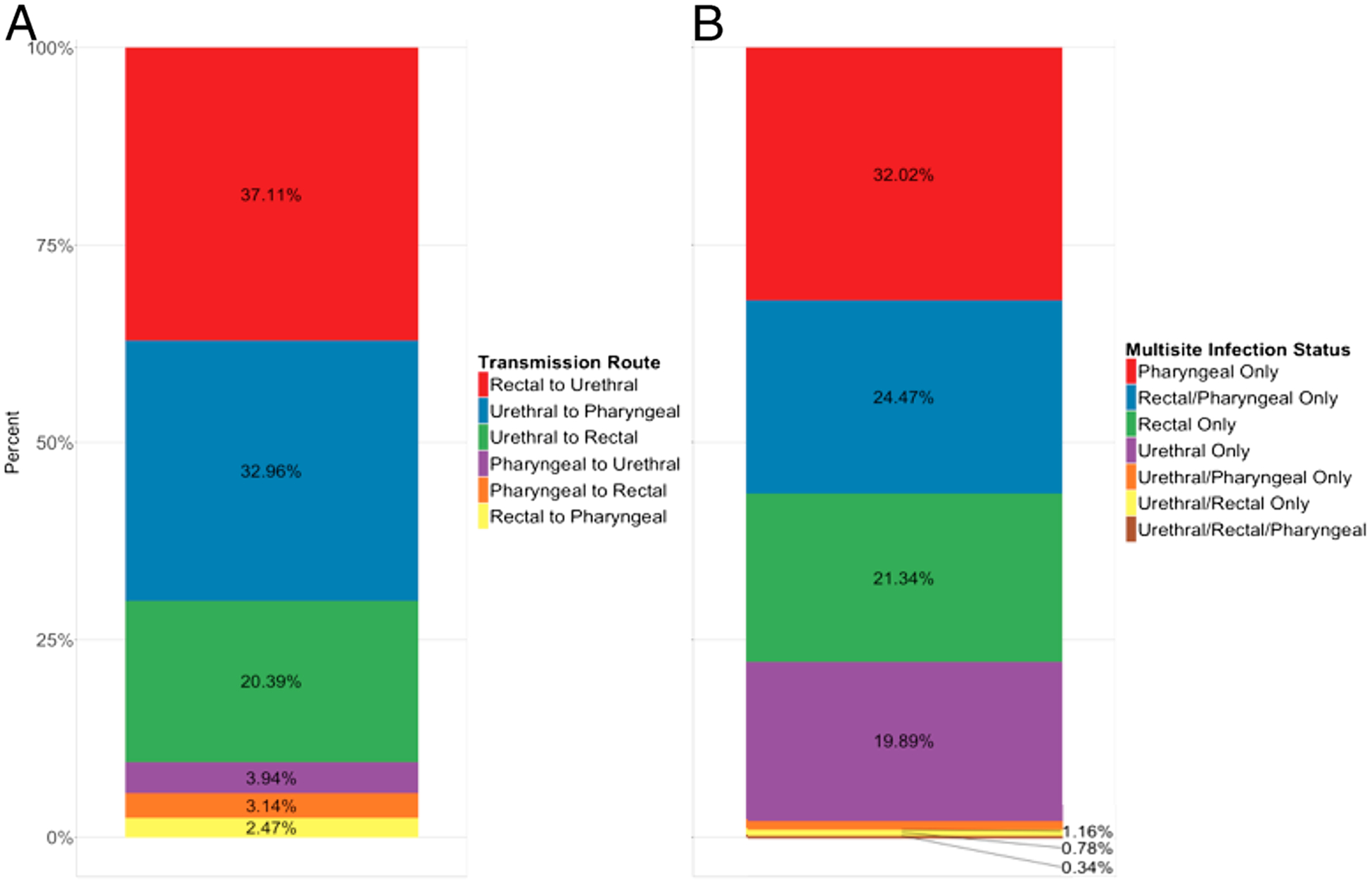
Mean weekly percentage of total transmissions by route (A) and prevalent infections (undiagnosed and diagnosed) by multisite infection status (B) in the calibrated baseline model.
Intervention Scenarios
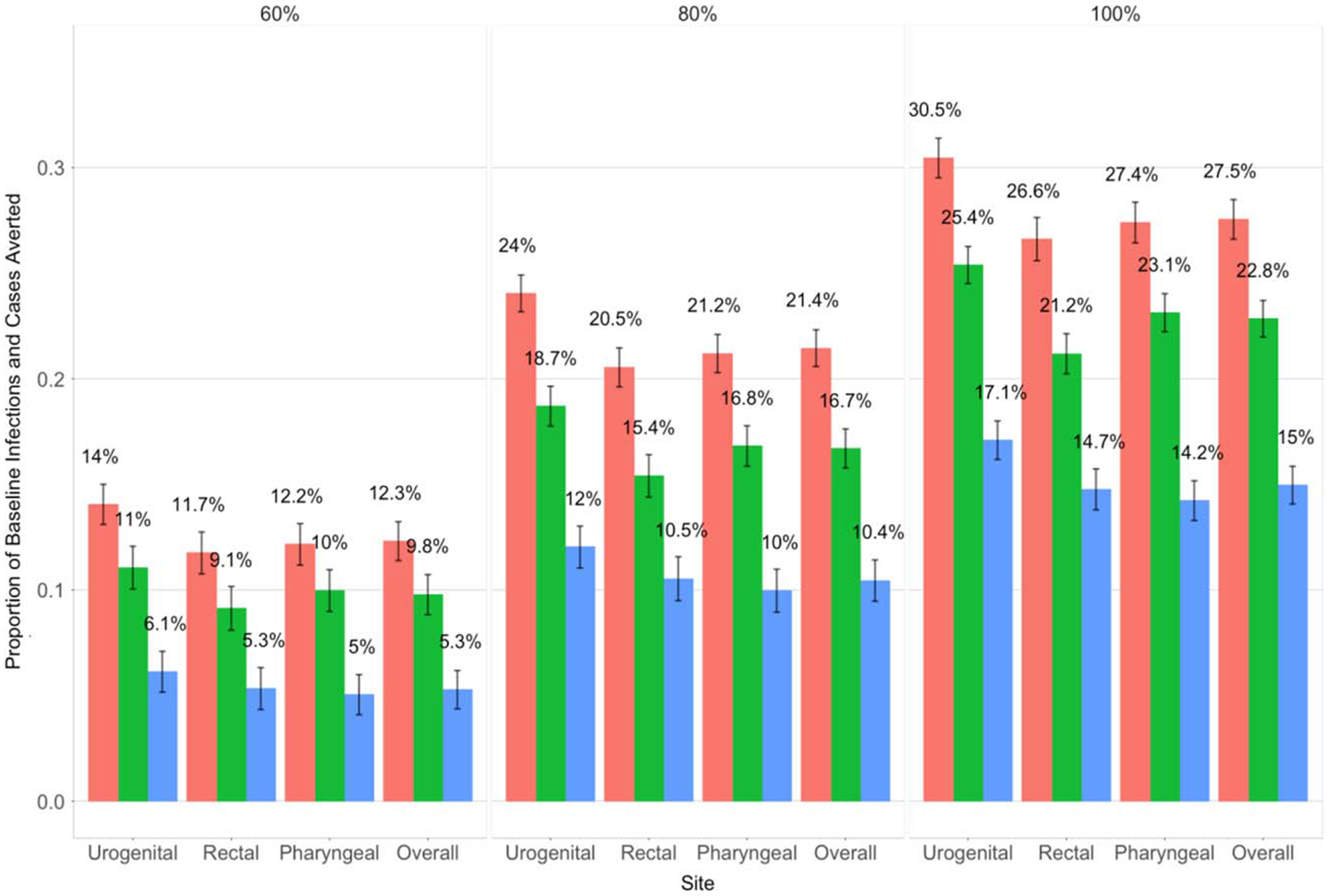
When assessing the impact of the scenarios in terms of decreasing prevalence and incidence and increasing the PIA and PCA, targeting both sites yielded the best results. Table 1 and Table 2 display the prevalence and incidence, respectively, for site-specific infections and overall cases by race, scenario, and screening level. At the 60% screening level, site-specific prevalence was reduced by an average of 18% (BMSM) and 23% (WMSM) when targeting both sites, 14% (BMSM) and 18% (WMSM) when targeting the rectum, and 8% (BMSM) and 11% (WMSM) when targeting the pharynx. At the 100% level, targeting both sites reduced site-specific prevalence by an average of 42% (BMSM) and 50% (WMSM), with these values dropping by approximately 10% and 20% for each race group when targeting only the rectum and only the pharynx, respectively (Table 1). We observed similar percent reductions in case prevalence, infection incidence, and case incidence. The scenario targeting both sites also resulted in the largest PIA and PCA (Fig. 3). For example, at the 100% screening level, the PCA for this scenario was 27.5% compared with 22.8% and 15% when targeting only the rectal and pharyngeal sites, respectively. The urogenital site had the highest PIA for all scenarios and screening levels. The PIA at the rectum and pharynx was relatively similar across scenarios and screening levels. The PIA was higher for WMSM than BMSM at each site (Fig. S-3A and S-3B in Appendix, http://links.lww.com/OLQ/A503).
TABLE 1.
Infection and Case Prevalence by Screening Scenario, Level*, and Race
| Site | Level | (1) Increase Both Rectal/Pharyngeal Screening | (2) Increase Only Rectal Screening | (3) Increase Only Pharyngeal Screening | |||
|---|---|---|---|---|---|---|---|
| Black MSM | White MSM | Black MSM | White MSM | Black MSM | White MSM | ||
| Infections: mean (95% CI) | |||||||
| Urogenital | 37.5%† | 3.0 (3.0–3.1) | 1.4 (1.3–1.4) | 3.0 (3.0–3.1) | 1.4 (1.3–1.4) | 3.0 (3.0–3.1) | 1.4 (1.3–1.4) |
| 60% | 2.5 (2.5–2.6) | 1.1 (1.0–1.1) | 2.6 (2.5–2.7) | 1.1 (1.1–1.2) | 2.8 (2.7–2.8) | 1.2 (1.2–1.3) | |
| 80% | 2.1 (2.0–2.1) | 0.9 (0.8–0.9) | 2.3 (2.2–2.4) | 1.0 (0.9–1.0) | 2.6 (2.5–2.6) | 1.1 (1.1–1.2) | |
| 100% | 1.8 (1.7–1.9) | 0.7 (0.7–0.8) | 2.0 (1.9–2.1) | 0.8 (0.8–0.9) | 2.4 (2.3–2.5) | 1.0 (1.0–1.0) | |
| Rectal | 37.5%† | 6.1 (6.0–6.3) | 3.2 (3.1–3.3) | 6.1 (6.0–6.3) | 3.2 (3.1–3.3) | 6.1 (6.0–6.3) | 3.2 (3.1–3.3) |
| 60% | 5.0 (4.9–5.2) | 2.4 (2.4–2.5) | 5.2 (5.1–5.3) | 2.6 (2.5–2.7) | 5.6 (5.5–5.7) | 2.9 (2.8–2.9) | |
| 80% | 4.1 (4.0–4.2) | 1.9 (1.9–2.0) | 4.6 (4.4–4.7) | 2.1 (2.1–2.2) | 5.2 (5.0–5.3) | 2.6 (2.5–2.7) | |
| 100% | 3.5 (3.4–3.7) | 1.6 (1.5–1.7) | 3.9 (3.8–4.0) | 1.8 (1.8–1.9) | 4.8 (4.7–4.9) | 2.3 (2.2–2.4) | |
| Pharyngeal | 37.5%† | 7.3 (7.1–7.4) | 4.1 (4.0–4.2) | 7.3 (7.1–7.4) | 4.1 (4.0–4.2) | 7.3 (7.1–7.4) | 4.1 (4.0–4.2) |
| 60% | 6.0 (5.9–6.2) | 3.2 (3.1–3.3) | 6.3 (6.2–6.5) | 3.5 (3.4–3.6) | 6.6 (6.4–6.7) | 3.7 (3.6–3.8) | |
| 80% | 4.9 (4.8–5.0) | 2.5 (2.5–2.6) | 5.6 (5.5–5.8) | 2.9 (2.8–3.0) | 6.0 (5.9–6.1) | 3.3 (3.2–3.4) | |
| 100% | 4.2 (4.1–4.4) | 2.1 (2.0–2.2) | 4.9 (4.7–5.0) | 2.6 (2.5–2.6) | 5.5 (5.4–5.7) | 2.9 (2.8–3.0) | |
| Cases: mean (95% CI) | |||||||
| Overall | 37.5% b | 12.8 (12.5–13.0) | 7.0 (6.9–7.2) | 12.8 (12.5–13.0) | 7.0 (6.9–7.2) | 12.8 (12.5–13.0) | 7.0 (6.9–7.2) |
| 60% | 10.6 (10.3–10.8) | 5.4 (5.3–5.6) | 11.0 (10.7–11.2) | 5.9 (5.7–6.0) | 11.6 (11.3–11.8) | 6.3 (6.1–6.4) | |
| 80% | 8.6 (8.4–8.9) | 4.3 (4.2–4.5) | 9.7 (9.5–10.0) | 4.9 (4.8–5.0) | 10.7 (10.4–10.9) | 5.7 (5.5–5.8) | |
| 100% | 7.4 (7.2–7.7) | 3.6 (3.5–3.7) | 8.4 (8.2–8.6) | 4.2 (4.1–4.4) | 9.9 (9.6–10.1) | 5.0 (4.9–5.2) | |
Extragenital screening level among men already being urogenitally screened.
37.5% is the baseline screening level.
TABLE 2.
Infection and Case Incidence Per 100 Person-Years by Screening Scenario, Level*, and Race
| Site | Level | (1) Increase Both Rectal/Pharyngeal Screening | (2) Increase Only Rectal Screening | (3) Increase Only Pharyngeal Screening | |||
|---|---|---|---|---|---|---|---|
| Black MSM | White MSM | Black MSM | White MSM | Black MSM | White MSM | ||
| Infections: mean (95% CI) | |||||||
| Urogenital | 37.5%† | 23.7 (23.1–24.3) | 10.6 (10.4–10.9) | 23.7 (23.1–24.3) | 10.6 (10.4–10.9) | 23.7 (23.1–24.3) | 10.6 (10.4–10.9) |
| 60% | 19.5 (19.0–19.9) | 8.2 (8.0–8.4) | 20.2 (19.8–20.7) | 8.8 (8.5–9.0) | 21.6 (21.0–22.1) | 9.5 (9.3–9.8) | |
| 80% | 16.0 (15.5–16.4) | 6.5 (6.2–6.7) | 17.7 (17.2–18.3) | 7.4 (7.2–7.6) | 20.0 (19.5–20.5) | 8.6 (8.4–8.9) | |
| 100% | 13.8 (13.3–14.4) | 5.4 (5.2–5.6) | 15.2 (14.7–15.7) | 6.3 (6.1–6.5) | 18.4 (17.9–18.9) | 7.7 (7.5–8.0) | |
| Rectal | 37.5%† | 13.6 (13.2–13.9) | 6.6 (6.5–6.8) | 13.6 (13.2–13.9) | 6.6 (6.5–6.8) | 13.6 (13.2–13.9) | 6.6 (6.5–6.8) |
| 60% | 11.5 (11.2–11.7) | 5.3 (5.1–5.4) | 11.8 (11.5–12.1) | 5.7 (5.5–5.8) | 12.5 (12.2–12.9) | 6.1 (5.9–6.3) | |
| 80% | 9.4 (9.2–9.7) | 4.3 (4.1–4.4) | 10.5 (10.2–10.9) | 4.8 (4.7–5.0) | 11.6 (11.3–11.9) | 5.5 (5.3–5.6) | |
| 100% | 8.3 (7.9–8.6) | 3.6 (3.5–3.8) | 9.2 (8.9–9.5) | 4.2 (4.0–4.3) | 10.9 (10.6–11.2) | 5.0 (4.8–5.1) | |
| Pharyngeal | 37.5%† | 20.1 (19.6–20.6) | 10.7 (10.4–11.0) | 20.1 (19.6–20.6) | 10.7 (10.4–11.0) | 20.1 (19.6–20.6) | 10.7 (10.4–11.0) |
| 60% | 16.8 (16.4–17.2) | 8.5 (8.2–8.7) | 17.2 (16.8–17.6) | 9.0 (8.8–9.3) | 18.4 (18.0–18.9) | 9.8 (9.5–10.1) | |
| 80% | 13.8 (13.4–14.2) | 6.8 (6.6–7.0) | 15.3 (14.8–15.7) | 7.6 (7.3–7.8) | 17.1 (16.7–17.6) | 8.9 (8.7–9.2) | |
| 100% | 12.0 (11.5–12.4) | 5.7 (5.5–6.0) | 13.2 (12.8–13.6) | 6.6 (6.3–6.8) | 15.9 (15.5–16.4) | 8.1 (7.8–8.3) | |
| Cases: Mean (95% CI) | |||||||
| Overall | 37.5%† | 41.5 (40.5–42.5) | 20.5 (20.0–21.1) | 41.5 (40.5–42.5) | 20.5 (20.0–21.1) | 41.5 (40.5–42.5) | 20.5 (20.0–21.1) |
| 60% | 34.2 (33.3–35.1) | 16.1 (15.6–16.5) | 35.4 (34.5–36.2) | 17.1 (16.7–17.6) | 37.7 (36.8–38.7) | 18.6 (18.1–19.1) | |
| 80% | 28.0 (27.2–28.8) | 12.8 (12.4–13.2) | 31.2 (30.2–32.2) | 14.4 (14.0–14.9) | 35.0 (34.2–35.9) | 16.8 (16.4–17.3) | |
| 100% | 24.3 (23.4–25.2) | 10.8 (10.4–11.3) | 26.8 (26.0–27.6) | 12.4 (12.0–12.9) | 32.3 (31.4–33.2) | 15.2 (14.7–15.6) | |
Extragenital screening level among men already being urogenitally screened.
37.5% is the baseline screening level.
Figure 3.
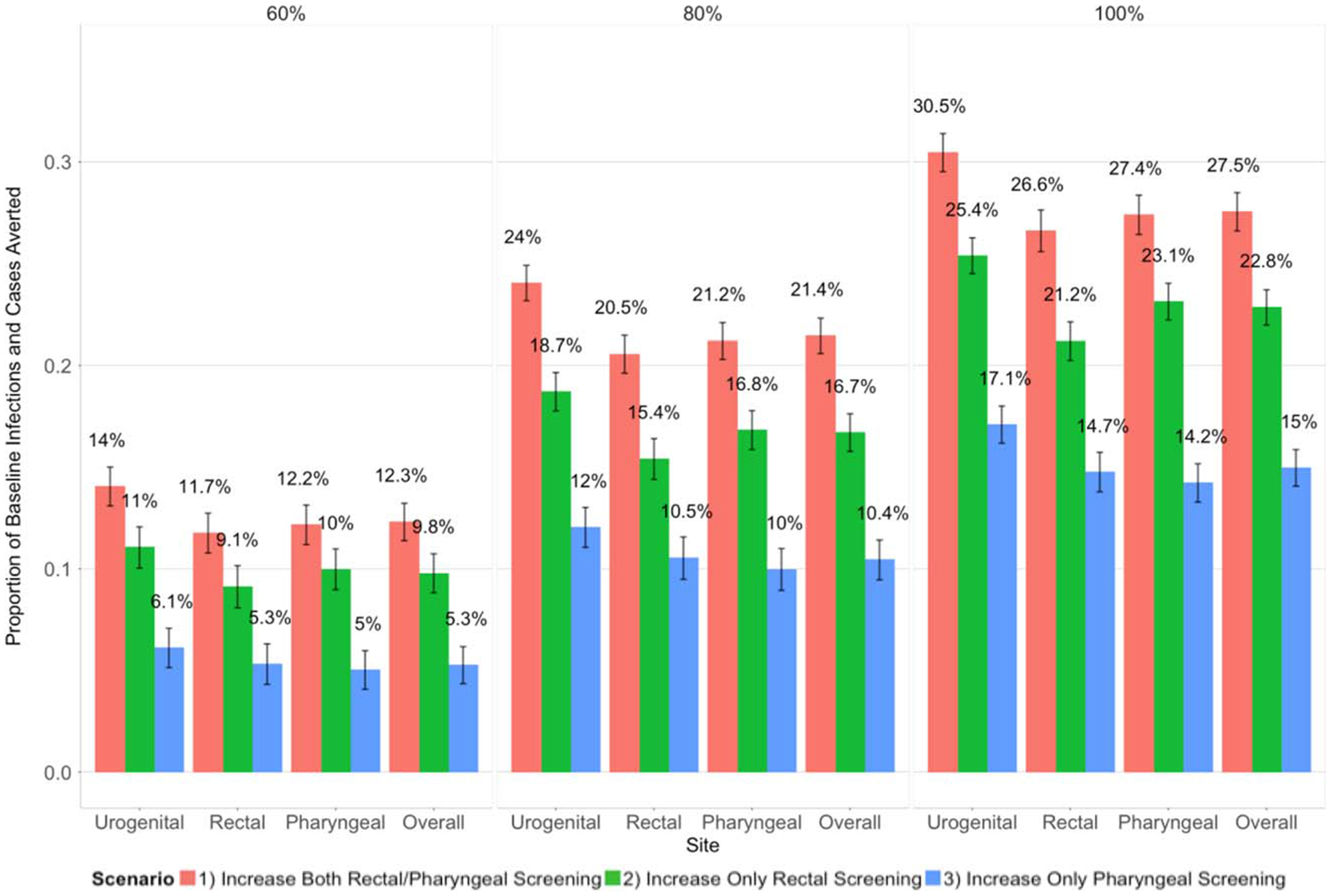
Proportion of baseline site-specific infections (urogenital, rectal and pharyngeal labels) and overall cases (overall label) averted over 10 years (mean and 95% confidence intervals) as the extragenital screening probability among men being urogenitally screened was increased from 37.5% (baseline) to 60%, 80%, and 100%.
We also evaluated scenario efficiency by assessing NTAI and NTAC. Increasing only rectal screening was the most efficient strategy, with the lowest mean NTAI and NTAC at each screening level for both race groups (Figs. 4 and 5). The comparative efficiency of the remaining scenarios varied by screening level. In general, at lower screening levels, targeting both sites had a lower NTAI and NTAC than targeting only the pharynx. For example, at the 60% screening level, targeting both sites yielded a NTAI of 3.47 (BMSM) and 5.6 (WMSM), whereas targeting only the pharynx had a NTAI of 4.18 (BMSM) and 6.87 (WMSM) (Fig. 4). As screening levels increased to 100%, this relationship flipped, and targeting only pharyngeal screening became more efficient for both race groups. Increasing screening levels generally yielded a higher NTAI and NTAC, with the scenario targeting the pharynx as the exception. In this case, the lowest NTAI and NTAC were at the 80% and 100% screening levels, although differences between screening levels for WMSM were negligible. Across all scenarios and screening levels, there was a lower NTAI and NTAC for BMSM versus WMSM.
Figure 4.
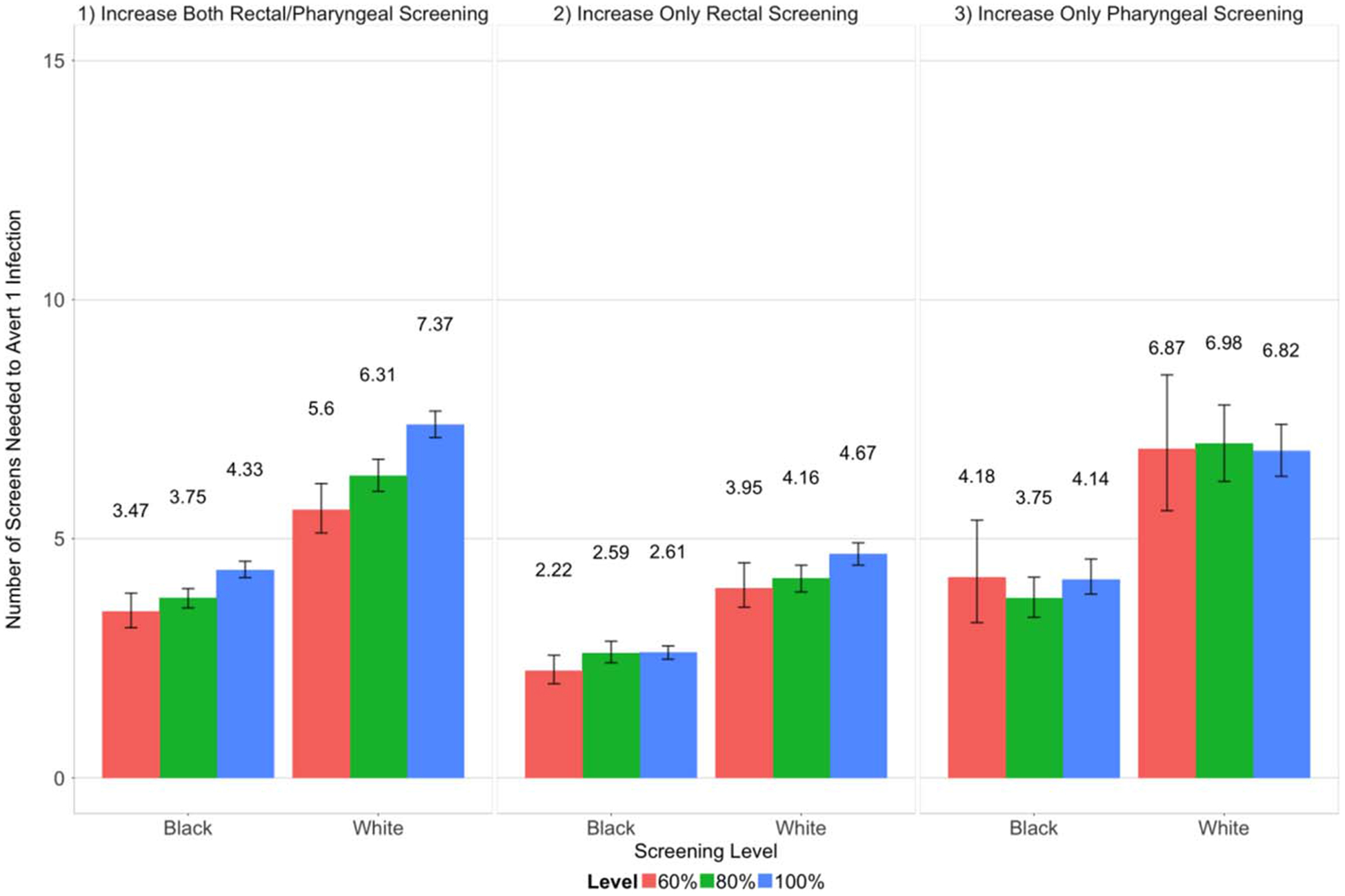
Number of screens needed to avert 1 infection (mean and 95% confidence intervals) as the extragenital screening probability among men being urogenitally screened was increased from 37.5% (baseline) to 60%, 80%, and 100%.
Figure 5.
Number of screens needed to avert 1 case (mean and 95% confidence intervals) as the extragenital screening probability among men being urogenitally screened was increased from 37.5% (baseline) to 60%, 80%, and 100%.
DISCUSSION
Most infections were extragenital-only, reflecting the high prevalence of extragenital gonorrhea, its asymptomatic nature, and lower screening rates at extragenital sites. This means that many extragenital infections would be missed without targeted screening. Multisite infection with the urethra was rare because urogenital infections were mostly symptomatic, leading men to promptly seek care. This (1) reduced the likelihood of men acquiring an extragenital infection while urethrally infected and (2) incidentally cleared any undetected extragenital coinfections, minimizing the prevalence of multisite infection with the urethra.
In our analysis, targeting both sites had the greatest impact because it directly cleared the most single-site extragenital infections. Targeting the rectal versus pharyngeal site was more effective because it reduced transmission from the rectum to urethra, and then outward from the urethra—all major transmission pathways. In contrast, outward transmission from the pharynx had relatively low transmission probabilities. Extragenital screening particularly affected the urogenital site because detecting and treating more rectal infections diminished the rectum to urethra pathway, which was responsible for the highest percentage of weekly transmissions. Even when increasing pharyngeal screening while holding rectal screening constant, we still observed a larger PIA and PCA at the rectum versus pharynx. Due to high levels of weekly prevalent rectal/pharyngeal multisite infection, detecting and treating more pharyngeal infections incidentally cleared more rectal infections in multisite infected MSM.
Targeting only the rectal site was the most efficient scenario because (1) screening both sites was often duplicative because rectal/pharyngeal coinfections were common and (2) screening only the pharynx averted far fewer onward transmissions. This scenario avoided these issues while also clearing many single-site rectal infections and multisite rectal/pharyngeal infections, resulting in relatively small NTAI and NTAC increases with higher screening levels. Comparing scenarios targeting both sites versus only the pharynx, the former was more efficient at lower screening levels, and the latter at higher levels. This suggests a threshold point at which the benefit from screening both sites began to be outpaced by duplicative screens in the case of multisite rectal/pharyngeal infection. Lower NTAI and NTAC for BMSM versus WMSM likely reflects the higher site-specific prevalence among BMSM. Under the scenario targeting the pharynx, the optimal screening level was 80% for BMSM and 100% for WMSM, meaning that more infections and cases were averted with the increase in screening compared with the 60% level.
There are knowledge gaps around site-specific infection, and results should be interpreted through the lens of our estimated parameters. Site-specific screening data were very limited, and a recent model using infection duration and sex act rates could not determine the relative transmission importance of different sites.33s Increasing screening at 1 extragenital site might not be done in isolation. For example, greater provider awareness of the benefits of rectal screening could result in a concurrent increase in pharyngeal screening, yielding a higher NTAI and NTAC than we observed. Screening may vary by HIV status or race, which we did not explore due to a lack of reliable data. We assumed that urogenitally screened men were equally likely to be extragenitally screened, and did not incorporate reporting behavior. Our burn-in model reproduced most of our calibration targets to within their 95% confidence intervals, but we were unable to reproduce 4 targets by a small margin. This is likely due to the limited data and complex interdependencies of calibrating a site-specific agent-based model. Our calibration data were sourced from separate studies due to data availability issues,36s, 38s–40s meaning that pharyngeal prevalence and incidence could differ from our estimations. Calibration would improve with site- and race-specific prevalence and incidence data measured within a single study. We did not find MSM network studies that collected orogenital and ororectal sex behavioral data in addition to anal sex. We modified our anal sex network to include orogenital and ororectal sex using scalars and assumptions, but directly measured data would yield stronger estimates. Our scenarios were universally administered, whereas in practice they would likely target higher-risk groups. However, our network was relatively high-risk due to the inclusion criteria of the study providing our data (men had to have at least 1 male sex partner in the past 3 months and be non-monogamous)40s and the CDC recommends annual screening of MSM at each anatomical site of reported exposure.4 We did not account for antibiotic resistance, which could eventually change infection dynamics. In our data sample, BMSM had significantly lower risk behaviors compared with WMSM but much higher HIV and gonorrhea prevalence and incidence,36s making it challenging to reproduce these observed disparities. However, we were able to do so by using priors for the act rate scalars and condom failure probability parameters based on their 95% confidence intervals provided in other studies using the same data.36s Multiple articles have explored the reasons for these persistent disparities, assessing factors such as differences in network structure, socioeconomic status, and access to care.35s, 36s, 40s
Our analysis showed that targeting both extragenital sites had the greatest overall impact. It also raised the question of whether rectal-only screening among MSM could be more efficient than triple-site screening given that (1) male urogenital infections are mostly detected via symptomatic testing and (2) pharyngeal infections may not substantially contribute to onward transmissions. This could reduce the burden of symptomatic infection and dampen overall transmission, which will become increasingly important with spreading antibiotic resistance.28 However, careful assessment of the risks of missing resistant pharyngeal infections will be needed. Future analyses could assess this strategy’s potential, the economic impact of increased extragenital screening, and the cost-effectiveness of targeted versus universally applied interventions.
Supplementary Material
Acknowledgments:
The authors thank Samuel Jenness for his substantial guidance in adapting the EpiModelHIV platform for use in this model. The computations in this article were run on the Odyssey cluster supported by the FAS Division of Science, Research Computing Group at Harvard University.
Sources of Funding: This work was supported by the U.S. Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (5NU38PS004644).
Footnotes
Conflict of Interest: none declared.
The findings and conclusions presented in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the authors’ other affiliated institutions.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.CDC, 2018 Sexually Transmitted Diseases Surveillance. 2019.
- 2.Whittles LK, Didelot X, Grad YH, et al. Testing for gonorrhoea should routinely Owusu-Eduseiinclude the pharynx. Lancet Infect Dis 2018; 18:716–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronn MM, Testa C, Tuite AR, et al. The potential population-level impact of different gonorrhea screening strategies in Baltimore and San Francisco: An exploratory mathematical modeling analysis. Sex Transm Dis 2020; 47:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workowski KA, Bolan GA, et alCenters for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015. 64(RR-03): p. 1–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Beck EC, Birkett M, Armbruster B, et al. A data-driven simulation of HIV spread among young men who have sex with men: Role of age and race mixing and STIs. J Acquir Immune Defic Syndr 2015; 70:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes KK, Johnson DW, Trostle HJ. An estimate of the risk of men acquiring gonorrhea by sexual contact with infected females. Am J Epidemiol 1970; 91:170–174. [DOI] [PubMed] [Google Scholar]
- 7.Hooper RR, Reynolds GH, Jones OG, et al. Cohort study of venereal disease. I: The risk of gonorrhea transmission from infected women to men. Am J Epidemiol 1978; 108:136–144. [DOI] [PubMed] [Google Scholar]
- 8.Hui B, Fairley CK, Chen M, et al. Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: A mathematical model. Sex Transm Infect 2015; 91:365–369. [DOI] [PubMed] [Google Scholar]
- 9.Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: A modeling study. Clin Infect Dis 2017; 65:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Regan DG, Chow EPF, et al. Neisseria gonorrhoeae transmission among men who have sex with men: An anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash. Sex Transm Dis 2017; 44:586–592. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein KT, Chesson H, Kirkcaldy RD, et al. Kiss and tell: Limited empirical data on oropharyngeal Neisseria gonorrhoeae among men who have sex with men and implications for modeling. Sex Transm Dis 2017; 44:596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow EP, Lee D, Tabrizi SN, et al. Detection of Neisseria gonorrhoeae in the pharynx and saliva: Implications for gonorrhoea transmission. Sex Transm Infect 2016; 92:347–349. [DOI] [PubMed] [Google Scholar]
- 13.Fairley CK, Hocking JS, Zhang L, et al. Frequent transmission of gonorrhea in men who have sex with men. Emerg Infect Dis 2017; 23:102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesner PJ, Tronca E, Bonin P, et al. Clinical spectrum of pharyngeal gonococcal infection. N Engl J Med 1973; 288:181–185. [DOI] [PubMed] [Google Scholar]
- 15.Willmott FE. Transfer of gonococcal pharyngitis by kissing? Br J Vener Dis 1974; 50:317–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page-Shafer K, Graves A, Kent C, et al. Increased sensitivity of DNA amplification testing for the detection of pharyngeal gonorrhea in men who have sex with men. Clin Infect Dis 2002; 34:173–176. [DOI] [PubMed] [Google Scholar]
- 17.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 2005; 41:67–74. [DOI] [PubMed] [Google Scholar]
- 18.Johnson Jones ML, Chapin-Bardales J, Bizune D, et al. Extragenital chlamydia and gonorrhea among community venue-attending men who have sex with men—five cities, United States, 2017. MMWR Morb Mortal Wkly Rep 2019; 68:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men—STD surveillance network, United States, 2010–2012. Clin Infect Dis 2014; 58:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbee LA, Tat S, Dhanireddy S, et al. Implementation and operational research: Effectiveness and patient acceptability of a sexually transmitted infection self-testing program in an HIV care setting. J Acquir Immune Defic Syndr 2016; 72:e26–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein KT. Systems approaches to improving rates of extragenital chlamydia and gonorrhea screening among men who have sex with men engaged in human immunodeficiency virus care. Sex Transm Dis 2015; 42:599–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunn RA, O’Brien CJ, Lee MA, et al. Gonorrhea screening among men who have sex with men: Value of multiple anatomic site testing, San Diego, California, 1997–2003. Sex Transm Dis 2008; 35:845–848. [DOI] [PubMed] [Google Scholar]
- 23.Lutz AR. Screening for asymptomatic extragenital gonorrhea and chlamydia in men who have sex with men: significance, recommendations, and options for overcoming barriers to testing. LGBT Health 2015; 2:27–34. [DOI] [PubMed] [Google Scholar]
- 24.Patel MR, Brooks JT, Tie Y, et al. Prevalence of gonorrhea and chlamydia testing by anatomical site among men who have sex with men in HIV medical care, United States, 2013–2014. Sex Transm Dis 2018; 45:25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss KM, Jones JS, Anderson EJ, et al. Optimizing coverage vs frequency for sexually transmitted infection screening of men who have sex with men. Open Forum Infect Dis 2019; 6:ofz405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA. FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea [press release]. 2019. [DOI] [PubMed]
- 27.Barbee LA, Dhanireddy S, Tat SA, et al. Barriers to bacterial sexually transmitted infection testing of HIV-infected men who have sex with men engaged in HIV primary care. Sex Transm Dis 2015; 42:590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiley DM, Jennison A, Pearson J, et al. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 2018; 18:717–718. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017. Atlanta: U.S: Department of Health and Human Services, 2018. [Google Scholar]
- 30.Buyze J, Vanden Berghe W, Hens N, et al. Current levels of gonorrhoea screening in MSM in Belgium may have little effect on prevalence: A modelling study. Epidemiol Infect 2018; 146:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


