Abstract
Background
As the use of herbal medicines increased worldwide, there has been concern about the risk of herb-induced liver injury (HILI). Many clinical studies have assessed the risk of HILI in Korea.
Methods
Therefore, we conducted a meta-analysis of the incidence of HILI in Korea, by analyzing nine clinical studies. These involved 8625 participants (3274 males; 5351 females), including 436 outpatients (three studies) and 8189 inpatients (six studies).
Results
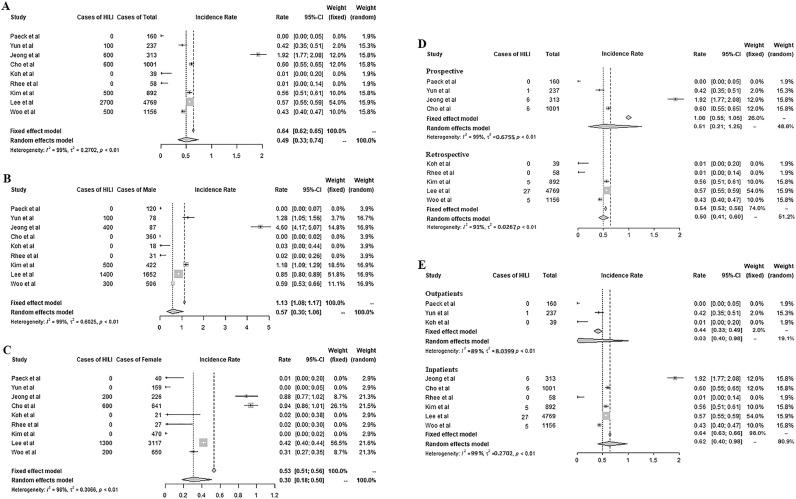
As a result, the overall incidence of HILI in Korea was 0.49% (95% CI 0.33–0.74%), and it was 0.57% in males and 0.30% in females. We found a similar incidence of HILI in prospective (0.51%) and retrospective (0.50%) studies. The incidence of HILI was higher in inpatients (0.62%) than outpatients (0.03%).
Conclusion
Although there are limitations regarding study quality and the number of participants, we systematically estimated the risk of HILI in Korea. We anticipate this study would be a helpful information for prescribing herbal medicines and researching the safety of herbs.
Keywords: Herb-induced liver injury, Meta-analysis, Korea
Adverse drug reactions (ADRs) are very common and are experienced by approximately 10% of patients treated in hospitals in Europe.1 Among ADRs, drug-induced liver injury (DILI) is the most important in terms of drug safety and is a major reason for acute liver failure in the US.2
As more people use herbal products worldwide, there has been concern about the risk of herb-induced liver injury (HILI).3 The role of herbal drugs in DILI has been controversial in China and Korea, where herbal remedies are very popular. Many clinical studies have assessed the risk of HILI in Korea. Therefore, we conducted a meta-analysis of the incidence of hepatotoxicity due to herbal drugs. Using domestic (KMBASE, https://kmbase.medric.or.kr/) and international (PubMed) databases, we surveyed publications investigating the incidence of HILI. Ultimately, nine studies (four prospective and five retrospective) were identified, including three reports in which 2006 was the first year (Fig. 1). These involved 8625 participants (3274 males; 5351 females), including 436 outpatients (three studies) and 8189 inpatients (six studies).
Fig. 1.
Incidence rate of HILI. Total incidence rate (A), in male (B), in female (C), by methods (D), and by subjects (E) are presented.
Meta-analysis by random-effects model was conducted to provide point estimates (95% CI) of prevalence with subgroup analysis to account for heterogeneity. To account for the potentially high inter-study heterogeneity, the pooled outcome measures and their corresponding 95% confidence intervals were calculated using a random-effects model fitted with the restricted maximum-likelihood estimator. The I2 statistic was used to evaluate the degree of heterogeneity between studies. Statistical analyses were performed using the "meta" packages (by Guido Schwarzer) in R (Ver. 4.0.2) & R Studio (Ver. 1.3.1073) software.
The overall incidence of HILI in Korea was 0.49% (95% CI 0.33–0.74%), and it was 0.57% in males and 0.30% in females (Fig. 1A–C). The incidence of ADRs including DILI can differ markedly depending on the country and study conditions. For example, the incidence of ADRs appears to be higher in prospective studies than in retrospective studies.4 However, we found a similar incidence of HILI in prospective (0.51%) and retrospective (0.50%) studies (Fig. 1D). As expected, the incidence of HILI was higher in inpatients (0.62%) than outpatients (0.03%) (Fig. 1E). This is a general tendency in other studies including our previous study, which analyzed hepatic ADRs in 6193 participants in 99 RCTs of herbal preparations and found an 0.08% incidence of HILI, which is comparable to our current finding for outpatients (0.03%).5 Regarding gender, females are generally believed to be more sensitive to DILI.6 However, there are conflicting data suggesting that in elder adults, HILI predominates in males (37.5%) and not females (10.5%),7 similar to our results for HILI (0.57% vs. 0.30%) (Fig. 1B and C). This HILI outcome (0.62% in inpatients) seems to be at the lower level of DILI due to conventional drugs among inpatients (0.7% to 1.4%).8,9
The medical issues regarding the safety of herbal drugs include calculating the risks of HILI, the clinical characteristics of HILI, listing the causative herbs, and exploring genetic susceptibility.10 Although there are limitations regarding study quality and the number of participants, we systematically estimated the risk of HILI in Korea. This information is important both for practices regarding herbal prescriptions in clinics and research on the safety of herbs.
Acknowledgments
Acknowledgement
The authors would like to thank Daejeon University (2020) for supporting this research.
Author contributions
Conceptualization: CG Son. Methodology: NH Lee. Software: YC Ahn. Validation: JH Cho. Formal Analysis: NH Lee. Investigation: NH Lee. Resources: GY Lee, CR Park, and SK Kim. Data Curation: YC Ahn. Writing the original draft: NH Lee. Writing the review & editing: GY Lee, CR Park, and SK Kim. Visualization: JH Cho. Supervision: CG Son. Project Administration: CG Son. Funding acquisition: CG Son.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This study was supported by Daejeon University (2020).
Ethical statement
Not applicable for this manuscript as this work did not involve human subjects or laboratory animals.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2020.100705.
Appendix. Supplementary materials
References
- 1.Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in europe: a review of recent observational studies. Drug Saf. 2015;38(5):437–453. doi: 10.1007/s40264-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byeon JH, Kil JH, YCh Ahn, Son CG. Systematic review of published data on herb induced liver injury. J Ethnopharmacol. 2019;233:190–196. doi: 10.1016/j.jep.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33(1):155–164. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Jun SA, Hong SS, Ahn YC, Lee DS, Son CG. Systematic review of adverse effects from herbal drugs reported in randomized controlled trials. Phytother Res. 2016;30(9):1412–1419. doi: 10.1002/ptr.5647. [DOI] [PubMed] [Google Scholar]
- 6.Medina-Caliz I, Robles-Diaz M, Garcia-Muñoz B. Spanish DILI registry. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol. 2016;65:532–542. doi: 10.1016/j.jhep.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin NH, Yang HW, Su YJ. Herb induced liver injury after using herbal medicine: a systemic review and case-control study. Med Baltim. 2019;98(13):e14992. doi: 10.1097/MD.0000000000014992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier Y, Cavallaro M, Roos M. Incidence of drug-induced liver injury in medical inpatients. Eur J Clin Pharmacol. 2005;61:135–143. doi: 10.1007/s00228-004-0888-z. [DOI] [PubMed] [Google Scholar]
- 9.Bagheri H, Michel F, Lapeyre-Mestre M. Detection and incidence of drug induced liver injuries in hospital: a prospective analysis from laboratory signals. Br J Clin Pharmacol. 2000;50:479–484. doi: 10.1046/j.1365-2125.2000.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haller CA, Dyer JE, Ko R, Olson KR. Making a diagnosis of herbal-related toxic hepatitis. West J Med. 2002;176(1):39–44. doi: 10.1136/ewjm.176.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.