Abstract
The main aim of the paper was to determine bioactive compounds in Pleione maculata extracts using gas chromatographic technique and to investigate their drug-likeness potential using molecular docking algorithm and ADME studies on the recent intractable disease, for example, SARS-CoV-2. Pleione maculata sample was prepared for GC–MS analysis. The peak components were identified based on the NIST Library. Molecular docking was performed using PatchDock, and energy refinement was carried out using the FireDock algorithm followed by drug-likeness analysis using the SwissADME tool. The mass spectrum revealed various pharmacologically important compounds and novel compounds 8-oxatetracyclo{5.2.1.1(2,6). 1(4,10)}dodecane, 7-tert-butyl-1,9,9-trimeth, docosane, 2,4-dimethyl, kryptogenin 2,4-dinitrophenyl hydrazine, and N-decyl-alpha,D-2-deoxyglycoside which are reported for the first time. Molecular docking using PatchDock illustrates GC–MS compounds Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy a good docking and high binding affinity with atomic contact energy -10.95 kcal/mol against SARS-CoV-2 spike protein S2 subunit. ADME analysis predicts Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy and andrographolide showed very high drug-likeness parameters with no metabolism disturbances. The random control antiviral drug arabidiol revealed a lower binding affinity and lower solubility compared to bioactive compounds of P. maculata. The study depicts the first and novel report on various pharmaceutical important GC–MS bioactive compounds and molecular docking study on Pleione maculata having potential against various intractable diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13721-020-00276-1.
Keywords: Gas chromatography, SARS-CoV-2, Pleione maculata, Molecular docking, Drug-likeness
Introduction
A worldwide viral outbreak of dreadful disease COVID-19 arose during December 2019 in Wuhan, China (Yang and Wang 2020; Yang et al. 2020). Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) as named by the International Committee on Taxonomy of Viruses (ICTV) and diseases cause was coronavirus disease 2019 (COVID-19). COVID-19 is no new viruses but a possible mutation of the long-known SARS-CoV-1. A genome variation analysis was analyzed using a detective genome computational tool, and it revealed SARS-CoV-2 shares 80% similarities with the gene pool of SARS-CoV-1 (Zhang et al. 2017) with nearly 17% variation which was a mutation occurring in spike protein and envelope protein (Sardar et al. 2020). The viral genome analysis of the SARS-CoV-1 open reading frame (orf) was observed to have a potential mutation to adapt to a new environment (Groneberg et al. 2005) and recombination (Li Fang 2016) which might cause severe virulence of the virus. The severe acute respiratory syndrome (SARS) is a crown-like virus which was spread widely in late 2000 over 25 countries causing thousands of cases and death (Wen et al. 2011). Since 2003, antiviral research has been evaluated for an anti-SARS-COV-1 activity to prevent re-emergence of the disease. The genome of SARS-CoV-1 encodes various vital target proteins: spike protein (S), 3CL protease, NTPase/helicase, RNA-dependent RNA polymerase, membrane protein (M), an envelope protein (E), and nucleocapsid phosphoprotein (N) which takes part in virus replication, transcription, and translation (Yang and Wang 2020). The main molecule that mediates coronavirus entry into a host cell is their spike protein (S) which is multi-functional (Fang 2016), where the attachment is initiated by the S1 subunit and conformation changes took place from pre-fusion to post-fusion or membrane fusion form. The virus incorporation is initiated by the S2 subunit of spike glycoprotein through membrane fusion with the host receptor (Rane et al. 2020). The viral 3-chymotrypsin-like protease responsible for replication complex (Anand et al. 2003) is considered highly conserved between SARS-CoV-1 and SARS-CoV-2 (Zhang et al. 2017; Donald and Hai-Feng 2020). The SARS-CoV-1 spike protein has a strong binding affinity towards human receptor angiotensin-converting enzyme 2 (ACE2) based on structure and interaction (Zhang et al. 2020a, b). The main agent for transmission is through respiratory droplets and can be transmitted from human to human through contact with droplets (Yang and Wang 2020). For early diagnosis of SARS-CoV-1, improved RT-PCR assays were carried out which is used worldwide for virus identification and its high specificity (Shen et al. 2020). The symptoms of SARS-CoV-1 were persistent fever, chills, dry cough, dizziness, headache, sore throat, sputum production, vomiting, and nausea; special attention was given for watery diarrhea, but the primary target for infection was respiratory epithelial cells. The viral effect immune-mediated mechanism and molecular studies showed epithelial cells of the gastrointestinal tract to be major target cells (Groneberg et al. 2020). In 2019, various health authorities of Hubei Province, China, reported novel COVID-19 disease as pneumonia (Wu et al. 2020) or COVID-19 pneumonia (Tian et al. 2020). Pneumonia is a type of fatal respiratory tract infection that is caused by either bacteria (Streptococcus pneumonia) or viruses, and symptoms are no different from the deadly virus SARS-CoV-2 such as high fever, shortness of breath, rapid breathing, and cough (Zafar 2016). Earlier, SARS-CoV-1 patients were treated with anti-inflammatory steroidal compounds such as methylprednisolone (Groneberg et al. 2005; Wu et al. 2017), and metabolite profiling for SARS-CoV-1 survivors was carried out after 12 years of recovery using ultra-high-performance liquid chromatography–mass spectrometry (UPLC-MS) and gas chromatography–mass spectrometry (GC–MS); a significant portion (64%) of recovered patients were prone to lung infections and various serum metabolic disorders associated with lipid metabolism including hyperlipidemia (HL), cardiovascular abnormality (CVA), and an abnormality in glucose metabolism (AGM). Single-stranded antisense as an antiviral compound has been a vital therapeutic area for emerging viruses (Gulam et al. 2016). Antisense therapy (antisense antivirals) treats diseases using single-stranded antisense oligonucleotides to target specific mRNA sequences and block translation of viral protein (Gulam et al. 2016) or modifies protein expression (Sharad 2019). To date, there are a lot of controversies regarding vaccine and drug development against SARS-CoV-2 pneumonia, though various antiviral drugs are being used for treatment there appeared to be many disadvantages and side effects caused. The transmission from person to person is highly contagious due to inadequate global healthcare facilities (Panda et al. 2020). Plants are the main source of natural medicines as they produce various biologically active secondary metabolites. Secondary metabolites provide nutritional and beneficial effects on human health (Lakshmi and Rajalakshmi 2011). According to World Health Organization (WHO), about 80–90% population relies on traditionally prepared medicinal plants for regular health care as it is safe and ready to use (Rizvi and Misra 2013; Ekor 2014). Orchids are one large kingdom of plants that are overexploited and also climatic changes; their illegal trading led to extinction and biodiversity loss (Pant 2003). About 50% of orchids are being used in traditional medicines apart from being sources of ornaments (Tsering et al. 2017). Epiphytic orchid growing on other living or non-living materials for physical supports tends to release bioactive secondary metabolites when they are exposed to disturbance (Lindley and Paxton 1851). Pleione maculata commonly known as peacock orchid is a rare unexplored epiphyte on the verge of extinction (Chauhan and Sharma 2017) growing on trees at a high elevations of about 600–1600 m (Lindley and Pacton 1851). The epiphyte is a well-known medicinal plant in the northeastern region of India, where local people use pseudobulbs or rhizomes to treat liver problems, stomach ailments, and headaches (Pant 2003; Teoh 2016).
Today, in silico studies are more favored for drug identification to find the interaction of the pharmaceutically important compounds with their targets. The most commonly known computational tool for drug target is molecular docking using various algorithms. PatchDock is highly efficient (Prabhu and Rajeswari 2016) accurate (Doss et al. 2014), fast transformational search, and free-online server in comparison to other computational molecular docking servers. PatchDock is a molecular docking algorithm based on the principle of shape complementarity. A complementarity molecular shape is a yield forming a conformational transformation of each docking complex known as induced fit. The transformed molecules can be further evaluated based on the scoring function which involves geometric-fit and binding affinity based on atomic contact energy (ACE). PatchDock calculates the amount of atomic contact energy (ACE) which is an atomic desolvation free energy required to replace ligand molecule from water contact to protein contact (Guo et al. 2012; Maiti and Banerjee 2020). PatchDock algorithms contain three stages, (a) molecular shape representation based on geometric patches (concave, convex, and flat surface pieces), (b) surface patch matching, and (c) filtering and scoring (Duhovny et al. 2002). There are various parameters involved for running a docking interaction between molecules such as root mean square deviation (RMSD) and a complex type. The RMSD is applied to prevent redundancy of molecule and is exact (Duhovny et al. 2002). PatchDock uses techniques such as geometry hashing and pose clustering which detects advance data structures and spatial pattern. In docking, energy refinement is required for further development of drug compounds for example FireDock (Andrusier et al. 2007; Surana et al. 2018). FireDock (fast iteration refinement in molecular docking) refinement of energy uses Monte Carlo binding score minimization, is highly efficient and easy to understand, and requires no prior knowledge in docking (Lipinski et al. 1997). The scores are atomic contact energy (ACE), softened van der Waals interaction, partial electrostatics, and additional estimation of binding energy. FireDock algorithm includes three refinement steps: (1) side-chain optimization, (2) rigid-body minimization, and (3) scoring and ranking (Lipinski et al. 1997). The docking analysis can be predicted base on higher binding affinity and lowest docked energy (Iyamah et al. 2017). Before clinical studies, the drug-likeness of compounds can be analyzed using computational tools that are cost-effective and less tedious. SwissADME is a free web tool available to evaluate pharmacokinetics based on different drug-likeness parameters such as physicochemical properties, solubility, and pharmacokinetics of molecule (Daina et al. 2003).
A compound separation technique gas chromatography–mass spectrophotometry will provide ideas on different bioactive compounds present in plants. Gas chromatography is an instrumental technique coupled with mass spectrometry applied for separation, identification, and quantification of organic compounds and chemical mixtures study. An inert gas such as helium is used as a mobile phase (carrier gas). The samples to be analyzed will be injected and interacted with glass or metal columns coated with the stationary phase and elute different compounds at the different times called retention time (Ghosal and Srivastava 2013). The GC–MS method is highly sensitive, reproducible, and high-speed resolution (Dua and Garg 2013). The study aimed to exploit safe, medicinal, nutraceutical effective natural compounds from Pleione maculata using GC–MS analysis as there seems to be an unreached target globally requiring active research against SARS-COV-2. The selected GC–MS compounds for docking were based on their bioactivity against symptoms related to SARS-CoV-2 pneumonia such as anti-inflammatory activity, preventing cardiac insufficiency, preventing fatigue, preventing shortness of breath, preventing gastrointestinal diseases, heartbeat improvement, antiviral activities, and a compound having repellent activity, larvicidal activity, based on earlier knowledge in using an antimalarial drug for reducing a viral load (Rane et al. 2020). In earlier years, steroidal compound and antisense target were also used for the treatment of viral diseases (Chidambaram et al. 1996; Kim et al 2009; Chen et al. 2014); therefore, with this prior knowledge, GC–MS analysis of Pleione maculata identified steroidal antiviral and an antisense target compound as a key compound to perform docking. The paper also evaluates the drug-likeness potential of compounds possessing multi-target bioactivity using a molecular docking algorithm to observe high binding affinity and conformational fit between bioactive compounds of P. maculata against target proteins of SARS-CoV-2. A random control antiviral drug arabidiol was used in severe cases of COVID-19 as it was observed to reduce the viral disease (Wang et al. 2020).
Materials and methods
Collection and extract preparation of plant material
Pleione maculata sample was collected from dense forest of Khliehriat, East Jaintia Hills regions of Meghalaya, and was processed. The parts of P. maculata were washed under running tap water, surface-sterilized with distilled water, 1% sodium hypochlorite, and re-washed with distilled water. The samples were shade-dried and crushed, and each plant part was soaked in three different solvents (ethanol, methanol, acetone) followed by 24–48-h incubation. The crude extract was filtered using Whatman filter paper Grade No-1 with circle size of 125 mm diameter (Cat No 1001125) (Vijisaral and Arumugam 2014). The solvent extracts are then evaporated using open-air evaporation in a laminar airflow hood for 24 h and centrifuged at 12,000 rpm for about 15 min at 4 °C. The concentrated extracts were transferred into a micro-centrifuge tube for GC–MS analysis.
GC–MS analysis
Gas chromatography–mass spectrometry of samples was analyzed in IIT Guwahati Biotech Park, Assam, using PerkinElmer (USA). The GC–MS system model was Clarus 680 GC & Clarus 600 C MS comprising a liquid auto-sampler with aid of Turbo mass ver. 5.4.2 software. The chromatography was performed on a capillary column Elite of 5MS 60.0 m × 250 μm, ID of 0.25 mm, and film thickness of 0.25 μm, and stationary phase was 5% diphenyl 95% dimethylpolysiloxane. The injection volume of the sample was 1 μl with a 0:1 split ratio. Helium (99.999%) was used as carrier gas throughout the column. The solvent delay was by 9.00 min, and transfer temperature was 200 °C. The injector and ion source temperature were 280 °C and 180 °C, respectively. The initial temperature in the oven was programmed from 110 °C for 3 min (Thomas et al. 2013), ramp 5 °C/min to 200 °C and hold for 3 min and was again increased for 5 °C/min to 300 °C and hold for 10 min (Darmasiwi et al. 2015). The sample was scanned from 40 to 600 Da.
Identification of components using NIST
The peaks were analyzed using the data analysis software NIST-2008. The National Institute of Standards and Technology (NIST) is a mass spectral search database for comparisons of the acquired and unknown spectrum with NIST/EPA (Environmental protection Agency)/NIH (National Institute of Health) databases. The components were identified based on standards employed by the National Institute Standards and Technology (NIST) Library (Darmasiwi et al. 2015). The detection was employed by comparing peaks with that of mass spectral standard reference data NIST having more than 62,000 patterns. A detailed chemical structure of the GC–MS graph of a molecule was presented using ChemDraw Ultra 8.0.3 by CambridgeSoft (www.cambridgesoft.com) (CS ChemOffice—Drawing, Modelling, and Information).
Molecular docking using PatchDock
Input
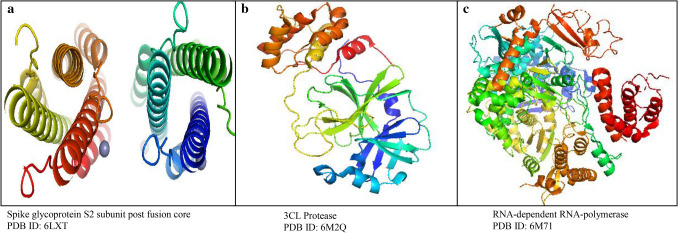
Protein structure retrieval: Protein receptor target was retrieved from RCSB (Research Collaboratory for Structural Bioinformatics) Protein Data Bank (PDB) (https://www.rcsb.org/) in 3D structure as shown in Fig. 1. The following three target receptors were (1) SARS-CoV-2-3CL protease (PDB ID: 6M2Q), (2) SARS-CoV-2 RNA-dependent RNA-polymerase (PDB ID: 6M71), and (3) SARS-CoV-2 spike glycoprotein receptor S2 subunit (PDB ID: 6LXT).
Fig. 1.
The 3D ribbon structures representation of SARS-CoV-2 target proteins a spike glycoprotein S2 subunit post-fusion core, b 3CL protease and c RNA-dependent RNA polymerase visualized using PyMOL
Ligand retrieval
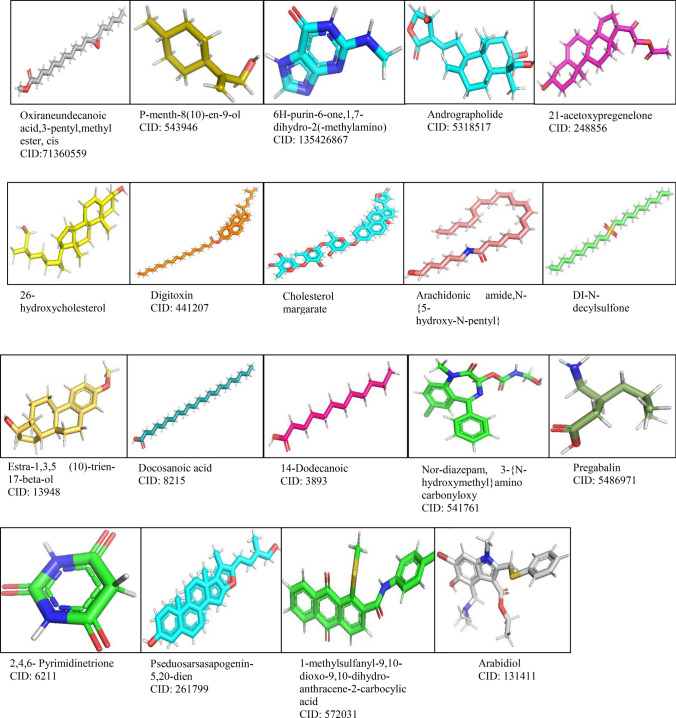
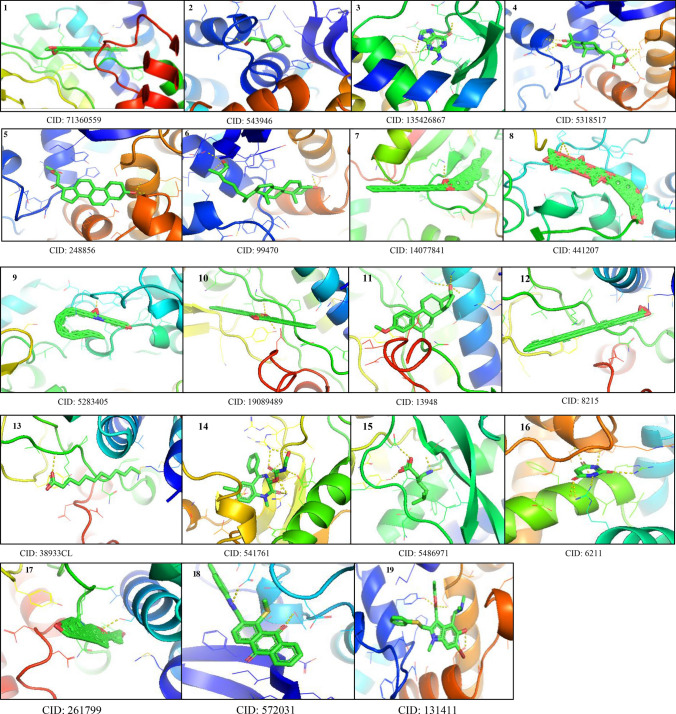
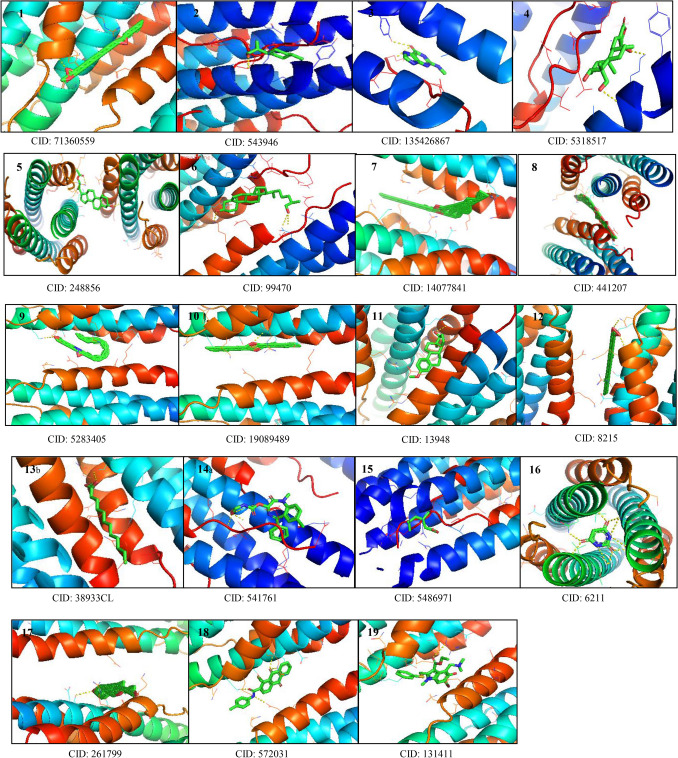
A total of 19 ligand molecules as shown in Fig. 2 were retrieved from PubChem (http://pubchem.ncbi.nlm.nih.gov/) database in SDF file format. The small ligand in SDF format was converted into PDB format using PyMOL a molecular modeling package (The PyMOL Molecular Graphics System, Version System, version 1.7.4 Schrödinger, LLC) (Prabhu and Rajeswari 2016; Yadav et al. 2017). The compound ID of the 3D structures of ligands was CID: 6211 (2,4,6-pyrimidinetrione), CID: 248,856 (21-acetoxypregenelone), CID: 543,946 (P-menth-8(10)-en-9-ol), CID: 541,761 (Nor-diazepam, 3-N-hydroxymethyl,aminocarbonyloxy), CID: 441,207 (digitoxin), CID: 19,089,489 (DI-N-decylsulfone), CID: 3893 (14-dodecanoic acid), CID: 13,948 (estra,13,5 (10)-trien-17-beta-ol), CID: 5,283,405 (arachidonic amide, N-5-hydroxy-N-pentyl), CID: 572,031 (1-methylsulfanyl-9,10-dioxo-9,10-dihydro-anthracene-2-carboxylic acid), CID: 14,077,841 (cholesterol margarate), CID: 8215 (docosanoic acid), CID: 99,470 (26-hydroxy cholesterol), CID: 5,318,517 (andrographolide), CID: 135,426,867 (6H-purin-6-one-,1,7-dihydro-2-methylamino), CID: 261,799 (pseduosarsasapogenin), CID: 71,360,559 (oxiraneundecanoic acid, 3-pentyl, methyl ester, cis), CID: 5,486,971 (pregabalin), and CID: 131,411(arabidol).
Fig. 2.
GC–MS compounds and positive control arabidol ligands downloaded from PubChem data bank with compound ID and visualized using PyMOL for molecular docking
Molecular Docking analysis
Protein–small ligand molecule docking was performed using the PatchDock algorithm (Prabhu and Rajeswari 2016; Yadav et al. 2017; Surana et al. 2018). The parameters were set, the complex type was selected, and clustering root mean square deviation (RMSD) was set to 1.5 Å for protein–ligand interaction. The protein receptor and small ligand molecule were uploaded in PDB format followed by form submission. The following output will be further sent by PatchDock to the given email ID. A 1000 transformed docking candidate generated from PatchDock was refined and re-scored using FireDock. The result was visualized using PyMOL a molecular visualization graphics system tool.
Drug-likeness analysis of bioactive compounds using SwissADME tool (Daina et al. 2003)
Chemical structure of compounds was downloaded from PubChem data bank (http://pubchem.ncbi.nlm.nih.gov/) in SDF (structure data format), SwissADME web page was opened, and files were imported from the external file option and were converted into molecular sketcher based on ChemAxon’s Marvin JS followed by ADME calculation using default parameters.
Results
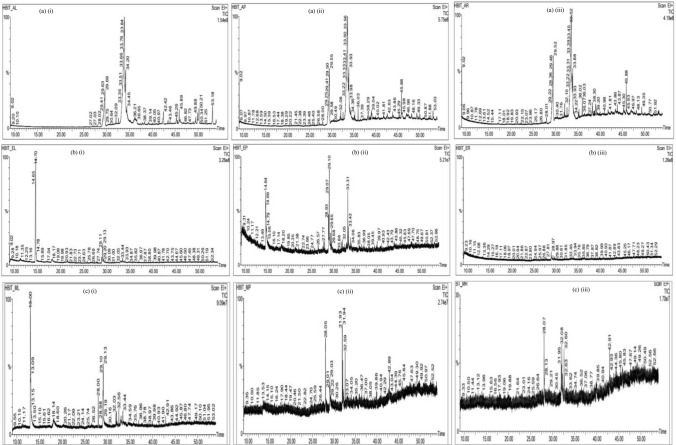
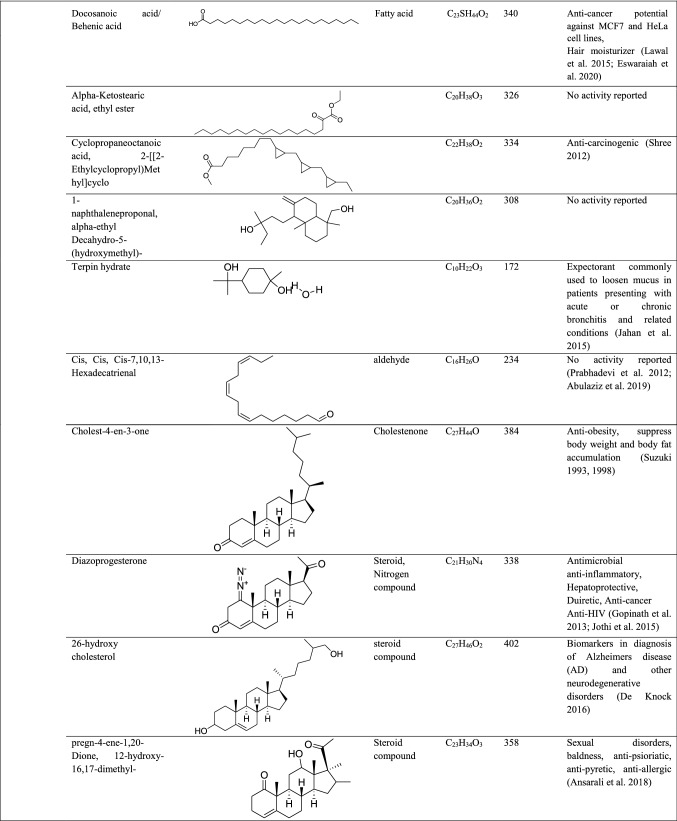
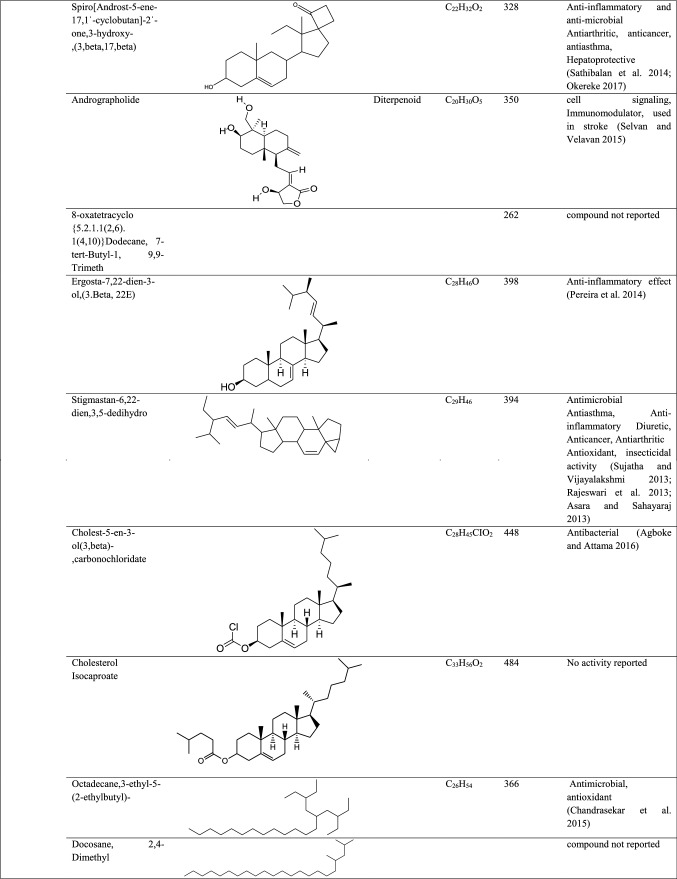
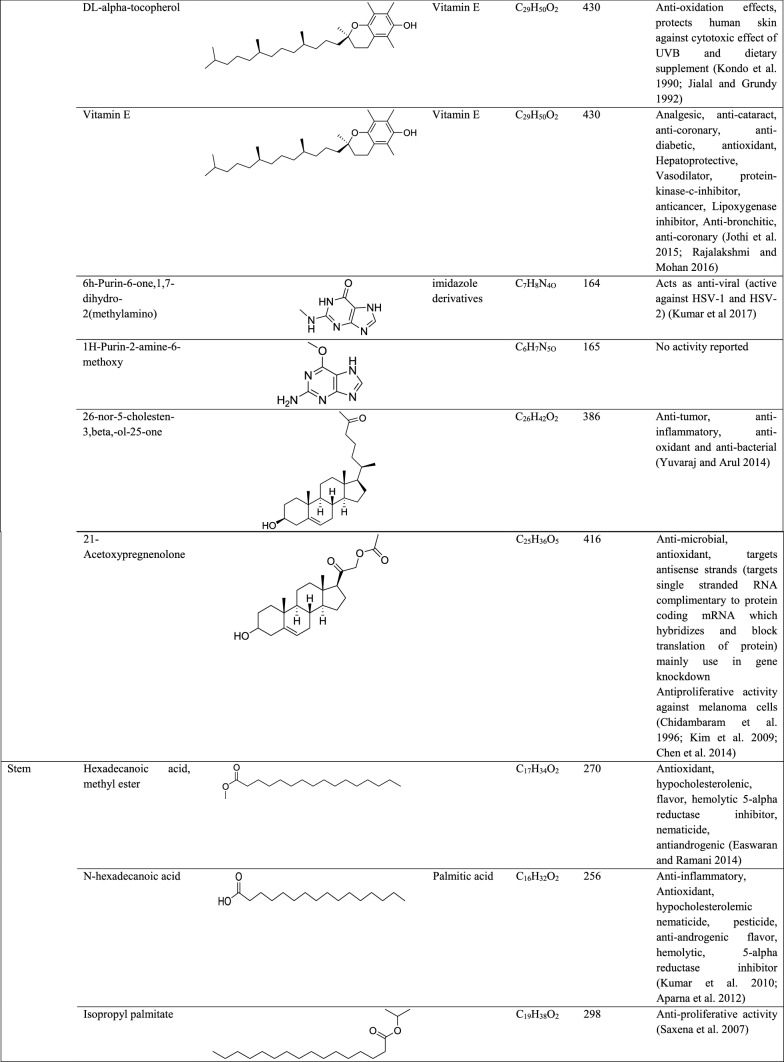
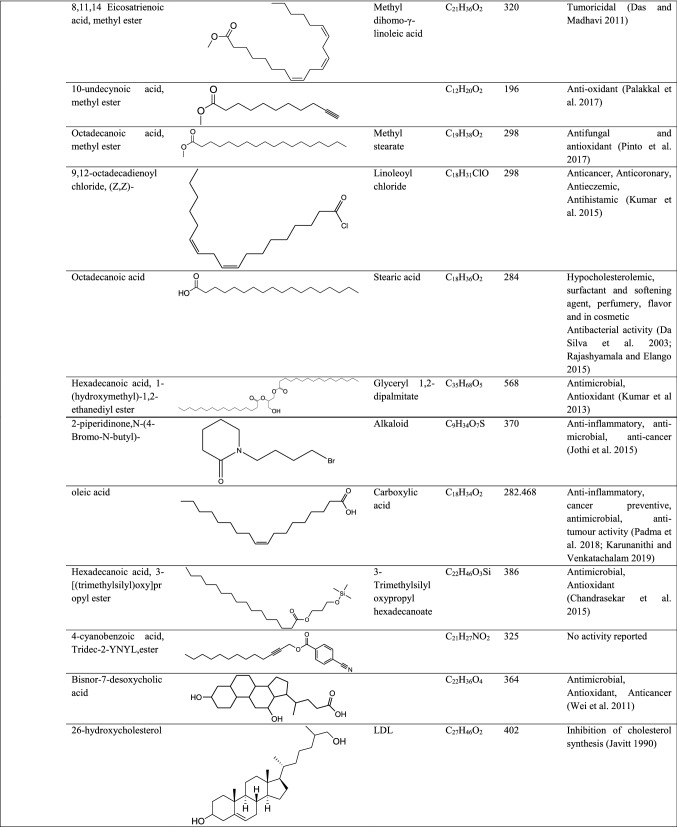
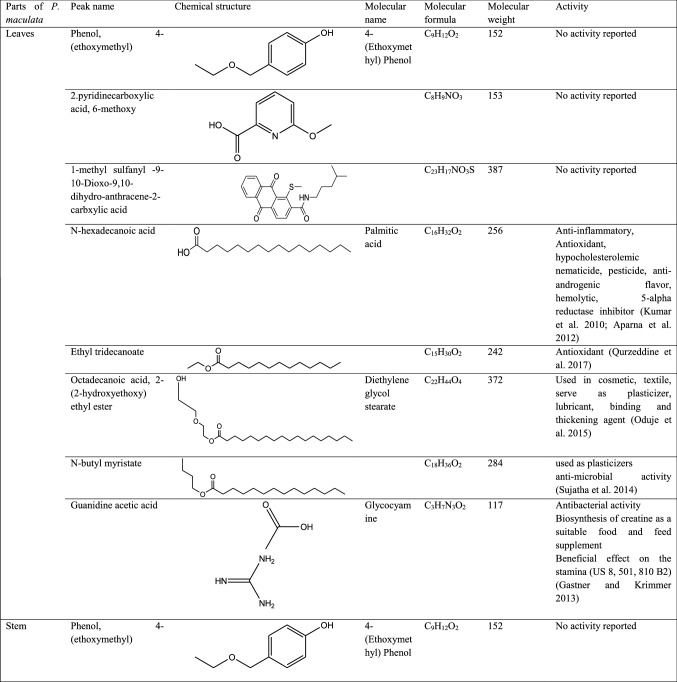
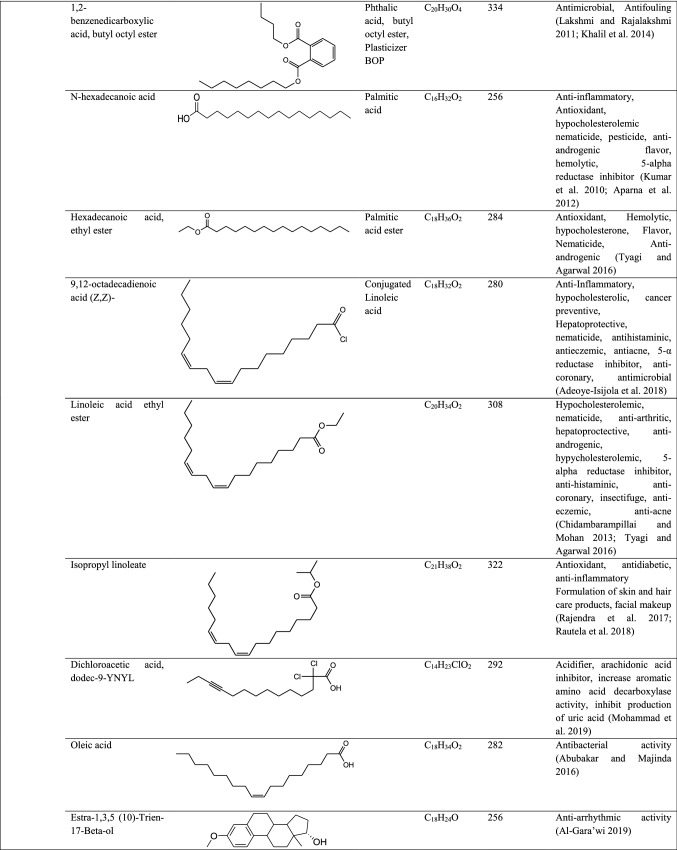
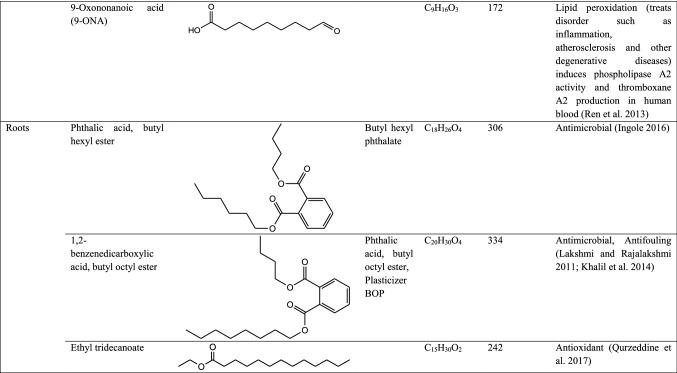
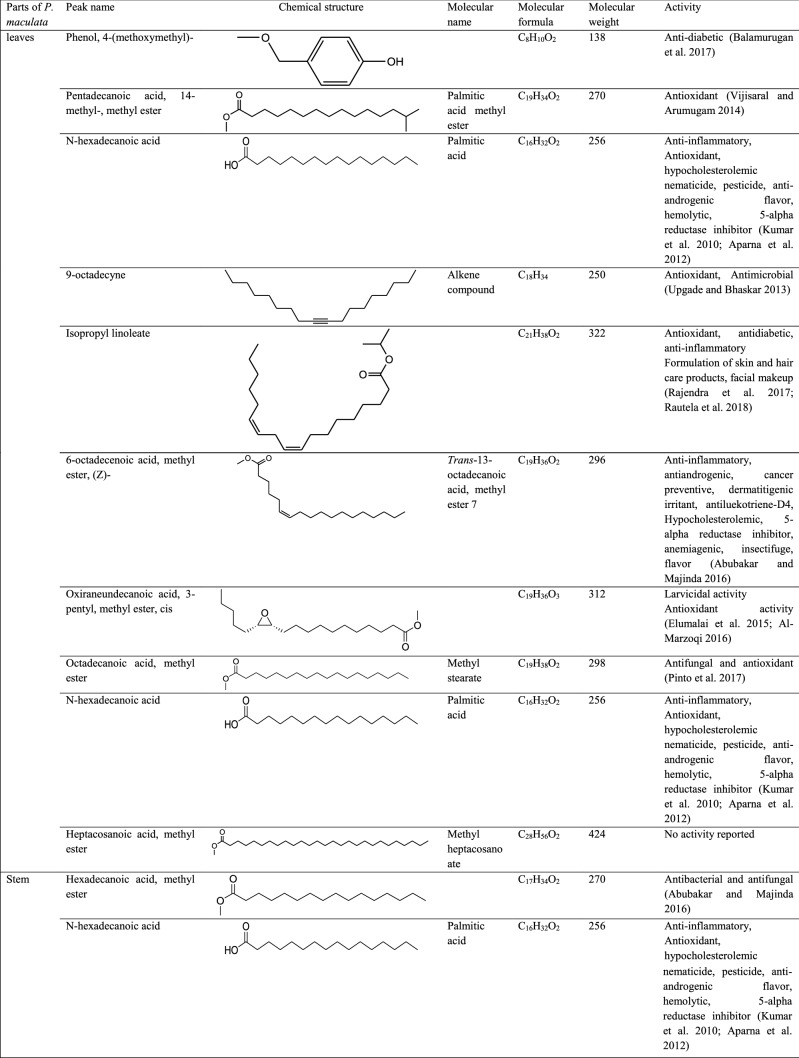
GC–MS analysis reported more than 146 hits and lead compounds from different parts of P. maculata (Fig. 3). The compounds identified from acetone leave extracts were more when compared to other solvent extracts. The following hit and lead compounds of P. maculata with their bioactivity are listed in Tables 1, 2, and 3.
Fig. 3.
GC–MS spectrograms showing peaks of compounds of Pleione maculata extracts (a) acetone, (b) ethanol (c) methanol extracts of (i) leave (ii) stem and (iii) root
Table 1.
GC–MS analysis of acetone extracts of Pleione maculata
Table 2.
GC–MS analysis of ethanol extracts of Pleione maculata
Table 3.
GC–MS analysis of methanol extracts of Pleione maculata
A molecular docking study performed for nineteen GC–MS compounds using PatchDock with energy minimization and structure refinement using FireDock was analyzed. The targets were docked properly as the binding affinity was shown to be negative and the docked ligand RMSD value was < 2.0 Å (Singh et al. 2017). The binding residues and atomic contact energy of ligands against the target proteins are listed in Table 4. The highest ACE of a docked molecule was considered below 6.00 kcal/mol (higher negative value); the higher the binding affinity value, the higher the binding potential between molecules. The different GC–MS compounds were interacted with numbers of residues on both side chain and backbone of the target protein as shown in Figs. 4, 5, and 6. A non-covalent (polar) interaction was observed between the docking molecules at a closer distance of 1.5 Å. The docking complex showed zero or one-to-three hydrogen bonding between them but formed a specific electrostatic, van der Waals interaction, and some interacted with positively charged functional groups of an amino acid (lysine, arginine, and histidine), while some interacted with hydroxyl groups of amino acid serine, threonine, and tyrosine. Amino acid residues such as serine, threonine, and tyrosine contribute to rotating hydroxyl groups in the docking complex are considered rigid (Pantsar and Poso 2018).
Table 4.
Docking analysis depicting the binding residues and their binding energy
| Protein Name | Drug-likeness ligands | Polar contact binding residues | Other intermolecular contact binding residues | Global energy (kcal/mol) | Attractive Vdw (kcal/mol) | Repulsive Vdw (kcal/mol) | ACE (kcal/mol) |
|---|---|---|---|---|---|---|---|
| RNA-dependent RNA-pol (6M71) | 2,4,6-Pyrimidinetrione | Cys 780, Arg 467, Leu 470, Arg 305 | All side bonded by polar contacts | − 23.59 | − 10.00 | 0.72 | − 5.79 |
| 21-Acetoxypregenelone | Ala 706, Leu 707, Asn 705 | Gly 774, Asp 135, Ala 130, Ser 709 | − 34.15 | − 20.12 | 10.12 | − 8.38 | |
| P-menth-8(10)-en-9-ol | Thr 141, Leu 142 | Ala 130, Asp 126 | − 31.56 | − 12.13 | 0.87 | − 7.18 | |
| Nor-diazepam, 3{n-hydroxymethyl}aminocarbonyloxy | Thr 556, Arg 555, Arg 624 | All side bonded by polar contact | − 35.35 | − 23.08 | 4.90 | − 3.16 | |
| Digitoxin | Lys 267, Ser 255, Thr 252 | Ser 255, Tyr 265, Thr 319 | − 79.90 | − 36.74 | 12.20 | − 22.96 | |
| DI-N-decylsulfone | Tyr 546, Val 410, Lys 141 | Lys 411, Asn 414, Gln 408 | − 44.31 | − 19.74 | 2.12 | − 11.57 | |
| 14-dodecanoic acid | Lys 411, Val 410 | Tyr 546, Ser 15, Asn 414 | − 29.99 | − 14.73 | 2.24 | − 6.96 | |
| Estra-1,3,5(10)-trien-17-beta-ol | Gln 18, Gln 19 | Ser 15, Asn 414 | − 36.68 | − 20.43 | 4.20 | − 6.57 | |
| Arachidonic amide,N-{5-hydroxy-N-Pentyl} | Tyr 268 | Lys 267 | − 46.08 | − 21.29 | 4.04 | − 12.04 | |
| 1-methylsulfanyl-9–10-dioxo-9,10-dihydro-Anthracene-2-carboxylic acid | Asp 218, Thr 206 | Tyr 217, Ile 37, Tyr 38 | − 37.97 | − 20.97 | 3.26 | − 6.72 | |
| Cholesterol margarate | Lys 391 | Thr 393, Lys 395, Asn 136 | − 70.69 | − 31.28 | 9.37 | − 21.60 | |
| Docosanoic acid | No polar contact | Ser 15, Asn 414, Tyr 546, Lys 411 | − 47.59 | − 23.22 | 8.71 | − 13.13 | |
| 26-hydroxycholesterol | Asn 781, Asp 126 | Ser 784, Lys 47, Thr 141, Lys 780 | − 42.36 | − 21.98 | 1.38 | − 7.11 | |
| Andrographolide | Leu 142, Asp 140, Tyr 129, Asn 781, Ser 709, Leu 708 | Cys 139, Asp 140, Thr 141, Lys 47 | − 37.42 | − 19.40 | 4.39 | − 8.43 | |
| 6H-purin-6-one,1,7-dihydro-2-(methylamino) | Tyr 530, Asn 534 | Asn 360, Val 342 | − 29.00 | − 12.14 | 0.14 | − 6.92 | |
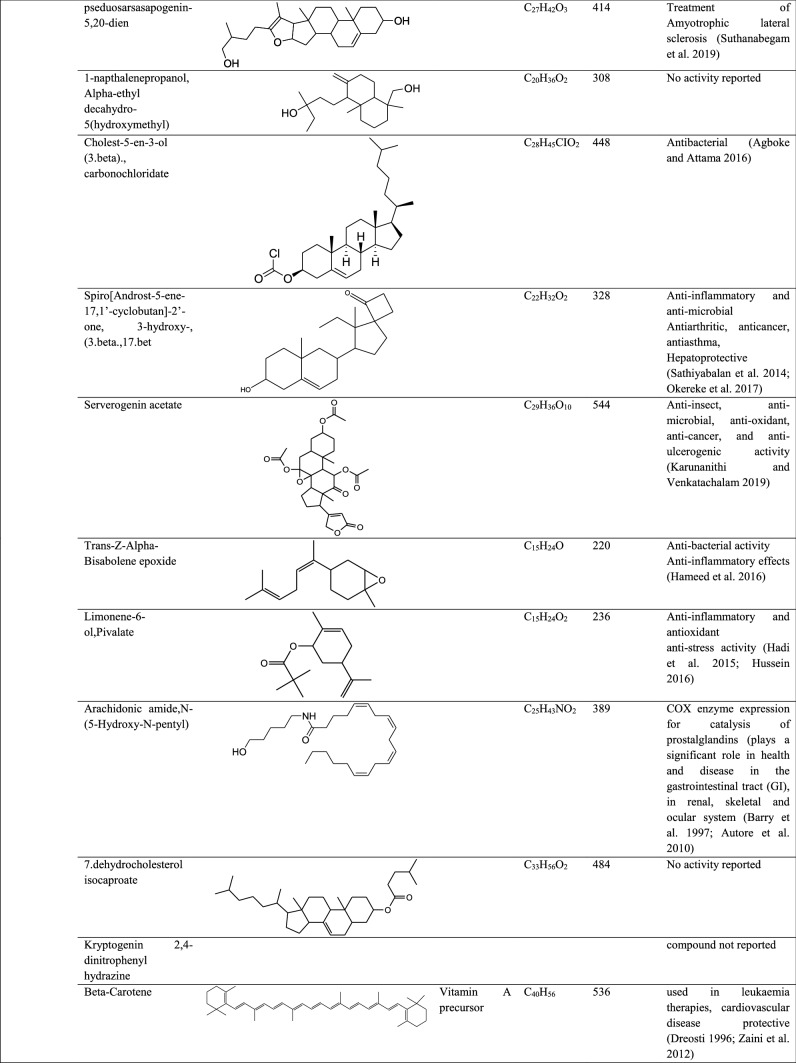
| Pseduosarsasapogenin-5,20-dien | Ser 15 | Val 12, Asp 846, Asn 414, Val 12 | − 56.41 | − 29.45 | 6.51 | − 12.54 | |
| Oxiraneundecanoic acid,3-pentyl, methyl ester, cis | No polar contact | Lys 411, Ser 15, Asn 414, Val 12 | − 46.14 | − 22.77 | 5.77 | − 11.38 | |
| Pregabalin | Asn 356, Tyr 530, Asp 377 | Thr 344, Ser 343 | − 30.35 | − 13.26 | 0.32 | − 6.85 | |
| Arabidol | Asn 781, Tyr 129 | Lys 780, Ser 709, Thr 710, Ser 784 | − 41.54 | − 20.55 | 5.40 | − 9.95 | |
| 3CL Protease (6M2Q) | 2,4,6-Pyrimidinetrione | Met 6, Asp 295, Gln 127, Val 296, Arg 298 | All side bonded by Polar contacts | − 16.68 | − 7.33 | 0.09 | − 4.42 |
| 21-Acetoxypregenelone | Arg 188, Gln 184, Thr 190, Met 165, Glu 166, Leu 167, Ser 46, Thr 45 | Thr 25, Thr 24, Thr 26 | − 28.57 | − 14.64 | 8.41 | − 9.22 | |
| P-menth-8(10)-en-9-ol | Asn 142 | Gly 143, Cys 145, His 163, Asn 143 | − 24.19 | − 11.51 | 2.27 | − .6.75 | |
| Nor-diazepam, 3{n-hydroxymethyl}aminocarbonyloxy | Met 165, Glu 166, Asn 142 | Ser 46, Met 49 | − 25.73 | − 11.42 | 0.80 | − 7.19 | |
| Digitoxin | Gly 116, Tyr 154 | Asn 151, Asp 153, Tyr 154, Phe 305, Phe 294 | − 54.15 | − 27.23 | 6.90 | − 13.27 | |
| DI-N-decylsulfone | Phe 294, Gln 110, Thr 111, Thr 292 | Asp 295, Pro 293 | − 28.23 | − 14.86 | 5.07 | − 7.39 | |
| 14-dodecanoic acid | Leu 253, Leu 250, Ile 249, Pro 252 | Phe 294, Val 297, Asp 248, Asp 245 | − 18.11 | − 9.92 | 4.01 | − 5.98 | |
| Estra-1,3,5(10)-trien-17-beta-ol | Phe 294, Pro 293, Thr 111, Gln 110, His 246 | All side bonded by polar contacts | − 19.00 | − 10.41 | 3.39 | − 5.43 | |
| Arachidonic amide,N-{5-hydroxy-N-Pentyl} | Asn 142, Gly 143 | Ser 144, Glu 166, Leu 167 | − 38.03 | − 17.95 | 5.31 | − 10.86 | |
| 1-methylsulfanyl-9–10-dioxo-9,10-dihydro-anthracene-2-carboxylic acid | Glu 166, Met 165 | Ser 46, Thr 25, Glu 47 | − 39.16 | − 16.63 | 4.12 | − 12.29 | |
| Cholesterol margarate | Gln 110, Gln 109 | Pro 108, Gln 240 | − 40.95 | − 23.94 | 9.46 | − 9.91 | |
| Docosanoic acid | no polar contacts | Phe 294, Gln 110, Thr 111, Asp 153 | − 28.31 | − 14.82 | 1.65 | − 6.51 | |
| 26-hydroxycholesterol | His 163, Met 165, Glu 166 | Asn 142, Cys 145, Ser 46, Thr 45 | − 40.85 | − 17.40 | 4.98 | − 12.61 | |
| Andrographolide | Thr 25, Ser 46, Glu 166, Met 165 | Asn 142, His 41 | − 24.51 | − 13.53 | 7.31 | − 7.17 | |
| 6H-purin-6-one,1,7-dihydro-2-(methylamino) | Glu 166, Met 165, Leu 167, Cys 145 | His 163, Asn 142, Gly 143 | − 21.58 | − 8.97 | 2.01 | − 6.60 | |
| Pseduosarsasapogenin-5,20-dien | Thr 243, Phe 294, Thr 111, Gln 110 | Thr 242, Asp 245 | − 34.40 | − 18.57 | 6.71 | − 8.41 | |
| Oxiraneundecanoic acid,3-pentyl, methyl ester, cis | Gln 110, Gly 109 | Phe 294, Pro 293, His 246, Asr 153 | − 24.73 | − 12.67 | 1.46 | − 5.53 | |
| Pregabalin | Arg 298, Met 6 | Val 303, Thr 304, Arg 298, Gln 299 | − 20.69 | − 10.29 | 3.12 | − 6.33 | |
| Arabidol | Glu 166, Met 165, His 164 | Thr 26, Thr 25, Thr 24, Thr 45 | − 37.06 | − 16.29 | 10.23 | − 14.02 | |
| Spike glycoprotein (6LXT) | 2,4,6-Pyrimidinetrione | Val 952, Asn 955, Asn 956, Asn 953 | All side bonded by polar contact | − 26.44 | − 10.31 | 0.16 | − 6.94 |
| 21-Acetoxypregenelone | Ala 942, Ser 940, Arg 1185 | Asn 1187, Ser 943, Lys 1181, Ile 183 | − 28.10 | − 19.75 | 5.74 | − 2.22 | |
| P- menth-8(10)-en-9-ol | Leu 922 | Ile 923, Ala 924, Asn 919, Gln 920 | − 24.89 | − 11.41 | 1.94 | − 6.68 | |
| Nor-diazepam, 3{n-hydroxymethyl}aminocarbonyloxy | Ile 1198, Asn 928, Leu 1197 | Asp 1199, Asn 925 | − 36.22 | − 17.30 | 4.48 | − 10.95 | |
| Digitoxin | Ser 940, Arg 1185 | Glu 1182, Ser 943, Asp 936, Lys 1191, Gln 935 | − 63.54 | − 42.37 | 17.15 | − 8.93 | |
| DI-N-decylsulfone | Ser 940 | Ser 943, Ser 939, Lys 1181, Lys 947 | − 41.11 | − 26.75 | 5.32 | − 4.29 | |
| 14-dodecanoic acid | Ser 939 | Asn 1194, Asn 1187, Glu 1188, Glu 1195, Gln 935 | − 23.19 | − 13.50 | 1.46 | − 3.08 | |
| Estra-1,3,5(10)-trien-17-beta-ol | Ala 1190, Lys 1191 | Arg 1185, Asn 1187, Gln 935 | − 32.36 | − 19.07 | 3.90 | − 4.34 | |
| Arachidonic amide,N-{5-hydroxy-N-Pentyl} | Lys 947 | Asp 1184, Glu 1188, Asn 1187, Asp 936, Lys 1191, Ser 939 | − 42.54 | − 27.63 | 3.87 | − 2.74 | |
| 1-methylsulfanyl-9–10-dioxo-9,10-dihydro-anthracene-2-carboxylic acid | Asp 184, Ser 940, Arg 1185, Lys 947 | Ser 943, Ser 949 | − 31.67 | − 21.43 | 1.25 | − 0.52 | |
| Cholesterol margarate | no polar contact | Asn 1184, Asn 1187, Asp 936, Gln 935 | − 54.12 | − 32.79 | 9.27 | − 8.23 | |
| Docosanoic acid | Asn 1178, Lys 1181 | Ser 943, Ser 939, Lys 947, Gln 935 | − 37.20 | − 26.13 | 3.97 | − 2.01 | |
| 26-hydroxycholesterol | Asn 925, Gln 926, Lys 1191 | Gln 1195, Asp 936, Gly 932, Asn 928 | − 41.67 | − 20.53 | 2.58 | − 8.23 | |
| Andrographolide | Gln 920, Lys 921, Tyr 917 | Glu 1202, Val 915 | − 29.04 | − 21.14 | 20.25 | − 9.23 | |
| 6H-purin-6-one,1,7-dihydro-2-(methylamino) | Tyr 917 | Leu 1200, Asp 1199, Tyr 917 | − 19.45 | − 8.27 | 0.66 | − 5.86 | |
| Pseduosarsasapogenin-5,20-dien | Ser 939 | Ser 940, Glu 1182, Lys 1181 | − 41.80 | − 28.41 | 2.51 | − 2.20 | |
| Oxiraneundecanoic acid,3-pentyl, methyl ester, cis | Lys 1181 | Arg 1181, Asp 936, Ser 943, Ser 939 | − 34.65 | − 25.21 | 6.80 | − 2.26 | |
| Pregabalin | no polar contacts | Leu 1200, Ile 923, Ala 924 | − 27.07 | − 13.01 | 2.00 | − 6.29 | |
| Arabidiol | Arg 1185, Leu 1186, Asp 1184 | Lys 1181, Asn 1187, Ser 943 | − 29.82 | − 22.67 | 4.74 | 0.13 |
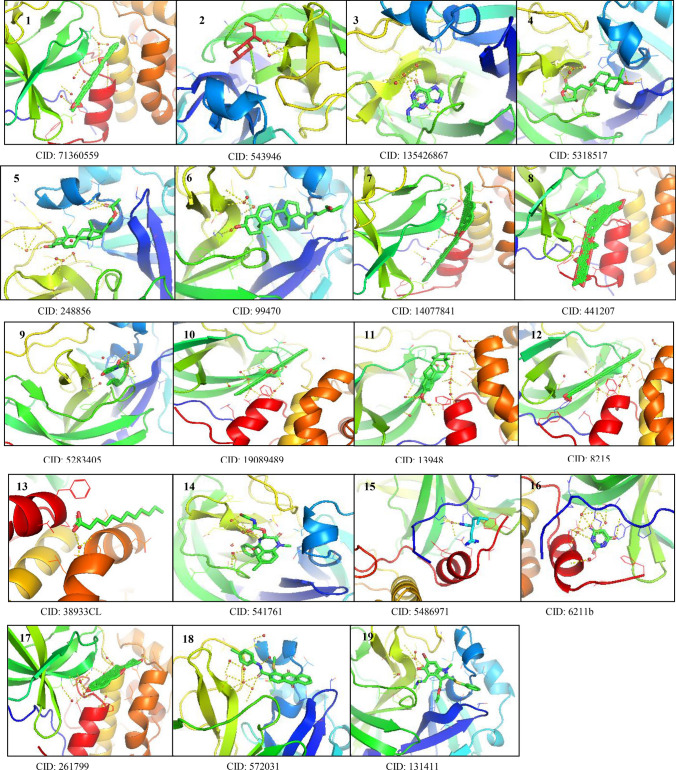
Fig. 4.
Molecular docking of target SARS-CoV-2 RNA-dependent RNA-polymerase (PDB ID- 6M71) with the S-CoV-2 RNA-dependent RNA polymerase (PDB ID-6M71) with the GC–MS bioactive compounds of Pleione maculata (1) oxiraneundecanoic acid,3-pentyl, methyl ester, cis (2) p-menth-8(10)-en-9-ol (3) 6H-purin-6-one,1,7-dihydro-(2-methylamino) (4) andrographolide (5) 21-acetoxypregenelone (6) 26-hydroxycholesterol (7) cholesterol margarate (8) digitoxin (9) arachidonic amide, N-{5-hydroxy-N-pentyl} (10) DI-N-decylsulfone (11) estra-1,3,5(10)-trien-beta-ol (12) docosanoic acid (13) 14-dodecanoic acid (14) Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy (15) pregabalin (16) 2,4,6-pyrimidinetrione (17) pseduosarsasapogenin acid, 3-pentyl, methyl ester, cis, (18) 1-methylsulfanyl-9,10-dioxo-9, 10-dihydro-anthracene-2-carboxylic acid and positive control (19) arabidiol
Fig. 5.
Molecular docking of target SARS-CoV-2 3CL protease (PDB ID- 6M2Q) with the GC–MS bioactive compounds of Pleione maculata (1) oxiraneundecanoic acid,3-pentyl, methyl ester, cis (2) p-menth-8(10)-en-9-ol (3) 6H-purin-6-one,1,7-dihydro-(2-methylamino) (4) andrographolide (5) 21-acetoxypregenelone (6) 26-hydroxycholesterol (7) cholesterol margarate (8) digitoxin (9) arachidonic amide, N-{5-hydroxy-N-pentyl} (10) DI-N-decylsulfone (11) estra-1,3,5(10)-trien-beta-ol (12) docosanoic acid (13) 14-dodecanoic acid (14) Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy (15) pregabalin (16) 2,4,6-pyrimidinetrione (17) pseduosarsasapogenin acid, 3-pentyl, methyl ester, cis, (18) 1-methylsulfanyl-9,10-dioxo-9, 10-dihydro-anthracene-2-carboxylic acid and positive control (19) arabidiol
Fig. 6.
Molecular docking of target SARS-CoV-2 spike glycoprotein S2 subunit (PDB ID- 6LXT) with the GC–MS bioactive compounds of Pleione maculata (1) oxiraneundecanoic acid,3-pentyl, methyl ester, cis (2) p-menth-8(10)-en-9-ol (3) 6H-purin-6-one,1,7-dihydro-(2-methylamino) (4) andrographolide (5) 21-acetoxypregenelone (6) 26-hydroxycholesterol (7) cholesterol margarate (8) digitoxin (9) arachidonic amide, N-{5-hydroxy-N-pentyl} (10) DI-N-decylsulfone (11) estra-1,3,5(10)-trien-beta-ol (12) docosanoic acid (13) 14-dodecanoic acid (14) Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy (15) pregabalin (16) 2,4,6-pyrimidinetrione (17) pseduosarsasapogenin acid, 3-pentyl, methyl ester cis, (18) 1-methylsulfanyl-9,10-dioxo-9, 10-dihydro-anthracene-2-carboxylic acid and positive control (19) arabidiol
Docking analysis of ligand against SARS-CoV-2 RNA-dependent RNA-polymerase (PDB ID—6M71) target
A high binding affinity was observed between GC–MS compounds digitoxin, cholesterol margarate, docosanoic acid, pseduosarsasapogenin-5,20-dien, arachidonic amide,N-{5-hydroxy-N-pentyl), DI-N-decylsulfone, oxiraneundecanoic acid, 3-pentyl, methyl ester, cis, andrographolide, 21-acetoxypregenelone, p-menth-8(10)-en-9-ol, 26-hydroxycholesterol, and pregabalin against target protein RNA-dependent RNA-polymerase with high atomic contact energies of − 22.96, − 21.60, − 13.13, − 12.54, − 12.04, − 11.57, − 11.38, − 8.43, 8.38, − 7.18, − 7.11, and − 6.85 kcal/mol. The most prominent and common amino acid residues binding to the target proteins were Asn 414, Lys 411, Tyr 141, Val 12, Tyr 546, and Asn 781.
Docking analysis of ligands against target SARS-CoV-2 3CL protease (3CL pro) (PDB ID—6M2Q)
GC–MS compounds of P. maculata digitoxin, 26-hydroxycholesterol, 1-methylsulfanyl-9,10-dioxo-dihydro-anthracene-2-carboxylic acid, arachidonic amide,N-{5-hydroxy-N-pentyl), cholesterol margarate, 21-acetoxypregenelone, pseduosarsasapogenin-5,20-dien, DI-N-decylsulfone, Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyl, andrographolide, p-menth-8(10)-en-9-ol, and pregabalin exhibited high binding affinity − 13,27, − 12.61, − 12.22, − 10.86, − 9.91, − 9.22, − 8.41, − 7.39, 7.19, − 7.17, 6.75, and − 6.33 kcal/mol against target SARS-CoV-2 3CL protease. The most commonly found amino acid residues binding to the target pocket were Gln 110, Phe 294, and Thr 111.
Docking analysis of ligands against target SARS-CoV-2 post-fusion core structure of spike glycoprotein S2 subunit (PDB ID- 6LXT)
The target spike glycoprotein-S2 subunit amino acid residues interacted with compounds, 1-methylsulfanyl-9,10-dioxo-9,10-dihydro-anthracene-2-carboxylic, Nor-diazepam, 3-{N-hydroxymethyl} aminocarbonyloxy, andrographolide, digitoxin, cholesterol margarate, 26-hydroxycholesterol, p-menth-8(10)-en-9-ol, and pregabalin with high binding affinity (ACE) of − 10.95, − 9.23, − 8.93, − 8.23, − 8.23, 6.68, and 6.29 kcal/mol. The most common residues binding to target proteins were Ser 943, Ser 940, Glu 1188, Ser 939, Asp 1184, Asp 936, Lys 1191, Gln 935, and Asn 1187.
Drug-likeness analysis of bioactive compound
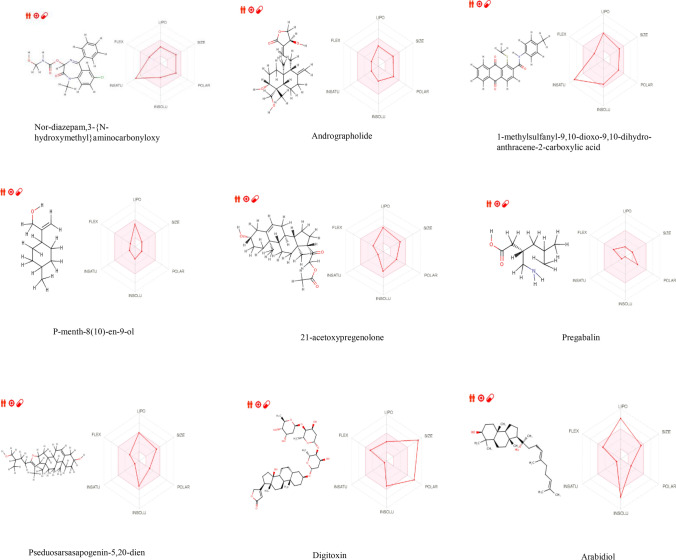
Drug-likeness was analyzed to check whether bioactive compounds possess favorable ADME (absorption, distribution, metabolism, and excretion) properties. Drug-likeness compounds should have a good aqueous solubility which is predicted by three methods ESOL, (ALI) logS, and (SILICOS-IT) logS (Shweta and Rashmi 2019). Orally active drug should obey Lipinski five rule in Table 5, molecular weight (MW) not more than 500 g/mol, hydrogen bond acceptors not more than 10, hydrogen bond donors not more than 5, LogP value less than 5, and number of rotatable bonds not less than 10 (Lipinski et al. 1997), and a violation of two or more rule depicts a molecule as not orally active. Drug-likeness analysis of bioactive compounds listed in Table 6 with their different parameters is shown in SwissADME bioavailability radar in Fig. 7. Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy bioactive compound showed good binding affinity against SARS-CoV-2 with high drug-likeness parameters such as good solubility and no excretion problems as there is no pharmacokinetics P-gp (permeability glycoprotein) interference and non-inhibitor of CYP enzymes and compound is specific in nature (zero alerts for PAINS (pan assay interference compounds)). 1-Methylsulfanyl-9,10-dioxo-9,10-dihydro-anthracene-2-carboxylic acid drug-likeness parameters is also good, since it follows Lipinski RO5, Ghose, Veber, Egan, Muegge rule, and Bioavailability score 0.55 but the PAINS (Pan assay interference compounds) exhibited some interference with Quinone A compound which indicates compound is not specific and is moderately soluble in nature. Digitoxin might have shown good binding affinity but does not obey any of the drug-likeness parameters. Pregabalin and p-menth-8(10)-en-9-ol bioactive compounds are highly soluble in nature but depict one or two violations as drug-likeness due to lower molecular weight (< 160). 21-Acetoxypregenolone an antisense target compound showing good binding affinity against SARS-CoV-2 shows moderate drug-likeness parameters, specific in nature, and no excretion problems but is an inhibitor of CYP2C9 enzyme. Pseduosarsasapogenin-5, 20-dien, and andrographolide drug-likeness activity are moderate with good solubility property. Compounds such as DI-N-decylsulfone, arachidonic amide, N-{5-hydroxy-N-pentyl}, 26-hydroxycholesterol, cholesterol margarate, and docosanoic acid showed poor solubility with various violations as a drug molecule. Arabidiol an already marketed antiviral drug does follow Lipinski RO5 but does not obey drug-likeness parameters and is poorly soluble compared to bioactive compounds of Pleione maculata with high solubility in nature.
Table 5.
Lipinski rule of five (RO5) violation
| Molecular weight | < 500 Daltons |
|---|---|
| Hydrogen bond donors | No > 5 |
| Hydrogen bond acceptors | No > 10 |
| Octanol–water partition coefficient (logP) | < 5 |
| Number of rotatable bonds | No < 10 |
Table 6.
Drug-likeness analysis of bioactive compounds showing good binding energy against SARS-CoV-2 proteins
| Drug-likeness compounds | Physicochemical properties (Lipinski rule of five) | Solubility | Pharmacokinetics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MW | HB donors | HB acceptors | No of rotatable bond | Consensus log P | Log S (ESOL) | Log S (Ali) | Log S (SILICOS-IT) | GI absorption | CYP enzymes inhibitors | |
| Digitoxin | 764 | 5 | 13 | 7 | 2.61 | Moderately soluble | Moderately soluble | Moderately soluble | Low | No |
| 26-hydroxycholesterol | 402 | 2 | 2 | 6 | 5.86 | Poorly soluble | Poorly soluble | Moderately soluble | High | No |
| 1-methylsulfanyl-9,10-dioxo-9,10-dihydro-anthracene-2-carboxylic acid | 387 | 1 | 3 | 4 | 4.10 | Moderately soluble | Poorly soluble | Poorly soluble | High | Yes |
| Arachidonic amide,N-{5-hydroxy-N-pentyl} | 389 | 2 | 2 | 20 | 6.27 | Moderately soluble | Poorly soluble | Poorly soluble | High | No |
| Cholesterol margarate | 639 | 0 | 2 | 22 | 12.43 | Insoluble | Insoluble | Insoluble | Low | No |
| 21-acetoxypregenolone | 374 | 1 | 4 | 4 | 3.77 | Moderately soluble | Soluble | Soluble | High | Only CYP2C9 inhibitor |
| DI-N-decylsulfone | 346 | 0 | 2 | 18 | 6.68 | Poorly soluble | Poorly soluble | Poorly soluble | Low | CYP2C9, CYP1A2 inhibitor |
| Pseduosarsasapogenin-5,20-dien | 414 | 2 | 3 | 4 | 4.86 | Moderately soluble | Moderately soluble | Moderately soluble | High | No |
| Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy | 373 | 2 | 5 | 5 | 2.16 | Soluble | Soluble | solUble | High | No |
| Andrographolide | 350 | 3 | 5 | 3 | 2.33 | Soluble | Soluble | Soluble | High | No |
| Pregabalin | 159 | 2 | 3 | 5 | 0.56 | Highly soluble | Highly soluble | Soluble | High | No |
| P-menth-8(10)-en-9-ol | 154 | 1 | 1 | 2 | 2.55 | Soluble | Soluble | Soluble | High | No |
| Docosanoic acid | 340 | 1 | 2 | 20 | 7.40 | Poorly soluble | Insoluble | Poorly soluble | Low | CYP1A2 inhibitor |
| Arabidol | 444 | 2 | 2 | 7 | 6.97 | Poorly soluble | Poorly soluble | Poorly soluble | Low | No |
Fig. 7.
SwissADME bioavailability radar of different bioactive drug-likeness molecules, where the pink areas represent each property (lipophilicity, molecular weight, solubility, and flexibility)
Discussion
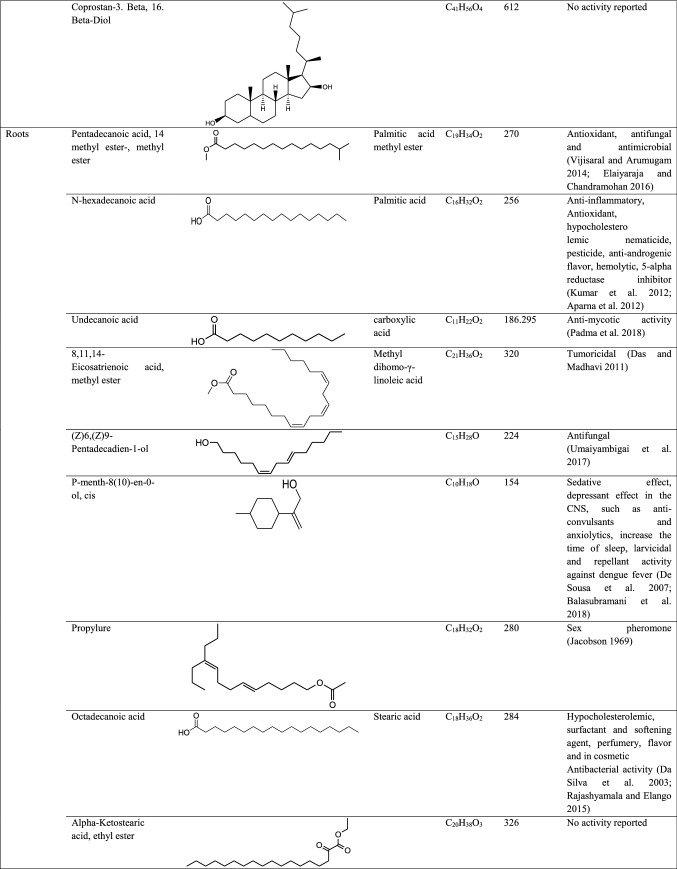
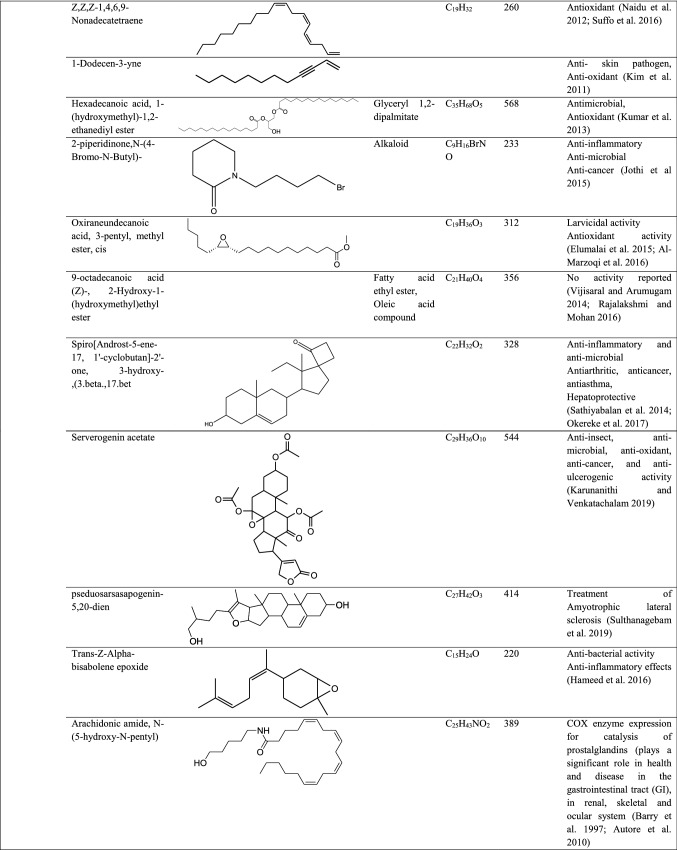
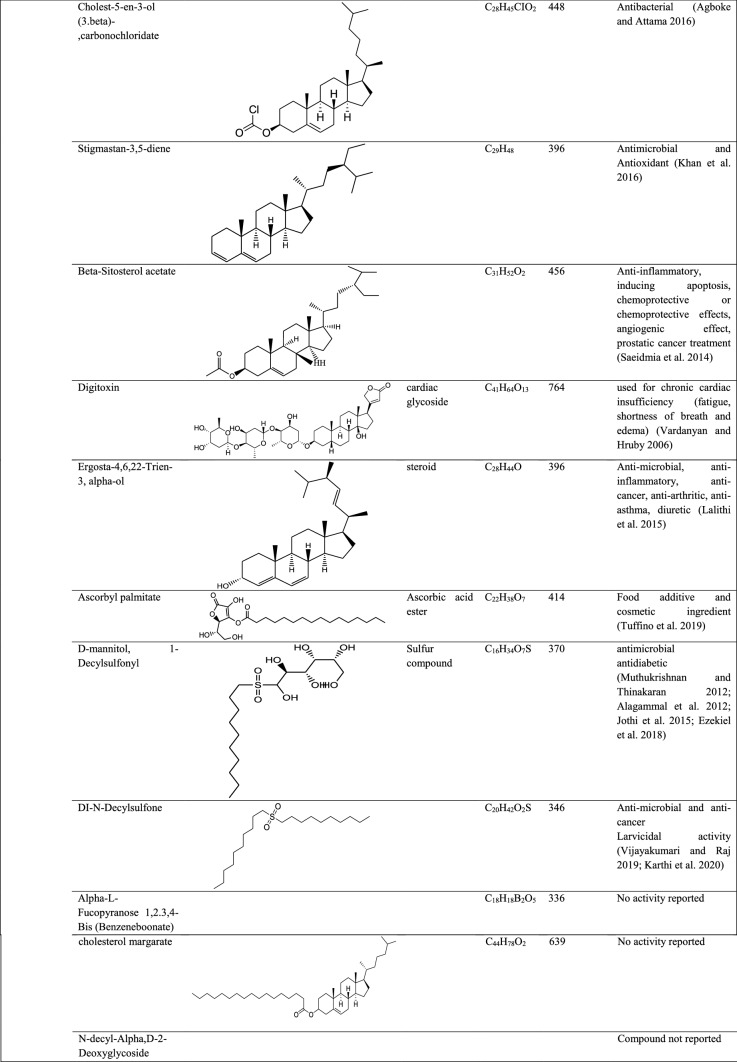
The GC–MS analysis of other orchids mostly focuses on flower scent profile rather than a therapeutic profile, for example, Vanda species (Darmasiwi et al. 2015), Rhynchostylis gigantean Ridl, Rhynchostylis gigantean var. harrisonianum Holtt., Vanda coerulea and Dendrobium parishii Rchb. F., (Julsrigival et al. 2013) and Dendrophylax lindenii (Sadler et al. 2011). In this paper, the GC–MS report of Pleione maculata mainly focused on total medicinal important compounds present in different parts. Compounds such as phenol 4-(ethoxymethyl), heptacosanoic acid methyl ester, 9-octadecanoic acid (Z)- 2-hydroxy-1-(hydroxymethyl) ethyl ester (Vijisaral and Arumugan 2014; Rajalakhsmi and Mohan 2016), nonanoic acid 9(3-hexemyldenecycyclopropylidene-2-hydroxy-1–1 (HYD) (Sahin et al. 2006), alpha-ketostearic acid ethyl ester, 1-naphthaleneproponal alpha-ethyl decahydro-5-(hydroxymethyl), cis, cis, cis-7,10,13-hexadecatrienal (Prabhadevi et al. 2012; Abdulaziz et al. 2019), 1H-purin-2-amine-6-methoxy, 4-cyanobenzoic acid Tridec-2-YNYL ester, alpha-L-fucopyranose 1,2.3,4-bis (benzeneboonate), cholesterol margarate, 2.pyridinecarboxylic acid 6-methoxy, 1-methyl sulfanyl -9–10-dioxo-9,10-dihydro-anthracene-2-carboxylic acid, pyrimidine-2,4,6(1H,3H,5H)-trione-1-octadecyl), E,E-1,9,17-docasatriene (Subavathy and Thilaga 2016; Kumaravel et al. 2019), N-decyl-alpha D-2-deoxyglycoside, cholesterol isocaproate, 7.dehydrocholesterol isocaproate, and pyrimidine-2,4,6(1H,3H,5H)-trione-1-octadecyl) are a few of the compounds which were identified but have no bioactivity reported so far. Compounds such as 8-oxatetracyclo{5.2.1.1(2,6). 1(4,10)}dodecane, 7-tert-butyl-1,9,9-trimeth, docosane, 2,4-dimethyl, kryptogenin 2,4-dinitrophenyl hydrazine, N-decyl-alpha,D-2-deoxyglycoside are some novel compounds which have not been reported earlier. Phytochemical studies and antimicrobial activity of Pleione maculata were also reported which depicts the presence of various phytochemical compounds in different parts of epiphyte and antibacterial activity showed a distinct zone of inhibition against Streptococcus pneumonia (Sympli et al. 2019).
Docking involves the interaction of various polar and non-polar groups, and both play a significant role in the stability of protein–ligand interactions. In docking, water solvent is removed by most programs as they tend to form hydrogen bonds with molecules either as donor or acceptor (Pantsar and Poso 2018). Protein–ligand interactions are strong on the removal of H-bond but in association with other non-covalent interactions (electromagnetic interactions, ionic interactions, van der Waals interaction, and hydrophobic interactions) play an important role in protein–ligand stability (Yadav et al. 2017). van der Waals interactions are non-covalent repulsive or attractive intermolecular interactions, bonding energy decreases from zero to its negative value when the distance of attraction between two molecules is close, and repulsive forces occurs when the distance of separation decreases and bonding energy increases (Singh 2016). van der Waals interaction may be considered weak, but they play a vital role in structure and biomolecules interaction. Hydrogen bonds are also weak non-covalent bond which induces thermal fluctuation in energies and tend to form or break rapidly causing conformational changes during binding (Bronowska 2011). GC–MS bioactive compound Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy, andrographolide, depicts a high binding affinity of − 10.95 and − 9.23Kcal/mol and very good drug-likeness properties when compared to the binding affinity of fisetin, quercetin, isorhamnetin, genistein, luteolin, resveratrol, and apigenin with − 8.5, − 8.5, − 8.3, − 8.2, − 8.2, − 7.9, − 7.7 kcal/mol, respectively, against S2 subunit spike glycoprotein (Rane et al. 2020). The atomic contact energy (ACE) was also reported higher in tea flavonoid catechin products epigallocatechin and theaflavin gallate without re-docking refinement used (Maiti and Banerjee 2020). A docking complex of epitope-based peptide vaccine component and toll-like receptor-5 (TLR-5) of SARS-COV-2 spike glycoprotein exhibits good binding affinity which might have the potential to activate immune cascades for destroying viral antigens (Bhattacharya et al. 2020). Molecular docking between structure-based drug design PC786 bearing ChEMBL ID 4291143 against SARS-COV-2 S protein and Mpro revealed a good binding affinity ranging from − 1.5 to 11.5 kcal/mol (Panda et al. 2020). Pleione maculata compounds Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy, andrographolide, pregabalin, P-menth-8(10)-en-9-ol, 21-acetoxypregenolone, and 1-methylsulfanyl-9,10-dioxo-9,10-dihydro-anthracene-2-carboxylic acid are few drug-likeness compounds obeying the Lipinski RO5 properties such as solubility, lipophilicity (Log P) less than 5, hydrogen bond not more than 5, good solubility, and absorption. The drug-likeness parameters described are the main criteria in primary drug development. The bioactive compounds of P. maculata screened using GC–MS have more potential as drug-likeness against various intractable diseases, for example, here SARS-CoV-2 pneumonia (COVID-19) because of its stronger binding affinity, closer interaction, and abide by ADME characteristics.
Conclusions
GC–MS analysis in methanol, ethanol, and acetone extracts of different parts (leaves, stem, and roots) of P. maculata identified major peaks indicating the presence of phytochemical constituents such as palmitic acid, vitamin E, vitamin A precursor, cardiac glycoside, ascorbic acid, linoleic acid, oleic acid, stearic acid, alkaloid, di-terpenoid, flavonoid, and phenolic and steroidal compound. Cholestenone compound which has the capability to suppress fat accumulation and antiobesity activity is not commonly found in other medicinal plants but reported in GC–MS study of P. maculata. The identified compounds were known to have various activities commonly antimicrobial, anti-inflammatory, antioxidant, cancer preventive, hypocholesterolemic, nematicide, antiasthma, antiarrhythmic activity (improve heartbeat), antiviral activity, larvicidal activity, anticonvulsants, and antiobesity activities. Molecular docking analysis is a structure-based design of drugs. The compounds of P. maculata showed a highly effective binding affinity (atomic contact energy). The spike glycoprotein S2 subunit of SARS-CoV-2 was one rigid target as it initiates membrane fusion with host receptor but bioactive compound Nor-diazepam,3-{N-hydroxymethyl}aminocarbonyloxy exhibits good docking and high binding affinity with atomic contact energy − 10.95 kcal/mol and very high drug-likeness properties. For centuries, plants are the main source of medicinally important natural products. The paper represents the first report on GC–MS analysis, molecular docking, and drug-likeness study on P. maculata. The findings are expected to contribute a significant and major therapeutic impact in the pharmaceutical and nutraceutical companies. An in vitro and in vivo analysis has to be implemented to understand the mechanism of action, cytotoxicity studies on the above effective bioactive compounds. In conclusion, a study on rare Pleione maculata highlights their prospective therapeutic potentialities against various intractable diseases and their bioactive components will enhance a sustainable rural livelihood in both primary and secondary health care and also to save them from extinction and over-exploitation. The effective drug-likeness compound does not have to be separated or isolated directly from the source plant, but the study will provide a basic idea on a synthetic production of effective bioactive compounds.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
I thank Dr. Mahua Gupta Choudhury, MSc. dissertation guide, and Dr. Vedant V. Borah, doctoral research guide, for their support. I would also like to thank Dr. Supriyo Sen for introducing me to Indian Institute of Technology (IIT), Biotech Park Guwahati, Assam, for GC-MS analysis. I sincerely acknowledge the School of Biosciences, Assam Don Bosco University, for permitting and providing me with basic requirements for the methodology part. Lastly, I acknowledge Dr. Rajiv Chandra Dev Goswami, IIT, Guwahati, for the guidance and information on GC-MS analysis. The work is financially supported by Ministry of tribal affair, Govt. of India (Ref: 201819-NFST-MEG-00850).
Compliance with ethical standards
Conflict of interest
The author declares no conflict of interest. The research work is original.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulaziz AA, Dabar MLG, Manting MM, Torres MAJ, Aranas AT, Mindo RAR, Cabrido CK, Demayo CG. Qualitative evaluation of the antimicrobial, antioxidant and medicinally important phytochemical constituents of the ethanolic extracts of the leaves of Gliricidia sepium (JACQ) WALP. Pharmacophore. 2019;10(4):72–83. [Google Scholar]
- Abubakar NM, Majinda TRR. GC-MS analysis amd Preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC) Medicines. 2016;3(3):1–9. doi: 10.3390/medicines3010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeoye-Isijola OM, Olajuyigbe OO, Jonathan GS, Coopoosamy MR. Bioactive compounds in ethanol extract of Lentinus squarrosulus Mont- A Nigerian medicinal macrofungus. Afr J Complement Altern Med. 2018;15(2):42–50. doi: 10.21010/ajtcamv15i2.6. [DOI] [Google Scholar]
- Agboke AA, Attama AA. Bioactive components and antibacterial activities of n-Hexane extract of Moringa oleifera root bark on clinical isolates of methicillin resistant Staphylococcus aureus. Int J Curr Res Chem Pharm Sci. 2016;3(3):1–9. [Google Scholar]
- Agrawal A, Srivastava K, Puri SKK, Chauhan PMS. Synthesis of 2,4,6-trisubstituted pyrimidines as anti-malarial agents. Bio Med chem. 2005;13(5):4645–4650. doi: 10.1016/j.bmc.2005.04.061. [DOI] [PubMed] [Google Scholar]
- Al-Gara’awi NI, Analysis of bioactive phytochemical compound of (Cyperus alternifolius L.) By using gas chromatography- mass spectrometry. IOP Conf Ser Mater Sci Eng. 2019;571:1–19. doi: 10.1088/1757-899X/571/1/012047. [DOI] [Google Scholar]
- Al-Marzoqi AH, Hadi MY, Hameed IH. Determination of metabolites products by Cassia angustifolia and evaluate antimicrobial activity. J Pharm Phytotherapy. 2016;8(2):25–48. doi: 10.5897/JPP2015.0367. [DOI] [Google Scholar]
- Alagammal M, Soris PT, Mohan VR. GC-MS analysis of Polygala rosmarinifolia Wights & Arn. J App Pharm Sci. 2012;2(4):188–190. doi: 10.7324/JAPS.2012.2504. [DOI] [Google Scholar]
- Anand K, Ziehbuhr J, Wandhwani P, Meters JR, Hilgenfield R. Coronavirus main proteinase (3CL pro) structure: basis for design of anti-SARS. Sciencexpress. 2003;300(5626):1763–1767. doi: 10.1126/cience.1085658. [DOI] [PubMed] [Google Scholar]
- Andrusier N, Nussinov R, Wolfson HJ. FireDock: Fast iteration refinement in molecular Docking. Proteins. 2007;69(1):139–159. doi: 10.1002/prot.21495. [DOI] [PubMed] [Google Scholar]
- Ansarali S, Manikandan S, Lakshmanan GMA. Identification of biological components from potential bone healer medicinal plants. J Drug Del Ther. 2018;8(3):32–41. doi: 10.22270/jddt.v8i3.1762. [DOI] [Google Scholar]
- Aparna V, Dileep VK, Mandal KP, Karthe P, Sadasivan C, Haridas M. Anti-inflammatory property of n-Hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Design. 2012;80(3):434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- Asaraja A, Sahayaraj K. Screening of insecticidal activity of brown macroalgal extracts against Dysdercus cingulatus (Fab.) (Hemiptera: Pyrrhocoridae) J Biopest. 2013;6(2):193–203. [Google Scholar]
- Autore G, Marzocco S, Palladino C, Saturnino C, Sinicropi S, Spagnuolo A, Vivacqua E, Capasso A (2010) New amides of arachidonic acid as potential anti-inflammatory drugs: a preliminary study. Biomed Res 21(4)
- Babar AG, Pande A, Kulkarni BG. Anti-fungal activity and investigation of bioactive compounds of marine intertidal bivalve Gafrarium divaricatum from West coast of India. Int J Pure App Bio. 2016;4(2):211–217. doi: 10.18782/2320-7051.2273. [DOI] [Google Scholar]
- Balamurugan TSB, Gunanithi M, Raja I, Vinothkumar D, Ramesh Babu NG, Sumanthi S, Aneesh N, Florida T. Antidiabetic potential of various traditional medicinal plants and in silico validation. Eur J Biotech Biosci. 2017;5(2):34–40. [Google Scholar]
- Balasubramani S, Sabapathi G, Moola AK, Solomon RV, Venuvanalingam P, Diana RKB. Evaluation of the leaf essential oil from Artemisia vulgaricus and its larvicidal and repellent activity against dengue fever vector Aedesaegypti- an experimental and molecular docking investigation. Am Chem Soc Pub. 2018;3:15657–15665. doi: 10.1021/acsomega.8b01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry OP, Practico D, Lawson JA, FitzGerald GA. Trancellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99(9):2118–1227. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Sharma AR, Patra P, Ghosh P, Sharma G, Patra BC, Lee S, Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. Med Virol. 2020;92:618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronowska AK (2011) Thermodynamics of ligand-protein interaction. Implication for molecular design thermodynamic-interaction studies-solid, liquid and gases. In Dr. Juan Carlos Moreno pirajajn (ed) InTech. Thermodynamics. 1–48. 10.5772/19447
- Chandrasekar T, Rao KRM, Kumar VR, Prabhu K, Kumar NS, Divya D. GC-MS analysis, antimicrobial, antioxidant activity of an Ayurvedic medicine, Nimbapatradi Choornam. J Chem Pharm Res. 2015;7(8):124–136. [Google Scholar]
- Chauhan S, Sharma S. Conservation of rare and endangered therapeutically important orchid Pleione maculata (Lindl.) Lindl. & Paxton through pseudobulb culture In-vitro. Ind J Sci Res. 2017;15(1):22–26. [Google Scholar]
- Chen C, Tong Z, Liao D, Li Y, Yang G, Li M. Chemical composition and antimicrobial and DPPH scavenging activity of essential oil of Toona sinensis (A. Juss) Roem from China. BioResources. 2014;9(3):5262–5278. doi: 10.15376/biores.9.3.5262-5278. [DOI] [Google Scholar]
- Chidambaram N, Zhou L, Cohen JS. Targeting of antisense: synthesis of steroid-linked and steroid-bridged oligonucleotides. Drug Deliv. 1996;3(1):27–33. doi: 10.3109/10717549609031378. [DOI] [Google Scholar]
- Chidambarampillai ST, Mohan VR. GC-MS analysis of bioactive components of aerial parts of Fluggea leucopyrus Willd (Euphorbiaceae) J App Pharm Sci. 2013;3(5):126–130. [Google Scholar]
- Da Silva DLL, Nascimento DSM, Silva SHD, Furlan M, Bolzani SV. Antibacterial activity of a stearic acid derivative from Stemodia foliosa. Planta Med. 2003;68(12):1137–1139. doi: 10.1055/s-2002-36346. [DOI] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Nat Sci Rep. 2003 doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmasiwi S, Indriani V, Innata D, Semiarti E (2015) The potential production of Aromatic Compounds in Flowers of Vanda tricolor. In: The 5th International conference on Mathematics and Natural Sciences 090006- 1- 090006–4. 10.1063/1.4930751 . Accessed 25 Apr 2020
- Das NU, Madhavi N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis. 2011;10(159):1–35. doi: 10.1186/1476-511X-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kock N. Targeted analysis of bioactive steroids and oxycholesterols. Method Dev Appl. 2016;1:41. [Google Scholar]
- De LC, Medhi RP. Orchid-A diversified component of farming systems for profitability and livelihood security of small and marginal farmers. J Glob Biosci. 2015;4(2):1393–1406. [Google Scholar]
- De Sousa DP, Raphael E, Brocksom U. Sedative effect of monoterpene alcohols in mice: a preliminary screening. Zeit fur Nat C J Biosci. 2007 doi: 10.1515/znc-2007-7-816. [DOI] [PubMed] [Google Scholar]
- Dinesh MD, Carmel A, George N, Ajma N, Anjana JC, Meenatchisundaram S. Terminaliachebula-A potential natural herbal drug against mastitis isolates. IOSR J Agri Vet Sci. 2016;9(10):64–69. [Google Scholar]
- Doss CGP, Chakraborty C, Chen L, Zhu H. Integrating In silico prediction methods, molecular docking and molecular dynamics simulation to predict the impact of ALK Missense mutations in structural perspective. BioMed Res Int. 2014;2014:1–15. doi: 10.1155/2014/895831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti IE. Bioactive ingredients: antioxidants and polyphenols in tea. Nutrit Rev. 1996;54(11):S51–S58. doi: 10.1111/j.1753-4887.1996.tb03819.x. [DOI] [PubMed] [Google Scholar]
- Dua S, Garg N. Biochemical methods of analysis. New Delhi: Narosa Publishing House; 2013. [Google Scholar]
- Duhovny D, Nussinov R, Wolfson HJ. Efficient unbound docking of rigid molecules. Algorith Bioinform Springer Ver. 2002;2452:185–200. doi: 10.1007/3-540-45784-4_14. [DOI] [Google Scholar]
- Easwaran L, Ramani AV. Phytochemical examination and GC–MS studies of the medicinal plant- Naravelia zeylanica. Int J Res Dev Pharm Life Sci. 2014;3(5):1180–1188. [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharm. 2014;4(177):1–10. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaiyaraja A, Chandramohan G. Comparative phytochemical profile of Indoneesiella echioides (L.) Nees leaves using GC–MS. J Pharm Phytochem. 2016;5(6):158–171. [Google Scholar]
- Elumalai D, Hemalatha P, Kaleena PK. Larvicidal activity and GC-MS analysis of Leucas aspera against Aedes aegyptiAnopheles stephensi and Culex quinquefasciatus. J Saud Soc Agri Sci. 2015;16(4):306–313. doi: 10.1016/j.jssas.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Eswaraiah G, Peele KA, Krupanidhi S, Kumar BR, Venkateswarulu TC. Identification of bioactive compounds in leaf extract of Avicennia alba by GC-MS analysis and evaluation of its in-vitro anticancer potential against MCF7 and HeLa cell lines. J King Saud Univ-Sci. 2020;32(1):740–744. doi: 10.1016/j.jksus.2018.12.010. [DOI] [Google Scholar]
- Ezekiel A, Zubai S, Abiodun O, Iqbal J. GC-MS analysis and anti-diabetic potentials of Bridelia micrantha crude extracts. Integr Mol Med. 2018;5(4):1–5. doi: 10.15761/IMM.1000337. [DOI] [Google Scholar]
- Fang Li. Strcuture, function, and evolution of Coronavirus spike protein. Annu ReV Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastner T, Krimmer PH (2013) US Patent 8,501,810 B2; 2013. Date of patent- 6 Aug 2013; 1–8
- George LO, Radha HR, Somasekariah BV. In vitro anti-diabetic activity and GC-MS analysis of bioactive compounds present in the methanol extract of Kalanchoe pinnata. Ind J Chem. 2018;57B:1213–1221. [Google Scholar]
- Ghosal S, Srivastava KA. Fundamentals of Bioanalytical techniques and instrumentation. New Delhi: Raj Press; 2013. [Google Scholar]
- Gideon AV. GC-MS analysis of phytochemical components of Pseudoglochidion anamalayanum Gamble: an endangered medicinal tree. As J Plant Sci Res. 2015;5(12):36–41. [Google Scholar]
- Gopinath S, Sakthidevi G, Muthukumaraswamy S, Mohan VR. GC-MS Analysis of bioactive constitutes of Hypericum mysorense (Hypericaceae) J Curr Chem Pharm Sci. 2013;3(1):6–15. [Google Scholar]
- Groneberg DA, Hilgenfield R, Zabel P. Molecular mechanism of severe acute respiratory syndrome (SARS) Resp Res. 2005;6(8):1–16. doi: 10.1186/1465-9921-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulam M, Ali NK, Kamal MK, Mayur P (2016) Antisense therapy and its application in biopharmaceutical research: past, present and future. As Pac J Pharm App Sci 61–69
- Guo F, Cheng Li S, Wang L, Zhu D (2012) Protein-protein binding site identification by enumerating the configurations. BMC Bioinform 13(158):1–25. http://www.biomedcentral.com/1471-2105/13/158 [DOI] [PMC free article] [PubMed]
- Gurnani N, Gupta M, Mehta D, Mehta KB. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.) J Taib Univ Sci. 2015 doi: 10.1016/j.jtusci.2015.06.011. [DOI] [Google Scholar]
- Hadi MY, Mohammed GJ, Hameed IH. Analysis of bioactive chemical compounds of Nigella sativa using Gas chromatography-mass spectrometry. J Pharm Phytotherapy. 2015;8(2):8–24. doi: 10.5897/JPP2015.0364. [DOI] [Google Scholar]
- Hall DC, Ji H-F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Trav Med Inf Dis. 2020 doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada HM, Awad M, El-Hefny M, Moustafa MAM. Insecticidal activity of garlic (Allium sativum) and ginger (Zingiber officinale) oils on the cotton leaf worm, Spodoptera littoralis (Boisd) (Lepidoptera:Noctuidae) Afri Entomo. 2018;26(1):84–94. doi: 10.4001/003.026.0084. [DOI] [Google Scholar]
- Hameed IH, Altameme HJ, Idan SA. Artemisia annua: bioachemical products analysis of methanolic aerial parts extract and anti-microbial capacity. Res J Pharm Biol Chem Sci. 2016;7(2):1843–1868. [Google Scholar]
- Hussein MH. Analysis of trace heavy metals and volatile chemical compounds of Lepidium sativum using atomic absorption spectroscopy, gas chromatography-mass spectrometric and fourier-transform infared spectroscopy. Res J Pharm Biol Chem Sci. 2016;7(4):2529–2555. [Google Scholar]
- Ingole NS (2016) Phytochemical analysis of leaf extract of Ocimum americanum L. (Lamiaceae) by GC-MS method. World scientific news 76–87
- Iyamah P, Famuti A, Idu M. GC-MS and molecular docking studies for identification of anti-malarial compounds in PMII-a polyherbal formulation. Chem Res J. 2017;2(1):46–56. [Google Scholar]
- Jacobson M. Sex pheromone of the pink bollworm moth: biological masking by its geometrical isomer. Science. 1969;163(3863):190–191. doi: 10.1126/science.163.3863.190. [DOI] [PubMed] [Google Scholar]
- Jahan Y, Mahmood T, Bagga P, Kumar A, Singh K, Mujahid M. Future prospects of cough treatment, herbal medicines v/s modern drugs. Int J Pharm Sci Res. 2015;6(9):1000–1009. [Google Scholar]
- Javitt NB. 26-Hydroxycholesterol: synthesis, metabolism and biologic acitivities. J Lipd Res. 1990;31:1527–1533. doi: 10.1016/S0022-2275(20)42337-5. [DOI] [PubMed] [Google Scholar]
- Jialal I, Grundy MS. Effect of dietary supplementation with alpha-tocopherol on the oxidative modification of low density lipoprotein. J Lipd Res. 1992;33(6):899–906. doi: 10.1016/S0022-2275(20)41515-9. [DOI] [PubMed] [Google Scholar]
- Jothi SR, Uttayakumari F, Bharathy V. Phytochemical profile of leaf samples of subspecies of Senna italic Mill. Bioscience Discovery. 2015;6(2):106–111. [Google Scholar]
- Julsrigival J, Songsak T, Kirdmanee C, Chansakaow S. Determination of volatile constituents of Thai fragrant orchids by gas chromatography-mass spectrometry with solid-phase microextraction. J Nat Sci. 2013;12(1):43–56. [Google Scholar]
- Karthi S, Vinothkumar M, Karthic U, Manigandan V, Saravanan R, Vasantha-Srinivasan P, Kamaraj C, Shivakumar MS, De Mandal S, Velusamy A, Krutmuang P, Senthil-Nathan S. Biological effects of Avicennia marina (Forssk.) Vierh Extractson physiological, biochemical, and anti-microbial activitiesagainst three challenging mosquito vectors and microbial pathogens. Env Sci Pol Res. 2020 doi: 10.1007/s11356-020-08055-1. [DOI] [PubMed] [Google Scholar]
- Karunanithi A, Venkatachalam S. Optimization of ultrasound-assisted extraction of phenolic compounds from wood apple pulp: identification of phytochemical using GC-MS. Chem Ind Chem Eng Quart. 2019;25(4):361–368. doi: 10.2298/CICEQ180828014K. [DOI] [Google Scholar]
- Khalil MN, Shalaby AE, Ali AIMD, Ali ME, Aboul-Enein MA. Biological activities of secondary metabolites from Emericella nidulans EGCU 312. Afri J Microbiol Res. 2014;8(20):2011–2021. doi: 10.5897/AJMR2014.6827. [DOI] [Google Scholar]
- Khan K, Firdous S, Ahmad A, Muhammad N, Rasheed M, Faizi S. GC-MS profile of antimicrobial and antioxidant fractions from Cordia rothii roots. Pharma Biol. 2016;54(11):2597–2605. doi: 10.3109/13880209.2016.1172320. [DOI] [PubMed] [Google Scholar]
- Kim TK, Chen J, Li W, Zjawiony J, Miller D, Janjetovic Z, Tuckey RC, Slominski A. A new steroidal 5,7-diene derivative, 3-beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid, shows potent anti-proliferative activity. Europe PMC. 2009;75(3):230–239. doi: 10.1016/j.steroids.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Kim JE, Hyun CGu, Lee NH. Neolitsea aciculate essential oil inhibits drug-resistant skin pathogen growth and Propionibacterium acnes-induced inflammatory effects on human monocyte leukemia. Nat Prod Com. 2011;6(8):1193–1198. [PubMed] [Google Scholar]
- Kondo S, Mamada A, Yamaguchi J, Fukuro S. Protective effect of DL-alpha-tocopherol on the cytotoxicity of ultraviolet B against human skin fibroblasts in vitro. Europe PMC. 1990;7(4):173–177. [PubMed] [Google Scholar]
- Kumar D, Arora S, Verma A. Fatty acid composition and antimicrobial and antioxidant activity of Cassla glauca seed extracts. Int J Phytopharm. 2013;4(2):113–118. [Google Scholar]
- Kumar R, Deka CB, Roy RA. Evaluation of orchid species under sub-tropical mid-hills of Meghalaya. HortFlora Res Spect. 2012;1(1):24–28. [Google Scholar]
- Kumar M, Kumar D, Raj V. Studies on Imidazole and its derivatives with particular emphasis on their chemical/biological applications as bioactive molecules intermediated to bioactive molecules. Curr Synth Syst Biol. 2017;5(1):1–10. doi: 10.4172/2332-0737.1000135. [DOI] [Google Scholar]
- Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolic s and GC-MS study of Vitex negundo. Afri J Biochem Res. 2010;4(7):191–195. [Google Scholar]
- Kumar NN, Ramakrishnaiah H, Krishna V, Deepalakshmi PA. GC-MS analysis and antimicrobial activity of seed oil of Broussonetia papyrifera (L.) Vent. Int J Pharm Sci Res. 2015;6:3954–3960. [Google Scholar]
- Kumaravel S, Bhabh PK, Singaravadivel K. GC-MS and FT-IR analysis of the spice ajwain (Trachyspermum ammi) Int J Mod Res Rev. 2016;4(3):1114–1120. [Google Scholar]
- Kumaravel S, Muthukumaran P, Thomas N. Phytochemical, GC-MS and FT-IR analysis of Papver somniferum L. J Pharm Biol Sci. 2019;7(1):1–8. doi: 10.18231/j.jpbs.2019.001. [DOI] [Google Scholar]
- Lakshmi VTP, Rajalakshmi P. Identification of phyto-components and its biological activities of Aloe vera through the gas chromatography–mass spectrometry. Int Res J Pharm. 2011;2(5):247–249. [Google Scholar]
- Lalithi S, Parthipan B, Mohan VR. Determination of bioactive components of Psychotria nilgiriensis Deb & Gang (Rubiaceae) by GC-MS analysis. Int J Pharm Phytochem Res. 2015;7(4):802–809. [Google Scholar]
- Lawal B, Shittu OK, AbdulRasheed-Adeleke T, Ossai PC, Ibrahim AM. Determination of bioactive constituents of giant African snail (Archachatina maginata) Haemolymph. J Pharm Biol Sci. 2015;10(2):59–64. [Google Scholar]
- Lindley, Paxton (1851) Pleione maculata. Flora of China 25: 326–327. http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=242424455.
- Lipinski CA, Lombardo F, Dominy BW, Feeny PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development setting. Adv Drug Del Rev. 1997;23(1–3):3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Maiti S, Banerjee A. Epigallocatechin gallate and theaflavin gallate interaction in SARS-COV-2 spike-protein central channel with reference to the hydroxychloroquine interaction: bioinformatics and molecular docking study. Drug Dev Res. 2020 doi: 10.1002/ddr.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiach E, Schneidman-Duhovny D, Andruisier N, Nussinov R, Wolfson HJ. FireDock: a web server for fast interaction refinement in molecular docking. Nucl Acids Res. 2008;36:w229–w232. doi: 10.1093/nar/gkn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad H, Prabhu K, Rao MRK, Sundram L, Dinakar S, Kumar MS, Vijayalakshmi N. The gas chromatography-mass spectrometry study of one Ayurvedic pain relieving oil ‘Karpooradi Thailam’. Drug Invent Today. 2019;12(7):1542–1546. [Google Scholar]
- Muthukrishnan S, Thinakaran R. Chemical investigation of Pseudarthria viscida root by GC-MS analysis. Pharmacog Comm. 2012;2(3):26–29. doi: 10.5530/pc.2012.3.6. [DOI] [Google Scholar]
- Naidu RJ, Ismail BR, Yeng C, Sasidharan S, Kumar P. Chemical composition and antioxidant activity of the crude methanolic extracts of Mentha spicata. J Physio. 2012;4(1):13–18. [Google Scholar]
- Oduje AA, Awode A, Edah A, Sagay I. Characterization and phytochemical screening of Hexane oil extract from Cissus aralioides seeds. Int J Sci Eng Res. 2015;6(1):112–116. [Google Scholar]
- Okereke SC, Ijeh II, Arunsi UO. Determination of bioactive constituents of Rauwolfia vomitoria Afzel (Asofeyeje) roots using gas chromatography-mass spectrometry (GC-MS) and Fourier transform infrared spectrometry (FT-IR) As J Pharm Pharmacol. 2017;11(2):25–31. doi: 10.5897/AJPP2016.4712. [DOI] [Google Scholar]
- Otaibi AA, McCluskey A (2013) Multicomponent reactions in Ionic liquids. Chapter-18, Ionic Liquids-New aspects for the future. IMTECH, pp 458–498
- Padma M, Ganesan S, Jayaseelan T, Azhagumadhavan S, Sasikala P, Senthikumar S, Mani P. Phytochemical screening and GC-MS analysis of bioactive compounds present in ethanolic leaves extract of Silybum marianum (L.) J Drug Del Therap. 2018;9(1):85–89. doi: 10.22270/jddt.v9i1.2174. [DOI] [Google Scholar]
- Palakkal L, Hukuman NHZ, Mullapally J. Anti-oxidant activities and chemical composition of various crude extracts of Lepidagathis keralensis. J App Pharm Sci. 2017;7(6):182–189. doi: 10.7324/JAPS.2017.7062. [DOI] [Google Scholar]
- Panda PK, Arul MN, Patel P, Verma SK, Luo W, Rubahn HG, Mishra YK, Suar M, Ahuja R. Structure-based drug designing and immunoinformatics approach for SARS-COV-2. Sci Adv. 2020;6:1–14. doi: 10.1126/sciadv.abb8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant B. Medicinal orchids and their uses: tissue culture a potential alternative for conservation. Afri J Plant Sci. 2003;7(10):445–467. doi: 10.5897/AJPS2013.1031. [DOI] [Google Scholar]
- Pantsar T, Poso A. Binding affinity via docking: fast and fiction. Molecules. 2018;23(1899):1–11. doi: 10.3390/molecules23081899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulpriya K, Tresina PS, Mohan VR. Assessment of bioactive constituents by GC-MS of Crotalaria longipes Wight & Arn: An endemic plant. Int J Pharm Phytochem Res. 2014;6(4):1043–1048. [Google Scholar]
- Pereira DM, Correia-da-silva G, Valentao P, Teixeira N, Andrade PB. Anti-inflammatory effect of unsaturated fatty acid acids and Ergosta-7,22-dien-3-ol from Marthasterias glacialis. Prevention of CHOP-Mediated ER-stress and NF-kB activation. PLoS ONE. 2014;9(2):e88341. doi: 10.1371/journal.pone.0088341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AEM, Araujo GS, Morais IM, Sa PN, Lima MC, Rosa AC, Siqueira PE, Johann S, Lima SRAL. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Ann Braz Acad Sci. 2017;89(3):1671–1681. doi: 10.1590/0001-376520172016090. [DOI] [PubMed] [Google Scholar]
- Plainfosse H, Burger P, Azoulay S, Landreau A, Verger-dubois G, Fernandez X. Development of a natural-age ingredient based on Quercus pubescens wild leaves extract—a case study. Cosmetics. 2017;1(15):1–22. doi: 10.3390/cosmetics5010015. [DOI] [Google Scholar]
- Prabhadevi V, Sathish S, Johnson M, Venkatramani B, Janakiraman N (2012) Phytochemical studies on Allamanda cathartica L. using GC-MS. As Pac J Trop Med S550–S554
- Prabhu DS, Rajeswari VD. In silico docking analysis of bioactive compounds from chinese medicine Jinqi Jiangtang Tablet (JQJTT) using Patch Dock. J Chem Pharm Res. 2016;8(5):15–21. [Google Scholar]
- Qamar MTU, Alqahtani SM, Alamri MA, Chen LL. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurzeddine W, Fadel H, Mechehoud Y, Chalchat JC, Figueredo G, Chalard P, Benayache F, Benayache S. Chemical composition and antioxidant activity of the fruit essential oil of Zizyphus lotus (L.) Desf. (Rhamanaceae) Int J Pharma Phytochem Res. 2017;9(2):228–232. [Google Scholar]
- Rajalakshmi K, Mohan VR. Determination of Bioactive components of Myxopyrum serratulum A. W. HILL (Oleaceae) stem by GC-MS analysis. Int Res J Pharm. 2016;38(1):30–35. [Google Scholar]
- Rajashyamala G, Elango V. Identification of bioactive components and its biological activities of Evolvulus alsinoides linn: a GC-MS study. Int J Chem Stud. 2015;3(1):41–44. [Google Scholar]
- Rajendra P, Bharathidasan R, Sureshkumar K. GC-MS analysis of phyto-components in raw and treated sugarcane juice. Int J Curr Microbiol App Sci. 2017;6(7):51–61. doi: 10.20546/ijcmas.2016.501.007. [DOI] [Google Scholar]
- Rajeswari G, Murugan M, Mohan RV. GC-MS analysis of bioactive components of Hugonia mystax L. bark (Linaceae) J Pharm Biomed Sci. 2013;29(29):818–824. [Google Scholar]
- Rane JS, Chatterjee A, Kumar A, Ray S (2020) Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an In silico study for drug development. ChemRxiv. 10.26434/chemrxiv.12094203.v1 [DOI] [PMC free article] [PubMed]
- Rautela I, Sharma MD, Sharma N, Kishor K, Singh K, Sharma N. Comparative GC-MS analysis of leaf and root extract of medicinal plant Withania somnifera. World J Pharm Res. 2018;7(2):956–972. [Google Scholar]
- Ren R, Hashimoto T, Mizuno M, Takigawa H, Yoshida M, Azuma T, Kanazawa K. A lipid peroxidation product 9-oxononanoic acid induces phospholipase A2 activity and thromboxane A2 production in human blood. J Clin Biochem Nutri. 2013;52(3):228–233. doi: 10.3164/jcbn.12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SI, Mishra N (2013) Traditional Indian medicines used for the management of Diabetes Mellitus. J Diab Res10.115/2013/712092 [DOI] [PMC free article] [PubMed]
- Sadler JJ, Smith MJ, Zettler WL, Alborn TH, Richardson WL. Fragrance composition of Dendrophylax lindenii (Orchidaceae) using a novel technique applied in situ. Euro J Env Sci. 2011;1(2):137–141. doi: 10.14712/23361964.2015.56. [DOI] [Google Scholar]
- Saeidnia S, Manayi A, Gohari AR, Abdollahi M. The story of Beta-sitosterol—a review. Euro J Med Plnt. 2014;4(5):590–609. doi: 10.9734/EJMP/2014/7764. [DOI] [Google Scholar]
- Sahin N, Kula I, Erdogan Y. Investigation of antimicrobial activities of nonanoic acid derivatives. Fresenius Environ Bull. 2006;15(2):141–143. [Google Scholar]
- Sardar R, Satish D, Birla S, Gupta D. Comaprative analyses of SARS-CoV2 genomes from different geographical locations and other coronavirus family genomes reveals unique features potentially consequential to host-virus interaction and pathogenesis. BioRxiv. 2020 doi: 10.1101/2020.03.21.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyabalan B, Packia LM, Muthukumarasamy S, Mohan VR. GC-MS Analysis of bioactive components of Petiveria alliacea L. whole plant (Phytolaccaceae) Int J Pharm Res Health Sci. 2014;2(5):387–392. [Google Scholar]
- Saxena M, Faridi U, Srivastava SK, Darokar MP, Mishra R, Pal A, Shisodia B, Khanuja SPS. A cytotoxic and Hepatoprotective agent from Withania somifera and biological evaluation of its Ester derivatives. Nat Prod Comm. 2007;2(7):775–778. doi: 10.1177/2F1934578X0700200714. [DOI] [Google Scholar]
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucl Acids Res. 2005;33:W363–367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvan PS, Velavan S. Analysis of bioactive compounds in methanol extracts of Cissus vitiginea leaf using GC-MS technique. Rasayan J Chem. 2015;8(4):443–447. [Google Scholar]
- Sharad S. Antisense therapy: an overview. Bethesda: Henry Jackson foundation for the advancement of military medicine; 2019. [Google Scholar]
- Sharma V, Chitranshi N, Agarwal AK. Significance and biological importance of pyrimidine in the microbial world. Int J Med Chem. 2014;2014:1–31. doi: 10.1155/2014/202784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Zhou Y, Ye J, Al-maskri AAA, Kang Y, Zeng S, Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shree LR. Efficacy of supplementation of flaxseed and soybean on post-operative cancer patients. Chennai: Thassim Beevi Abdul Kader College for Women; 2012. [Google Scholar]
- Shweta M, Rashmi D. In-vitro ADME studies of TUG-891, a GPR-120 inhibitor using Swiss ADME. J Drug Del Therap. 2019;9(2):266–369. doi: 10.22270/jddt.v9i2-s.2710. [DOI] [Google Scholar]
- Singh K, Kaur T. Pyrimidine-based antimalarials: design stragtegies and antiplasmodial effects. MedChem Comm. 2016 doi: 10.1039/C6MD00084C. [DOI] [Google Scholar]
- Singh S, Srivastava HK, Kishor G, Singh H, Agrawal P, Raghava GPS. Evaluation of protein-ligand docking methods on peptide-ligand complexes for docking small ligands to peptides. BioRxiv. 2017 doi: 10.1101/212514. [DOI] [Google Scholar]
- Singh AK (2016) Structure, synthesis and application of nanoparticles. Engineered nanoparticles, Structure, properties and mechanisms of toxicity
- Subavathy P, Thilaga RD. GC-MS analysis of bioactive compounds from whole body tissue methanolic extract of Cypraea arabica (L.1758) World J Pharma Res. 2016;5(3):800–806. [Google Scholar]
- Suffo AKL, Ashish R, Tedonkeng PE, Kuiate JR. Effect of processing methods on chemical composition and antioxidant activities of two Amaranthus Sp. Harvested in West Region of Cameroons. J Nutri Food Sci. 2016;6(2):1–9. [Google Scholar]
- Sujatha K, Sivakamasundari MC. GC-MS analysis of phytocomponents and total anti-oxidant activity of hexane extract of Sinapis alba. Int J Pharm Chem Biol Sci. 2014;4(1):112–117. [Google Scholar]
- Sujatha K, Vijayalakshmi V. Foliar application of Caulerpa racemosa seaweed extract as bio-stimulant for enhancement of growth and yield of blackgram (Vigna mungo L.) Int J Adv Res Tech. 2013;2(10):216–230. [Google Scholar]
- Sulthanabegam M, Palani S, Senthikumar B. Phytoconstituents screening by GC-MS and evaluation of In-vitro Gastroprotective and antioxidant activity of Vulva lactuca. Int J Pharm Chem Biol Sci. 2019;9(2):939–950. [Google Scholar]
- Surana P, Singh S, Manokaran S, Reddy AHM. The interactions between variants of GALT in Galactosemia: In silico approach. Int J Fund App Sci. 2018;7(3):14–18. [Google Scholar]
- Suzuki K. Anti-obesity effect of cholest-4-en-3-one, an intestinal catabolite of cholesterol, on mice. J Nutri Sci Vit (Tokyo) 1993;39(5):537–543. doi: 10.3177/jnsv.39.537. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Shimizu T, Nakata T. The cholesterol metabolite cholest-4-en-3one and its-oxo derivatives suppress body weight gain, body fat accumulation and serum lipid concentration in mice. Bioorg Med Chem Lett. 1998;8(16):2133–2138. doi: 10.1016/S0960-894X(98)00362-X. [DOI] [PubMed] [Google Scholar]
- Sympli HD, Paul S, Borah VV, Choudhury M. Phytochemical and anti-bacterial activity of an endangered orchid, Pleione maculata (Orchidaceae) of Meghalaya. Int J Res Anal Rev. 2019;6(2):221–232. doi: 10.1729/Journal.21437. [DOI] [Google Scholar]
- Teoh ES (2016) Orchids of Asia. Times Editions/Marshall Cavendish Singapoore 367
- Thomas E, Aneesh TP, Thomas DG, Anandan R. GC-MS analysis of phytochemical compounds present in the rhizomes of Nervilia aragoana Gaud. As J Pharm Clin Res. 2013;6(3):68–74. [Google Scholar]
- Tian S, Hu W, Niu Li, Lui H, Xu H, Xiao S (2020) Pulmonary pathology of early-phase 2019 Novel Coronavirus (COVID-19) Pneumonia in two patients with lung cancer. J Thorac Oncol 1–5 [DOI] [PMC free article] [PubMed]
- Tsering J, Tam N, Tag H, Gogoi JB, Apang O. Medicinal orchids of Arunachal Pradesh: a review. Bull Arunachal For Res. 2017;32(1& 2):1–16. [Google Scholar]
- Tufino C, Bernal C, Ottone C, Romero O, Illanes A, Wilson L. Synthesis with immobilized lipases and downstream processing of Ascorbyl palmitate. Molecules. 2019;24(3227):1–13. doi: 10.3390/molecules24183227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi T, Agarwal M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pista stratiotes L. and Eichhornia crassipes (Mart.) solms. J Pharm Phytochem. 2016;6(1):195–206. [Google Scholar]
- Ukil S, Laskar S, Roy NR. Physicochemical characterization and antibacterial activity of the leaf oil of Crotalaria pallida Aiton. J Taib Univ Sci. 2015 doi: 10.1016/j.jtusci.2015.07.001. [DOI] [Google Scholar]
- Umaiyambigai D, Saravanakumar K, Raj AG (2017) Phytochemical profile and antifungal activity of leaves methanol extract from the Psydrax dicoccos (Gaertn) Teys. & Binn. Rubiaceace family. Int J Pharma Phytochem Ethnomed 7: 53–6. 10.18052/www.scipress.com/IJPPE.7.53.
- Upgade A, Bhaskar A. Characterization and medicinal importance of phytoconstituents of C. papaya from down South Indian region using Gas Chromatography and Mass Spectroscopy. As J Pharm Clin Res. 2013;6(4):101–106. [Google Scholar]
- Valiei M, Shafaghat A, Salimi F. Chemical composition and antimicrobial activity of the flower and root hexane extracts of Althaea officinalis in Northwest Iran. J Med Plnts Res. 2011;5(32):6972–6976. doi: 10.5897/FJMPR11.963. [DOI] [Google Scholar]
- Vardanyan RS, Hruby VJ (2006) Digitoxin.Synthesis of essential drug. Synth Essent Drugs Cardiotonic Drugs. https://doi.org/10.1016/B978-044452166-8/50017-0
- Vijayakumari J, Raj TLS (2019) GC-MS analysis of secondary metabolites from acetone and chloroform extract of Dicranopteris linearis (burm.F.)Underw. Int Res J Biol Sci 8(9): 39–43.
- Vijisaral ED, Arumugam S. GC-MS analysis of bioactive constituents of Indigofera suffruticosa leaves. J Chem Pharm Res. 2014;6(8):294–300. [Google Scholar]
- Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel corona-virus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agent. 2020;105948:1–7. doi: 10.1016/j.ijantimicag.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SL, Wee W, Siong FYJ, Syamsumir FD. Characterization of antimicrobial, antioxidant, anticancer property and chemical composition of Michelia champaca seed and flower extract. Stam J Pharm Sci. 2011;4(1):19–24. doi: 10.3329/sjps.v4i1.886. [DOI] [Google Scholar]
- Wen C, Shyur L, Jan J, Liang P, Kuo C, Arulselvan P, Wu J, Yang N. Traditional chineses medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-COV replication. J Trad Compl Med. 2011;1(1):41–50. doi: 10.1016/s2225-4110(16)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Critical review report: Pregabalin. Geneva: Expert committee on Drug Dependence forty-first meeting; 2018. [Google Scholar]
- Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Inf Dis. 2020 doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, Xu L, Li X, Liu H, Yin P, Zhao J, Li Y, Wang X, Li Y, Zhang Q, Xu GCH. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rept. 2017;7:1–12. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Pandey SK, Singh VK, Goel Y, Kumar A, Singh SM. Molecular docking studies of 3-bromopyruvate and its derivaties to metabolic regaltory enzymes: Implication in deisgning of novel anti-cancer therapeutic strategies. PLoS ONE. 2017;2017:1–15. doi: 10.1371/journal.pone.0176403.g005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-New Coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wang X (2020) COVID-19: a new challenge for human beings. Cell Mol Immunol 1–3 [DOI] [PMC free article] [PubMed]
- Yusufzai SK, Khan MS, Hanry EL, Rafatullah M, Elison BB. GC-MS analysis of chemical constituents and in-vitro antioxidant activity of the organic extracts from the stem of Bridelia stipularis. Sans Malaysiana. 2019;48(5):999–1009. doi: 10.17576/JSM-2019-4805-08. [DOI] [Google Scholar]
- Yuvaraj N, Arul V. In vitro anti-tumor, anti-inflammatory, anti-oxidant, and antibacterial activities of marine brown alga Sargassum wightii collected from Gulf of Mannar. Glob J Pharm. 2014;8(4):566–577. doi: 10.5829/idosi.gjp.2014.8.4.84281. [DOI] [Google Scholar]
- Zafar MZ. A case study: pnuemonia. Occu Med Health Aff. 2016;4(4):1–3. [Google Scholar]
- Zaini RG, Brandt K, Clench MR, Le Maire CL. Effects of bioactive compounds from carrots (Daucus carota L.), Polyacetylenes, Beta-carotene and Lutein on human lymphoid leukaemia cells. Anti-canc Agnts Med Chem. 2012;12:1–13. doi: 10.2174/187152012800617704. [DOI] [PubMed] [Google Scholar]
- Zhang C, Vasmatzis G, Cornette JL, DeLisi C. Determination of atomic desolvation energies from the structures of crystallized proteins. J Mol Bio. 1997;267(3):707–726. doi: 10.1006/jmbi.1996.0859. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun H, Chen H, Chen S, Li Zeng, Wang T (2017) Anti-fungal activity, mechanism studies on α- Phellandrene and nonanal against Penicillium cyclopium. Bot Stud 58(13): 1–9. 10.1186/2Fs40529-017-0168-8 [DOI] [PMC free article] [PubMed]
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Inten Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu WY, Hou JJ, Zhang LL, Li FF, Gao L, Wu XD, Shi JY, Zhang R, Long HI, Lei M, Wu WY, Guo DA, Chen KX, Hofman LA, Ci ZH. Active constituents and mechanisms of respiratory detox shot, a traditional chinese medidine prescription, for COVID-19 control and prevention: network-molecular docking LC-MS analysis. J Int Med. 2020 doi: 10.1016/j.joim.2020.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.