Abstract
Background:
The purpose of this study was to systematically review the prevalence of class 1 integrons, antibiotic resistance pattern in Pseudomonas aeruginosa (P. aeruginosa) isolated from clinical samples other than burn samples.
Methods:
The Web of Science, PubMed, Scopus, and Science Direct databases were searched using keywords based on the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. The cross-sectional studies published from 1st January 2000 until 1st January 2019 were included which addressed the prevalence of class 1 integrons and antibiotic-resistance in P. aeruginosa isolated from clinical samples other than burn samples. Meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) software. The random-effects model, Cochran’s Q and I2 tests were applied for statistical analyses.
Results:
Eight articles met the eligibility standards for including in the present meta-analysis. The combined prevalence of class 1 integrons in P. aeruginosa isolated from clinical samples other than burn samples was reported by 40% (95% CI:26.1–55.8%). The pooled prevalence of Multi-Drug Resistant (MDR) P. aeruginosa isolates was 70.1%. The highest prevalence of combined antibiotic resistance was related to carbenicillin with a resistance rate of 79.9%. In general, 6 (75%) out of the 8 included studies showed the correlation between the presence of class 1 integrons and antibiotic resistance.
Conclusion:
Regarding the correlation between the presence of integrons and the high antibiotic resistance reported by studies included in the present review, there is the need for preventive measures to prevent the spread of resistance by integrons and transferring to other micro-organisms.
Keywords: Burns, Drug resistance, Integrons, Pseudomonas aeruginosa
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is an aerobic non-fermentative gram-negative bacillus and a common nosocomial pathogen. P. aeruginosa is one of the dreadful causes of severe infections in clinical settings particularly in immunocompromised patients, as well as individuals hospitalized in Intensive Care Unit -(ICU), patients with cystic fibrosis, and those with severe burns 1.
The selective pressure imposed by inappropriate antibiotic use on one hand, and the increasing use of antibiotics on the other hand are probably the main causes for the development of MultiDrug-Resistant (MDR) P. aeruginosa in hospital settings 2.
Infections caused by MDR- P. aeruginosa strains present clinically significant challenges. The empiric antibiotic therapy for MDR P. aeruginosa has represented poor outcomes with high morbidities, mortalities, long hospital stays and high economic and therapeutic burden on both health systems and the patients 3.
According to the reports of the European Centre for Disease Prevention and Control (ECDC), P. aeruginosa comprises 9% of all hospital-based infections presenting the fourth most common hospital pathogen in Europe 4. Also, CDC reported similar findings in the United States with the frequency about 7% for infections caused by P. aeruginosa in hospital settings 5. A survey in Spain in 2016 showed a higher prevalence about 13% for this microorganism in ICU of hospitals6. However, the rate of MDR P. aeruginosa isolates in Iran has been reported between 30–100% 7.
Some possible antimicrobial resistance mechanisms in P. aeruginosa include the production of beta-lactamases, overexpression of efflux pumps, down regulation of outer membrane porins, production of AmpC or loss of OprD, genetic mutations, and finally expression of integrons on plasmids and transposons 4,8. The integrons are specialized genetic structures by which bacteria can acquire resistance genes through horizontal transmission 9.
Integrons can acquire external drug resistance gene cassettes and integrate them by site-specific recombination 10,11. Generally, integrons are comprised of an integrase gene, two conserved sequences called sul1 and int1, and a variable region harboring gene cassettes between the two conserved fragments 12.
Based on the structure of integrase gene, several classes of integrons have been recognized. Three main classes of integrons (i.e. class 1, 2, and 3) have been identified in gram-negative bacteria including Enterobacteriaceae, and Pseudomonas. Among these, class 1 integrons are the most frequent. Class 1 integrons usually carry one or several gene cassettes conferring resistance to a broad spectrum of antibacterial agents such as β-lactams, aminoglycosides and fluoroquinolones 13,14.
Regarding the significance of P. aeruginosa in hospital acquired infections, and the lack of a comprehensive study on the prevalence of class 1 integrons in P. aeruginosa isolates and antimicrobial resistance patterns of this organism in Iran, an attempt was made to study the prevalence of class 1 integrons, antibiotic resistance pattern in P. aeruginosa isolated from clinical samples other than burn samples.
Materials and Methods
Search strategy
The studies addressing the prevalence of class 1 integrons and antibiotic resistance patterns of P. aeruginosa isolates from Iranian patients’ clinical specimens other than burn samples published from 1st January 2000 till 1st January 2019 were included. The search was carried out in Web of Science, Cochrane Library, Scopus, PubMed, and Google Scholar databases. The search was restricted to the English original articles reporting the prevalence of class 1 integrons in P. aeruginosa. The following keywords from medical subject headings or titles or abstracts were used with the help of Boolean operators (AND, OR). “P. aeruginosa”, “P. aeruginosa”, “clinical sample”, “clinical specimen”, “drug susceptibility”, “drug-resistance”, “antibiotic resistance”, “integrons”, “prevalence”, “Int1”, “Iran”, “correlation” and “local studies” were used alone or in combination to conduct a complete search on antibiotic resistance.
Eligibility criteria
All the original articles reporting the prevalence of integrons in P. aeruginosa isolated from clinical specimens of Iranian patients were considered. Only those studies that performed antibiotic susceptibility tests based on Clinical and Laboratory Standards Institute (CLSI) guidelines were considered and selected. Foreign studies (i.e. not performed in Iran) and those studies that did not follow CLSI guidelines in performing antibiotic susceptibility tests were excluded. Also, all review types were removed. Studies published in languages other than English, articles only available in abstract form and also duplicate publications, letters, case reports and congress abstracts were excluded.
Data extraction
The intended data including first author’s name, year of publication, location of study, sample size, the frequencies of MDR and class 1 integrons, hospital wards, and correlation between the presence of integrons and antibiotic resistance were recorded in a data extraction form designed by the researchers.
Qualification of the studies
The strengthening the reporting of observational studies in epidemiology (STROBE) checklist was utilized for qualifying the methodology of the included studies 15. Based on the qualification criteria, the studies were categorized as high, medium or low quality. For quality assessment, a scoring system was used, in which ten questions were designed and for each question one score was considered. If the answer was positive, a score of 1 and if the answer was negative or doubtful, a score of zero was given. Accordingly, the studies were divided into three groups of weak (Score between 1–4), moderate (Score between 5–8) and strong (Score above 8).
Statistical analysis
Meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) software (Version 3.3.070). The random-effects model, Cochran’s Q and I2 tests were applied for statistical analyses. The prevalence of class 1 integrons in P. aeruginosa isolates was reported with 95% Confidence Interval (CI). To evaluate possible publication bias, the funnel plot and quantitative Egger weighted regression test were applied. p-value <0.05 was considered a statistically significant cut off for detecting any publication bias.
Results
Inclusion process and characteristics of eligible studies
A total of 911 papers were identified through literature search process (Supplementary figure). Out of these, 456 duplicate studies were recognized and removed. Also, 90 records with irrelevant titles were excluded. The abstracts of 365 remained articles were considered and 259 were excluded owing to justified reasons. Then, 106 full texts of studies were assessed of which, 98 studies were omitted due to either lack of data accessibility, missing data, or not reporting the frequency of class 1 integrons. Finally, 8 articles were included in the quantitative evaluation (Meta-analysis).
Supplementary figure.
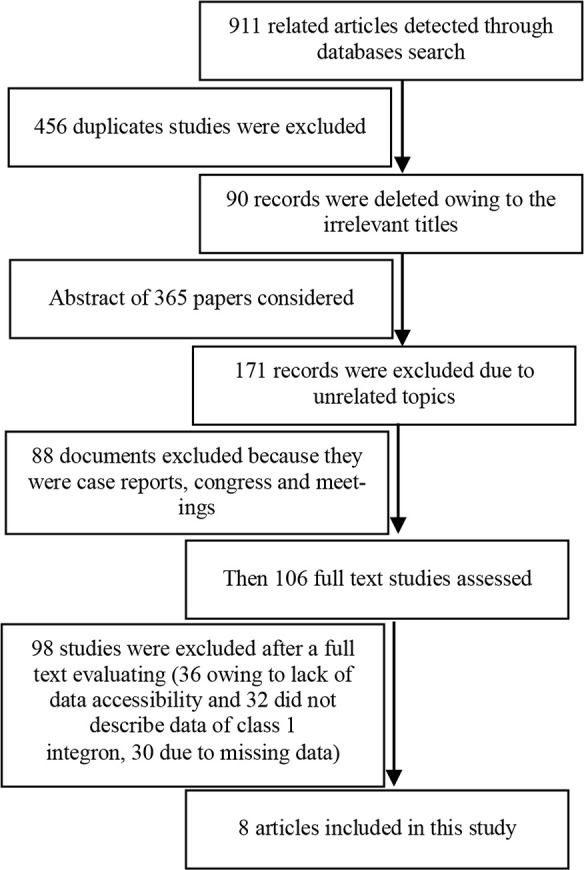
Flow chart diagram of selection studies.
As shown in figure 1, the prevalence of class 1 integrons in P. aeruginosa isolated from clinical specimens varied from 13.3 to 99.1%. The clinical samples were mostly collected from ICU. The pooled prevalence of MDR P. aeruginosa isolates varied from 13.1 to 100%. Overall, 6 (75 %) out of the 8 included studies in the present review reported the correlation between the presence of class 1 integrons and antibiotic resistance pattern (Table 1, p<0.05).
Figure 1.
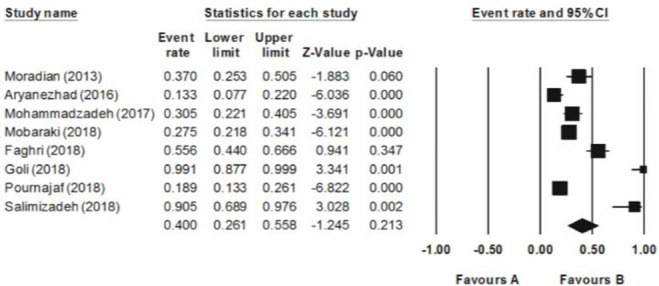
Forest plot of the meta-analysis of epidemiology of class 1 integron in P. aeruginosa isolated from clinical specimens.
Table 1.
Characteristics of selected studies from Iranian patients
| Studies | Publication year | Location | Sample size (N) | MDR N (%) | Int1 in total N (%) | Units | Correlation* |
|---|---|---|---|---|---|---|---|
| Moradian Kouchaksaraei F, et al (23) | 2013 | Babol | 54 | 53 (98.1) | 20 (37) | ICU | Yes |
| Aryanezhad M, et al (24) | 2016 | Bandar Abbas | 90 | 12(13.3) | 12(13.3) | ICU, non-ICU | yes |
| Mohammadzadeh A, et al (36) | 2017 | Gonabad | 95 | - | 29 (30.5) | Different wards | - |
| Mobaraki S, et al (2) | 2018 | Tabriz | 200 | 106(53) | 55 (27.5) | Different wards | Yes |
| Faghri J, et al (18) | 2018 | Isfahan | 72 | - | 40(55.6) | ICU, non-ICU | Yes |
| Goli HR, et al (26) | 2018 | Tabriz | 57 | 57(100) | 57(99.1) | ICU | Yes |
| Pournajaf A, et al (37) | 2018 | Different | 143 | 12(8.4) | 27(18.9) | CF Patient (Iran) | - |
| Salimizadeh Z, et al (38) | 2018 | Tehran | 21 | 21(100) | 19(90.5) | Different wards | Yes |
Correlation between Int1 and antibiotic resistance, ICU: Intensive care unit, CF: Cystic fibrosis.
Overall effects
There was a statistically significant heterogeneity among the included studies (Q2=74.1, I2=90.5, t=1.8, p=0.12). Accordingly, the random effects model was applied to combine the prevalence of class 1 integrons in P. aeruginosa isolates. The combined prevalence of class 1 integrons was obtained as 40% (95% CI: 26.1–55.8%) in Iranian patients’ clinical specimens other than burn samples (Table 2). The pooled prevalence of MDR P. aeruginosa isolates was reported as 70.1% (95% CI: 32.6–91.9%).
Table 2.
Analysis of epidemiology of class 1 integron, and MDRs in P. aeruginosa isolated from clinical specimens
| Subgroups overall effect | Number of studies | Heterogeneity test | Egger’s test | Random model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence (95% CI) (%) | Z | p | Q | p | I2 | T | p | ||
| Int1 | 8 | 40(26.1–55.8) | 1.2 | 0.00 | 74.1 | 0.00 | 90.5 | 1.8 | 0.12 |
| MDR | 6 | 70.1(32.6–91.9) | 1 | 0.00 | 119.1 | 0.00 | 95.8 | 0.43 | 0.68 |
Note: Int1: Class 1 integron; MDR: Multi-drug resistant.
Publication bias
The publication bias was checked using funnel plot. Concerning possible asymmetrical data distribution in the selected studies, the Egger’s linear regression test was used to further evaluate any publication bias. Nevertheless, the results of the Egger’s linear regression test revealed no publication bias (p=0.12) (Figure 2).
Figure 2.
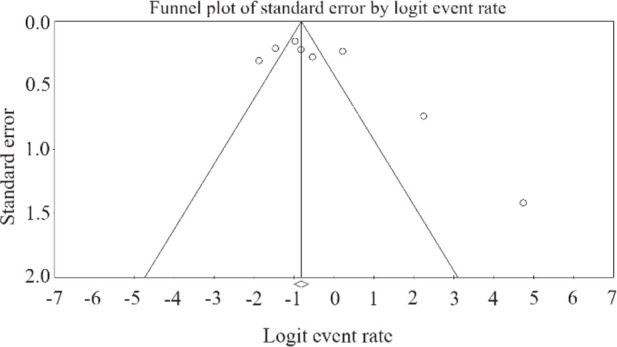
Funnel plot of meta-analysis on the epidemiology of class 1 integron in P. aeruginosa isolated from clinical samples.
Subgroups analysis
Subgroups analysis revealed that the highest combined antibiotic resistance belonged to carbenicillin following by cloxacillin and cefotaxime with respective resistance rates of 79.9, 77.4, and 76.6%. On the other hand, the most effective antibiotics against P. aeruginosa were polymyxin b and colistin with no resistance rate (Figure 3, Table 3).
Figure 3.
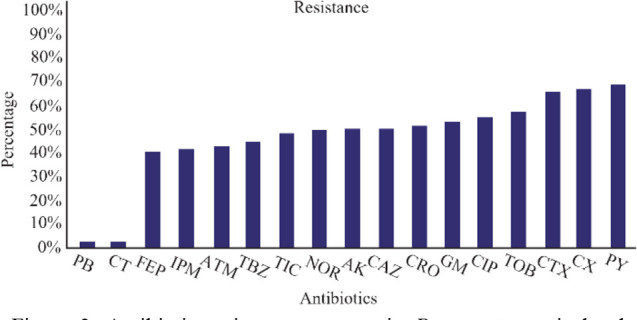
Antibiotic resistance patterns in P. aeruginosa isolated from clinical samples. Imipenem (IPM), Ciprofloxacin (CIP), Gentamicin (GM), Amikacin (AK), Ceftriaxone (CRO), Ceftazidime (CAZ), Cefepime (FEP), Piperacillin/tazobactam (TBZ), Aztreonam (ATM), Cloxacillin (CX), Tobramycin (TOB), Ticarcillin (TIC), Carbenicillin (PY), Cefotaxime (CTX), Norfloxacin (NOR), Polymyxin B (PB), Colistin (CT).
Table 3.
Subgroups analysis for antibiotic resistance in P. aeruginosa isolated from clinical samples other than burn samples
| Subgroups | Number of studies | Heterogeneity test | Egger’s test | Random model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence (95% CI) (%) | Z | P | Q | P | I2 | T | p | ||
| Imipenem | 7 | 47.5(32.8–62.7) | 0.31 | 0.00 | 352.6 | 0.42 | 96.3 | 0.83 | 0.75 |
| Ciprofloxacin | 8 | 62.4(48.6–74.5) | 1.7 | 0.00 | 406.7 | 0.2 | 96.06 | 1.1 | 0.07 |
| Gentamicin | 7 | 60.5(47.4–72.2) | 1.5 | 0.11 | 288.9 | 0.37 | 95.1 | 0.9 | 0.11 |
| Amikacin | 8 | 57.4(46.4–67.8) | 1.3 | 0.00 | 262.2 | 0.9 | 94.2 | 0.1 | 0.18 |
| Ceftriaxone | 5 | 58.6(29.8–82.5) | 0.56 | 0.00 | 129.8 | 0.72 | 96.9 | 0.37 | 0.57 |
| Ceftazidime | 6 | 57.8(44.4–70.2) | 1.14 | 0.00 | 289.6 | 0.99 | 95.5 | 0.00 | 0.25 |
| Cefepime | 5 | 46.4(26.6–67.4) | 0.32 | 0.00 | 250.9 | 0.34 | 96.8 | 1 | 0.74 |
| Piperacillin/tazobactam | 6 | 50.6(40.4–60.8) | 0.11 | 0.00 | 102.9 | 0.35 | 91.2 | 0.97 | 0.9 |
| Aztreonam | 5 | 48.1(27.9–68.9) | 0.17 | 0.00 | 180.7 | 0.1 | 96.6 | 1.9 | 0.86 |
| Cloxacillin | 3 | 77.4(62.8–87.4) | 3.4 | 0.00 | 32.8 | 0.23 | 90.8 | 1.6 | 0.001 |
| Tobramycin | 6 | 65.8(51.2–77.9) | 2.1 | 0.00 | 160.8 | 0.78 | 95 | 0.28 | 0.035 |
| Ticarcillin | 8 | 55.4(40.1–69.7) | 0.68 | 0.00 | 102.5 | 0.70 | 93.1 | 0.39 | 0.49 |
| Carbenicillin | 3 | 79.9(72.7–85.6) | 6.7 | 0.00 | 2.7 | 0.80 | 28.3 | 0.32 | 0.00 |
| Cefotaxime | 3 | 76.6(72.1–80.6) | 9.7 | 0.00 | 9.6 | 0.96 | 79.3 | 0.05 | 0.00 |
| Norfloxacin | 4 | 57.3(11.5–93.3) | 0.24 | 0.00 | 180.9 | 0.45 | 98.3 | 0.91 | 0.81 |
| Polymyxin B | 3 | 1(1–2.5) | 6.4 | 0.00 | 0.001 | 0.05 | 0.00 | 121 | 0.00 |
| Colistin | 3 | 1(1–1.25) | 4.1 | 0.00 | 0.001 | 0.06 | 0.00 | 102 | 0.00 |
Note: Cl: Confidence interval, Q: Cochran's Q test, P: p value, T: t student test, I2: I-square, Z: Z-value.
Discussion
In the present study, the combined prevalence of class 1 integrons in P. aeruginosa isolates recovered from clinical samples other than burn samples was obtained as 40% ranging from 13.3 to 99.1%. A systematic review and meta-analysis conducted on isolates recovered from Iranian burn samples at similar times showed a higher pooled prevalence of class 1 integrons in P. aeruginosa (69%) compared to the present review16.
Seventy-five percent of the included studies in the current review described a correlation between the presence of class 1 integrons and antibiotic resistance. Similarly, a significant correlation was found between the presence of class 1 integrons and antibiotic resistance in 55.5% of studies selected for review on burn samples from Iran 16.
This is while most of P. aeruginosa isolates represented the high resistance toward carbenicillin, and cloxacillin. Polymyxin B and colistin are among the most important anti-pseudomonal antibiotics with the highest effects against MDR P. aeruginosa isolates. Likely, a review conducted on burn samples from Iran at the similar time 16 and also another study from North of Iran (Guilan) 17 showed the same pattern, as the highest combined resistance was reported against cloxacillin, followed by carbenicillin and ceftriaxone. There was no difference between antibiotic resistance pattern, MDR isolates, and prevalence of integrons in isolates recovered from both burn and non-burn samples, while these parameters were high in either of them which can be considered by clinicians and physicians.
The noticeable differences and variability in the distribution of class 1 integrons among the Iranian studies included in the current systematic review and meta-analysis may be partly due to the heterogeneities in the patterns of antibiotics use, as well as the sources of infections and geographical locations 18. Integrons carrying gene cassettes provide a powerful vehicle for the fast horizontal transmission of antibiotic resistance enes among different bacterial populations, and in this manner, contribute to the dissemination of antibiotic resistance in hospital settings which creates a serious concern for human health services 9. Similar to our findings, several researchers such as Ren et al. in the United States 19, Cholley et al in France 20, and Taccone et al in Brooklyn 21 have reported a relationship between the expression of class 1 integrons and antibiotic resistance in bacterial strains. In accordance with our findings, the class 1 integrons have been reported as the most common integrons expressed in P. aeruginosa isolates in studies conducted in Japan 22, Spain9, and China 10.
The high frequency of integrons particularly class 1 integrons among resistant P. aeruginosa isolates highlights a role for these genetic elements in resistance to antimicrobial agents. In this study, relatively high rates of resistant P. aeruginosa in ICU and other units of hospitals were reported, and also patients in these units were particularly susceptible to infections caused by MDR P. aeruginosa. Thus, particular strategies should be implemented to prevent the colonization of gram-negative bacteria such as P. aeruginosa and transmission of resistance genes among these micro-organisms through integrons in hospital settings 23.
A study carried out by Aryanezhad et al 24, included in the present review, showed a significant correlation between antibiotic resistance pattern and the presence of integron class 1 among isolates recovered from ICU’s patients compared to ones retrieved from other wards. The resistance to third generation of cephalosporins, carbapenems, aminoglycosides, and amino-penicillins was possibly caused by integron class 1, leading to clonal spread of these resistance genes in different hospital units and consequently among patients hospitalized in clinical settings 25. Also, similar to other studies mentioned in the present review, a high level of resistance in positive isolates for class 1 and 2 integrons was observed against ceftazidime and ciprofloxacin, with prevalence rate of 82.5, and 76.2%, respectively in a study conducted by Faghri et al 18. Interestingly, they reported a significant correlation between presence of class 1 and 2 integrons and the high antibiotic resistance 18. For further confirming the role of integrons in antibiotic resistance, another study conducted by Goli et al described the significant relationship between the presence of class 1 integrons and resistance against aminoglycosides and beta-lactams. Moreover, they reported that all burn isolates were integron-positive and MDR 26.
Nevertheless, some included studies showed no relationship between the presence of integrons and antibiotic resistance in P. aeruginosa. This may partially be related to the contribution of other mechanisms involved in antibiotic resistance such as the overexpression of AmpC beta lactamase (which is encoded by chromosomal genome), the repression or inactivation of Carbapenem porin OprD, and the up-regulation of different efflux pumps 4.
In the present review, the pooled prevalence of MDR P. aeruginosa isolates recovered from clinical specimens of Iranian patients was 70.1%. In comparison, the frequency of MDR P. aeruginosa isolates was reported 30% in eastern European countries 27 that was lower than the ratio obtained here. In line with our findings, however, other studies in China 28 and Brazil29 reported the high frequency of MDR P. aeruginosa isolates. In accordance with our results, Fonseca et al showed that more than half of imipenem-resistant and almost all MDR P. aeruginosa isolates expressed class 1 integrons 29.
In the current study, the most effective antibiotics against P. aeruginosa isolates were polymyxin B and colistin with susceptibility rate of 100%. This is while most of P. aeruginosa isolates represented the high resistance toward carbenicillin (79.9%), cloxacillin (77.4%), and cefotaxime (76.6%). Polymyxin B and colistin are among the most important anti-pseudomonal antibiotics with the highest effects against MDR P. aeruginosa isolates. However, both of these antibiotics have been associated with side effects and toxicities 4. In fact, the restricted prescription of polymyxins because of their toxicity is probably the most important reason for the high susceptibility rate (100%) of P. aeruginosa isolates in exposure to these antibiotics 30. Accordingly, a report from Spain in 2015 revealed a high rate of combined resistance to three or more frequently prescribed antimicrobial agents (Piperacillin– tazobactam, ceftazidime, fluoroquinolones, aminoglycosides and carbapenems) among P. aeruginosa isolates 31. Also, polymyxins have shown the highest antibacterial activity among XDR P. aeruginosa isolates 27 which is in agreement with our results.
To the best of our knowledge, fluoroquinolones (such as ciprofloxacin) are among the most effective available antibiotics for the treatment of P. aeruginosa infections, particularly urinary tract infections 32. In this study, however, up to 50% of P. aeruginosa isolates showed the resistance against ciprofloxacin. According to the studies conducted in Latin America and Europe, 25–30% of P. aeruginosa isolates were also resistant to ciprofloxacin 33,34. In addition, in this study, a broad resistance against beta-lactams and aminoglycosides was observed in P. aeruginosa strains which is in line with the results of previous studies 28,35. On the contrary, other researchers have described low resistance rates of P. aeruginosa against aminoglycosides23,29. Overall, the prevalence of MDR P. aeruginosa isolates was high in clinical samples other than burn samples obtained from Iranian patients especially those hospitalized in critical care units. Furthermore, a high penetrance of class 1 integrons was noted in the MDR P. aeruginosa isolates delineating their association with antibiotic resistance in these bacteria. Timely reporting of antibiotic resistance patterns in these bacteria is recommended to prescribe appropriate antibiotics. Also, it is recommended to develop hospital-based infection control committees and educate their members regarding the nosocomial infections control programs. The current review showed the polymyxin B and colistin as the effective antibiotics; however, the toxic adverse effects are seen in cases treated with polymyxins.
As it is clear from the results, the Pseudomonas strains showed more than 50% resistance against most antibiotics, which indicates an increase in resistance against P. aeruginosa in Iran. Gradually, special antibiotics will not be available to treat Pseudomonas infections and there is a serious challenge for the control of infections created by Pseudomonas. Furthermore, the high prevalence of class 1 integrons and consequently the higher prevalence of MDR strains show the significant role that class 1 integrons play in the transmission of antibiotic resistance, which has become a serious problem for health systems in our country that should be seriously considered.
Conclusion
Results of the present review indicate a high rate of antibiotic resistance among P. aeruginosa isolates recovered from clinical samples. Also, 70% of isolates were MDR and the prevalence of class 1 integrons was almost high. These integrons had genes for resistance to different family members of antibiotics and caused multi drug resistance in our isolates. A significant correlation between the presence of integrons and the high antibiotic resistance was reported by most studies included in the present review. Therefore, existence of class 1 integrons shows high risk of resistance transmission and spreading of MDR isolates in clinical settings. Antibiotic consumption control and antibiotic stewardship are required for decreasing resistance in clinical strains. There is a necessity for preventive measures (safe food preparation, immunization, hand washing, and taking antibiotics as directed and only when needed) to prevent the spread of these resistances by integrons and transferring to other micro-organisms.
Footnotes
Conflict of Interest
None declared.
References
- 1.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen–host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005;171(11): 1209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mobaraki S, Aghazadeh M, Barhaghi MHS, Memar MY, Goli HR, Gholizadeh P, et al. Prevalence of integrons 1, 2, 3 associated with antibiotic resistance in Pseudomonas aeruginosa isolates from Northwest of Iran. BioMedicine 2018;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haidar G, Philips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017;65(1):110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Garbajosa P, Canton R. Epidemiology of antibiotic resistance in Pseudomonas aeruginosa. Implications for empiric and definitive therapy. Rev Esp Quimioter 2017; 30(Suppl 1):8–12. [PubMed] [Google Scholar]
- 5.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care–associated infections. New Engl J Med 2014;370(13):1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palomar M, Álvarez-Lerma F, Olaechea P, Insausti J, López-Pueyo M. Estudio nacional de vigilancia de infección nosocomial en servicios de medicina intensiva. SEMICYUC.[Consultado el 10 dic 2014] Disponible en: URL: http://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI202012.
- 7.Vaez H, Salehi-Abargouei A, Ghalehnoo ZR, Khademi F. Multidrug resistant Pseudomonas aeruginosa in Iran: A systematic review and metaanalysis. J Glob Infect Dis 2018;10(4):212–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carattoli A. Importance of integrons in the diffusion of resistance. Veterinary Res 2001;32(3–4):243–59. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Martínez L, López-Jiménez L, Fusté E, Vinuesa T, Martínez J, Viñas M. Class 1 integrons in environmental and clinical isolates of Pseudomonas aeruginosa. Int J Antimicrob Agents 2011;38(5):398–402. [DOI] [PubMed] [Google Scholar]
- 10.Gu B, Tong M, Zhao W, Liu G, Ning M, Pan S, et al. Prevalence and characterization of class I integrons among Pseudomonas aeruginosa and Acinetobacter baumannii isolates from patients in Nanjing, China. J Clin Microbiol 2007;45(1):241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazel D, Dychinco B, Webb VA, Davies J. Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrob Agents Chemother 2000;44(6):1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillings MR, Gaze WH, Pruden A, Smalla K, Tiedje JM, Zhu YG. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J 2015;9(6): 1269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y, Bao X, Ji L, Chen L, Liu J, Miao J, et al. Resistance integrons: class 1, 2 and 3 integrons. Ann Clin Microbiol Antimicrob 2015;14(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall RM, Collis CM. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist Updat 1998;1(2):109–19. [DOI] [PubMed] [Google Scholar]
- 15.Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12 (12):1500–24. [DOI] [PubMed] [Google Scholar]
- 16.Heidarzadeh S, Enayati Kaliji Y, Pourpaknia R, Mohammadzadeh A, Ghazali-Bina M, Saburi E, et al. A meta-analysis of the prevalence of class 1 integron and correlation with antibiotic resistance in Pseudomonas aeruginosa recovered from Iranian burn patients. J Burn Care Res 2019;40(6):972–8. [DOI] [PubMed] [Google Scholar]
- 17.Nikokar I, Tishayar A, Flakiyan Z, Alijani K, Rehana-Banisaeed S, Hossinpour M, et al. Antibiotic resistance and frequency of class 1 integrons among Pseudomonas aeruginosa, isolated from burn patients in Guilan, Iran. Iran J Microbiol 2013;5(1):36–41. [PMC free article] [PubMed] [Google Scholar]
- 18.Faghri J, Nouri S, Jalalifar S, Zalipoor M, Halaji M. Investigation of antimicrobial susceptibility, class I and II integrons among Pseudomonas aeruginosa isolates from hospitalized patients in Isfahan, Iran. BMC Res Notes 2018;11(1):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren CL, Konstan MW, Yegin A, Rasouliyan L, Trzaskoma B, Morgan WJ, et al. Multiple antibiotic-resistant Pseudomonas aeruginosa and lung function decline in patients with cystic fibrosis. J Cystic Fibros 2012;11(4): 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cholley P, Thouverez M, Hocquet D, Van Der Mee-Marquet N, Talon D, Bertrand X. Most multidrug-resistant Pseudomonas aeruginosa isolates from hospitals in eastern France belong to a few clonal types. J Clin Microbiol 2011;49(7):2578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taccone FS, Cotton F, Roisin S, Vincent JL, Jacobs F. Optimal meropenem concentrations to treat multidrug-resistant Pseudomonas aeruginosa septic shock. Antimicrob Agents Chemother 2012;56(4):2129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata N, Doi Y, Yamane K, Yagi T, Kurokawa H, Shibayama K, et al. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J Clin Microbiol 2003;41(12):5407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moradian Kouchaksaraei F, Shahandashti EF, Molana Z, Moradian Kouchaksaraei M, Asgharpour F, Mojtahedi A, et al. Molecular detection of Integron genes and pattern of antibiotic resistance in Pseudomonas aeruginosa strains isolated from intensive care unit, Shahid Beheshti Hospital, North of Iran. Int J Mol Cell Med 2012;1(4): 209–17. [PMC free article] [PubMed] [Google Scholar]
- 24.Aryanezhad M, Shakibaie MR, Karmostaji A, Shakibaie S. Prevalence of Class 1, 2, and 3 Integrons and Biofilm Formation in Pseudomonas aeruginosa and Acinetobacter baumannii among ICU and non-ICU Patients. Infect Epidemiol Med 2016;2(4):1–7. [Google Scholar]
- 25.Jabbari Amiri MR, Siami R, Khaledi A. Tuberculosis status and coinfection of pulmonary fungal infections in patients referred to reference laboratory of health centers Ghaemshahr city during 2007–2017. Ethiop J Health Sci 2018;28(6):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goli HR, Nahaei MR, Rezaee MA, Hasani A, Kafil HS, Aghazadeh M, et al. Role of MexAB-OprM and Mex-XY-OprM efflux pumps and class 1 integrons in resistance to antibiotics in burn and Intensive Care Unit isolates of Pseudomonas aeruginosa. J Infect Public Health 2018;11(3):364–72. [DOI] [PubMed] [Google Scholar]
- 27.Oliver A, Mulet X, Lopez-Causape C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 2015;21–22: 41–59. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Su Z, Liu Y, Wang S, Dai X, Li Y, et al. Identification and characterization of class 1 integrons among Pseudomonas aeruginosa isolates from patients in Zhenjiang, China. Int J Infect Dis 2009;13(6):717–21. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca ÉL, Vieira VV, Cipriano R, Vicente AC. Class 1 integrons in Pseudomonas aeruginosa isolates from clinical settings in Amazon region, Brazil. FEMS Immunol Med Microbiol 2005;44(3):303–9. [DOI] [PubMed] [Google Scholar]
- 30.Fernández L, Álvarez-Ortega C, Wiegand I, Olivares J, Kocíncová D, Lam JS, et al. Characterization of the polymyxin B resistome of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013;57(1):110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelband H, Miller-Petrie M, Pant S, Gandra S, Levinson J, Barter D, et al. The state of the world's antibiotics 2015. Center for Disease Dynamics, Economics & Policy 2015. CDDEP: Washington, D.C. [Google Scholar]
- 32.Gales A, Jones R, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis 2001;32(Supplement_2):S146–S155. [DOI] [PubMed] [Google Scholar]
- 33.Bouza E, Garcia-Garrote F, Cercenado E, Marin M, Diaz M. Pseudomonas aeruginosa: a survey of resistance in 136 hospitals in Spain. Antimicrob Agents Chemother 1999;43(4):981–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown PD, Izundu A. Antibiotic resistance in clinical isolates of Pseudomonas aeruginosa in Jamaica. Rev Panam Salud Publica 2004;16(2):125–30. [DOI] [PubMed] [Google Scholar]
- 35.Poonsuk K, Tribuddharat C, Chuanchuen R. 2012. Class 1 integrons in Pseudomonas aeruginosa and Acinetobacter baumannii isolated from clinical isolates. Southeast Asian J Trop Med Public Health 2012;43(2):376–84. [PubMed] [Google Scholar]
- 36.Mohammadzadeh A, Mardaneh J, Ahmadi R, Adabi J. Evaluation of the virulence features and antibiotic resistance patterns of pathogenic Pseudomonas aeruginosa strains isolated from hospitalized patients in Gonabad, Iran. Arch Pediatr Infect Dis 2017;5(3): e41267. [Google Scholar]
- 37.Pournajaf A, Razavi S, Irajian G, Ardebili A, Erfani Y, Solgi S, et al. Kafshgari R. 2018. Integron types, antimicrobial resistance genes, virulence gene profile, alginate production and biofilm formation in Iranian cystic fibrosis Pseudomonas aeruginosa isolates. Infez Med 2018;26(3):226–36. [PubMed] [Google Scholar]
- 38.Salimizadeh Z, Karouei H, Masoud S, Hosseini F. Dissemination of Class 1 integron among different multidrug resistant Pseudomonas aeruginosa strains. Medical Laboratory Journal 2018;12(4):36–42. [Google Scholar]


