Abstract
MicroRNA (miR)-874-3p is a newly identified miRNA that is involved in several pathological processes, including cancer, myocardial infarction, bone formation and erectile dysfunction. However, the role of miR-874-3p in polycystic ovary syndrome (PCOS) and granulosa cell (GC) apoptosis is not completely understood. The present study investigated the expression profile of miR-874-3p in PCOS by reverse transcription- quantitative PCR and the GC apoptosis by flow cytometry analysis. miR-874-3p expression was significantly upregulated in GCs isolated from patients with PCOS compared with patients without PCOS. In addition, miR-874-3p expression was positively correlated with GC apoptosis and testosterone levels in both patients with PCOS and patients without PCOS. Therefore, the present study also aimed to investigate the effects of miR-874-3p on testosterone-induced GC apoptosis. Compared with vehicle-treated GCs, miR-874-3p expression levels were significantly increased in testosterone-treated GCs, which was inhibited by the androgen receptor antagonist flutamide. GCs were transfected with either the miR-874-3p mimic or a miR-874-3p inhibitor. Compared with the control group, miR-874-3p mimic significantly enhanced GC apoptosis, whereas miR-874-3p inhibitor significantly decreased GC apoptosis. Moreover, histone deacetylase (HDAC) activity and HDAC1 expression levels were decreased in testosterone-treated GCs compared with vehicle-treated GCs. HDAC1 overexpression significantly attenuated the proapoptotic effects of testosterone. Additionally, miR-874-3p mimic and inhibitor significantly decreased and increased HDAC1 expression levels, respectively, compared with the control group. miR-874-3p inhibitor failed to attenuate HDAC1 overexpression-induced GC apoptosis. Furthermore, compared with the control group, testosterone treatment notably increased p53 expression and acetylation. Compared with the control group, western blotting analysis showed that miR-874-3p mimic notably increased p53 expression and acetylation, whereas miR-874-3p inhibitor markedly decreased p53 expression and acetylation. However, miR-874-3p inhibitor did not further decrease p53 acetylation and expression in cell overexpressing HDAC1. Collectively, the results of the present study indicated that miR-874-3p was upregulated in PCOS and promoted testosterone-induced GC apoptosis by suppressing HDAC1-mediated p53 deacetylation. Therefore, the present study improved the current understanding of the pathogenesis of PCOS and GC apoptosis.
Keywords: granulosa cell apoptosis, histone deacetylase 1, microRNA-874-3p, p53, polycystic ovary syndrome, testosterone
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine and metabolic disorder in females of reproductive age worldwide, with a prevalence of 6-20%, depending on the diagnostic criteria applied (1). The key features of PCOS include hyperandrogenism, polycystic ovarian morphology, ovulatory dysfunction and irregular menstrual cycles (2). PCOS is a primary cause of infertility, resulting in abnormal follicle development and a high rate of follicular atresia in females with PCOS (3). Increasing evidence indicates that granulosa cell (GC) apoptosis serves an important role in follicular atresia and PCOS development (4,5). GC apoptosis is increased in patients with PCOS and in PCOS model animals, compared with control patients with normal ovarian function and healthy animals respectively (6,7), and inhibition of GC apoptosis improved the ovary functions of PCOS model rats (8). The hyperandrogenic condition is a key pathogenic factor that mediates GC apoptosis in PCOS (9). It was reported that androgens induce GC apoptosis via activation of the intrinsic and extrinsic apoptotic signaling pathways (7,9). However, the mechanisms underlying androgen-mediated induction of GC apoptosis are not completely understood.
MicroRNAs (miRs/miRNAs) are short non-coding RNA molecules that are widely present in different tissues (10). miRNAs regulate gene expression by destabilizing target mRNAs or inhibiting translation (10). miR-874-3p, a newly identified miRNA, is associated with several pathological processes, including cancer, myocardial infarction, bone formation and erectile dysfunction (11-15). However, the role of miR-874-3p in PCOS and GC apoptosis is yet to be elucidated. Several targets of miR-874-3p have been identified, one of which is histone deacetylase (HDAC)1(14). Although the effects of HDAC1 on apoptosis have been widely studied (16,17), to the best of our knowledge, the role of HDAC1 in GC apoptosis, especially in hyperandrogenic conditions, has not been previously reported.
The present study assessed the miR-874-3p expression profile in PCOS and testosterone-induced GC apoptosis. Additionally, the present study investigated the role of miR-874-3p in testosterone-induced GC apoptosis, as well as the underlying mechanisms.
Materials and methods
Subjects and collection of human GCs
The present study was approved by the Medical Ethics Committee of Zaozhuang Maternal and Child6 Health Care Hospital. All patients were female and hospitalized in Zaozhuang Maternal and Child Health Care Hospital (Zaozhuang, China) from February 2016 to May 2018. Written informed consent was obtained from all patients. A total of 16 patients diagnosed with PCOS according to the Rotterdam criteria (18) were recruited into the PCOS group. The control group consisted of 11 patients with normal ovarian function and regular periods, where infertility was ascribed to tubal factors or male infertility. Individuals with ovarian tumors, congenital adrenal hyperplasia, androgen secreting tumors, Cushing's syndrome and endometriosis were excluded. All patients received controlled ovarian hyperstimulation as previously reported (6). Briefly, follicular growth was promoted with recombinant follicle stimulating hormone (FSH; LiShenBao, Lizhu Pharmaceutical; https://en.livzon.com.cn/product/14.html) on the third day of the menstrual cycle. The dosages were adjusted according to the follicular size and serum hormonal level of each patient. When at least three of the follicles displayed diameters of 18 mm, 10,000 IU human chorionic gonadotropin (Lizhu Pharmaceutical; https://en.livzon.com.cn/product/9.html) was administered to induce ovulation. At 36 h post-administration, oocyte retrieval was performed via ultrasound-guided puncture. Follicular fluids (diameter, >18 mm) were carefully collected and immediately transported to the laboratory on ice.
Human GCs were isolated using a previously described strainer methodology (19). First, the follicular fluids were filtered through a 40-µm cell strainer. The strainer was rinsed with PBS and then backwashed with PBS to collect GCs. Following incubation for 5 min at 4˚C, the cell suspension was repeatedly aspirated using Pasteur pipettes to mechanically break up aggregates. The resulting cell suspension was filtered through a 70-µm cell strainer to remove unwanted and undispersed material. Finally, the filtrate was centrifuged at 600 x g for 5 min at 4˚C. GCs in the cell pellet were used for subsequent experiments. Human GCs were cultured under the same conditions as mouse GCs, which was in DMEM/F12 (HyClone; Cytiva) supplemented with 10% FBS (HyClone; Cytiva) and 1% penicillin/streptomycin (HyClone; Cytiva) at 37˚C with 5% CO2
Isolation, culture, and treatment of mouse GCs
All animal procedures were approved by the Animal Ethics Committee of Zaozhuang Maternal and Child Health Care Hospital. In total, 84 C57BL/6 mice (weight, 10.3±0.9 g; age, 21 days; total number, 84; immature females) were from Shanghai SLAC Laboratory Animal Co., Ltd. The mice were housed in a temperature (22-24˚C) and humidity (50-60%) controlled environment with a 12-h light/dark cycle and given ad libitum access to food and water. The mice were intraperitoneally injected with 8 IU pregnant mare serum gonadotrophin (Ningbo Sansheng Pharmaceutical Industry Co., Ltd.). At 47 h post-injection, mice were sacrificed by cervical dislocation, the ovaries were collected and the surrounding tissues removed. GCs were isolated from the ovaries by puncturing the follicles with 26-gauge needles, followed by centrifugation at 300 x g for 10 min at 4˚C. GCs were cultured in DMEM/F12 (HyClone; Cytiva) supplemented with 10% FBS (HyClone; Cytiva) and 1% penicillin/streptomycin (HyClone; Cytiva) at 37˚C with 5% CO2. At 1 day post-transduction, cells were incubated with 10 µM testosterone (Sigma-Aldrich; Merck KGaA) or vehicle (0.1% DMSO) for 24 h at 37˚C to induce apoptosis. In addition, cells were treatment with 10 µM testosterone together with 1 µM flutamide (Sigma-Aldrich; Merck KGaA) at 37˚C for 24 h.
Lentiviral vectors
miR-874-3p mimic non-targeting negative control (NC; 5'-ACUACUGAGUGACAGUAGA-3') or miR-874-3p mimic (5'-CUGCCCUGGCCCGAGGGACCGA-3') were inserted into the GV309 vector (Shanghai GeneChem Co., Ltd.); miR-874-3p inhibitor non-targeting NC (5'-CAGUACUUUUGUGUAGUACAA-3') or miR-874-3p inhibitor (5'-UCGGUCCCUCGGGCCAGGGCAG-3') were inserted into the GV280 vector (Shanghai GeneChem Co., Ltd.). For HDAC1 overexpression, the coding sequence of HDAC1 was inserted into the GV358 vector (Shanghai GeneChem Co., Ltd.). The empty vector was used as the NC for HDAC1 overexpression. 293T cells (American Type Culture Collection) were transfected with 20 µg the GV vector (GV309, GV280 or GV358) together with 15 µg pHelper 1.0 and 10 µg pHelper 2.0 vectors (both from Shanghai GeneChem Co., Ltd.) and then cultured at 37˚C in a humidified incubator in an atmosphere of 5% CO2 for 48 h to produce lentiviral particles. Transduction was performed when cells were growing exponentially and were 70-80% confluent. Polybrene (8 µg/ml, Sigma-Aldrich; Merck KGaA) and appropriate lentiviral particles (108 TU/ml, 5 µl) were added to cells for 16 h at 37˚C in a humidified incubator in an atmosphere of 5% CO2. The medium containing lentiviral particles were removed from wells and fresh medium were added. Subsequent experiments were performed 24 h later.
Flow cytometry analysis
GC apoptosis (early and late) was quantified by flow cytometry using an Annexin V-FITC Apoptosis Detection kit (cat. no. 556547; BD Bioscience) according to the manufacturer's instructions. Briefly, cells were harvested, washed twice with cold PBS and re-suspended in the binding buffer. The cell suspension (100,000 cells/ml) was incubated with 5 µl Annexin V-FITC and 10 µl PI for 15 min at room temperature in the dark. Subsequently, flow cytometry was performed using a flow cytometer (CytoFlex S; Beckman Coulter, Inc.). The data were analyzed with FlowJo software (v10; FlowJo, LLC.).
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using an RNeasy Plus Mini kit (Qiagen GmbH) according to the manufacturer's instructions. Total RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.). The reverse transcription reactions were performed at 37˚C for 15 min, followed by inactivation of the reverse transcriptase at 85˚C for 5 sec. Subsequently, qPCR was performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.). The following thermocycling conditions were used for qPCR: 95˚C for 10 min; followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min. The following primers were used for qPCR: miR-874-3p forward, GAACTCCACTGTAGCAGAGATGGT and reverse, CATTTTTTCCACTCCTCTTCTCTC; HDAC1 (human) forward, GAATCCGCATGACTCATAAT and reverse, GCTGTGGTACTTGGTCATCT; HDAC1 (mouse) forward, CTGAATACAGCAAGCAGATGCAGAG and reverse, TCCCGTGGACAACTGACAGAAC; U6 (human and mouse) forward, CTCGCTTCGGCAGCACA and reverse, AACGCTTCACGAATTTGCGT; GAPDH (human) forward, GCACCGTCAAGGCTGAGAAC and reverse, TGGTGAAGACGCCAGTGGA; and GAPDH (mouse) forward, AACGGGAAGCTCACTGGCAT and reverse, GCTTCACCACCTTCTTGATG. miRNA and mRNA expression levels were quantified using the 2-ΔΔCq method (20) and normalized to the internal reference genes U6 and GAPDH, respectively.
Western blotting
Proteins were extacted using a Western Cell lysis Buffer kit (Beyotime Institute of Biotechnology) and the concentrations were determined by a bicinchninic acid (BCA) Protein Assay Kit (Beyotime Institute of Biotechnology). In total, 30 µg total proteins were loaded per lane. Proteins were separated via 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). Following blocking with 5% non-fat milk at room temperature for 1 h, the membranes were incubated at 4˚C overnight with the following primary antibodies: Anti-androgen receptor (AR; cat. no. 5153; 1:1,000; Cell Signaling Technology, Inc.); Anti-p53 (cat. no. ab26; 1:1,000; Abcam), anti-acetylated p53 (Lys379; cat. no. 2570; 1:1,000; Cell Signaling Technology, Inc.), anti-HDAC1 (cat. no. 34589; 1:1,000; Cell Signaling Technology, Inc.), anti-sirtuin 1 (SIRT1; cat. no. 8469; 1:1,000; Cell Signaling Technology, Inc.) and anti-GAPDH (cat. no. ab8245; 1:1,000; Abcam). After washing by TBS with 0.05% Tween-20, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (cat. no. ab7090 and ab97040; 1:5,000; Abcam) at room temperature for 1 h. Protein bands were visualized using an ECL kit (Beyotime Institute of Biotechnology).
Measurement of HDAC activity
GC nuclear extracts were obtained and quantified using a Nuclear Extract kit (Beyotime Institute of Biotechnology) and a BCA protein assay kit (Beyotime Institute of Biotechnology), respectively. HDAC activity in the nuclear extracts was determined using an HDAC Activity Colorimetric Assay kit (cat. no. K331; BioVision, Inc.) according to the manufacturer's instructions. The absorbance was measured at a wavelength of 405 nm using a microplate reader.
Statistical analysis
All experiments were performed in triplicate. Data are presented as the mean ± SEM. Comparisons between two groups were analyzed using the Student's unpaired t-test. Comparisons among multiple groups were analyzed using one-way ANOVA followed by Bonferroni's post hoc test. For correlation analyses, Pearson's correlation coefficient was used. Statistical analyses were performed using SPSS 13.0 software (SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-874-3p is upregulated in GCs isolated from patients with PCOS
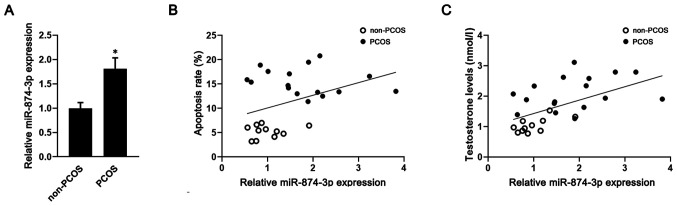
miR-874-3p expression in the GCs of patients with or without PCOS were assessed. A total of 16 patients with PCOS and 11 patients with PCOS were recruited in the present study. The clinical features of the patients are presented in Table I. There was no significant difference in age between the two groups. However, compared with patients without PCOS, patients with PCOS displayed significantly higher basal testosterone levels, basal luteinizing hormone (LH) levels and LH/FSH ratios. The rate of apoptosis of GCs isolated from patients with PCOS was significantly higher compared with patients without PCOS (Fig. S1). Meanwhile, the RT-qPCR results demonstrated that miR-874-3p expression was significantly upregulated in GCs isolated from patients with PCOS compared with patients without PCOS (Fig. 1A). Moreover, miR-874-3p expression levels were positively correlated with GC apoptosis (r=0.39; Fig. 1B) and testosterone levels (r=0.55; Fig. 1C) in both patients with PCOS and patients without PCOS.
Table I.
Clinical features of patients with or without PCOS.
| Non-PCOS (n=11) | PCOS (n=16) | P-value | |
|---|---|---|---|
| Age (year) | 30.18±3.16 | 30.50±2.90 | >0.05 |
| BMI (kg/m2) | 23.84±1.91 | 23.76±1.97 | >0.05 |
| Basal T (nmol/l)a | 1.04±0.24 | 2.05±0.53 | 0.03 |
| Basal E2 (pmol/l) | 136.57±47.29 | 153.66±49.18 | >0.05 |
| Basal PRL (µIU/ml) | 278.33±87.45 | 301.72±90.33 | >0.05 |
| Basal FSH (mIU/ml) | 6.95±1.02 | 6.77±1.21 | >0.05 |
| Basal LH (mIU/ml)a | 4.55±1.37 | 12.51±5.93 | 0.01 |
| LH/FSH ratioa | 0.68±0.28 | 1.57±0.58 | 0.02 |
PCOS, polycystic ovary syndrome; T, testosterone; E2, E2 estradiol; PRL, prolactin; FSH, follicle stimulating hormone; LH, luteinizing hormone.
aP<0.05.
Figure 1.
miR-874-3p is upregulated in GCs isolated from patients with PCOS. (A) miR-874-3p expression levels in GCs isolated from patients with or without PCOS were detected via reverse transcription-quantitative PCR. *P<0.05 vs. non-PCOS. Human GCs were isolated and subjected to flow cytometry to detect cell apoptosis. (B) Correlation between miR-874-3p expression and apoptosis in GCs isolated from patients (r=0.39; P<0.05). (C) Correlation between miR-874-3p expression and testosterone levels (r=0.55; P<0.05). miR, microRNA; GC, granulosa cell; PCOS, polycystic ovary syndrome.
miR-874-3p serves a role in testosterone-induced GC apoptosis
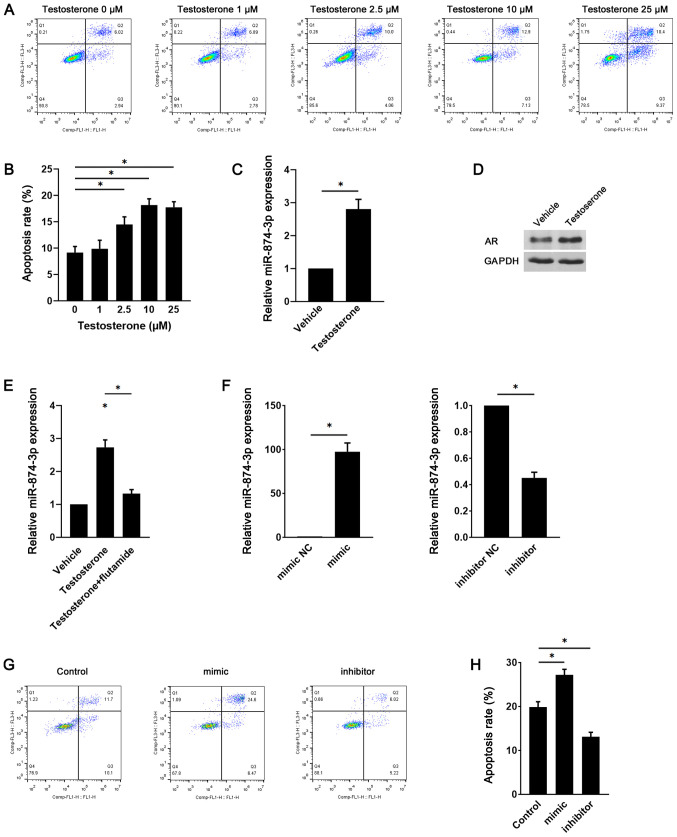
Since hyperandrogenic conditions are a key pathogenic factor that mediate GC apoptosis in PCOS (9), the role of miR-874-3p in testosterone-induced GC apoptosis was investigated. Compared with the control group (0 µM testosterone), testosterone significantly increased GC apoptosis at concentrations ≥2.5 µM (Fig. 2A and B). Moreover, miR-874-3p expression levels were significantly increased in testosterone-treated GCs compared with vehicle-treated GCs (Fig. 2C). The protein expression levels of androgen receptor were also notably upregulated in testosterone-treated GCs compared with vehicle-treated GCs (Fig. 2D). To examine whether the induction of miR-874-3p was mediated by the androgen receptor, GCs were treated with testosterone in the presence or absence of the androgen receptor antagonist flutamide. In GCs, testosterone-induced miR-874-3p upregulation was significantly inhibited by flutamide (Fig. 2E). Furthermore, GCs were transduced with lentiviral vectors expressing miR-874-3p mimic or inhibitor. Compared with the mimic NC group, miR-874-3p mimic significantly increased miR-874-3p expression levels, whereas compared with the inhibitor NC group, miR-874-3p inhibitor significantly decreased miR-874-3p expression levels (Fig. 2F). Moreover, compared with the control group, miR-874-3p mimic significantly increased GC apoptosis and miR-874-3p inhibitor significantly decreased GC apoptosis (Fig. 2G and H). The results indicated that miR-874-3p may serve a role in testosterone-induced GC apoptosis.
Figure 2.
miR-874-3p serves a role in testosterone-induced GC apoptosis. Following treatment with different doses of testosterone for 24 h, mouse GC apoptosis was (A) determined via flow cytometry and (B) quantified. (C) Following treatment with vehicle or 10 µM testosterone for 24 h, miR-874-3p expression levels were detected via RT-qPCR. (D) AR protein expression levels were detected via western blotting. (E) Following treatment with 10 µM testosterone in the presence or absence of 1 µM flutamide for 24 h, miR-874-3p expression levels in mouse GCs were detected via RT-qPCR. (F) Transduction efficiency of miR-874-3p mimic and miR-874-3p inhibitor in mouse GCs. Following transduction with lentiviral vectors expressing miR-874-3p mimic or miR-874-3p inhibitor for 1 day, then treatment with 10 µM testosterone for 24 h, mouse GC apoptosis was (G) determined via flow cytometry and (H) quantified. *P<0.05. miR, microRNA; GC, granulosa cell; RT-qPCR, reverse transcription-quantitative PCR; AR, androgen receptor; NC, negative control.
HDAC1 is a target of miR-874-3p during GC apoptosis
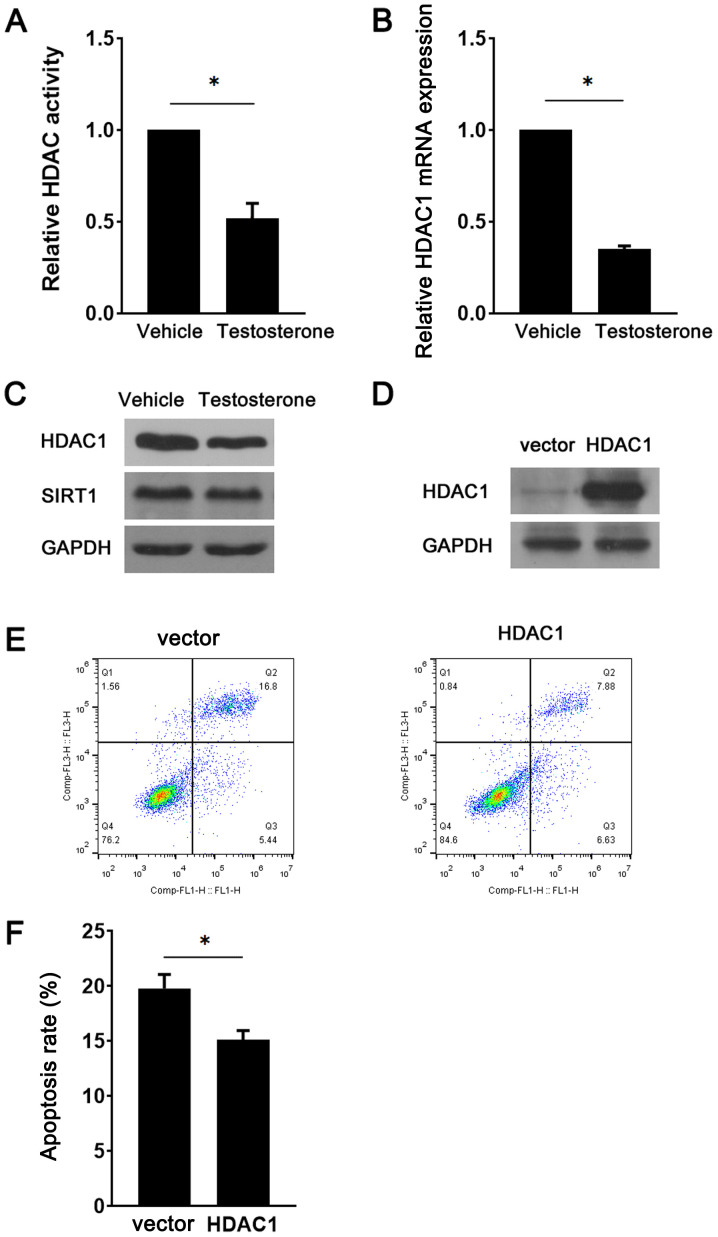
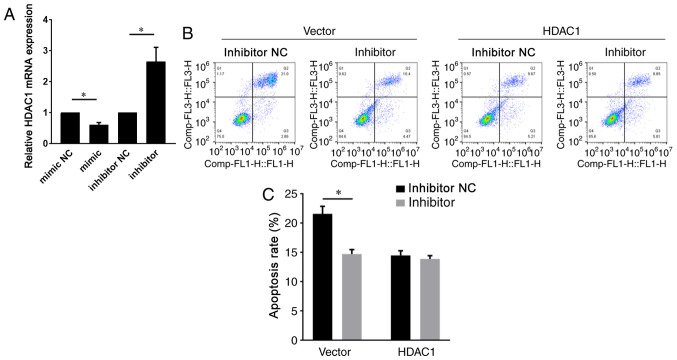
HDAC1 was reported to be a target of miR-874-3p (12). Therefore, HDAC1 expression during GC apoptosis was examined. Compared with the vehicle group, testosterone treatment significantly decreased HDAC activity (Fig. 3A). Similarly, HDAC1 expression was markedly downregulated in testosterone-treated GCs compared with vehicle-treated GCs (Fig. 3B and C). However, there were no notable alterations in the expression levels of SIRT1, a HDAC family member, between the vehicle and testosterone groups (Fig. 3C). To determine the role of HDAC1 downregulation in testosterone-induced GC apoptosis, a lentiviral vector was used to overexpress HDAC1 in the presence of testosterone. The western blotting results indicated that HDAC1 protein expression levels were notably increased in the HDAC1 overexpression group compared with the vector group, demonstrating successful transduction (Fig. 3D). GC apoptosis was significantly decreased in the HDAC1 overexpression group compared with the vector group (Fig. 3E and F). Subsequently, the present study investigated whether miR-874-3p regulated HDAC1 in GCs. Compared with the control group, miR-874-3p mimic significantly decreased HDAC1 expression, whereas miR-874-3p inhibitor significantly increased HDAC1 expression in GCs (Fig. 4A). By contrast, miR-874-3p inhibitor could not attenuate the apoptosis of GCs with HDAC1 overexpression further (Fig. 4B and C), suggesting that miR-874-3p mediated GC apoptosis via HDAC1. Collectively, the aforementioned results indicated that miR-874-3p downregulated HDAC1 to promote GC apoptosis.
Figure 3.
HDAC1 inhibits testosterone-induced GC apoptosis. Following treatment with 10 µM testosterone for 24 h, (A) HDAC activity, (B) HDAC1 mRNA expression levels, and (C) HDAC1 and SIRT1 protein expression levels in GCs were assessed. (D) At 48 h post-transduction with the HDAC1 overexpression vector or the control empty vector, HDAC1 protein expression levels in GCs were detected via western blotting. Following transduction with the HDAC1 overexpression vector or control empty vector and treatment with 10 µM testosterone for 24 h, mouse GC apoptosis was (E) determined via flow cytometry and (F) quantified. *P<0.05. HDAC, histone deacetylase; GC, granulosa cell; SIRT1, sirtuin 1.
Figure 4.
miR-874-3p mediates GC apoptosis via HDAC1 inhibition. (A) Following transduction with lentiviral vectors expressing miR-874-3p mimic, mimic NC, inhibitor or inhibitor NC for 24 h, HDAC1 mRNA expression levels in mouse GCs were measured via reverse transcription-quantitative PCR. Following transduction with the HDAC1 overexpression vector or the control empty vector for 24 h, then transduction with miR-874-3p inhibitor or inhibitor NC for 24 h, mouse GC apoptosis was (B) determined via flow cytometry and (C) quantified. *P<0.05. miR, microRNA; GC, granulosa cell; HDAC, histone deacetylase.
miR-874-3p promotes p53 acetylation and accumulation by suppressing HDAC1
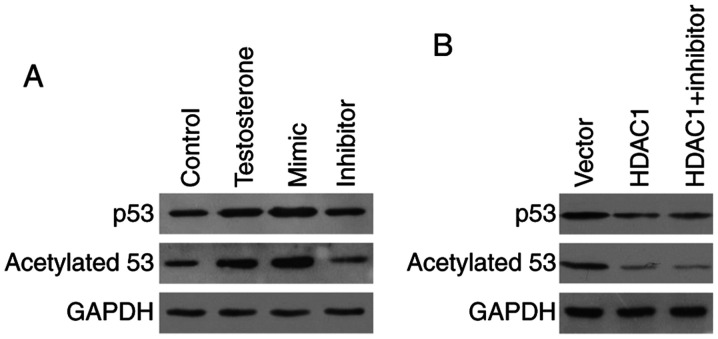
To investigate how miR-874-3p mediated GC apoptosis, the expression of several apoptosis-related proteins was examined. Compared with the control group, the protein expression levels of the proapoptotic protein p53 were notably upregulated in testosterone-treated GCs. Moreover, compared with the control group, p53 protein expression levels were markedly increased miR-874-3p mimic, but notably decreased by miR-874-3p inhibitor (Fig. 5A). Since acetylation serves a positive role in p53 protein accumulation (21), the expression levels of acetylated p53 were also detected. The western blotting results demonstrated that p53 acetylation was markedly increased by testosterone treatment compared with the control group. Moreover, miR-874-3p mimic increased p53 acetylation, whereas miR-874-3p inhibitor decreased p53 acetylation, compared with the testosterone group (Fig. 5A). To determine whether miR-874-3p regulated p53 acetylation via HDAC1, GCs were treated with miR-874-3p inhibitor in the presence of HDAC1. HDAC1 overexpression markedly decreased p53 acetylation compared with the vector group. However, in HDAC1-overepression GCs, miR-874-3p inhibitor did not further decrease p53 acetylation and expression (Fig. 5B). The results suggested that miR-874-3p promoted p53 acetylation and expression via inhibition of HDAC1.
Figure 5.
miR-874-3p promotes p53 acetylation and expression via HDAC1 inhibition. (A) Following transduction with lentiviral vectors expressing miR-874-3p mimic or inhibitor for 24 h, and treatment with 10 µM testosterone for 24 h, western blotting was performed to detect the protein expression levels of p53 and acetylated p53 in mouse GCs. Cells in the control group were treated with 0.1% DMSO for 24 h. (B) Following transduction with the control empty vector, the HDAC1 overexpression vector or the HDAC1 overexpression vector + the miR-874-3p inhibitor lentiviral vector for 24 h, western blotting was performed to detect the protein expression levels of p53 and acetylated p53 in mouse GCs. miR, microRNA; HDAC, histone deacetylase; GC, granulosa cell.
Discussion
To the best of our knowledge, the present study was the first to investigate miR-874-3p expression in PCOS and its role in GC apoptosis. Recently, the role of miRNAs in the pathogenesis of PCOS has attracted increasing attention (22). Altered miRNA expression profiles in patients with PCOS have been regarded as biomarkers and therapeutic targets for PCOS (22,23). Testosterone-induced GC apoptosis is involved in the pathological development of PCOS (7). However, the role of miRNAs in hyperandrogen-induced GC apoptosis is not completely understood. The present study demonstrated that miR-874-3p was positively correlated with testosterone levels, and miR-874-3p expression was upregulated by testosterone treatment compared with the vehicle group, which suggested that miR-874-3p may serve as a diagnostic marker for PCOS. Moreover, gain- and loss-of-function experiments indicated that miR-874-3p mediated the proapoptotic effects of testosterone, suggesting that miR-874-3p may serve a role in testosterone-induced GC apoptosis. Since miR-874-3p is a newly identified miRNA, the present study furthered the current understanding of the role of miR-874-3p.
HDACs are enzymes that catalyze the deacetylation of lysine residues of both histone and non-histone proteins (24). Abnormal HDAC activity is involved in a number of diseases, including cancer, neurological disease, metabolic disorders, cardiac disease and inflammatory diseases (25). To the best of our knowledge, the present study was the first to demonstrate that HDAC activity and HDAC1 expression were notably decreased in testosterone-treated GCs compared with vehicle-treated GCs. A previous study also reported that the expression of SIRT1, another HDAC family member, was also downregulated in a PCOS model (26). Moreover, females receiving HDAC inhibitor displayed polycystic ovaries (27). Therefore, low HDAC activity may serve as a mechanism underlying the development of PCOS. However, in the present study, SIRT1 expression was not altered in testosterone-treated GCs compared with vehicle-treated GCs, suggesting that HDAC1 might be the primary target of testosterone/miR-874-3p and that SIRT1 might be regulated by other factors in PCOS. The present study indicated that the androgen receptor antagonist flutamide inhibited testosterone-induced upregulation of miR-874-3p, an upstream factor of HDAC1. Interestingly, it has also been reported that HDAC1 can regulate the function of the androgen receptor (28,29). Thus, there might be a feedback mechanism controlling the effects of HDAC1 and androgen receptor. In the present study, HDAC1 was overexpressed in GCs and the results indicated that HDAC1 protected against GC apoptosis. Therefore, HDAC1 may serve as a potential therapeutic target for PCOS.
The p53 protein is a proapoptotic protein that serves a central role in the cellular response to DNA damage and other genomic aberrations (30). The present study demonstrated that compared with the control group, p53 accumulation was markedly increased by testosterone treatment, which might explain the proapoptotic role of testosterone. However, another study reported that testosterone reduced p53 expression levels during oxidative stress damage (31). The contradiction between the results of the present study and the aforementioned study suggested that testosterone might differentially regulate p53 expression depending on the pathological circumstances and cell type. Acetylation is one of the characteristics of stabilized p53, and upon deacetylation, p53 expression is maintained at low levels by degradation (21). The results of the present study indicated that both testosterone treatment and miR-874-3p overexpression induced p53 acetylation, whereas miR-874-3p knockdown and HDAC1 overexpression lead to p53 deacetylation. The results suggested that testosterone/miR-874-3p/HDAC1 signaling regulated p53 acetylation. Moreover, miR-874-3p inhibitor did not further decrease p53 acetylation and expression in HDAC1-overexpression GCs, indicating that miR-874-3p regulated p53 expression by suppressing HDAC1-mediated deacetylation of p53 but not via other mechanisms. Previous studies demonstrated that HDAC1 mediated the deacetylation of p53 (32,33), confirming that p53 could be deacetylated by HDAC1. Collectively, the aforementioned results indicated that testosterone/miR-874-3p promoted p53 acetylation and expression to induce GC apoptosis.
The present study demonstrated that miR-874-3p was upregulated in PCOS and promoted testosterone-induced GC apoptosis by suppressing HDAC1-mediated p53 deacetylation. Therefore, the present study furthered the current understanding of the pathogenesis of PCOS.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the Natural Science Foundation of China (grant no. 81601255).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YW and CL designed the experiments, drafted the manuscript and authenticated the data in this study. YW, ZW and CL contributed to patient recruitment and data collection. YW, ZW, LW, SL and XQ performed cell culture, reverse transcription-quantitative PCR, western blotting and flow cytometry experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of Zaozhuang Maternal and Child Health Care Hospital and the Animal Ethics Committee of Zaozhuang Maternal and Child Health Care Hospital [ZFYYLL2016(001) and ZFYYLL2016(002)]. Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Escobar-Morreale HF. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 2.Neven ACH, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: Diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med. 2018;36:5–12. doi: 10.1055/s-0038-1668085. [DOI] [PubMed] [Google Scholar]
- 3.Cai L, Ma X, Liu S, Liu J, Wang W, Cui Y, Ding W, Mao Y, Chen H, Huang J, et al. Effects of upregulation of Hsp27 expression on oocyte development and maturation derived from polycystic ovary syndrome. PLoS One. 2013;8(e83402) doi: 10.1371/journal.pone.0083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen H, Wang Y. Activation of TGF-β1/Smad3 signaling pathway inhibits the development of ovarian follicle in polycystic ovary syndrome by promoting apoptosis of granulosa cells. J Cell Physiol. 2019;234:11976–11985. doi: 10.1002/jcp.27854. [DOI] [PubMed] [Google Scholar]
- 5.Yeung CK, Wang G, Yao Y, Liang J, Tenny Chung CY, Chuai M, Lee KK, Yang X. BRE modulates granulosa cell death to affect ovarian follicle development and atresia in the mouse. Cell Death Dis. 2017;8(e2697) doi: 10.1038/cddis.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikaeili S, Rashidi BH, Safa M, Najafi A, Sobhani A, Asadi E, Abbasi M. Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet. 2016;294:185–192. doi: 10.1007/s00404-016-4068-z. [DOI] [PubMed] [Google Scholar]
- 7.Azhary JMK, Harada M, Takahashi N, Nose E, Kunitomi C, Koike H, Hirata T, Hirota Y, Koga K, Wada-Hiraike O, et al. Endoplasmic reticulum stress activated by androgen enhances apoptosis of granulosa cells via induction of death receptor 5 in PCOS. Endocrinology. 2019;160:119–132. doi: 10.1210/en.2018-00675. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang Y, Liu S, Tan J, Yan Q. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 2017;8(e3145) doi: 10.1038/cddis.2017.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao KK, Cui YG, Jiang YQ, Wang J, Li M, Zhang Y, Ma X, Diao FY, Liu JY. Effect of HSP10 on apoptosis induced by testosterone in cultured mouse ovarian granulosa cells. Eur J Obstet Gynecol Reprod Biol. 2013;171:301–306. doi: 10.1016/j.ejogrb.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Pal I, Safari M, Jovanovic M, Bates SE, Deng C. Targeting translation of mRNA as a therapeutic strategy in cancer. Curr Hematol Malig Rep. 2019;14:219–227. doi: 10.1007/s11899-019-00530-y. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Xue H, Guo S, Chen Y, Zhang X, Tang X. doi: 10.23736/S0031-0808.19.03682-6. miR-874-3p suppresses cell proliferation and invasion by targeting ADAM19 in nasopharyngeal carcinoma. Panminerva Med: Jul 19, 2019 (Epub ahead of print). doi: 10.23736/S0031-0808.19.03682-6. [DOI] [PubMed] [Google Scholar]
- 12.Yuan RB, Zhang SH, He Y, Zhang XY, Zhang YB. miR-874-3p is an independent prognostic factor and functions as an anti-oncomir in esophageal squamous cell carcinoma via targeting STAT3. Eur Rev Med Pharmacol Sci. 2018;22:7265–7273. doi: 10.26355/eurrev_201811_16261. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y, Song X, Li Z, Zhang J, Ren J, Wu J, Li Y, Guan Y, Wang J. Elevated levels of granzyme B correlated with miR-874-3p downregulation in patients with acute myocardial infarction. Biomark Med. 2017;11:761–767. doi: 10.2217/bmm-2017-0144. [DOI] [PubMed] [Google Scholar]
- 14.Kushwaha P, Khedgikar V, Sharma D, Yuen T, Gautam J, Ahmad N, Karvande A, Mishra PR, Trivedi PK, Sun L, et al. MicroRNA 874-3p exerts skeletal anabolic effects epigenetically during weaning by suppressing Hdac1 expression. J Biol Chem. 2016;291:3959–3966. doi: 10.1074/jbc.M115.687152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo W, Li H, Zhang Y, Li H. Epigenetic silencing of microRNA-874-3p implicates in erectile dysfunction in diabetic rats by activating the Nupr1/Chop-mediated pathway. FASEB J. 2020;34:1695–1709. doi: 10.1096/fj.201902086R. [DOI] [PubMed] [Google Scholar]
- 16.Wang XQ, Bai HM, Li ST, Sun H, Min LZ, Tao BB, Zhong J, Li B. Knockdown of HDAC1 expression suppresses invasion and induces apoptosis in glioma cells. Oncotarget. 2017;8:48027–48040. doi: 10.18632/oncotarget.18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demyanenko SV, Dzreyan VA, Neginskaya MA, Uzdensky AB. Expression of histone deacetylases HDAC1 and HDAC2 and their role in apoptosis in the penumbra induced by photothrombotic stroke. Mol Neurobiol. 2020;57:226–238. doi: 10.1007/s12035-019-01772-w. [DOI] [PubMed] [Google Scholar]
- 18.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1016/j.fertnstert.2003.10.004. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. [DOI] [PubMed] [Google Scholar]
- 19.Ferrero H, Delgado-Rosas F, Garcia-Pascual CM, Monterde M, Zimmermann RC, Simón C, Pellicer A, Gómez R. Efficiency and purity provided by the existing methods for the isolation of luteinized granulosa cells: A comparative study. Hum Reprod. 2012;27:1781–1789. doi: 10.1093/humrep/des096. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell. 2011;2:456–462. doi: 10.1007/s13238-011-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B, Xu P, Wang J, Zhang C. The role of miRNA in polycystic ovary syndrome (PCOS) Gene. 2019;706:91–96. doi: 10.1016/j.gene.2019.04.082. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Ou H, Wu H, Wu P, Mo Z. Role of microRNA in the pathogenesis of polycystic ovary syndrome. DNA Cell Biol. 2019;38:754–762. doi: 10.1089/dna.2019.4622. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Wang Z, Liu J. Role of HDACs in normal and malignant hematopoiesis. Mol Cancer. 2020;19(5) doi: 10.1186/s12943-019-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seto E, Yoshida M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(a018713) doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao X, Zhang X, Ge SQ, Zhang EH, Zhang B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int J Clin Exp Pathol. 2015;8:8276–8283. [PMC free article] [PubMed] [Google Scholar]
- 27.Isojarvi JI, Laatikainen TJ, Pakarinen AJ, Juntunen KT, Myllyla VV. Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med. 1993;329:1383–1388. doi: 10.1056/NEJM199311043291904. [DOI] [PubMed] [Google Scholar]
- 28.Gaughan L, Logan IR, Cook S, Neal DE, Robson CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277:25904–25913. doi: 10.1074/jbc.M203423200. [DOI] [PubMed] [Google Scholar]
- 29.Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N, Sawyers CL. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res. 2009;69:958–966. doi: 10.1158/0008-5472.CAN-08-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou HL, Schumacher B. DNA damage responses and p53 in the aging process. Blood. 2018;131:488–495. doi: 10.1182/blood-2017-07-746396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pronsato L, Milanesi L. Effect of testosterone on the regulation of p53 and p66Shc during oxidative stress damage in C2C12 cells. Steroids. 2016;106:41–54. doi: 10.1016/j.steroids.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408:377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Yao X, Li Y, Saifudeen Z, Bachvarov D, El-Dahr SS. Histone deacetylase 1 and 2 regulate Wnt and p53 pathways in the ureteric bud epithelium. Development. 2015;142:1180–1192. doi: 10.1242/dev.113506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.