Abstract
Rare variants in the coding sequence of triggering receptor expressed on myeloid cells 2 (TREM2) have been identified in Alzheimer's disease (AD). They have been reported to be causative or confer risk of AD in several populations. However, the results are not conclusive. Therefore, a meta-analysis was performed to investigate the association between rare variants of TREM2 and the susceptibility to AD. Case-control studies meeting the inclusion criteria were identified by searching the PubMed, Embase and Web of Science databases. The association between four commonly analyzed variants of TREM2, p.Arg47His (R47H), p.Arg62His (R62H), p.Asp87Asn (D87N) and p.His157Tyr (H157Y), and the risk of AD were evaluated by meta-analyses with the fixed-effects model. Finally, a total of 26 datasets comprising 28,007 cases and 45,121 controls were included. There was no or low between-study heterogeneity in all comparisons. A significantly increased risk of AD was observed in carriers of R47H compared with non-carriers [odds ratio (OR)=3.88, 95% CI: 3.17-4.76, P<0.001], R62H (OR=1.37, 95% CI: 1.11-1.70, P=0.004) and H157Y (OR=4.22, 95% CI: 1.93-9.21, P<0.001). However, R62H only conferred a mild risk compared to R47H and H157Y (OR=1.37 vs. 3.88 and 4.22, respectively). D87N was not associated with AD susceptibility. Sensitivity analysis indicated that the association identified for R62H was not significant (P=0.192) when excluding a large-sample study. Subgroup analysis according to ethnicity revealed significant associations (R47H and H157Y) in Caucasians but not in Asians. In conclusion, rare coding variants of TREM2 were associated with an elevated risk of AD, particularly in Caucasians.
Keywords: TREM2, R47H, Alzheimer's disease, susceptibility, meta-analysis
Introduction
Alzheimer's disease (AD), the most prevalent form of dementia in the elderly, is characterized by the progressive loss of cognitive function. It is pathologically characterized by the extracellular accumulation of amyloid β proteins and intracellular formation of neurofibrillary tangles (1). Accumulating evidence has suggested an important role of the genetic component, which is estimated to be 60-80%, in the pathogenesis of AD (2). Numerous susceptible loci containing common variants [minor allele frequency (MAF) >5%] have been identified in recent decades (3). However, only the Ɛ4 allele of apolipoprotein E (APOE) is confirmed to be the most important variant conferring a 3- to 4-fold risk to AD (4). Furthermore, since most of the loci reside in the intronic or intergenic regions, the biological mechanisms remain difficult to interpret. With the advent of next-generation sequencing, more and more rare coding variants (MAF<1%) with a moderate effect on the risk of AD, including those in phospholipase Cγ2 (PLCG2), ABI family member 3 (ABI3) and triggering receptor expressed on myeloid cells 2 (TREM2), have been identified (5).
TREM2 is located on chromosome 6p21.1 and encodes a transmembrane protein on microglial cells. The protein is involved in the innate immunity within the central nervous system (CNS) by stimulating phagocytosis and inhibiting cytokine production (6). The association between TREM2 and AD was first suggested by findings indicating that homozygous loss-of-function variants of TREM2 caused autosomal recessive Nasu-Hakola disease, which is characterized by polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy and early-onset dementia (7). Subsequent genome-wide and exome-wide sequencing revealed that rare coding variants of TREM2 may confer a risk of AD (4,8). A rare missense variant, p.Arg47His (R47H, rs75932628), has been identified as a risk factor of AD in Caucasians (4,8), but not in African-American or Asian populations (9,10). Additional rare variants, including p.Arg62His (R62H, rs143332484), p.Asp87Asn (D87N, rs142232675) and p.His157Tyr (H157Y, rs2234255), have also been detected in AD subjects and indicated to increase the susceptibility to AD (11,12). Furthermore, certain TREM2 mutations, such as p.Gln33stop, p.Tyr38Cys and Thr66Met, have been identified only in patients with AD but not in normal controls, and appear to be causative (13), However, due to the low frequency of these variants, studies with a small sample size may not have adequate power to identify the genetic associations (14-16). The results of association studies from different regions and ethnicities are not always consistent. Therefore, a meta-analysis for the genetic association between TREM2 rare coding variants and AD risk involving 73,128 individuals was performed.
Materials and methods
Search strategy
The PubMed, Embase and Web of Science databases were searched for articles investigating the association between TREM2 variants and the risk of AD using the following key words: (‘TREM2’ OR ‘triggering receptor expressed on myeloid cells 2’) and (‘AD’ OR ‘Alzheimer's disease’ OR ‘Alzheimer disease’) from inception to December 31, 2019. Additional articles were obtained by manually searching the reference lists of review and research articles.
Inclusion and exclusion criteria
For inclusion in the present meta-analysis, studies were required to meet the following criteria: i) Investigation of the association of TREM2 variants with the risk of AD in human subjects; ii) reporting on genotype data of at least one of the following four variants: R47H, R62H, D87N and H157Y; iii) studies with a case-control design with unrelated samples; and iv) providing sufficient data to calculate an odds ratio (OR) and corresponding 95% CI. If studies had overlapping samples, only the study with a larger sample size was included. Studies were excluded if they were based on family samples, used imputed data or provided insufficient genotype data.
Data extraction
A total of two independent researchers (RL and XW) extracted the following information: First author, publication year, ethnicity, sample size, genotype distribution in the case and control group and the genotyping method. Discrepancies between researchers were resolved by discussion.
Quality assessment
The present study used the Newcastle-Ottawa Scale (NOS) (17), which includes 8 items in 3 categories (selection, comparability and exposure), to assess the quality of all included studies. There are 9 stars in total, and studies having 7 or more stars were considered of high quality.
Statistical analysis
Since the four variants examined are rare in the general population, only the AD risk of carriers (heterozygous + homozygous variants) vs. non-carriers (homozygous wild-type) was compared. The OR was calculated as follows: OR=(no. of case carriers/no. of case non-carriers)/(no. of control carriers/no. of control non-carriers) and the 95% CI was determined to estimate the association strength of each variant with AD risk. Between-study heterogeneity was assessed by I2 statistics, which indicated low, intermediate and high heterogeneity if I2<25, 25-50 and >50%, respectively. The random-effects model was applied if there was high heterogeneity; otherwise, the fixed-effects model was used. Sensitivity analysis was performed to evaluate the impact of each study on the pooled effect size by excluding one study at a time. Publication bias was detected by Begg's funnel plots and Egger's test. All statistical analyses were performed by using STATA 12.0 (StataCorp LP). P<0.05 was considered to indicate statistical significance.
Results
Study selection
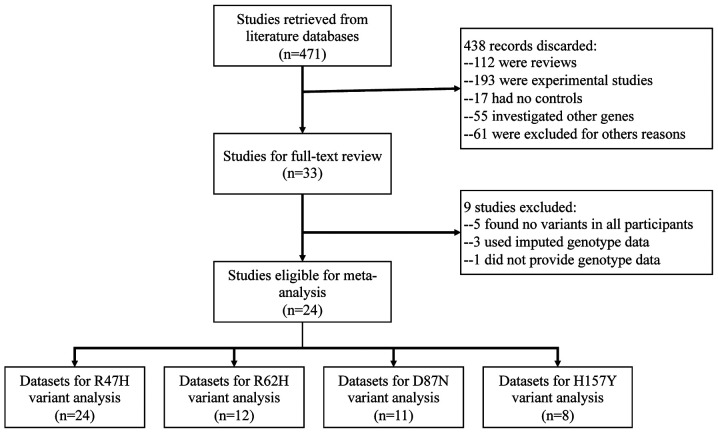
A total of 33 studies investigating the genetic association between rare TREM2 variants (R47H, R62H, D87N and H157Y) and the risk of AD were identified. However, 9 studies were excluded for the following reasons: A total of 5 did not provide any variant of interest in neither the case nor control groups (18-22), 3 used imputed data (5,23,24) and 1 did not provide genotype data (25). A total of two studies used a multi-stage strategy (8,26), and the datasets from discovery and replication stages were independently included in the quantitative synthesis. Finally, a total of 26 datasets from 24 studies (4,8-12,14-16,26-40) comprising 28,007 cases and 45,121 controls were eligible for the meta-analysis. Among these studies, 24 were for R47H (4,8-11,14-16,26-37,39,40), 12 for R62H (8,9,11,14-16,27,28,31,32,38,39), 11 for D87N (8,9,11,15,26-28,31,32,36) and eight for H157Y (8-12,26,31,36). A flowchart of the literature search is provided in Fig. 1. Regarding ethnicity, the majority of screened individuals were Caucasians (20 datasets) and the others were from African (2 datasets), Asian (2 datasets) or mixed populations (2 datasets). All studies were of high quality (≥7 stars) according to the NOS. The characteristics of all datasets included in the quantitative synthesis are listed in Table I.
Figure 1.
Flowchart of the literature search. TREM2, triggering receptor expressed on myeloid cells 2.
Table I.
Characteristics of all datasets included in the quantitative synthesis.
| First author | Year | Ethnicity | Genotyping method | Sample size (cases/controls) | Variant | NOS score | Refs. |
|---|---|---|---|---|---|---|---|
| Jonsson | 2013 | Caucasian | Assay, Taqman | 2037/9727 | R47H | 9 | (4) |
| Guerreiro (discovery) | 2013 | Caucasian | NGS, Sequencing | 1091/1105 | R47H, R62H, D87N, H157Y | 9 | (8) |
| Guerreiro (replication) | 2013 | Caucasian | Taqman | 1887/4061 | R47H | 9 | (8) |
| Pottier | 2013 | Caucasian | Sequencing | 726/783 | R47H, R62H, D87N | 7 | (27) |
| Bertitez | 2013 | Caucasian | Sequencing | 504/550 | R47H, R62H, D87N | 7 | (28) |
| Gonzalez | 2013 | Caucasian | Taqman | 427/2540 | R47H | 7 | (29) |
| Ruiz | 2014 | Caucasian | Taqman, HRM, KASPar | 3172/2169 | R47H | 8 | (30) |
| Cuyvers | 2014 | Caucasian | Sequencing | 1216/1094 | R47H, R62H, D87N, H157Y | 7 | (31) |
| Miyashita | 2014 | Asian | Taqman | 2190/2498 | R47H, H157Y | 8 | (10) |
| Jin | 2014 | Caucasian | Taqman | 2082/1648 | R47H, R62H, D87N, H157Y | 7 | (11) |
| Slattery | 2014 | Caucasian | Sequencing | 971/534 | R47H, R62H, D87N | 8 | (32) |
| Finelli | 2015 | Caucasian | Sequencing | 474/608 | R47H | 7 | (33) |
| Roussos | 2015 | Caucasian | Sequencing | 265/225 | R47H | 7 | (34) |
| Jin | 2015 | African | Sequencing | 906/2487 | R47H, R62H, D87N, H157Y | 8 | (9) |
| Mehrjoo | 2015 | Caucasian | Sequencing | 131/157 | R47H, R62H | 7 | (14) |
| Rosenthal | 2015 | Caucasian | Taqman | 1613/2927 | R47H | 8 | (35) |
| Jiang | 2016 | Asian | NGS | 988/1354 | H157Y | 8 | (12) |
| Ghani | 2016 | Caucasian | NGS | 210/233 | R47H, D87N, H157Y | 7 | (36) |
| Sirkis (discovery) | 2016 | Caucasian | NGS | 31/245 | R47H, D87N | 7 | (26) |
| Sirkis (replication) | 2016 | Caucasian | NGS | 2927/2633 | R47H, D87N, H157Y | 8 | (26) |
| Bellenguez | 2017 | Caucasian | NGS | 1779/1273 | R47H | 8 | (37) |
| Peplonska | 2018 | Caucasian | Sequencing | 274/208 | R47H, R62H, D87N | 7 | (15) |
| Landoulsi | 2018 | African | Sequencing | 172/158 | R62H | 7 | (38) |
| Arboleda-Bustos | 2018 | Caucasian | KASPar | 358/329 | R47H, R62H | 7 | (16) |
| Dalmasso | 2019 | Mixed | Taqman | 419/486 | R47H, R62H | 7 | (39) |
| Ayer | 2019 | Mixed | Taqman | 1157/5089 | R47H | 8 | (40) |
HRM, high-resolution melting; KASPar, KBioscience competitive allele-specific polymerase chain reaction genotyping system; NGS, next-generation sequencing; NOS: Newcastle-Ottawa Scale.
R47H and risk of AD
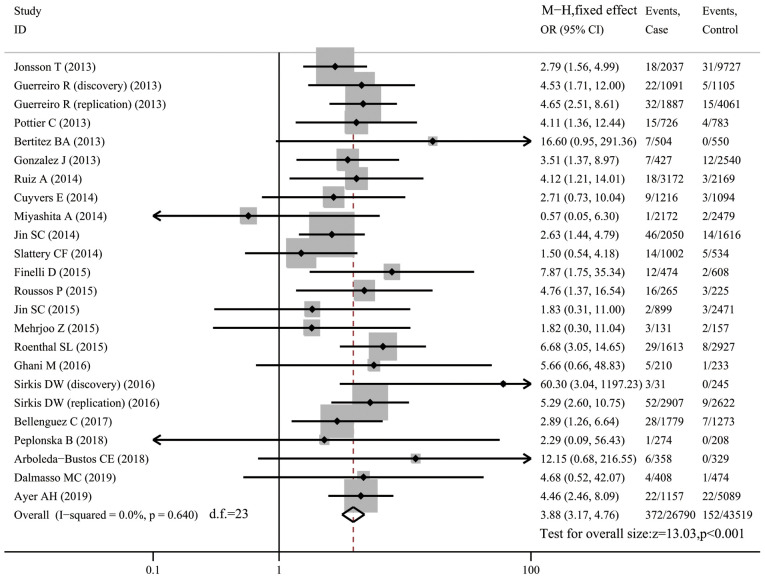
A total of 24 datasets (26,847 cases and 43,609 controls) investigated the association between the R47H variant and risk of AD. Carriers of R47H accounted for 1.39% of AD patients and 0.35% of controls. There was no between-study heterogeneity (I2=0) and the fixed-effects model was used. Compared with non-carriers, R47H carriers were more susceptible to AD (OR=3.88, 95% CI: 3.17-4.76, P<0.001; Fig. 2). Subgroup analysis of the datasets of Caucasian populations (20 datasets with 22,175 cases and 33,049 controls) revealed an increased risk of AD in R47H carriers (OR=3.93, 95% CI: 3.15-4.90, P<0.001, I2=0; Fig. S1).
Figure 2.
Forest plot for the association of triggering receptor expressed on myeloid cells 2 variant R47H with susceptibility to Alzheimer's disease. OR, odds ratio; M-H, Mantel-Haentzel method; d.f., degree of freedom.
There were three studies reporting on the association between the R47H variant and the susceptibility to early-onset AD (EOAD), which onsets in patients below the age of 65 years. These 3 studies comprised a total of 1,758 cases of EOAD and 2,606 controls (26,27,36). The variant occurred in 2.22% of EOAD cases but only in 0.42% of controls, thus conferring a 4.86-fold increased risk (95% CI: 2.52-9.36, P<0.001, I2=0; Fig. S2) to EOAD.
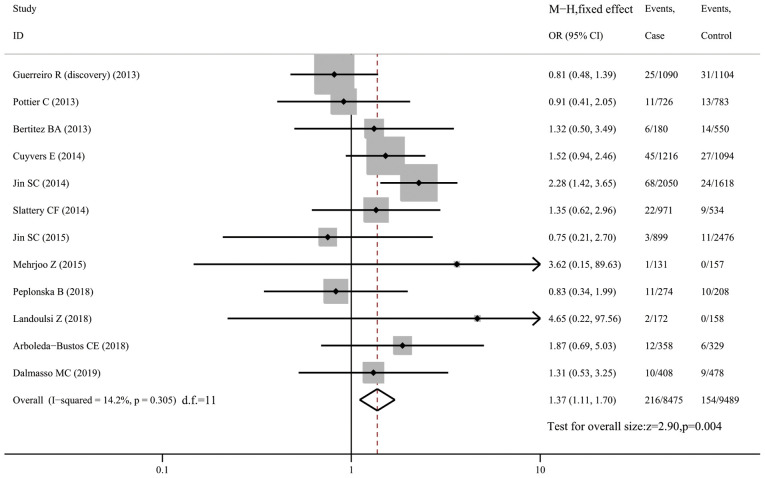
R62H and risk of AD
The R62H variant was genotyped in 12 datasets comprising 8,525 AD cases and 9,539 controls. Pooled analysis using the fixed-effects model demonstrated a higher risk of AD in carriers than in non-carriers of R62H in the whole sample (OR=1.37, 95% CI: 1.11-1.70, P=0.004, I2=14.2%; Fig. 3) and in Caucasians only (OR=1.39, 95% CI: 1.11-1.74, P=0.004; Fig. S3). However, sensitivity analysis revealed that the association became insignificant (OR=1.17, 95% CI: 0.92-1.50, P=0.192, I2=0; Fig. S4) when the study by Jin et al (11) was excluded, indicating that the study had a significant impact on the pooled effect size and was the main source of heterogeneity. In a subgroup analysis of Caucasian subjects after excluding the study by Jin et al (eight datasets with 13,436 subjects), no association was identified between the R62H variant and AD susceptibility (OR=1.17, 95% CI: 0.90-1.52, P=0.231, I2=0; Fig. S5).
Figure 3.
Forest plot for the association of triggering receptor expressed on myeloid cells 2 variant R62H with susceptibility to Alzheimer's disease. OR, odds ratio; M-H, Mantel-Haentzel method; d.f., degree of freedom.
D87N and risk of AD
A total of 11 datasets (10,614 cases and 11,520 controls) were included in the meta-analysis on the association between the D87N variant and AD susceptibility. However, no significant associations were observed in the whole sample (OR=1.61, 95% CI: 0.94-2.76, P=0.081, I2=0; Fig. 4) or in the Caucasian subgroup (OR=1.62, 95% CI: 0.94-2.82, P=0.084, I2=0; Fig. S6).
Figure 4.
Forest plot for the association of triggering receptor expressed on myeloid cells 2 variant D87N with susceptibility to Alzheimer's disease. OR, odds ratio; M-H, Mantel-Haentzel method; d.f., degree of freedom.
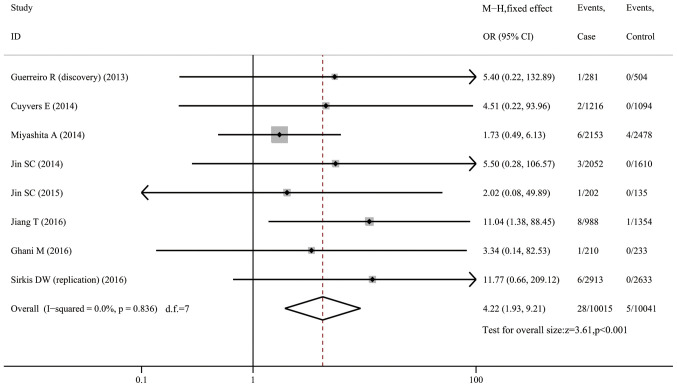
H157Y and risk of AD
The H157Y variant was rare in AD cases (0.28%) and controls (0.05%). When pooling 8 datasets (10,096 cases and 10,099 controls) together, the analysis indicated that carriers of the H157Y variant were more predisposed to AD (OR=4.22, 95% CI: 1.93-9.21, P<0.001, I2=0; Fig. 5). The variant conferred a 6.20-fold risk to Caucasians (95% CI: 1.60-24.04, P=0.008, I2=0; Fig. S7) from 5 datasets, but was not associated with AD risk in Asians (OR=3.61, 95% CI: 0.61-21.37, P=0.157, I2=55.1%; Fig. S8) from two datasets. Of note, in Caucasians, the variant was only found in AD patients (13/6,672) but was completely absent in normal controls (0/6,074).
Figure 5.
Forest plot for the association of triggering receptor expressed on myeloid cells 2 variant H157Y with susceptibility to Alzheimer's disease. OR, odds ratio; M-H, Mantel-Haentzel method; d.f., degree of freedom.
Sensitivity analysis and publication bias
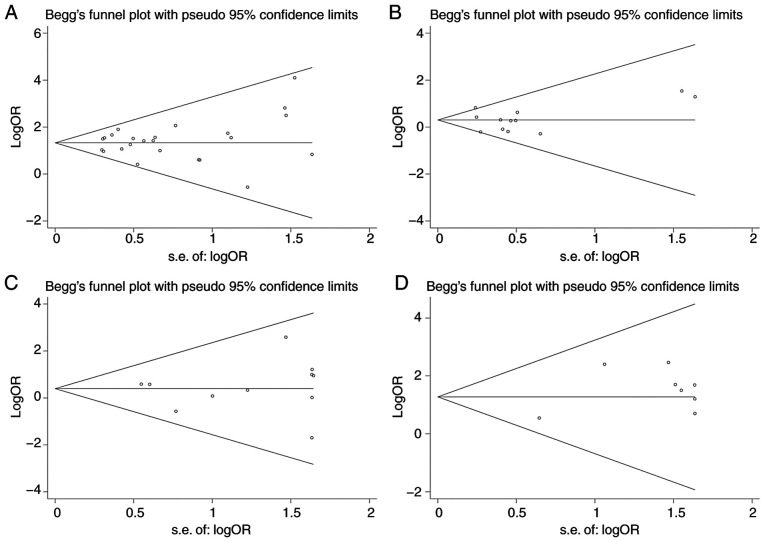
Sensitivity analysis demonstrated that none of the included datasets significantly affected the pooled effect size except the study of Jin et al (11) for the R62H variant as mentioned above. The funnel plots were symmetric for each mutation (Fig. 6) and Egger's test indicated that there was no publication bias (P>0.05).
Figure 6.
Symmetric funnel plots for the meta-analyses of (A) R47H, (B) R62H, (C) D87N and (D) H157Y. OR, odds ratio; M-H, Mantel-Haentzel method; d.f., degree of freedom.
Discussion
The present meta-analysis systematically investigated the genetic associations of four frequently reported rare variants of TREM2 with AD susceptibility. The results indicated that carriers of the R47H, R62H or H157Y variants were more vulnerable to AD.
TREM2 encodes a transmembrane immune receptor on microglial cells and exerts its effect by regulating the number of myeloid cells, enhancing phagocytosis and modulating the inflammation response in the CNS (6,41). Its pivotal role in neuroimmunology and neuroinflammation may indicate an implication of TREM2 in the pathogenesis of neurodegenerative disease (41). Association studies have identified rare variants in the coding sequence of TREM2 as susceptibility markers for amyotrophic lateral sclerosis (42), Parkinson's disease and frontotemporal dementia (43), as well as AD. The involvement of TREM2 in the etiology of neurodegenerative diseases, particularly AD, is complex. Numerous studies have revealed that disease-associated variants (mainly R47H) of TREM2 impact amyloid pathology (44), modulate neuritic dystrophy (34), influence tau hyperphosphorylation and aggregation (45) and affect synaptic and neuronal loss (46). Although the pathogenic mechanism of TREM2 in AD may not have been conclusive, TREM2 variants are conclusively susceptibility markers for AD, as demonstrated by the present meta-analysis.
R47H is the most frequently investigated variant in TREM2. The substitution of Arg by His leads to a marked reduction in soluble TREM2 levels (47) and the binding ability to cells, APOE and various lipids (45,48,49). However, whether it reduces the mRNA and protein expression in humans remains controversial (50). Furthermore, experiments have revealed that the heterozygous R47H variant may confer a loss of TREM2 function and enhance neuritic dystrophy around plaques, thus increasing the risk of AD (51), which was ascertained by epidemiological investigations in various populations (4,8,40). The R47H variant is enriched in AD cases (1.39%) but less prevalent in cognitively normal controls (0.35%). Most of the identified variants are heterozygous (98%, 514/524) and homozygous variants are detected in AD cases only. The present meta-analysis indicated an approximately 4-fold risk of AD in R47H carriers compared to non-carriers, indicating an effect size similar to that of the APOE Ɛ4 allele (4). However, APOE Ɛ4 is much more prevalent in populations and should still be the main genetic determinant of AD susceptibility.
In addition to risk, the R47H variant may also contribute to an earlier age of onset in AD. Slattery et al (32) reported a markedly younger age at onset in patients with the R47H variant than in those without the variant (55.2 vs. 61.7 years on average). Similar results were obtained in Icelandic and Dutch populations (4), implying an association between R47H and susceptibility to EOAD. In the present study, pooled analysis of 3 studies (27,28,37) suggested a higher prevalence (2.22%) of R47H in EOAD cases. Carriers had 4.86-fold risk of developing EOAD, which was even higher than the effect size observed in the total AD samples.
The R62H variant disrupts TREM2 recognition of cells and APOE (52) but does not alter the binding to lipid ligands (53). Jin et al (11) determined that it is associated with an increased risk of AD in a population of Americans of European descent (11). The present study revealed a mildly increased risk of AD in individuals harboring the R62H variant (OR=1.38, 95% CI: 1.11-1.70, P=0.004). However, the effect size may be largely attributed to the aforementioned study (11), as indicated by the sensitivity analysis. The association was no longer significant after excluding this study (P=0.192). A significant association was only observed in the study by Jin et al (11), but not in any other study. It should be noted that the study by Jin et al (11) had a large sample size (>3,700 subjects), and thus, it may have more statistical power than the other studies to identify the association of a rare variant with AD susceptibility. Therefore, more studies with a large sample size are required to confirm the association.
The D87N variant resides within the ectodomain proximal to the stalk region of TREM2. The mutation leads to enhanced interaction of TREM2 with certain ligands (53). However, the present study did not indicate any association of the variant with AD susceptibility when 11 datasets were pooled together. Of note, D87N was identified in 4 out of 5 patients in a family affected by AD (54). It may be enriched in familial cases in contrast to sporadic cases. Another variant, H157Y, is located on the stalk domain of TREM2 but has no impact on binding activity (53). Specifically, it is located at the TREM2 cleavage site by metalloprotease ADAM metallopeptidase domain 10 and results in increased shedding of TREM2 and reduced cell surface expression of TREM2(55). In addition, in silico programs [SIFT (http://sift.jcvi.org/) and Polyphen2 (http://genetics.bwh.harvard.edu/pph2/)] predicted that the variant may have a deleterious/possibly damaging impact on functions of TREM2. The present analysis, comprising 10,096 cases and 10,099 controls, revealed a significantly increased AD susceptibility in carriers of H157Y, although the variant is rare in AD (0.28%).
In the present study, a subgroup analysis was performed with regard to ethnicity, indicating an ethnicity-specific pattern of TREM2 variants. R47H and R62H were mostly identified in Caucasians but rare in Asians. Only one study from Asian (10) reported a low frequency of R47H (0.06%), whereas the other studies did not (18-22). Therefore, R47H and R62H mutations were only associated with an increased risk of AD in Caucasians. Contrary to R47H and R62H, H157Y was more frequent in Asians. Miyashita et al (10) reported 10 carriers out of 4,631 participants, while Jiang et al (12) identified 9 carriers among 2,352 participants. However, the H157Y variant was not associated with AD risk in Japanese (10) but increased the risk in a Han Chinese population (12). Of note, in Caucasians, the H157Y variant was only harbored by AD cases but by none of the controls, indicating that H157Y was more likely to be a causative variant of AD in Caucasians.
In summary, the present meta-analysis, involving >73,000 individuals, supported that rare coding variants of TREM2 are associated with AD susceptibility and may be used as predictive markers for neurodegenerative disease.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RL designed and supervised the study; RL and XW performed literature search, study selection, data curation and formal analysis; RL and PH performed quality control of studies; RL and XW prepared the original draft; RL, XW and PH critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Castellani RJ, Rolston RK, Smith MA. Alzheimer disase. Dis Mon. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiat. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 3.Naj AC, Schellenberg GD. Genomic variants, genes, and pathways of Alzheimer's disease: An overview. Am J Med Genet B Neuropsychiatr Genet. 2017;174:5–26. doi: 10.1002/ajmg.b.32499. Alzheimer's Disease Genetics Consortium (ADGC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. New Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, Bis JC, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohn TT. The triggering receptor expressed on myeloid cells 2: ‘TREM-ming’ the inflammatory component associated with Alzheimer's disease. Oxid Med Cell Longev. 2013;2013(860959) doi: 10.1155/2013/860959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin SC, Carrasquillo MM, Benitez BA, Skorupa T, Carrell D, Patel D, Lincoln S, Krishnan S, Kachadoorian M, Reitz C, et al. TREM2 is associated with increased risk for Alzheimer's disease in African Americans. Mol Neurodegener. 2015;10(19) doi: 10.1186/s13024-015-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyashita A, Wen Y, Kitamura N, Matsubara E, Kawarabayashi T, Shoji M, Tomita N, Furukawa K, Arai H, Asada T, et al. Lack of genetic association between TREM2 and late-onset Alzheimer's disease in a Japanese population. J Alzheimers Dis. 2014;41:1031–1038. doi: 10.3233/JAD-140225. [DOI] [PubMed] [Google Scholar]
- 11.Jin SC, Benitez BA, Karch CM, Cooper B, Skorupa T, Carrell D, Norton JB, Hsu S, Harari O, Cai Y, et al. Coding variants in TREM2 increase risk for Alzheimer's disease. Hum Mol Genet. 2014;23:5838–5846. doi: 10.1093/hmg/ddu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang T, Tan L, Chen Q, Tan MS, Zhou JS, Zhu XC, Lu H, Wang HF, Zhang YD, Yu JT. A rare coding variant in TREM2 increases risk for Alzheimer's disease in Han Chinese. Neurobiol Aging. 2016;42:217.e1–e3. doi: 10.1016/j.neurobiolaging.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Dardiotis E, Siokas V, Pantazi E, Dardioti M, Rikos D, Xiromerisiou G, Markou A, Papadimitriou D, Speletas M, Hadjigeorgiou GM. A novel mutation in TREM2 gene causing Nasu-Hakola disease and review of the literature. Neurobiol Aging. 2017;53:194.e13–194.e22. doi: 10.1016/j.neurobiolaging.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Mehrjoo Z, Najmabadi A, Abedini SS, Mohseni M, Kamali K, Najmabadi H, Khorram Khorshid HR. Association study of the TREM2 gene and identification of a novel variant in exon 2 in iranian patients with late-onset Alzheimer's disease. Med Prin Pract. 2015;24:351–354. doi: 10.1159/000430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peplonska B, Berdynski M, Mandecka M, Barczak A, Kuzma-Kozakiewicz M, Barcikowska M, Zekanowski C. TREM2 variants in neurodegenerative disorders in the Polish population. Homozygosity and compound heterozygosity in FTD patients. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:407–412. doi: 10.1080/21678421.2018.1451894. [DOI] [PubMed] [Google Scholar]
- 16.Arboleda-Bustos CE, Ortega-Rojas J, Mahecha MF, Arboleda G, Vásquez R, Pardo R, Arboleda H. The p.R47H variant of TREM2 gene is associated with late-onset Alzheimer disease in Colombian population. Alzheimer Dis Assoc Disord. 2018;32:305–308. doi: 10.1097/WAD.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Yu JT, Jiang T, Wang YL, Wang HF, Zhang W, Hu N, Tan L, Sun L, Tan MS, Zhu XC, Tan L. Triggering receptor expressed on myeloid cells 2 variant is rare in late-onset Alzheimer's disease in Han Chinese individuals. Neurobiol Aging. 2014;35:937.e1–e3. doi: 10.1016/j.neurobiolaging.2013.10.075. [DOI] [PubMed] [Google Scholar]
- 19.Chung SJ, Kim MJ, Kim J, Kim YJ, You S, Koh J, Kim SY, Lee JH. Exome array study did not identify novel variants in Alzheimer's disease. Neurobiol Aging. 2014;35:1958.e13–e14. doi: 10.1016/j.neurobiolaging.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Zhou Y, Xu J, Liu X, Wang Y, Deng Y, Wang G, Xu W, Ren R, Liu X, et al. Association study of TREM2 polymorphism rs75932628 with late-onset Alzheimer's disease in Chinese Han population. Neurol Res. 2014;36:894–896. doi: 10.1179/1743132814Y.0000000376. [DOI] [PubMed] [Google Scholar]
- 21.Jiao B, Liu X, Tang B, Hou L, Zhou L, Zhang F, Zhou Y, Guo J, Yan X, Shen L. Investigation of TREM2, PLD3, and UNC5C variants in patients with Alzheimer's disease from mainland China. Neurobiol Aging. 2014;35:2422.e9–2422.e11. doi: 10.1016/j.neurobiolaging.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Guo Q, Zhou Y, Chen K, Xu Y, Ding D, Hong Z, Zhao Q. Lack of association between triggering receptor expressed on myeloid cells 2 polymorphism rs75932628 and late-onset Alzheimer's disease in a Chinese Han population. Psychiat Genet. 2018;28:16–18. doi: 10.1097/YPG.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giraldo M, Lopera F, Siniard AL, Corneveaux JJ, Schrauwen I, Carvajal J, Muñoz C, Ramirez-Restrepo M, Gaiteri C, Myers AJ, et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer's disease. Neurobiol Aging. 2013;34:2077.e11–e18. doi: 10.1016/j.neurobiolaging.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertram L, Parrado AR, Tanzi RE. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369(1565) doi: 10.1056/NEJMc1306509. [DOI] [PubMed] [Google Scholar]
- 25.Tosto G, Vardarajan B, Sariya S, Brickman AM, Andrews H, Manly JJ, Schupf N, Reyes-Dumeyer D, Lantigua R, Bennett DA, et al. Association of variants in PINX1 and TREM2 with late-onset Alzheimer disease. JAMA Neurol. 2019;76:942–948. doi: 10.1001/jamaneurol.2019.1066. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirkis DW, Bonham LW, Aparicio RE, Geier EG, Ramos EM, Wang Q, Karydas A, Miller ZA, Miller BL, Coppola G, Yokoyama JS. Rare TREM2 variants associated with Alzheimer's disease display reduced cell surface expression. Acta Neuropathol Commun. 2016;4(98) doi: 10.1186/s40478-016-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pottier C, Wallon D, Rousseau S, Rovelet-Lecrux A, Richard AC, Rollin-Sillaire A, Frebourg T, Campion D, Hannequin D. TREM2 R47H variant as a risk factor for early-onset Alzheimer's disease. J Alzheimers Dis. 2013;35:45–49. doi: 10.3233/JAD-122311. [DOI] [PubMed] [Google Scholar]
- 28.Benitez BA, Cooper B, Pastor P, Jin SC, Lorenzo E, Cervantes S, Cruchaga C. TREM2 is associated with the risk of Alzheimer's disease in Spanish population. Neurobiol Aging. 2013;34:1711.e15–e17. doi: 10.1016/j.neurobiolaging.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez Murcia JD, Schmutz C, Munger C, Perkes A, Gustin A, Peterson M, Ebbert MT, Norton MC, Tschanz JT, Munger RG, et al. Assessment of TREM2 rs75932628 association with Alzheimer's disease in a population-based sample: The cache county study. Neurobiol Aging. 2013;34:2889.e11–e13. doi: 10.1016/j.neurobiolaging.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz A, Dols-Icardo O, Bullido MJ, Pastor P, Rodríguez-Rodríguez E, López de Munain A, de Pancorbo MM, Pérez-Tur J, Alvarez V, Antonell A, et al. Assessing the role of the TREM2 p.R47H variant as a risk factor for Alzheimer's disease and frontotemporal dementia. Neurobiol Aging. 2014;35:444.e1–e4. doi: 10.1016/j.neurobiolaging.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Cuyvers E, Bettens K, Philtjens S, Van Langenhove T, Gijselinck I, van der Zee J, Engelborghs S, Vandenbulcke M, Van Dongen J, Geerts N, et al. Investigating the role of rare heterozygous TREM2 variants in Alzheimer's disease and frontotemporal dementia. Neurobiol Aging. 2014;35:726.e11–e19. doi: 10.1016/j.neurobiolaging.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Slattery CF, Beck JA, Harper L, Adamson G, Abdi Z, Uphill J, Campbell T, Druyeh R, Mahoney CJ, Rohrer JD, et al. R47H TREM2 variant increases risk of typical early-onset Alzheimer's disease but not of prion or frontotemporal dementia. Alzheimers Dement. 2014;10:602–608.e4. doi: 10.1016/j.jalz.2014.05.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finelli D, Rollinson S, Harris J, Jones M, Richardson A, Gerhard A, Snowden J, Mann D, Pickering-Brown S. TREM2 analysis and increased risk of Alzheimer's disease. Neurobiol Aging. 2015;36:546.e9–e13. doi: 10.1016/j.neurobiolaging.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Roussos P, Katsel P, Fam P, Tan W, Purohit DP, Haroutunian V. The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer's dementia. Alzheimers Dement. 2015;11:1163–1170. doi: 10.1016/j.jalz.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal SL, Bamne MN, Wang X, Berman S, Snitz BE, Klunk WE, Sweet RA, Demirci FY, Lopez OL, Kamboh MI. More evidence for association of a rare TREM2 mutation (R47H) with Alzheimer's disease risk. Neurobiol Aging. 2015;36:2443.e21–e26. doi: 10.1016/j.neurobiolaging.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghani M, Sato C, Kakhki EG, Gibbs JR, Traynor B, St George-Hyslop P, Rogaeva E. Mutation analysis of the MS4A and TREM gene clusters in a case-control Alzheimer's disease data set. Neurobiol Aging. 2016;42:217.e7–217.e13. doi: 10.1016/j.neurobiolaging.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellenguez C, Charbonnier C, Grenier-Boley B, Quenez O, Le Guennec K, Nicolas G, Chauhan G, Wallon D, Rousseau S, Richard AC, et al. Contribution to Alzheimer's disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1,779 cases and 1,273 controls. Neurobiol Aging. 2017;59:220.e1–220.e9. doi: 10.1016/j.neurobiolaging.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Landoulsi Z, Ben Djebara M, Kacem I, Sidhom Y, Kefi R, Abdelhak S, Gargouri-Berrechid A, Gouider R. Genetic Analysis of TREM2 variants in tunisian patients with Alzheimer's disease. Med Prin Pract. 2018;27:317–322. doi: 10.1159/000489779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalmasso MC, Brusco LI, Olivar N, Muchnik C, Hanses C, Milz E, Becker J, Heilmann-Heimbach S, Hoffmann P, Prestia FA, et al. Transethnic meta-analysis of rare coding variants in PLCG2, ABI3, and TREM2 supports their general contribution to Alzheimer's disease. Transl Psychiatry. 2019;9(55) doi: 10.1038/s41398-019-0394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayer AH, Wojta K, Ramos EM, Dokuru D, Chen JA, Karydas AM, Papatriantafyllou JD, Agiomyrgiannakis D, Kamtsadeli V, Tsinia N, et al. Frequency of the TREM2 R47H variant in various neurodegenerative disorders. Alzheimer Dis Assoc Disord. 2019;33:327–330. doi: 10.1097/WAD.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jay TR, von Saucken VE, Landreth GE. TREM2 in neurodegenerative diseases. Mol Neurodegener. 2017;12(56) doi: 10.1186/s13024-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cady J, Koval ED, Benitez BA, Zaidman C, Jockel-Balsarotti J, Allred P, Baloh RH, Ravits J, Simpson E, Appel SH, et al. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:449–453. doi: 10.1001/jamaneurol.2013.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, et al. TREM2 in neurodegeneration: Evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson's disease. Mol Neurodegener. 2013;8(19) doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan P, Condello C, C Keene D, Wang Y, Bird TD, Paul SM, Luo W, Colonna M, Baddeley D, Grutzendler J. TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron. 2016;92:252–264. doi: 10.1016/j.neuron.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lue LF, Schmitz CT, Serrano G, Sue LI, Beach TG, Walker DG. TREM2 Protein expression changes correlate with Alzheimer's disease neurodegenerative pathologies in post-mortem temporal cortices. Brain Pathol. 2015;25:469–480. doi: 10.1111/bpa.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Q, Danao J, Talreja S, Wen P, Yin J, Sun N, Li CM, Chui D, Tran D, Koirala S, et al. TREM2-activating antibodies abrogate the negative pleiotropic effects of the Alzheimer's disease variant Trem2R47H on murine myeloid cell function. J Biol Chem. 2018;293:12620–12633. doi: 10.1074/jbc.RA118.001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kober DL, Alexander-Brett JM, Karch CM, Cruchaga C, Colonna M, Holtzman MJ, Brett TJ. Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms. Elife. 2016;5(e20391) doi: 10.7554/eLife.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey CC, DeVaux LB, Farzan M. The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J Biol Chem. 2015;290:26033–26042. doi: 10.1074/jbc.M115.677286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang X, Piers TM, Wefers B, Zhu K, Mallach A, Brunner B, Kleinberger G, Song W, Colonna M, Herms J, et al. The Trem2 R47H Alzheimer's risk variant impairs splicing and reduces Trem2 mRNA and protein in mice but not in humans. Mol Neurodegener. 2018;13(49) doi: 10.1186/s13024-018-0280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng-Hathaway PJ, Reed-Geaghan EG, Jay TR, Casali BT, Bemiller SM, Puntambekar SS, von Saucken VE, Williams RY, Karlo JC, Moutinho M, et al. The Trem2 R47H variant confers loss-of-function-like phenotypes in Alzheimer's disease. Mol Neurodegener. 2018;13(29) doi: 10.1186/s13024-018-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 2016;91:328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Song W, Hooli B, Mullin K, Jin SC, Cella M, Ulland TK, Wang Y, Tanzi RE, Colonna M. Alzheimer's disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimer Dement. 2017;13:381–387. doi: 10.1016/j.jalz.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghani M, Lang AE, Zinman L, Nacmias B, Sorbi S, Bessi V, Tedde A, Tartaglia MC, Surace EI, Sato C, et al. Mutation analysis of patients with neurodegenerative disorders using NeuroX array. Neurobiol Aging. 2015;36:545.e9–e14. doi: 10.1016/j.neurobiolaging.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlepckow K, Kleinberger G, Fukumori A, Feederle R, Lichtenthaler SF, Steiner H, Haass C. An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol Med. 2017;9:1356–1365. doi: 10.15252/emmm.201707672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.