Abstract
Given their genetic and anatomic similarities to humans, nonhuman primates (NHPs) may serve as animal models for urogenital diseases of humans. The purpose of this study was to examine the frequency of spontaneous urogenital lesions occurring over a 30-year period at the Yerkes and Southwest National Primate Research Centers and to compare and contrast lesions occurring in Old World versus New World primates. Lesions occurring in the chimpanzee (Pan troglodytes), baboon (Papio spp.), rhesus macaque (Macaca mulatta), cynomolgus macaque (Macaca fascicularis), pig-tailed macaque (Macaca nemestrina), sooty mangabey (Cercocebus atys), common marmoset (Callithrix jacchus), cotton-top tamarin (Sanguinus oedipus) and squirrel monkey (Saimiri sciureus) are discussed.
The most common lesions of the kidney were medullary amyloidosis, renal cysts, renal tubular degeneration, glomerulonephritis or glomerulopathy, nephritis, nephrocalcinosis, pyelonephritis and hydronephrosis. Specific causes of renal tubular disease included pigmentary nephrosis and tubular lipidosis. Renal tumors, including renal adenoma and carcinoma, lymphoma, and nephroblastoma, were infrequent diagnoses in all species. Endometriosis was the most frequently diagnosed lesion of the female genital tract, occurring primarily in Old World primates. Leiomyoma was the most common uterine tumor. Granulosa cell tumor was the most frequently observed neoplasm of the ovaries, followed by teratoma. Of animals included in the study, most ovarian tumors occurred in baboons. Neoplasms of the male reproductive tract included interstitial cell tumor, seminoma, penile squamous cell carcinoma, penile papilloma and histiocytoma. In New World monkeys, renal lesions were reported more frequently than genital lesions.
Keywords: Primate, Urinary, Reproductive
Nonhuman primates (NHPs) are used as laboratory animal models in biomedical, pharmacological and toxicological studies. The number of NHPs used in research studies has continued to rise annually since 2014, with rhesus and cynomolgus macaques being the most commonly used species.24 Diseases of the urinary and reproductive tracts are a significant cause of morbidity and mortality in humans worldwide; urologic diseases and reproductive health are among the top primary research areas for studies involving NHPs.24,49 In order to effectively utilize these invaluable animals in research, it is critical for veterinary pathologists and researchers involved in NHP study design and interpretation to have a thorough understanding of species-specific, commonly occurring spontaneous diseases.
The microscopic anatomy of the NHP kidney closely resembles that of humans and other laboratory animals.20 Age-associated structural and functional abnormalities have been characterized in a few NHP species.43 Among the order Primates, humans and members of the family Atelidae (spider and woolly monkeys) are unique in that they have multipapillate, multipyramidal kidneys, in which urine flows through a system of calyces to reach the renal pelvis and ureter.20,23 The remaining primate species have unipapillate kidneys.20 Despite these anatomical differences, similar functional renal capacity has been reported for humans and NHPs, so they purportedly serve as excellent models of renal transplantation.20
Most male NHPs have an external, fibrovascular penis with a rounded tip (glans), an os penis (baculum), prepuce, and scrotal testes. The male accessory sex glands are located within the pelvic cavity, and typically consist of paired seminal vesicles, a single- or bi-lobed prostate, and paired bulbourethral glands.20 Testicular size varies with the reproductive season in some species of NHPs.36
The normal anatomical and histological features of the female genital system are noteworthy. New and Old World monkeys (NWMs and OWMs) have a simple uterus, while prosimians have a bicornuate uterus.53 Normal ovarian histology of NWMs is notable for prominent interstitial tissue, luteal tissue and granulosa cell nests or clusters.21,79 Macaques and baboons have well-developed perineal sex skin that displays marked swelling and erythema during estrus. Old World primates have a similar endometrial physiology to that of humans, with regular, roughly monthly menstrual cycles occurring in great apes and macaques; they also exhibit prominent keratinization of the vaginal mucosa under the influence of estrogen, and the vaginal mucosa may become permanently thickened with age.21,61 The cervix of rhesus and cynomolgus macaques is sigmoid with blind folds, making transcervical uterine access extremely difficult, while baboons and marmosets have a straight cervix.4,31,84 This anatomical difference is of particular importance for insemination procedures and gives the baboon an advantage as a model for reproductive research. Unlike OWMs, the majority of NWMs do not exhibit menses.41 The reproductive cycles of rhesus macaques and marmosets are closely linked to social structure, frequently resulting in ovarian cycle suppression of subordinate females in group settings.1,19
The purpose of this study is to provide veterinary pathologists and researchers with an overview of spontaneous urogenital lesions occurring in NHPs during a defined period (30 years) and to compare urogenital lesions occurring in OWMs versus NWMs. Spontaneous lesions are defined as those occurring naturally (i.e. not experimentally induced). To this end, we conducted a 30-year retrospective study of the Yerkes National Primate Research Center (YNPRC) and Southwest National Primate Research Center (SNPRC) pathology records from January 1989 to February 2019. For the purposes of this review, the term “study period” will refer to the >30-year period (January 1989 to February 2019). Data from both Primate Centers were pooled.
Methods
Pathology records from the YNPRC and SNPRC were reviewed to identify all spontaneous lesions occurring in the male and female reproductive and urinary tissues of NHPs. A combination of manual and automated data mining techniques was employed using keywords relating to the urinary system (kidney, ureter, urinary bladder and urethra) and male and female reproductive systems (testis, epididymis, prostate, seminal vesicles, penis, prepuce, scrotum, ovary, oviduct, uterus, cervix, vagina, and vulva) of NHPs. All spontaneous lesions were included, including those interpreted to be clinically significant and those deemed to be insignificant or background lesions. Lesions in the tissues of interest that have a known association with experimental manipulation [i.e. nephritis or glomerulonephritis associated with simian immunodeficiency virus (SIV) infection in macaques] and experimental NHP renal transplant cases were excluded from the data analysis.27 Spontaneously occurring cases of SIV infection were also excluded.
Many animals had lesions affecting more than one tissue; if these lesions were considered separate processes, they were counted individually. However, if the same process was present in multiple tissues within the same animal (i.e. endometriosis or metastatic/multifocal neoplasms), this lesion was counted once. If the same, non-metastatic primary neoplasm was diagnosed in paired organs in the same animal (i.e. bilateral ovarian or testicular neoplasms) it was considered as a separate lesion in each paired organ. If a tumor had multiple sites within an organ, it was counted as one neoplasm (i.e. multiple uterine leiomyomas). If an animal had two identical diagnoses, one from a previous biopsy and one from necropsy (SNPRC data only), only the necropsy diagnosis was counted. Procedures involving animals at each institution were conducted in compliance with applicable regulations, including oversight by the YNPRC and SNPRC Institutional Animal Care and Use Committees.
When reviewing the data from female NHPs, we focused on lesions of the non-gravid uterus, oviducts, cervix, ovaries, vagina, and vulva. Diseases of the postpartum uterus either resulting from normal parturition, abortion or stillbirth were excluded. The spectrum of normal uterine changes across the menstrual cycle were not described in this study.19,20
For each necropsy case included in the analyses, standard necropsy procedures were followed at each institution. A full postmortem exam was completed for all necropsy cases, including gross examination of vital organs. Histopathology was performed for further characterization of most of gross lesions.
Results
The most frequent non-neoplastic and neoplastic lesions are summarized in Tables 1 and 2. Species-specific data for the macaque and New World monkey species are included in Supplemental Tables S1 and S2.
Table 1.
Non-neoplastic Lesions of the Urogenital Tract in Nonhuman Primates Observed Over a >30-year Period (1989–2019) at Two Primate Centers
| Frequency of Diagnosis | |||||
|---|---|---|---|---|---|
| Diagnosis (total) | Chimpanzee | Baboon | Macaque | Sooty Mangabey | NWM |
| Kidney | |||||
| Amyloidosis (244) | 2 | 139 | 68 | 6 | 29 |
| Cysts (82) | 6 | 200 | 63 | 4 | 7 |
| Degeneration, tubular (81) | 1 | 13 | 59 | 4 | 4 |
| GN/Glomerulopathy (228) | 22 | 97 | 20 | 5 | 84 |
| Nephritis (1261) | 64 | 569 | 281 | 40 | 307 |
| Nephrocalcinosis (120) | 4 | 69 | 34 | - | 13 |
| Nephropathy or nephrosis (156) | 7 | 47 | 66 | 2 | 34 |
| Pyelonephritis (164) | 5 | 133 | 18 | 4 | 4 |
| Hydronephrosis (73) | 3 | 49 | 19 | 1a | 1 |
| Ureter | |||||
| Hydroureter (34) | 1 | 29 | 3 | 1 | - |
| Urinary bladder | |||||
| Cystitis (169) | 4 | 112 | 43 | 5 | 5 |
| Cyst (10) | 1 | 7 | 2 | - | - |
| Endometriosis (45) | - | 30 | 13 | 2 | - |
| Urethra | |||||
| Urethritis (15) | 2 | 6 | 5 | - | 2 |
| Ovary | |||||
| Cyst (188) | 10 | 90 | 76 | 6 | 6 |
| Endometriosis (71) | - | 45 | 26 | - | - |
| Mineralization (17) | - | 6 | 11 | - | - |
| Atrophy (12) | 2 | 8 | 2 | - | - |
| Oophoritis (5) | - | 4 | 1 | - | - |
| Oviduct | |||||
| Endometriosis (13) | - | 11 | 2 | - | - |
| Cyst (7) | 1 | 1 | 2 | - | 3 |
| Salpingitis (4) | - | 1 | 2 | - | 1 |
| Ectopic decidua (4) | - | 2 | 2 | - | - |
| Uterus & Cervix uteri | |||||
| Endometriosis (472) | 1 | 298 | 152 | 18 | 3 |
| Adenomyosis (292) | 10 | 226 | 46 | 3 | 7 |
| Metritis/endometritis (133) | 3 | 65 | 54 | 6 | 5 |
| Uterine prolapse (12) | - | 2 | 10 | - | - |
| Uterine rupture (11) | - | 10 | 1 | - | - |
| Cervicitis (18) | - | 4 | 13 | 1 | - |
| Vagina & Vulva | |||||
| Vaginitis/vulvitis (49) | 2 | 39 | 6 | 2 | - |
| Ulcer (vaginal, vulvar, perineal) (30) | 1 | 29 | - | - | - |
| Obstruction, stricture, stenosis | - | 40 | - | - | - |
| (vaginal, vulvar) (40) | - | ||||
| Vaginal prolapse (9) | - | 3 | 6 | - | - |
| Testis & Epididymis | |||||
| Atrophy, testicular (80) | 6 | 62 | 14 | - | 8 |
| Degeneration (13) | 2 | 6 | 4 | 1 | - |
| Orchitis (25) | 1 | 16 | 7 | - | 1 |
| Male Accessory Sex Glands | |||||
| Atrophy, prostatic (21) | 1 | 9 | 1 | - | 10 |
| Prostatitis, NS (170) | 5 | 95 | 64 | - | 6 |
| Seminal vesiculitis (4) | - | 3 | 1 | - | - |
| Penis, Prepuce & Scrotum | |||||
| Balanitis/balanoposthitis (8) | - | 4 | 4c | - | - |
| Dermatitis, scrotal (9) | 1 | 7 | 1d | - | - |
| Edema, scrotal or preputial (9) | 5b | 1 | 2 | 1 | - |
| Paraphimosis with penile necrosis (1) | - | - | - | - | 1 |
| Ulcer, penile (9) | - | 7 | 2e | - | - |
Abbreviations and footnotes: NWM, New World monkey; GN, glomerulonephritis
Secondary to endometriosis lesions;
Four of 5 cases secondary to congestive heart failure;
Vesicular balanoposthitis due to Herpes B virus infection in one case (a rhesus macaque);
Exudative scrotal dermatitis in a rhesus macaque;
Due to self-injurious behavior.
Table 2.
Hyperplastic and Neoplastic Lesions of the Urogenital Tracts in Nonhuman Primates Observed Over a >30-year Period (1989–2019) at Two Primate Centers
| Age at Diagnosis (years) | |||||
|---|---|---|---|---|---|
| Diagnosis (frequency) | Chimpanzee | Baboon | Macaque | Sooty Mangabey | NWM |
| Kidney | |||||
| Adenoma (6) | - | 40,45,38 | 6a,8b,22 | - | - |
| Carcinoma (4) | 41,42 | - | 31,35 | - | - |
| Carcinoma, metastatic (2) | - | 3 (HCC) | - | - | |
| - | 17 (Adrenal) | ||||
| Ectopic adrenocortical adenoma (1) | 47 | - | - | - | - |
| Hemangioma (2) | 54 | - | - | 24 | - |
| Lymphoma (8) | - | 19,29,38,45 | 6,6,10 | 16 | |
| Nephroblastoma (4) | - | 3,13,21,29 | - | - | - |
| TC/urothelial carcinoma, renal pelvis (1) | - | - | 25 | - | - |
| Tumor, NS (2) | 41 | - | 16 | - | - |
| Urinary bladder | |||||
| TC/urothelial carcinoma (0) | - | - | - | - | - |
| Endometrial stromal tumor (1) | - | 39 | - | - | - |
| Carcinoma, metastatic (3) | - | - | 10,19(ICACA) | - | 14c |
| Ovary | |||||
| Adenoma (10) | - | 21*,30,37,38d,39, 47 | 18,28;Unke | - | - |
| Adenocarcinoma (3) | - | 36,37,49 | - | - | - |
| Benign serous ovarian tumor (1) | - | 19 | - | - | - |
| Brenner tumor (4) | 56 | 28,33,40 | - | - | - |
| Carcinoma (7) | - | 18,21,26,34,34,40,49 | - | - | - |
| Carcinoma, metastatic (1) | - | - | 19g | - | - |
| Dermoid cyst (1) | - | 34 | - | - | - |
| Dysgerminoma (2) | - | 33,37 | - | - | - |
| Endometrioid tumor (1) | - | - | 21f | - | - |
| Fibroadenoma (1) | - | 40 | - | - | - |
| Fibroma-thecoma (1) | - | - | 28 | - | - |
| Granulosa cell tumor (63) | 46,46 | 27 (average of 54 animals; range = 5 – 53 years) | 3,11,21h,26,28,31 | - | 20 |
| Hyperplasia (11) | 32 | 7,7,13,19,20,22,38,39 | - | - | 5,13 |
| Leiomyoma (1) | - | 21 | - | - | - |
| Leydig cell tumor (3) | - | 32,41,48 | - | - | - |
| Luteoma (1) | - | 37 | - | - | - |
| Neoplasm, NS (4) | 55 | 24,39 | - | - | |
| Sertoli cell tumor (1) | - | 29 | - | - | - |
| Sex cord-Stromal tumor (4) | - | 12,18,19,22 | - | - | - |
| Steroid tumor (2) | - | 22,37 | - | - | - |
| Teratoma (12) | 39 | 18,18,19,22,28,29,37,38 | 7,11,27 | - | - |
| Oviduct | |||||
| Adenocarcinoma, well-differentiated | - | - | 17 | - | - |
| Fimbrial (1) | |||||
| Adenofibroma (1) | - | 19 | - | - | - |
| Leiomyoma (2) | 55 | 31 | - | - | - |
| Papilloma (1) | - | - | 26 | - | - |
| Hyperplasia (10) | 32 | 22,22 | 6,11,13,15,21,21,28 | - | - |
| Uterus/Cervix | |||||
| Adenocarcinoma, uterine (4) | - | 46 | 22l | 17k,26m | - |
| Adenoma, uterine, likely (1) | - | 24 | - | - | - |
| Muellerian/paramesonephric origin | |||||
| Atypia, endocervical (1) | - | - | 13 | - | - |
| Carcinoma, cervical (2) | - | 21 | 20o | - | - |
| Carcinoma, endocervical (1) | - | - | 22p | - | - |
| Carcinoma, metastatic (2) | - | - | 1q,28r | - | - |
| Carcinoma, uterine (2) | - | 22 | - | 27n | - |
| Carcinoma, uterine with giant cells (1) | - | - | 15 | - | - |
| Cystadenoma, endometrial (1) | - | - | 19 | - | - |
| Dysplasia, endocervical (1) | - | - | 13 | - | - |
| Fibroleiomyoma, uterine with (1) | - | - | 18 | - | - |
| squamous metaplasia | - | ||||
| Fibroma, uterine (1) | 36 | - | 15s | - | - |
| Granulosa cell tumor, uterine (6) | - | 13,14,16,22,33,33 | - | - | - |
| Hemangioma, uterine (2) | - | - | 13,17 | - | |
| Hyperplasia, cervical (10) | - | 12,36 | 9,9,17,23,Unk | - | 5,9,14 |
| Hyperplasia, endometrial (37) | 46 | 39 (average of 21 animals; range 21 – 52) | 9,9,11,14,21,22,23,27,28,15,22,28,29,31 | 12 | - |
| Leiomyoma, cervical (2) | - | - | 26 (also uterus) | 19 | - |
| Leiomyoma, uterine (110) | 44 (average of 24 animals; range 29–61) | 35 (average of 48 animals; range 3–54; 1 Unk) | 31 (average of 29 animals; range 4–40; 3 Unk) | 22 (average of 8 animals; range 15–31) | 14 |
| Leiomyosarcoma, uterine (5) | - | 37 | 2i,19j | 17k,23 | - |
| Neoplasm, other cervical (1) | - | - | 16 | - | - |
| Neoplasm, other uterine (7) | 41 | - | 15t,15,15,20,24,28 | - | - |
| Papilloma, cervical (1) | - | 39 | - | - | - |
| Papilloma, uterine (1) | - | - | 1s | - | - |
| Polyp, uterine or cervical (75) | 28,35,36,39,52 | 31 (average of 48 animals; range 13–49) | 20 (average of 21 animals; range 7–28) | - | 12 |
| SCC, cervical (1) | - | - | 22p | - | - |
| SCC, uterine (1) | - | - | 13 | - | - |
| Vagina, vulva | |||||
| Fibroma (1) | - | - | 23 | - | - |
| Leiomyoma (2) | - | - | - | 16,31u | - |
| Myxoma (1) | - | 37 | - | - | - |
| Papilloma (3) | 49 | 17,29 | - | - | - |
| Squamous cell carcinoma, vulvar (1) | - | 38 | - | - | - |
| Testis | |||||
| Interstitial cell tumor (1) | - | - | - | - | 11 |
| Mass, NS (1) | - | - | - | - | 20 |
| Seminoma (1) | - | Unk | - | - | - |
| Male Accessory Sex Glands | |||||
| Adenoma, prostatic (1) | - | 30 | - | - | - |
| Hyperplasia, prostatic (15) | - | 23 (average of 12 animals; range 14–42) | 7,14,31 | - | - |
| Mass, peri-prostatic (1) | - | - | 2 | - | - |
| Adenoma, seminal vesicle (1) | - | - | 1 | - | - |
| Penis, Prepuce & Scrotum | |||||
| Histiocytoma, penile (1) | - | 40 | - | - | - |
| Papilloma(s), penile (4) | - | 1,4 | 16,17 | - | - |
| SCC, penile (2) | - | - | 4, 21 | - | - |
| SCC, preputial (1) | - | - | - | 17 | - |
| Melanocytoma, scrotal (1) | - | - | 4 | - | - |
Abbreviations and footnotes: NWM, New World monkey; HCC, hepatocellular carcinoma; ICACA, ileocecal adenocarcinoma; TC, transitional cell; Unk, unknown
Papillary subtype;
Tubulopapillary subtype;
Hepatocellular carcinoma with metastasis to uterus and urinary bladder;
Papillary cystadenoma;
Serous papillary adenoma;
Within a focus of endometriosis;
Metastatic carcinoma, most likely ileocecal adenocarcinoma origin; also metastasized to urinary bladder;
Intravascular metastases in liver;
Leiomyosarcoma with metastasis to regional lymph nodes;
Leiomyosarcoma with metastasis to omentum;
Animal had uterine adenocarcinoma, leiomyosarcoma, and uterine torsion;
Animal was euthanized for dysmenorrhea of >1 year duration;
Uterine adenocaricnoma with metastasis (not further specified);
Uterine carcinoma with metastasis to lungs;
Cervical carcinoma (incidental finding);
Endocercival carcinoma with metastasis to liver and lung;
Metastatsis/carcinomatosis of ileocecal adenocarcinoma to uterus, lungs and diaphragm;
Metastatsis/carcinomatosis of ileocecal adenocarcinoma to uterus and mesentery;
Animal had uterine hemangioma, fibroleiomyoma, and endometriosis;
Mass involved uterus and vagina.
Animal had endometrial carcinoma and uterine leiomyoma.
Bilateral serous ovarian cystadenomas.
The Urinary Tract (Kidneys, Ureters, Urinary Bladder and Urethra)
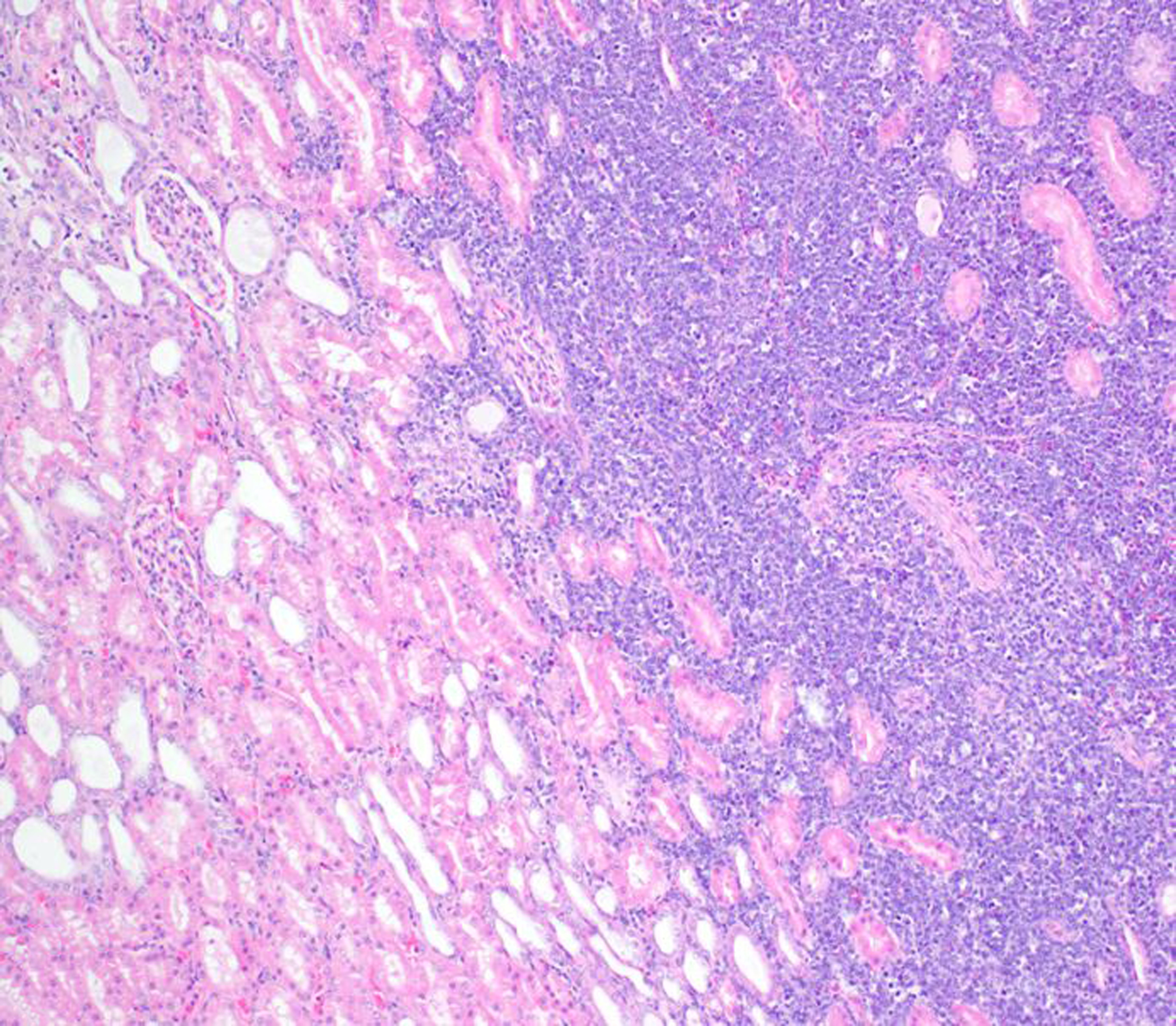
The most common lesions of the kidney were amyloidosis, cysts, tubular degeneration, glomerulonephritis or glomerulopathy, nephritis (including chronic renal disease), nephrocalcinosis, nephropathy or nephrosis, pyelonephritis and hydronephrosis (Fig. 1). Animals with chronic renal disease resulting in renal failure were included in the diagnostic category of nephritis (21 cases; Fig. 2). Ureteral lesions were rare; the most frequent diagnosis was hydroureter (34 cases; Fig. 1), and this was observed more frequently in females compared to males and was occasionally attributed to endometriosis-related stricture of the ureter.
Figure 1.
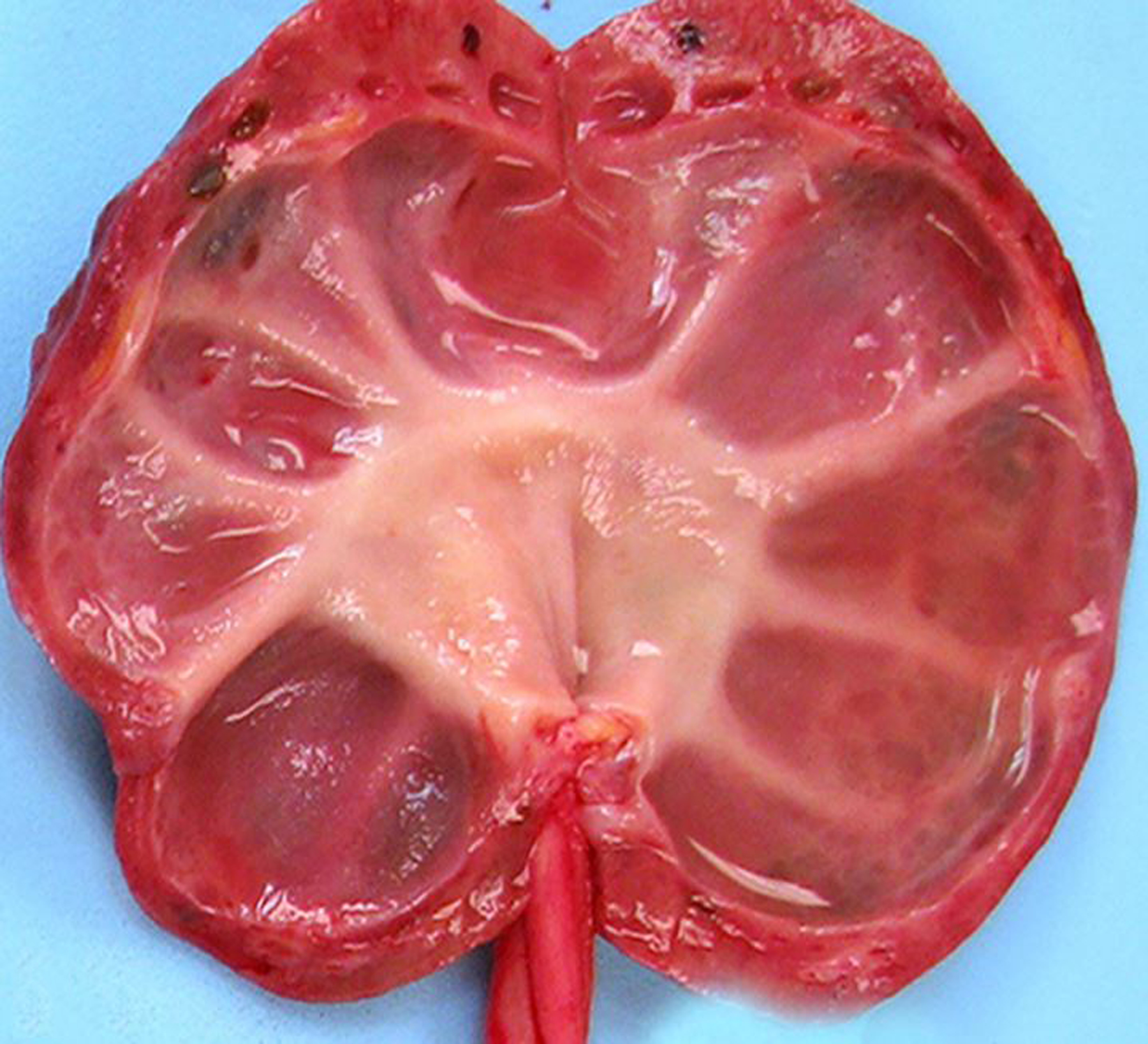
Hydronephrosis and hydroureter with secondary renal atrophy, kidney and ureter, chimpanzee.
Figure 2.
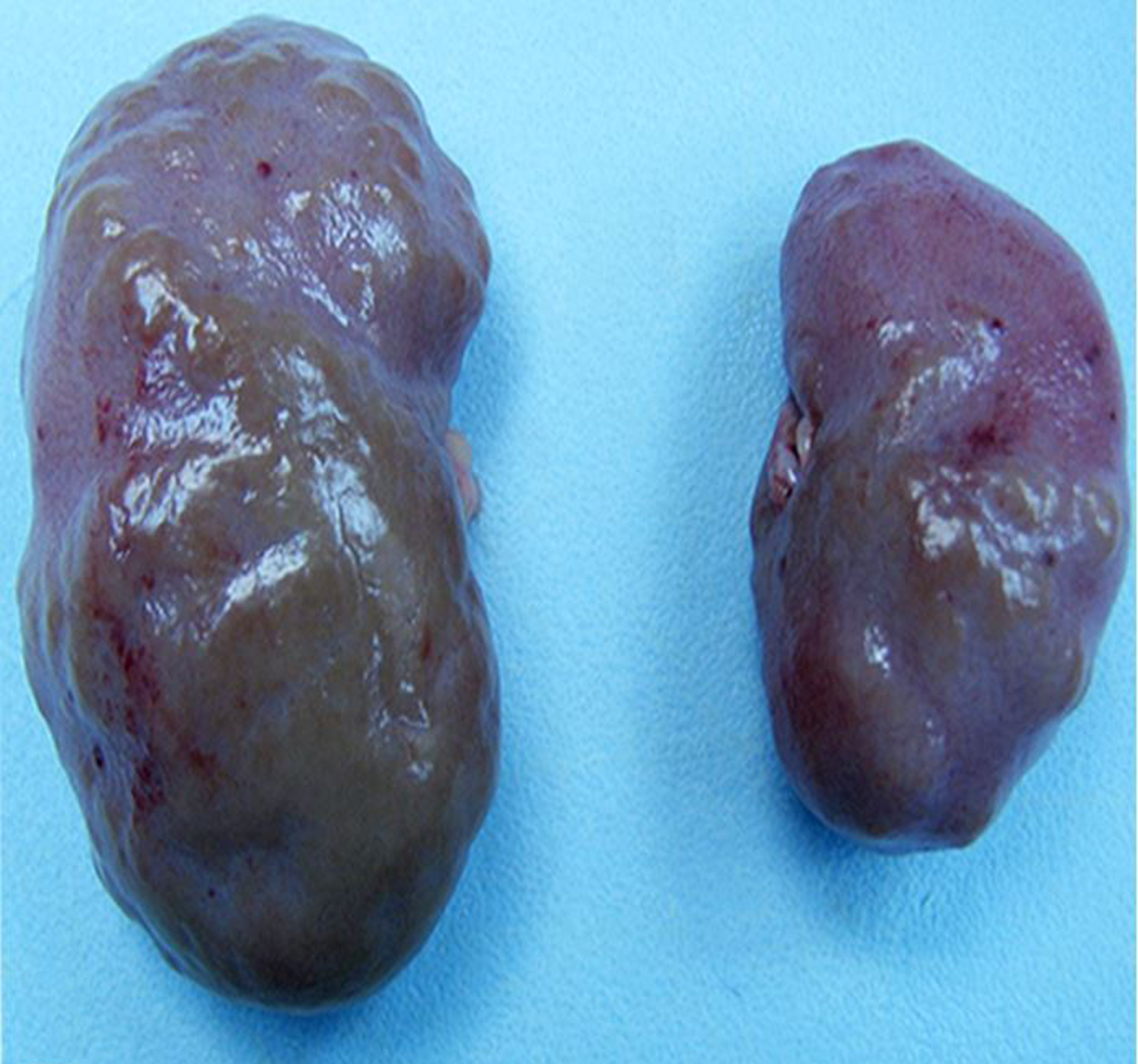
Chronic renal disease, kidneys, rhesus macaque. Parenchymal loss, fibrosis, and severe unilateral renal atrophy are evident.
There were 16 cases of rhabdomyolysis-associated pigmentary nephrosis (15 rhesus macaques and 1 sooty mangabey; Figs. 3 & 4). Many additional animals had nephrosis or nephropathy of undetermined cause. We identified one rhesus macaque with severe renal and hepatic lipidosis at necropsy, suggestive of fatal fasting syndrome; this animal had a history of obesity, rapid significant weight loss, and severe azotemia. There were 41 additional cases of renal tubular lipidosis or steatosis, the cause of which was not further specified.
Figure 3. Rhabdomyolysis-associated pigmentary nephropathy following trauma, kidney, rhesus macaque.
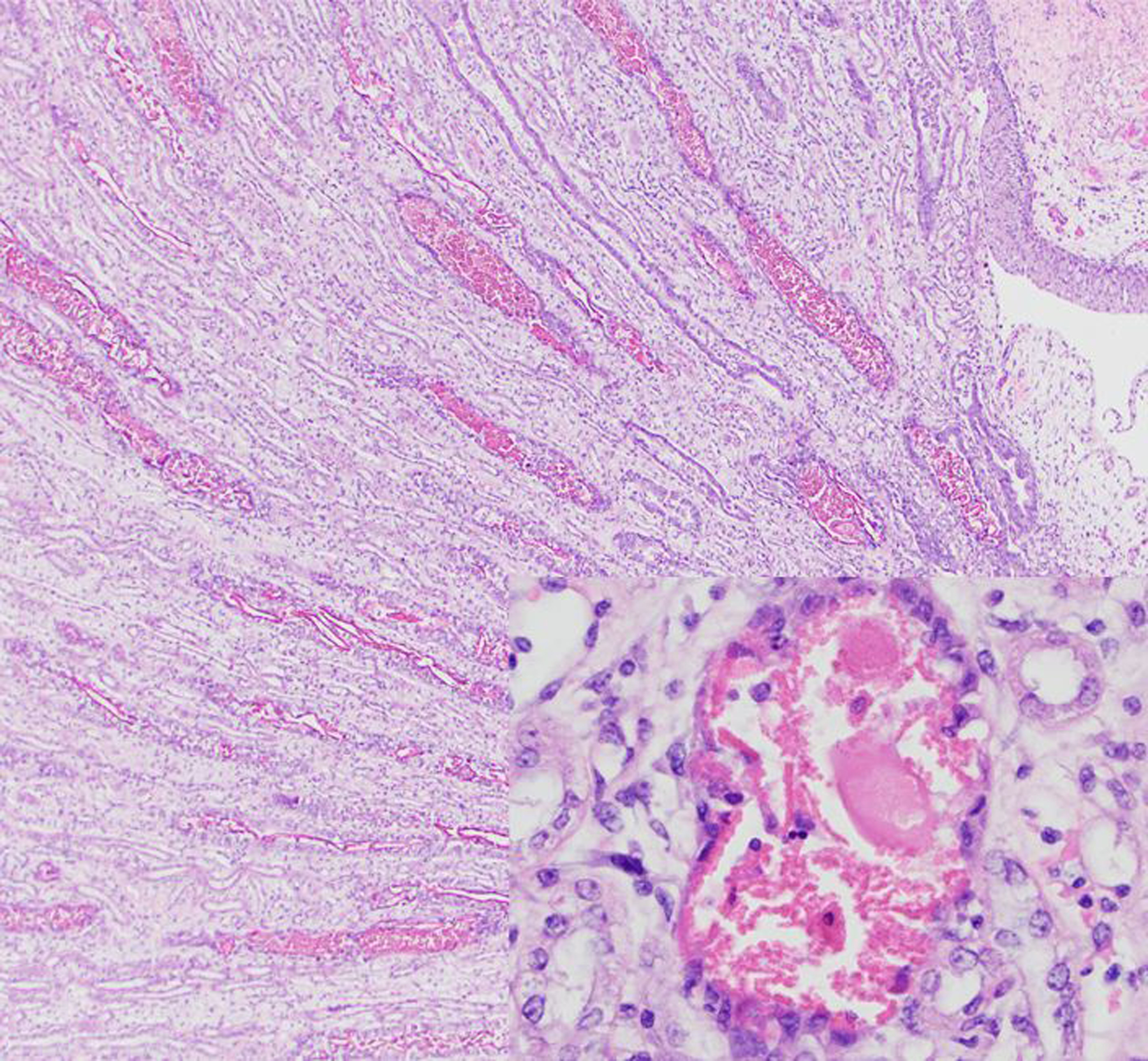
Within the medulla, there are numerous degenerate or necrotic tubules containing intratubular granular to globular eosinophilic casts. Hematoxylin and eosin (HE).
Figure 4. Rhabdomyolysis-associated pigmentary nephropathy following trauma, kidney, rhesus macaque.
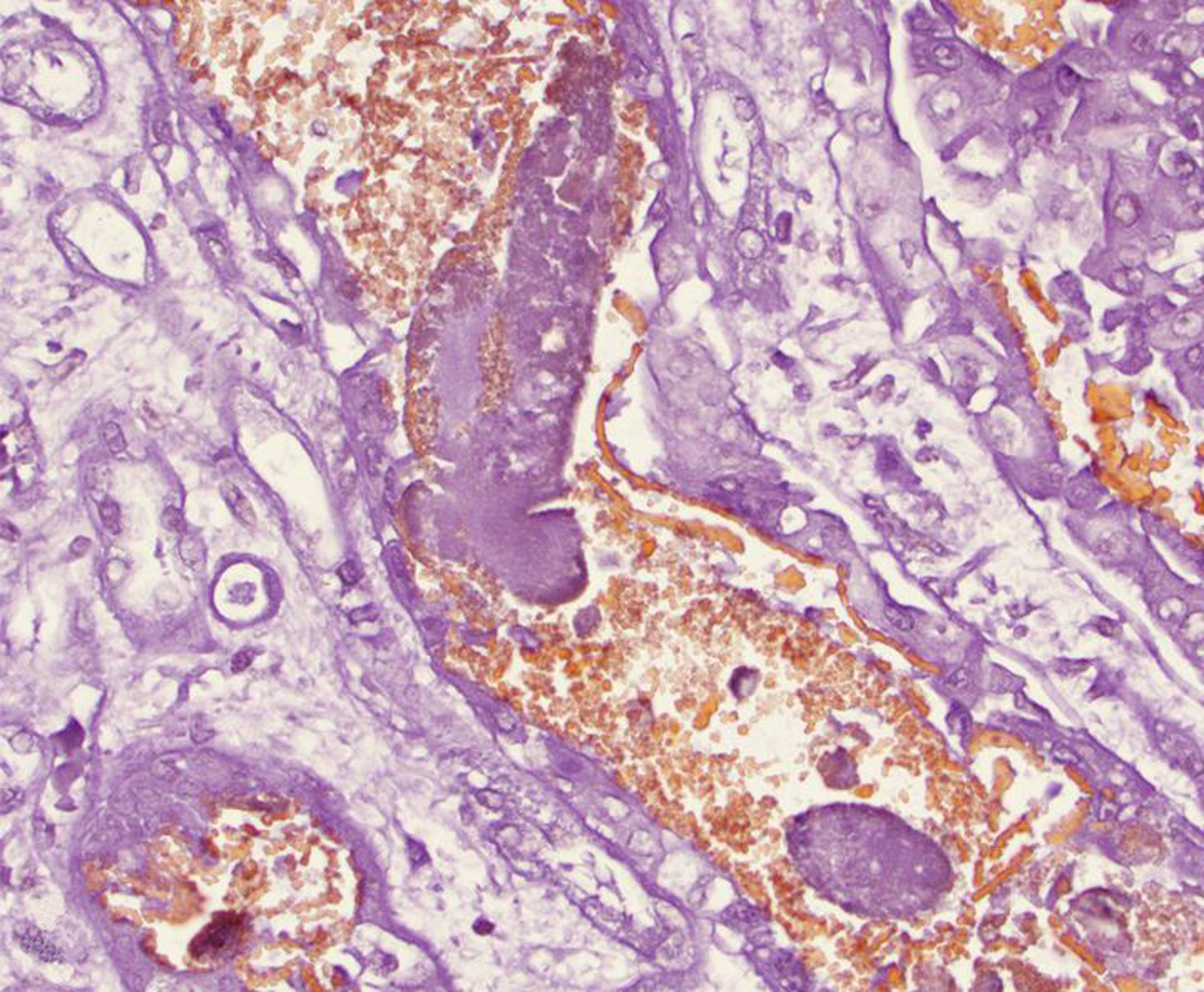
Intratubular casts are often non-staining, consistent with myoglobin. Casts are mixed with orange-staining granular material, consistent with hemoglobin. Okajima stain.
Glomerular disease was uncommon in NHPs during the study period. Of the animals with glomerulopathy or glomerulonephritis, baboons and common marmosets were highly represented. One female chimpanzee had both glomerular and medullary renal amyloidosis, along with extensive hepatic and splenic amyloidosis. This animal had hypoproteinemia (total serum protein 2.4 g/dL), and ascites and hydropericardium at necropsy. Protein loss was largely attributed to the glomerular amyloidosis, but urine protein was not measured, so hypoproteinemia due to decreased liver function as a result of hepatic amyloidosis, or a combination of the two, could not be ruled out.
Congenital renal lesions observed during the study period included polycystic kidneys (3 adult rhesus macaques and 1 baboon), ectopic adrenal tissue (3 rhesus macaques and 1 chimp), renal ectopia (2 rhesus macaques), adrenal-renal fusion (1 baboon), and unilateral renal aplasia (1 cynomolgus and 2 rhesus macaques).
The most frequent lesions of the urinary bladder were cystitis, endometriosis and urinary bladder cysts. The cause of cystitis was most often undetermined, and the character of inflammation was not specified for most cases. In a minority of cases (16 rhesus macaques), the inflammation was specified as lymphocytic, lymphoplasmacytic, lymphofollicular, eosinophilic or suppurative. Suppurative bacterial cystitis was associated with Escherichia coli, Klebsiella pneumonia, and Proteus mirabilis infections. Other findings in urinary bladders included polyarteritis nodosa (1 baboon), mineralization (1 baboon) and cystolithiasis (7 cases). In rhesus macaques, 6 cases of cystolithiasis were associated with retrograde ejaculation (Figs. 5 & 6), while the 7th case was of unknown mineral composition in a pig-tailed macaque.
Figure 5. Urinary bladder, rhesus macaque. Semen matrix calculi associated with retrograde ejaculation.
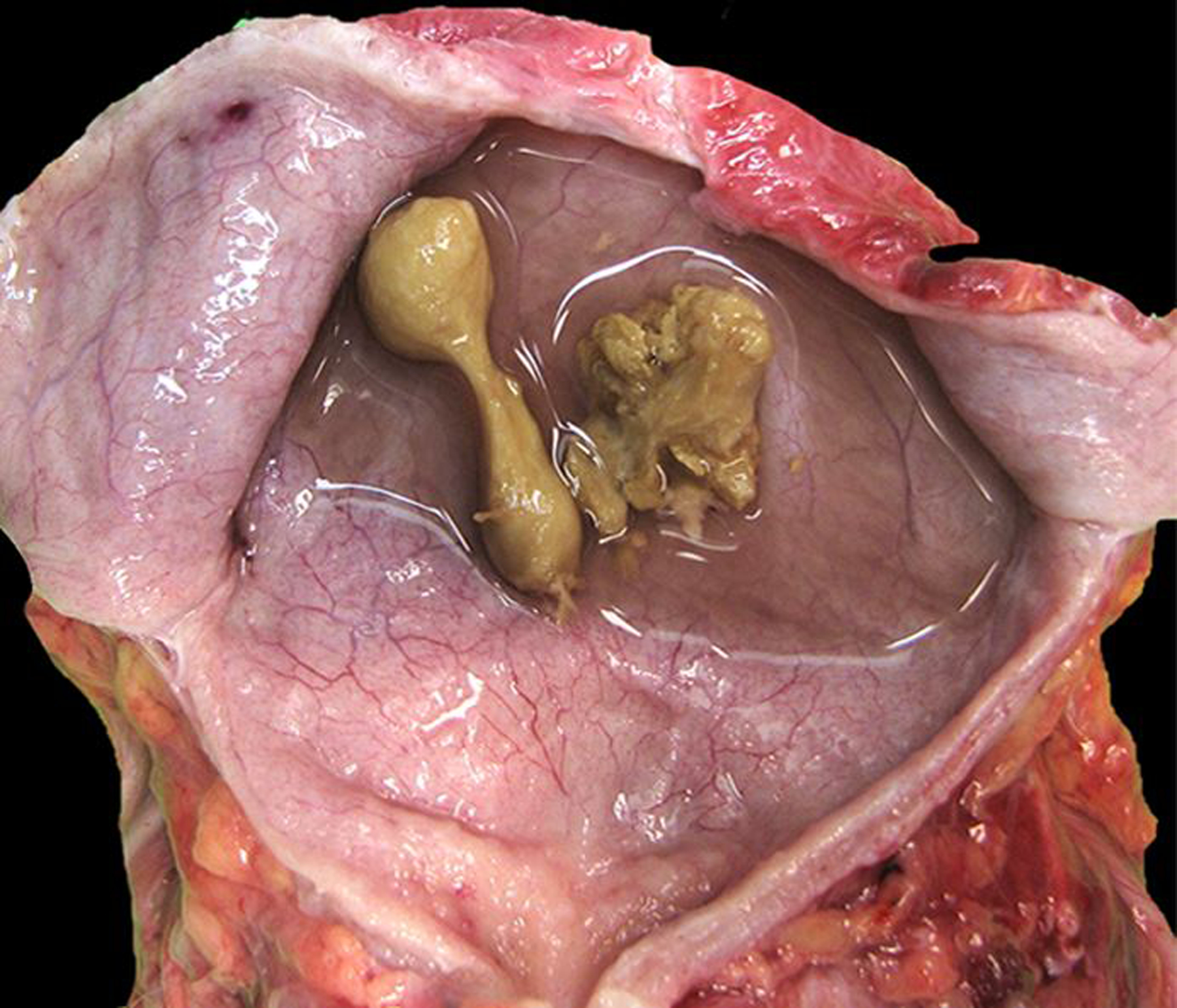
The calculi are yellow-to-green, firm, and irregular to dumbbell-shaped with spiculated surface projections.
Figure 6. Urinary bladder, rhesus macaque. Semen matrix calculi associated with retrograde ejaculation.
The calculus is composed of eosinophilic semen matrix material with numerous embedded spermatozoa. HE.
Lesions of the urethra were rare. Specific causes of ulcerative urethritis included retrograde ejaculation (4 rhesus macaques), bacterial infections (2 male baboons and a female chimpanzee), and trauma (1 male chimpanzee and 1 female rhesus macaque).
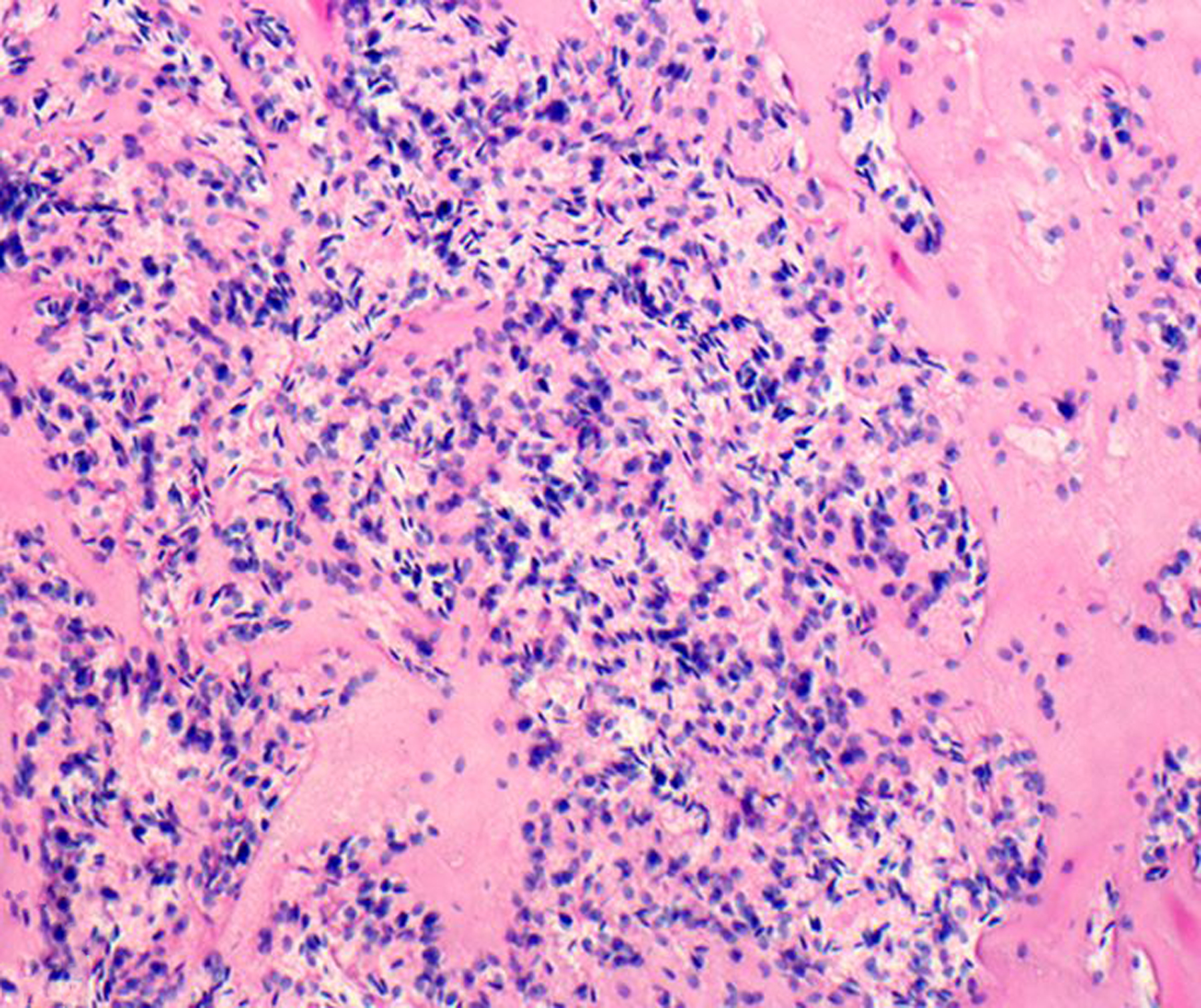
Thirty neoplasms involving the kidney were diagnosed during the study period (Table 2). These included renal lymphoma (Figs. 7, 8, 9), carcinoma, adenoma, nephroblastoma, urothelial (transitional cell) carcinoma of the renal pelvis, renal hemangioma, ectopic adrenocortical carcinoma, and renal sarcoma. Renal lymphoma was the most frequent tumor. One rhesus macaque had T-cell lymphoma affecting the kidney, liver, tonsils, thymus, and lymph nodes; SIV and Epstein-Barr virus infections were ruled out in that case. Two additional SIV-negative macaques had multicentric lymphoma affecting the kidneys; their simian T-lymphotropic virus (STLV) and lymphocryptovirus (LCV) serologic statuses are unknown. Four baboons and one common marmoset were diagnosed with renal lymphoma; serologic information for those animals was not available. Metastatic carcinomas involved the kidneys of 2 rhesus macaques (ileocecal adenocarcinoma) and one common marmoset (hepatocellular carcinoma).
Figure 7. Renal lymphoma (CD8+ T cell), kidney, rhesus macaque.
A well demarcated mass focally effaces and expands the renal parenchyma.
Figure 8. Renal lymphoma (CD8+ T cell), kidney, rhesus macaque.
The unencapsulated neoplasm is composed of sheets of lymphocytes, infiltrates the renal interstitium, and focally replaces the renal parenchyma. HE.
Figure 9. Renal lymphoma (CD8+ T cell), kidney, rhesus macaque.
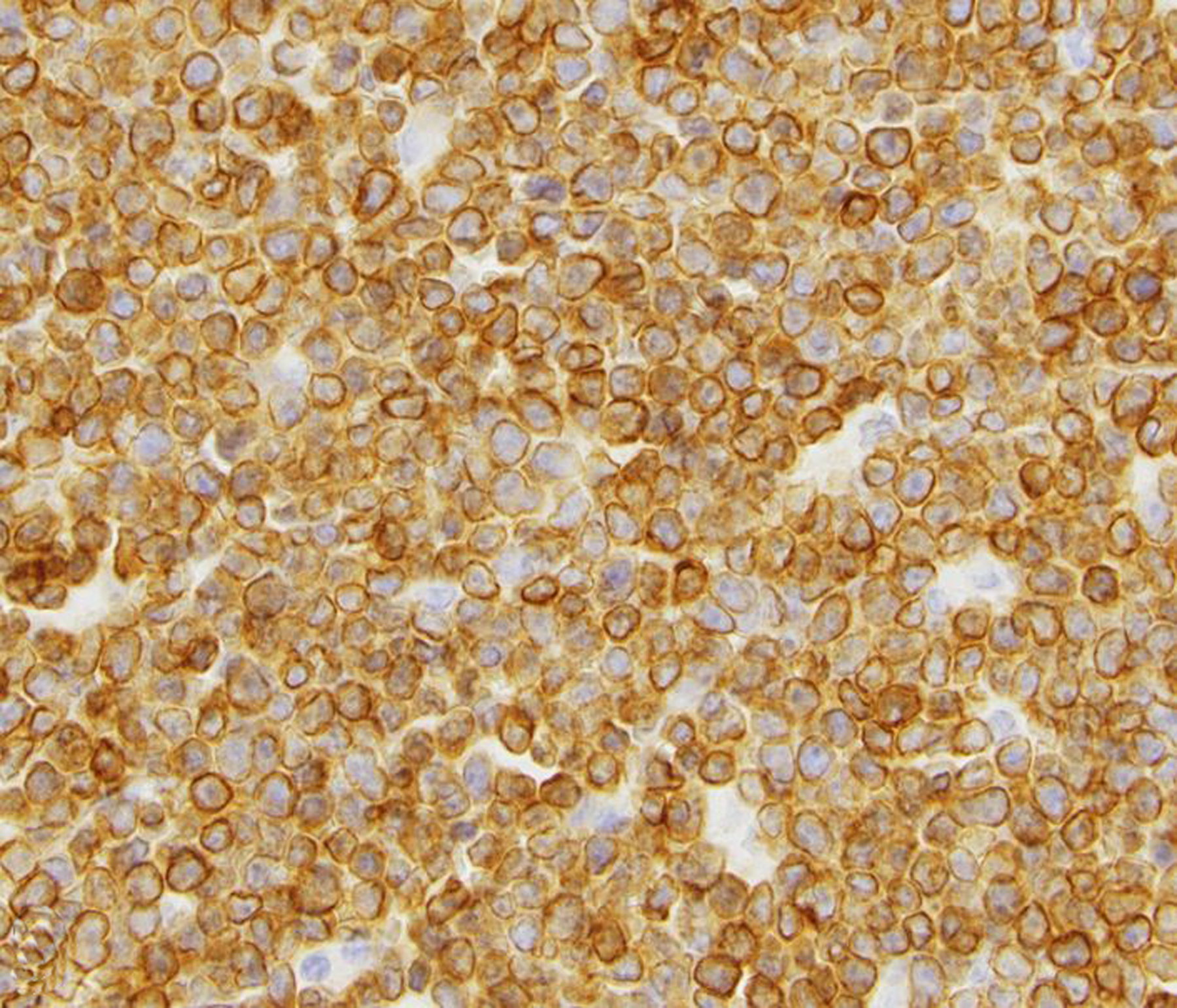
Neoplastic lymphocytes exhibit strong membranous to cytoplasmic immunolabeling for CD3.
Primary neoplasia of the urinary bladder and urethra was not diagnosed during the study period. Non-primary tumors of the urinary bladder included an endometrial stromal tumor in a baboon, metastatic ileocecal adenocarcinoma in 2 rhesus macaques, and metastatic hepatocellular carcinoma in a common marmoset.
Female Reproductive Tract
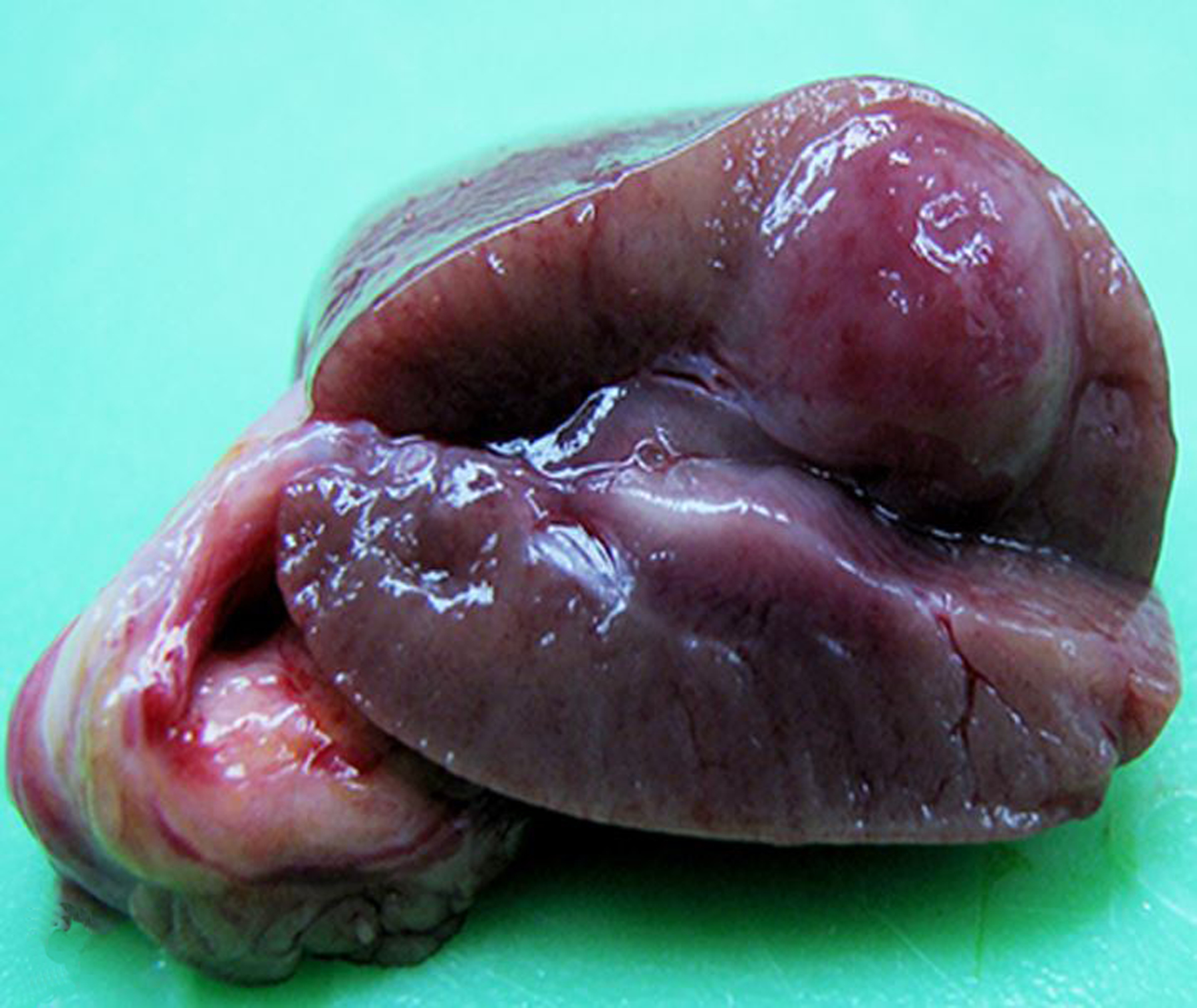
Endometriosis was by far the most common diagnosis of the female genital tract (Figs 10 & 11). Adenomyosis, defined as the presence of endometrial glands within the myometrium, and endometrial or cervical polyps (Fig. 12) were frequent lesions. Other uterine lesions included endometrial hyperplasia (Figs. 13 & 14), metritis or endometritis, uterine prolapse, uterine rupture and cervicitis (Table 1). Ovarian cysts were the most frequent non-neoplastic lesion of the ovary (Table 1). Ovarian atrophy, aplasia (Fig. 15), hypoplasia, mineralization, oophoritis, ectopic adrenal tissue, ectopic decidua, and ovotestes were occasionally diagnosed.
Figure 10. Endometriosis; ovary, uterus and colon; 18-year-old female rhesus macaque.
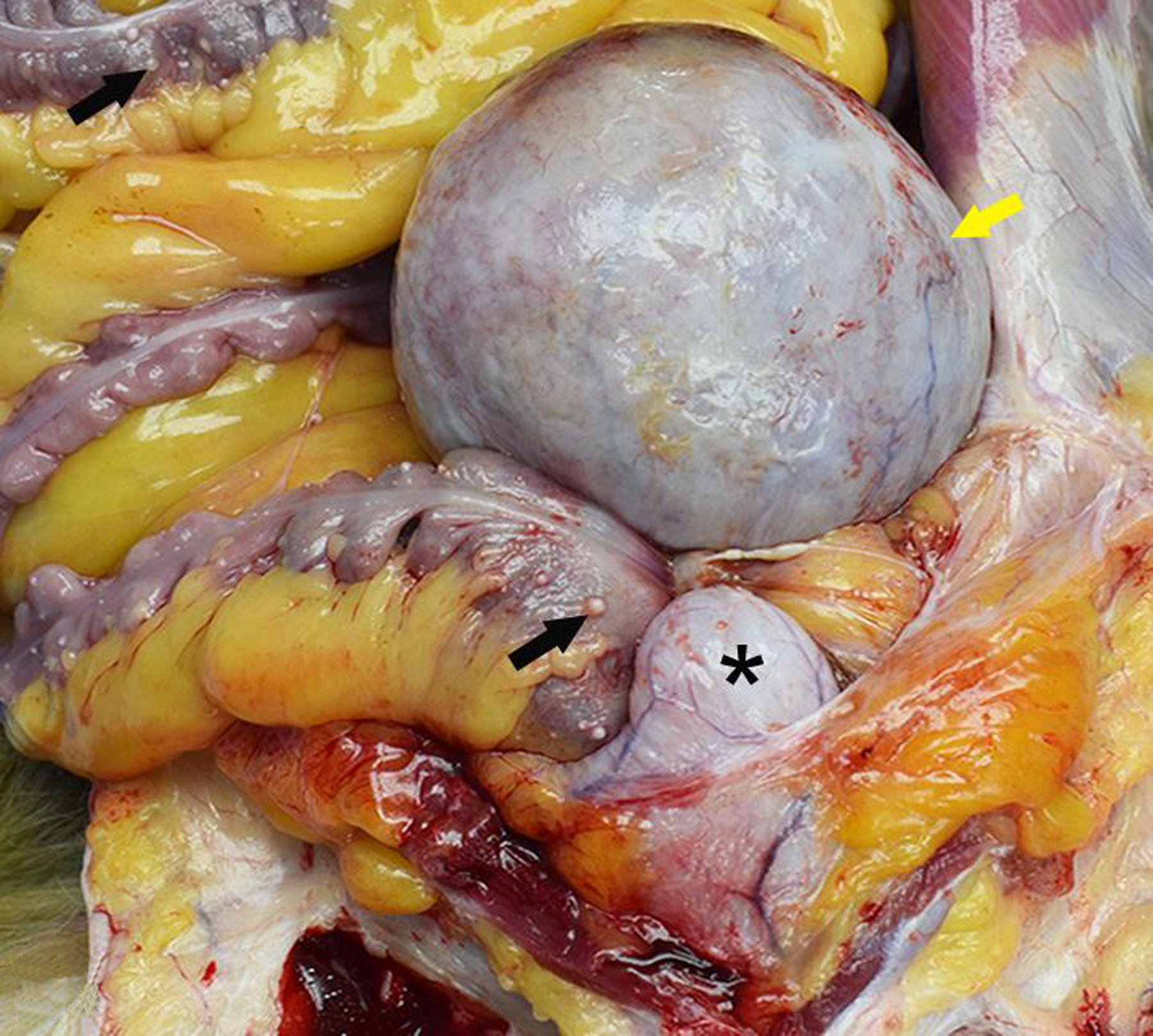
A single, large “chocolate fluid”-filled cyst (endometrioma; yellow arrow) severely expands the left ovary and is adhered to the uterus, urinary bladder (*) and colon. There are numerous, smaller serosal cysts on the colon (black arrows).
Figure 11. Endometriosis; ovary, uterus and colon; 18-year-old female rhesus macaque.
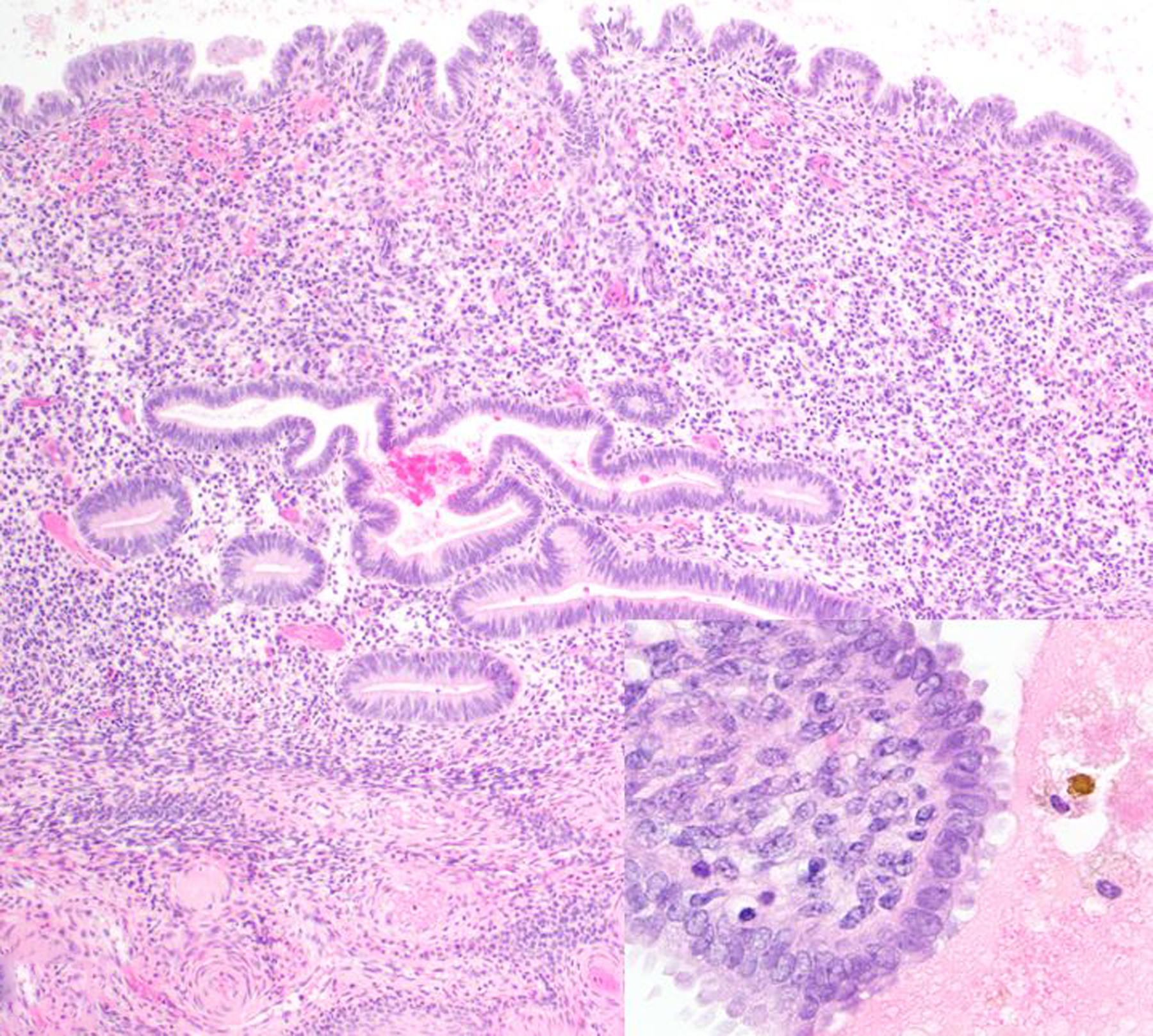
Lesions of endometriosis in the peri-ovarian tissue contain endometrial glands and stroma as well as hemorrhage and hemosiderin-laden macrophages (inset). Hematoxylin and eosin (HE).
Figure 12.
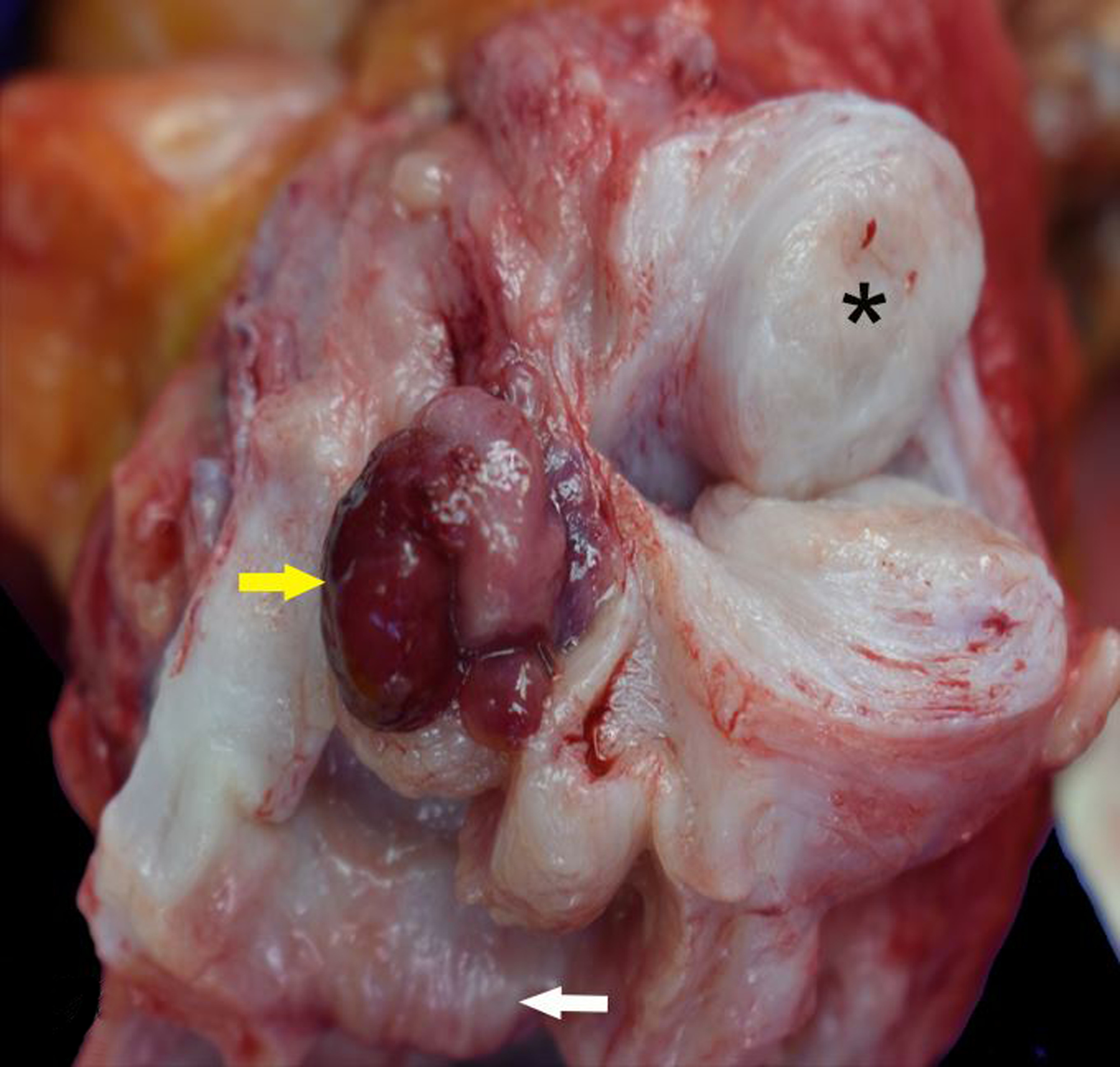
Endocervical polyp and leiomyoma, cervix and uterus, rhesus macaque. A fleshy, red, sessile to polypoid mass arises from the endocervix and protrudes into the cervical canal (yellow arrow). The cervical and uterine myometrium are focally expanded by a leiomyoma (*). The external cervical os is at the bottom of the image (white arrow).
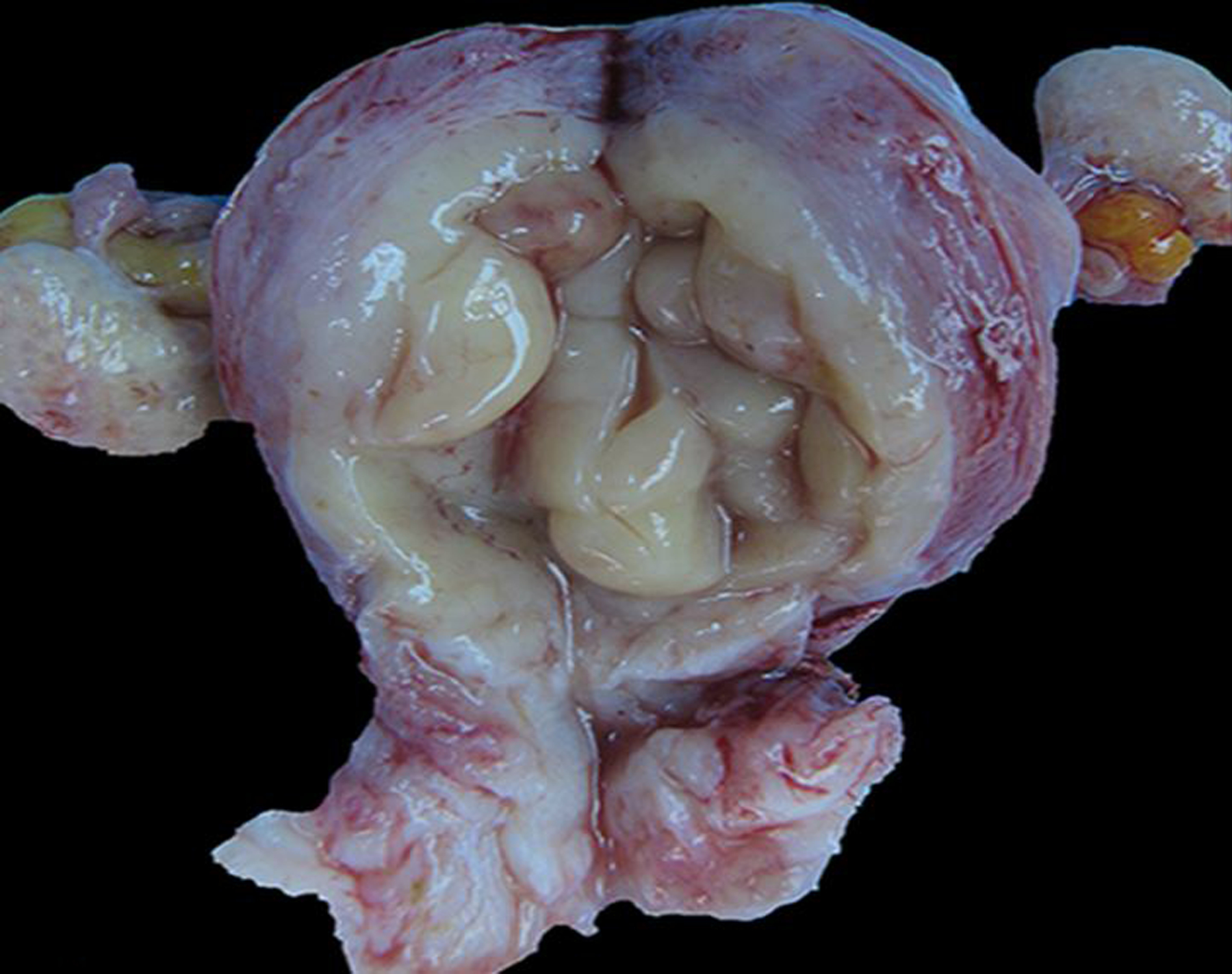
Figure 13. Endometrial hyperplasia, uterus, sooty mangabey.
The endometrium is thickened, pale tan, and rugose.
Figure 14. Endometrial hyperplasia, uterus, sooty mangabey.
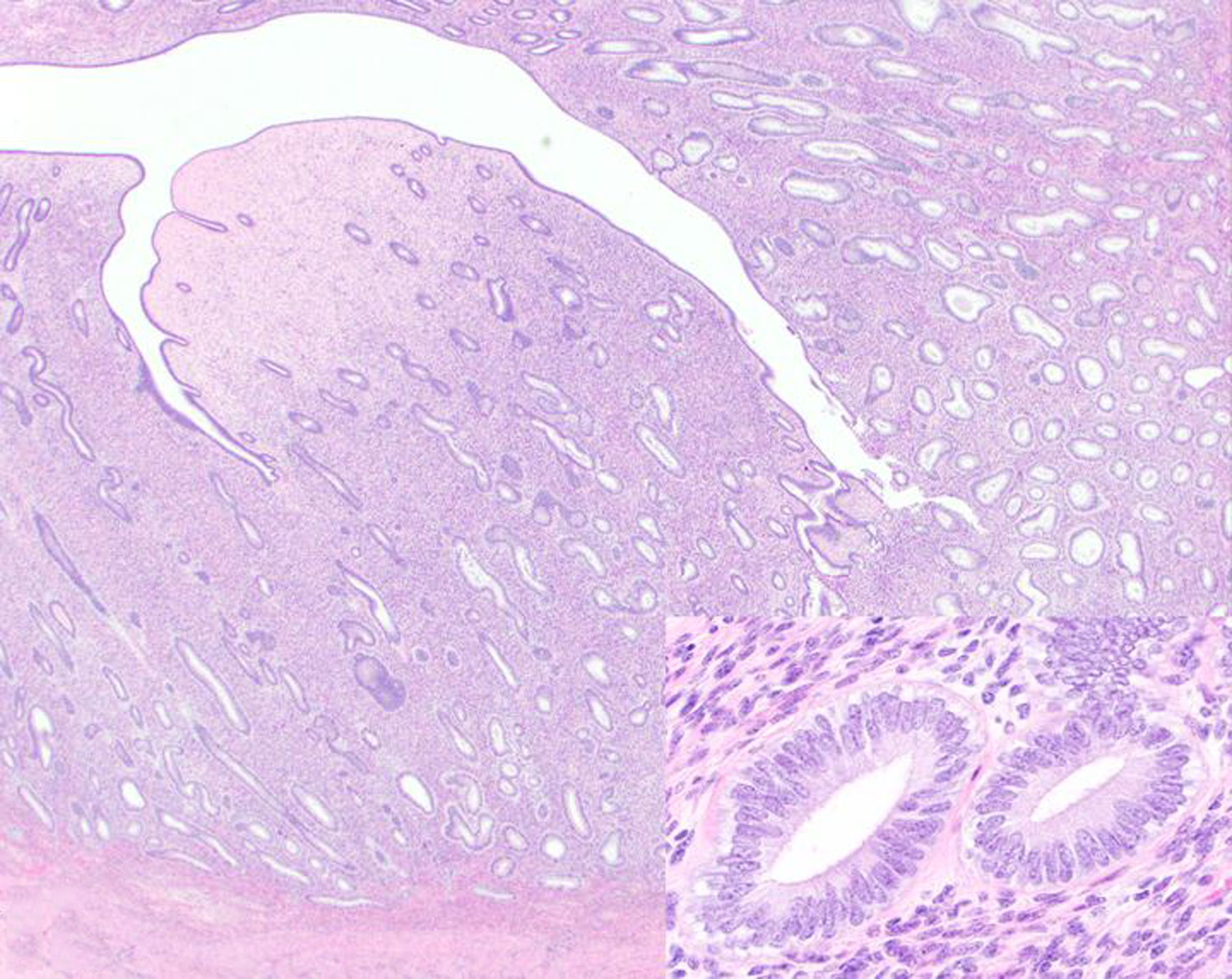
The thickened endometrium is composed of endometrial glands (inset) embedded in stroma. HE.
Figure 15.
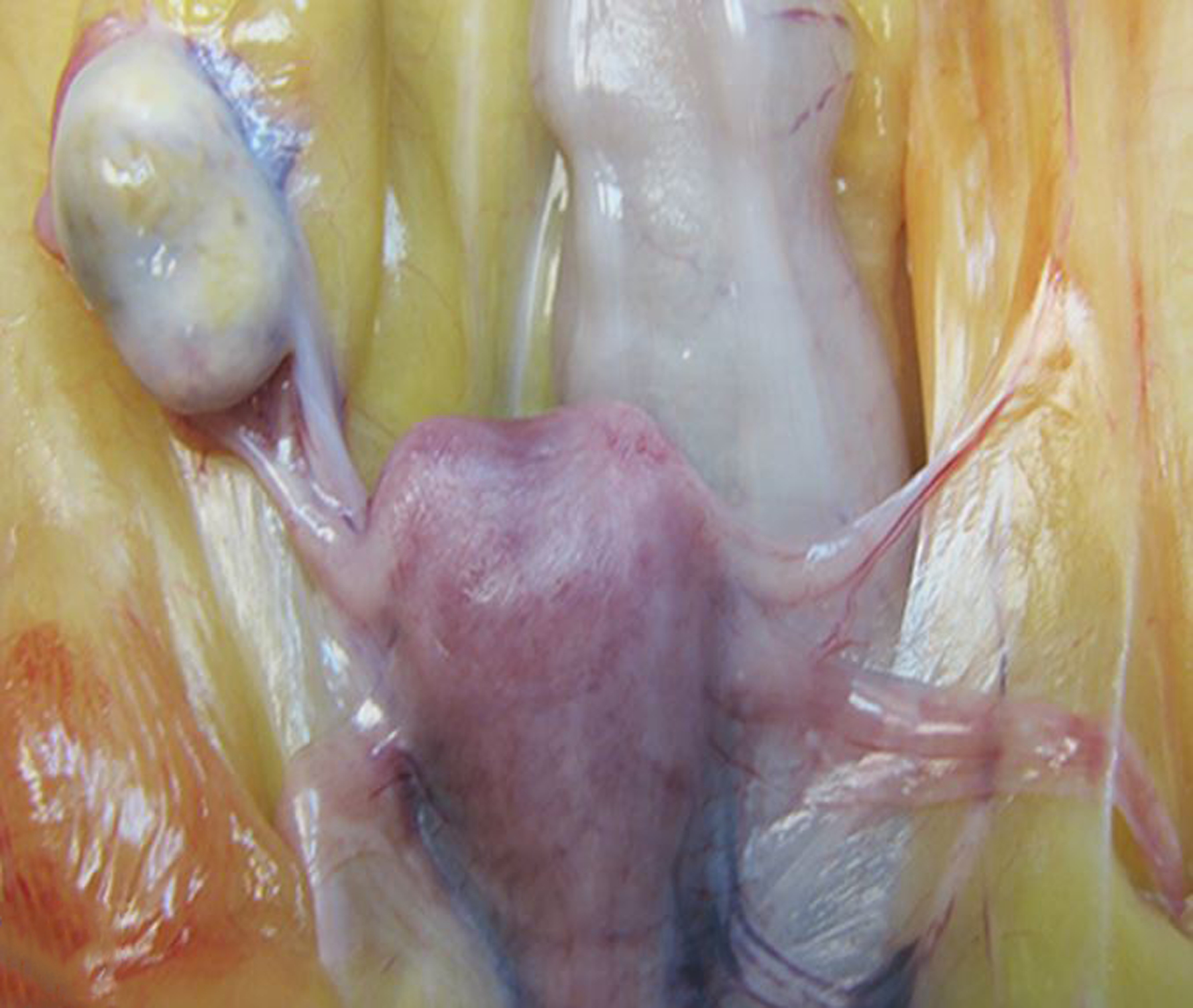
Ovarian agenesis, left ovary, rhesus macaque.
In baboons, vaginal, vulvar or perineal ulceration, vaginal obstruction, stenosis or stricture, and vulvitis was often attributed to herpesvirus papio 2 infection. Mild vaginitis or vulvitis were the most frequent diagnoses of the lower female genital tract. Chronic vaginal prolapse was seen in three baboons and six rhesus macaques; at least one of these macaques was a breeding female.
Leiomyoma was the most frequently diagnosed uterine neoplasm during the study period (Fig. 12; Table 2); this was most often an incidental finding. An unusual presentation of malignant uterine neoplasia was a leiomyosarcoma in a 2-year-old rhesus macaque with extension to the cervix and vagina.
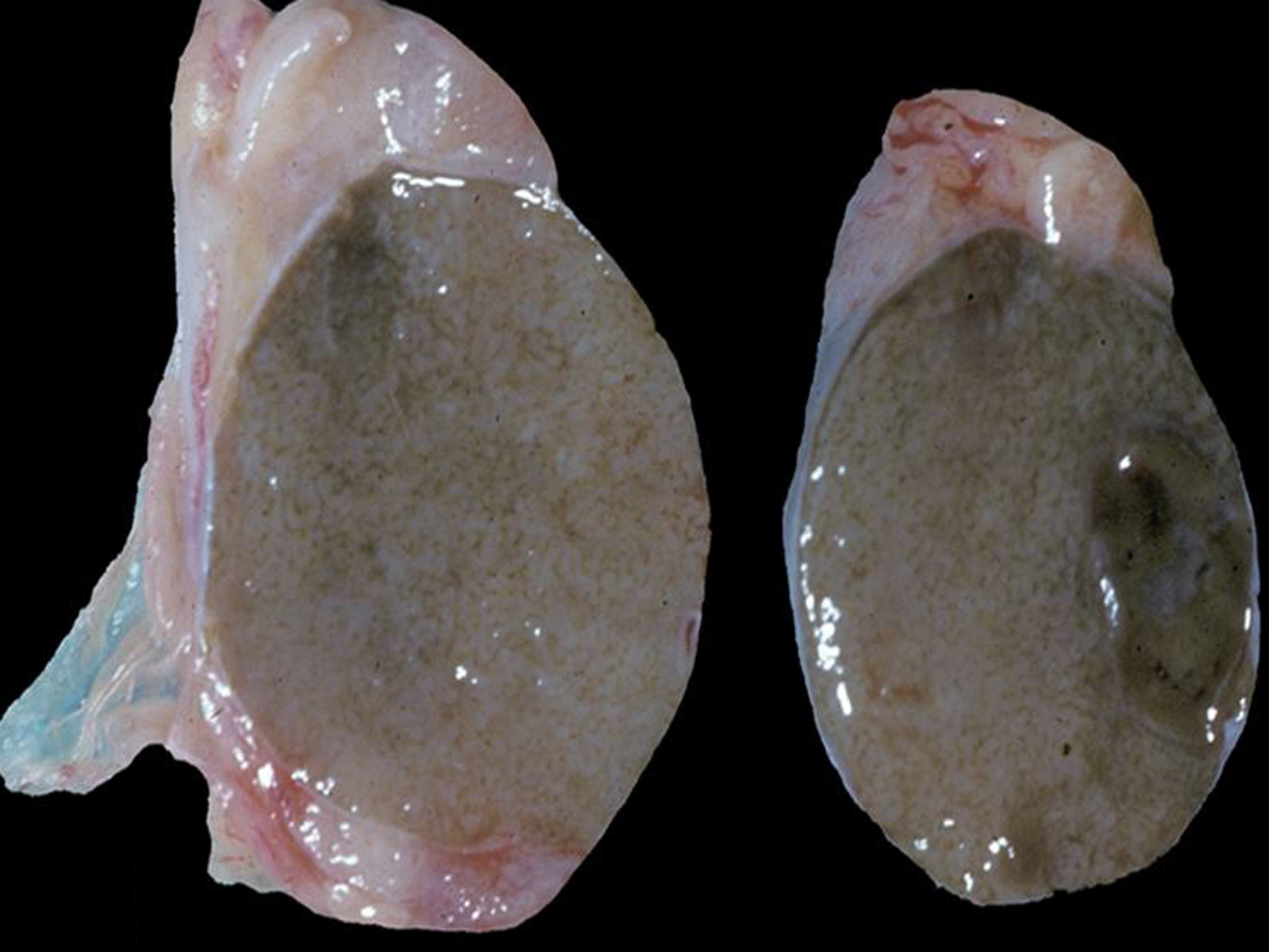
Granulosa cell tumors (GCTs; Figs. 16 & 17) accounted for 63 of 123 (51%) ovarian neoplasms diagnosed during the study period. The second most common ovarian tumor was teratoma (1 chimpanzee, 8 baboons and 3 macaques; Figs. 18 & 19). Baboons accounted for a majority of the ovarian tumors (108/134; 80%).
Figure 16. Granulosa cell tumor, left ovary, 21-year-old female rhesus macaque.
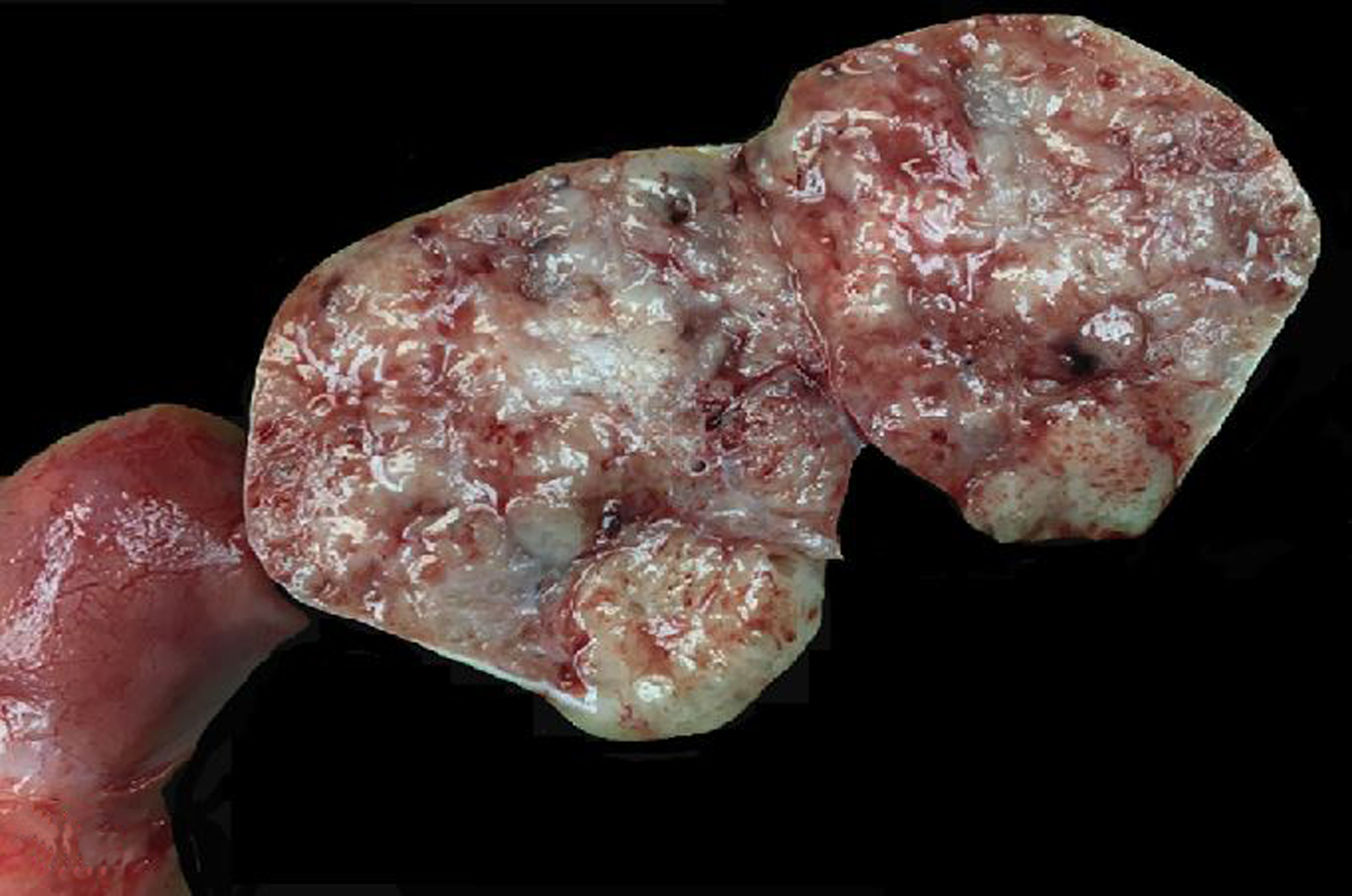
The left ovary is expanded and replaced by a solid tan to red neoplasm.
Figure 17. Granulosa cell tumor, left ovary, 21-year-old female rhesus macaque.
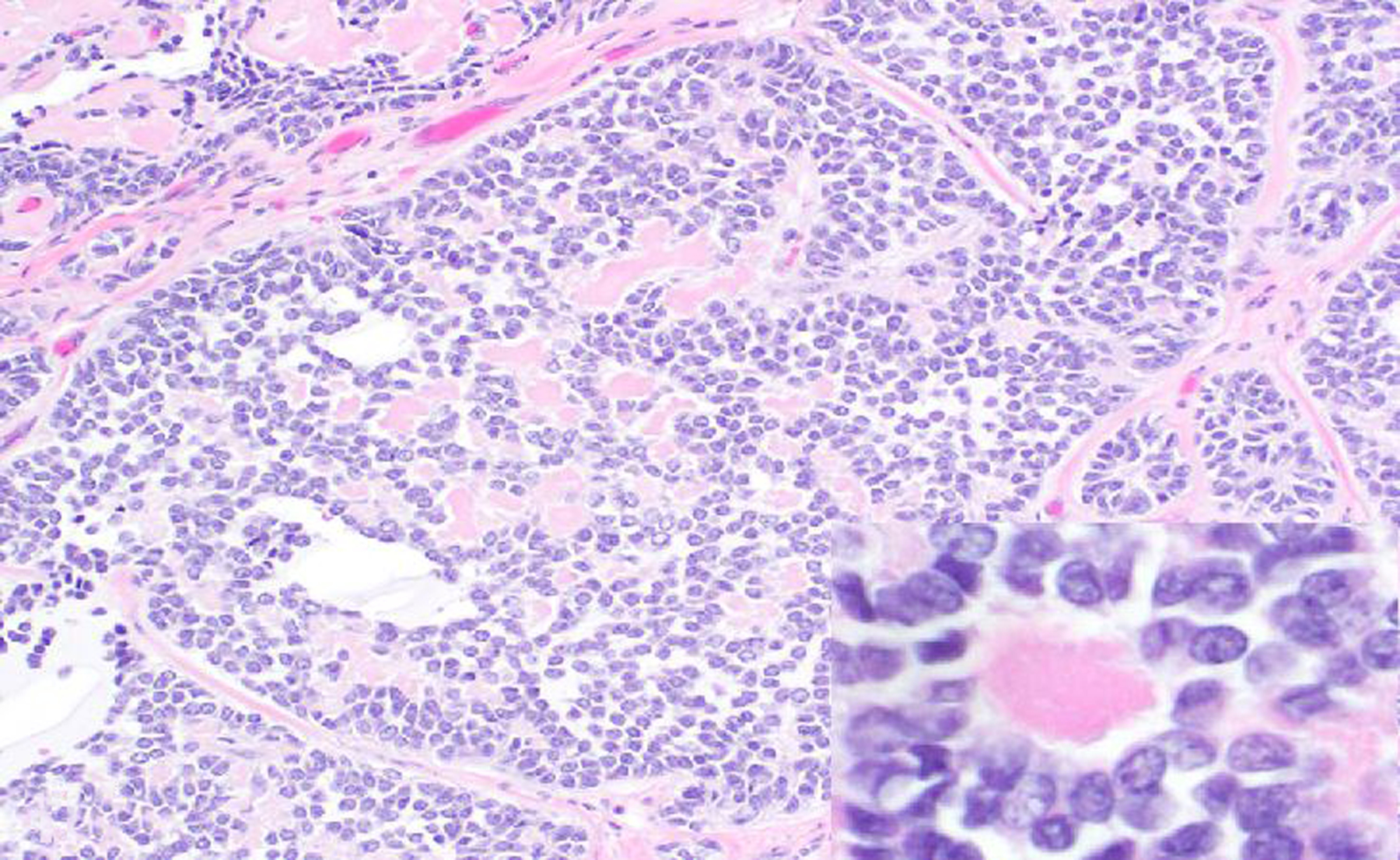
The neoplasm is composed of sheets of round to polygonal cells with abundant eosinophilic cytoplasm, which occasionally form follicle-like structures containing extracellular eosinophilic material (Call-Exner bodies; inset). HE.
Figure 18. Teratoma, left ovary, rhesus macaque.
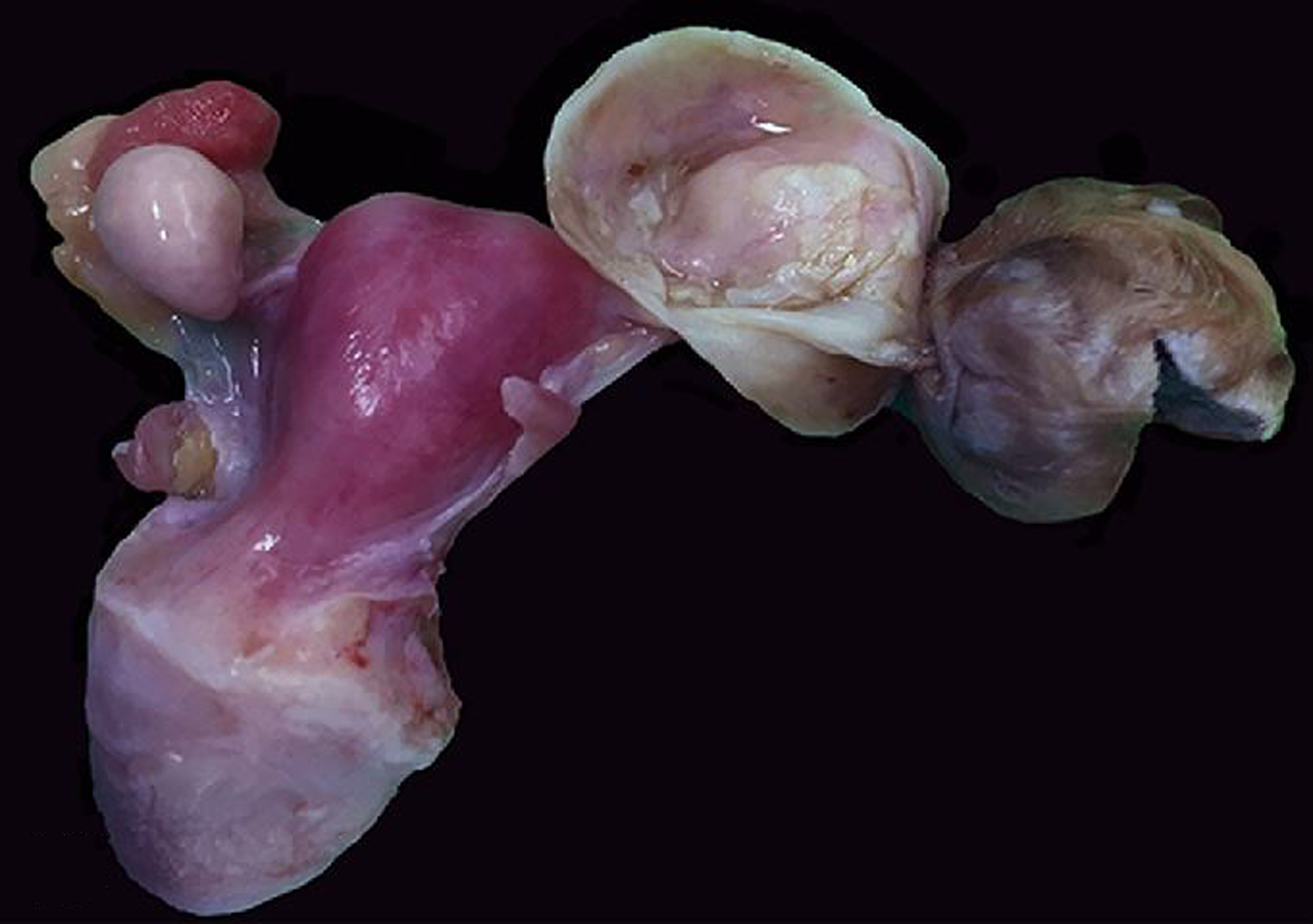
The left ovary is replaced by a 4 cm-diameter solid soft neoplasm, the surface of which is covered with haired skin.
Figure 19. Teratoma, left ovary, rhesus macaque.
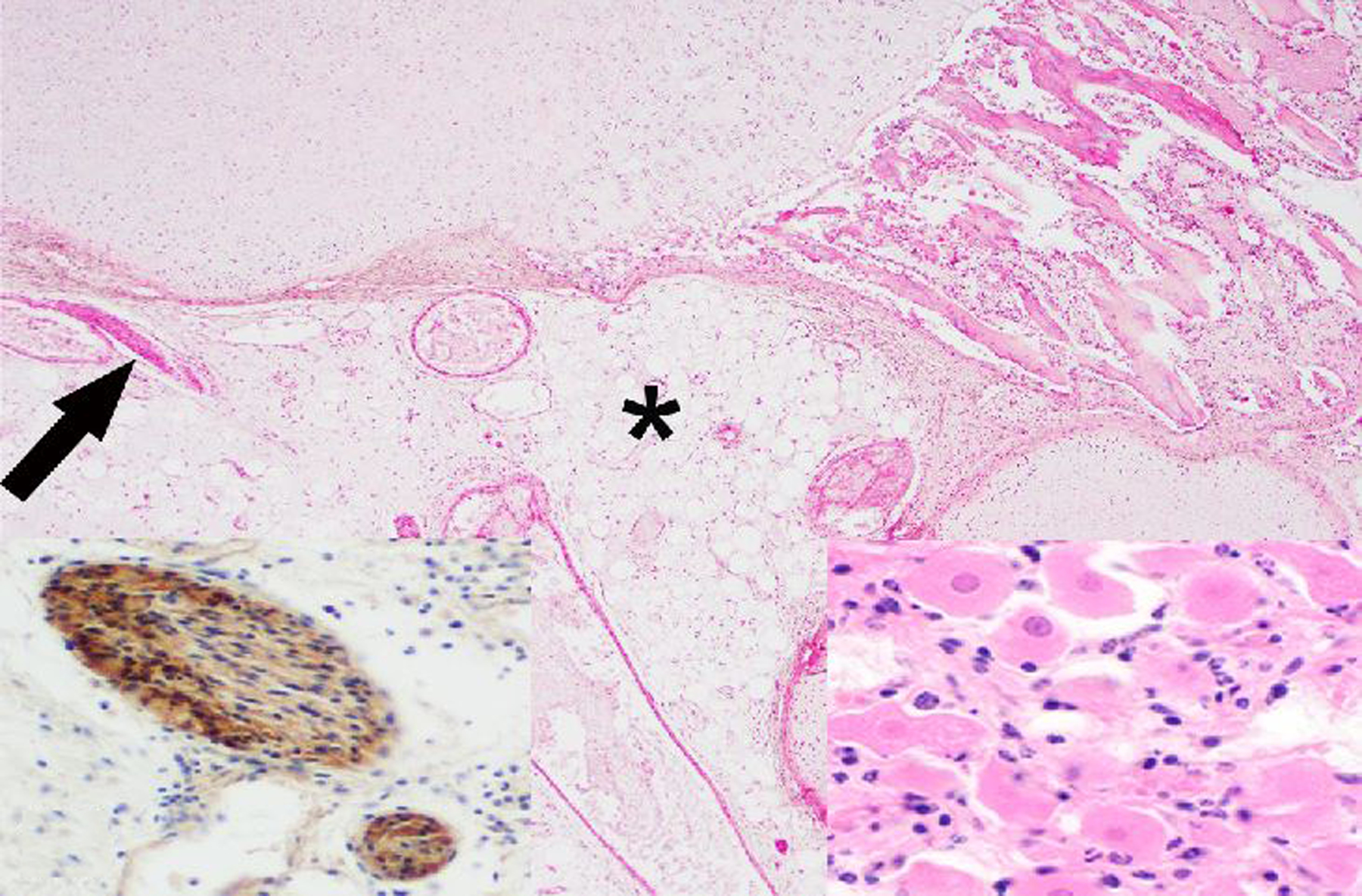
The core of the neoplasm is composed of disorganized adipose tissue (*), blood vessels, few nerves (arrow), bone, cartilage (top of image) and a small cluster of neurons (right inset). HE. Left inset: Diffuse cytoplasmic immunolabeling of nerve fibers for S-100.
During the study period, there were 4 benign and one malignant oviductal tumor, including 1 fimbrial adenocarcinoma in a rhesus macaque (Figs. 20 & 21). Ten cases of oviductal hyperplasia were observed during the study period in OWMs. The hyperplastic change was further specified as fimbrial in 2 cases (1 rhesus macaque and 1 baboon).
Figure 20. Adenocarcinoma, right oviduct, 17-year-old female rhesus macaque.
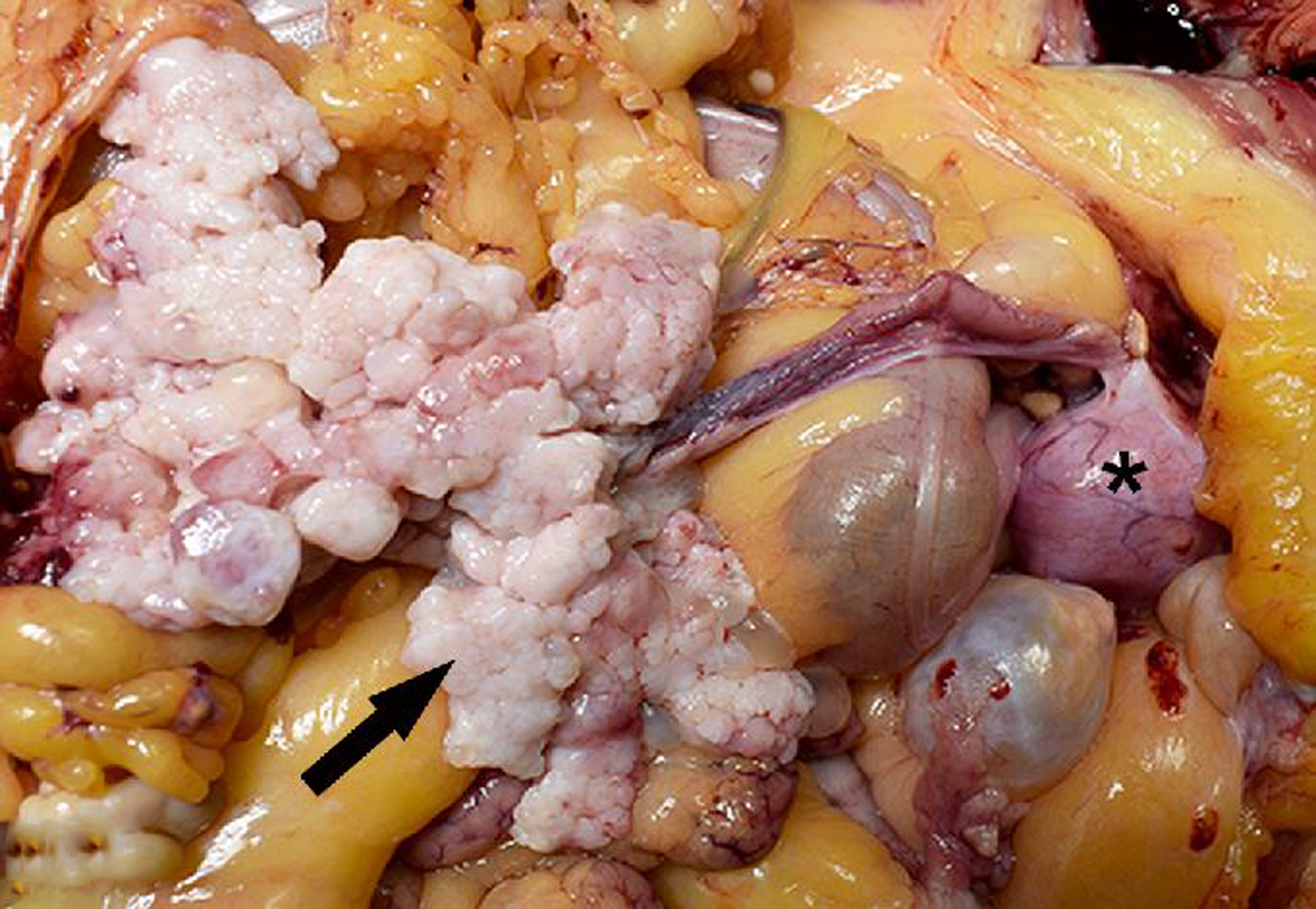
An unencapsulated, expansile, firm, multilobular tan neoplasm (arrow) expands and infiltrates the right oviduct and mesenteric adipose tissue. *, uterus.
Figure 21. Adenocarcinoma, right oviduct, 17-year-old female rhesus macaque.
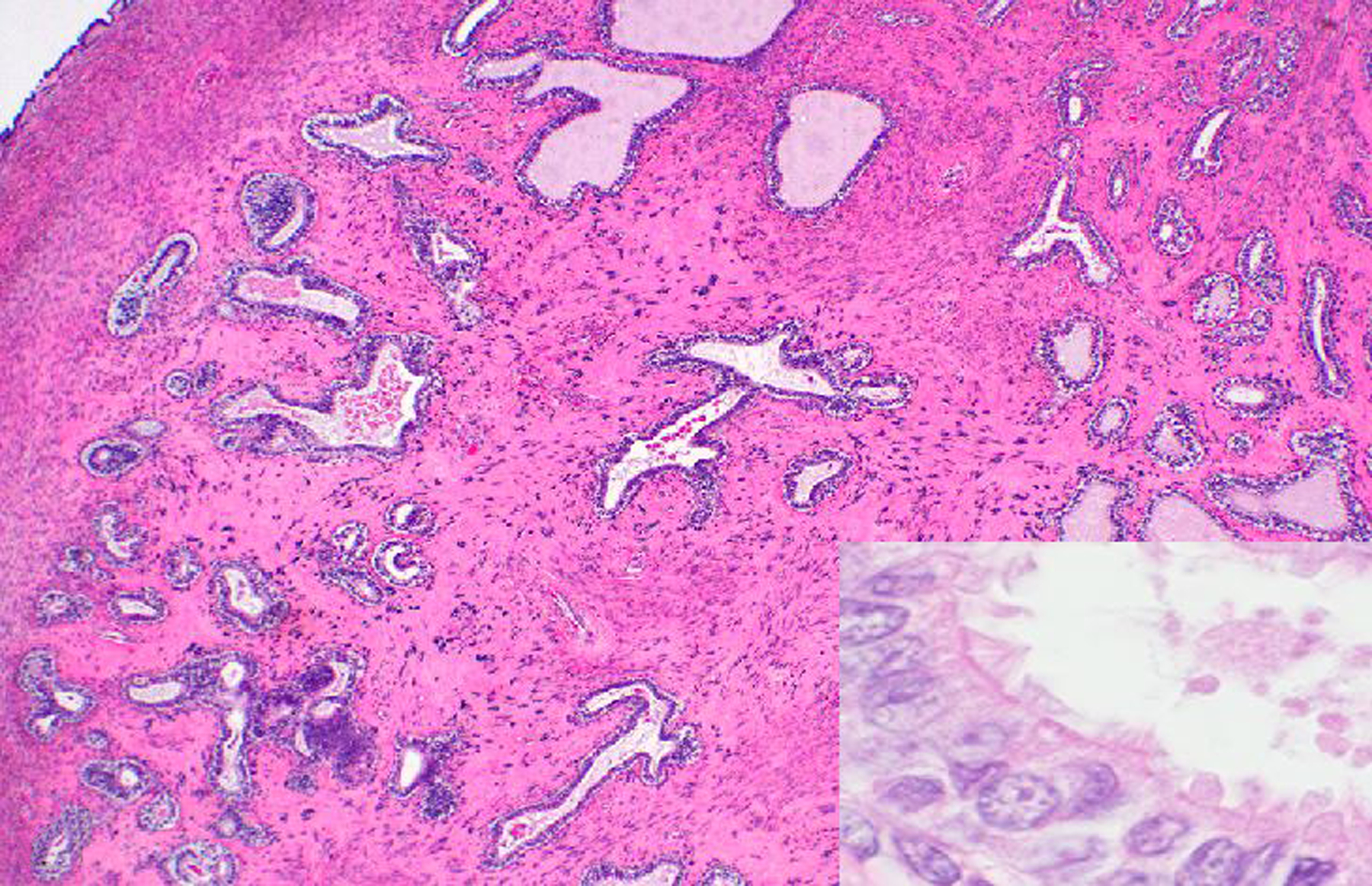
Neoplastic cells are well-differentiated, tall columnar, form irregular tubules or small clusters of isolated cells, and have apical cilia (inset) consistent with oviductal epithelial origin. Neoplastic tubules are embedded in abundant, dense fibrous connective tissue. HE.
Six benign vaginal and vulvar neoplasms were diagnosed, including a fibroma in a rhesus macaque, 2 leiomyomas in cynomolgus macaques, a papilloma in a chimp, and a myxoma and 2 papillomas in baboons (Table 2). The PV status of the chimpanzee and 2 baboons with vaginal papillomas is unknown.
Developmental anomalies of the female genital tract included uterus didelphys (1 rhesus macaque), uterine hamartoma (1 rhesus macaque and 1 common marmoset), ectopic ovarian tissue (1 rhesus and 1 baboon) and an imperforate vulva in a stump-tailed macaque fetus, who also had atresia coli, imperforate anus, pulmonary hypoplasia and right-sided hydronephrosis.
The Male Genital Tract
Testicular atrophy (80 cases) and minimal to mild prostatitis (170 cases) were the most frequently diagnosed lesions of the male genital tract (Table 1). These were almost always incidental findings. Herpesvirus papio 2 was the single most frequent confirmed or suspected cause of infectious posthitis or balanoposthitis in baboons during the study period (10 cases). A single case of B virus-associated ulcerative balanitis associated with macacine alphaherpesvirus 1 (herpes B virus) infection was diagnosed in a 7-year-old rhesus macaque.
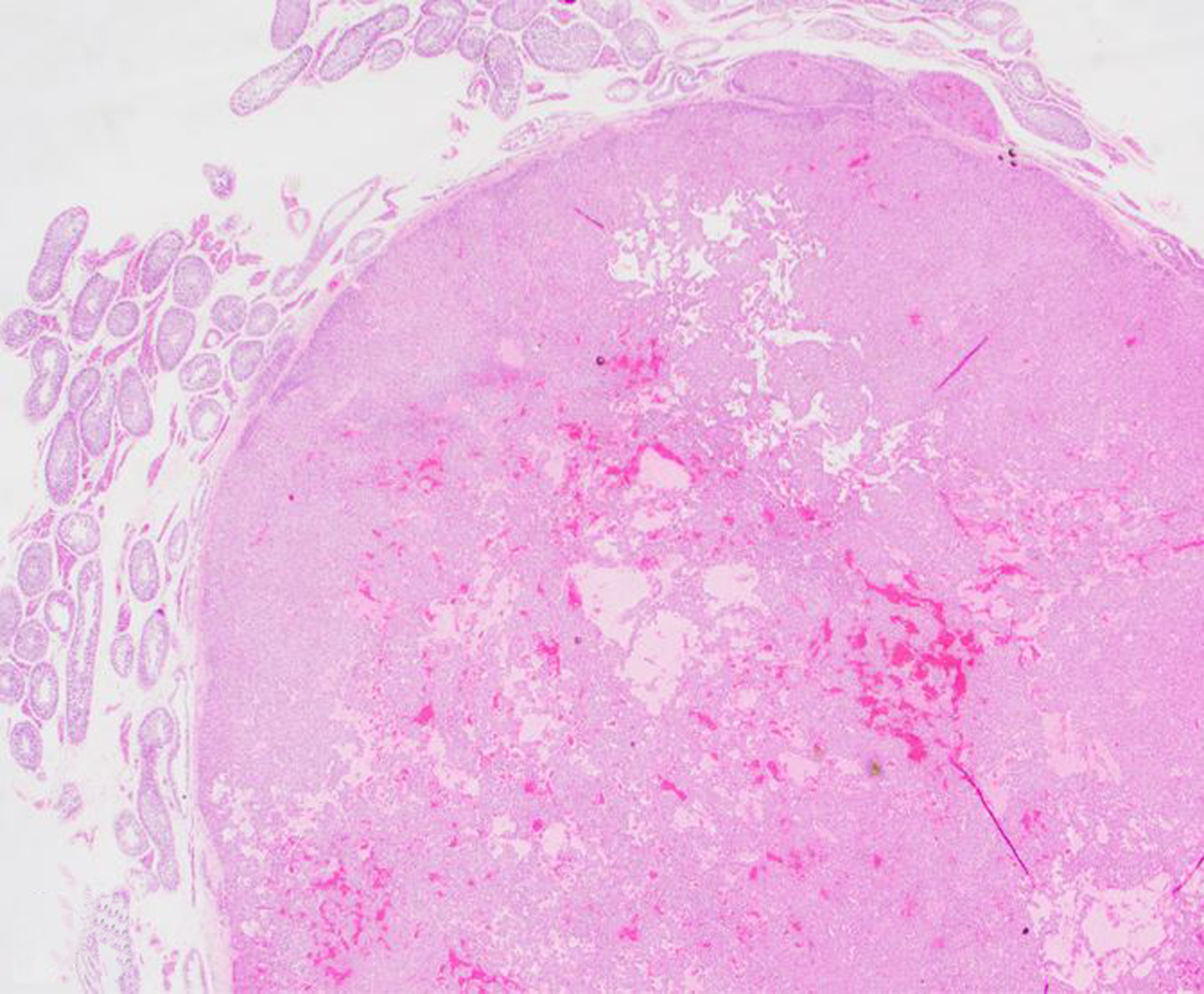
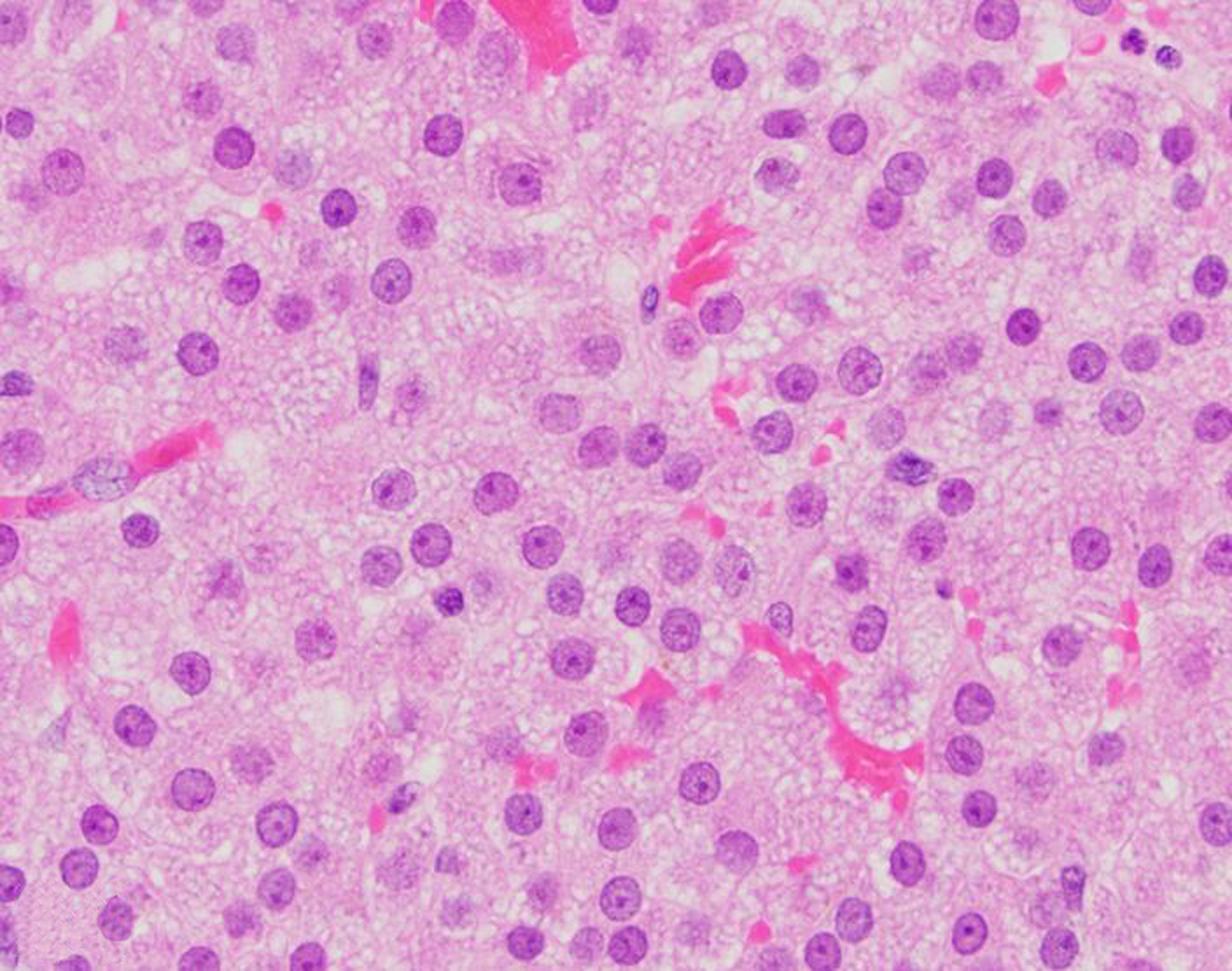
Neoplasia of the testes, epididymis, accessory sex glands and external genitalia was rare during the 30-year period, with a total of 15 tumors. One interstitial cell tumor was diagnosed in a squirrel monkey (Figs. 22, 23, 24). Malignant prostatic neoplasia and neoplasia of the seminal vesicles were not observed during the study period. Prostatic hyperplasia was a rare diagnosis across all NHP species (12 baboons and 3 rhesus macaques). The most common penile neoplasm during the study period was squamous cell carcinoma (3 cases; Figs. 25, 26, 27); the papillomavirus (PV) status of these cases is undetermined.
Figure 22. Interstitial (Leydig) cell tumor, testis, 20-year-old male squirrel monkey.
The testicular parenchyma contains a well-demarcated, round, dark brown mass.
Figure 23. Interstitial (Leydig) cell tumor, testis, 20-year-old male squirrel monkey.
The mass is well demarcated, thinly encapsulated, and composed of sheets of polygonal cells with abundant eosinophilic cytoplasm that occasionally line dilated blood- or fluid-filled spaces. Hematoxylin and eosin (HE).
Figure 24. Interstitial (Leydig) cell tumor, testis, 20-year-old male squirrel monkey.
The neoplastic cells have abundant, finely vacuolated cytoplasm, and minimal anisocytosis and anisokaryosis. HE.
Figure 25. Squamous cell carcinoma, penis, 4-year-old male rhesus macaque.
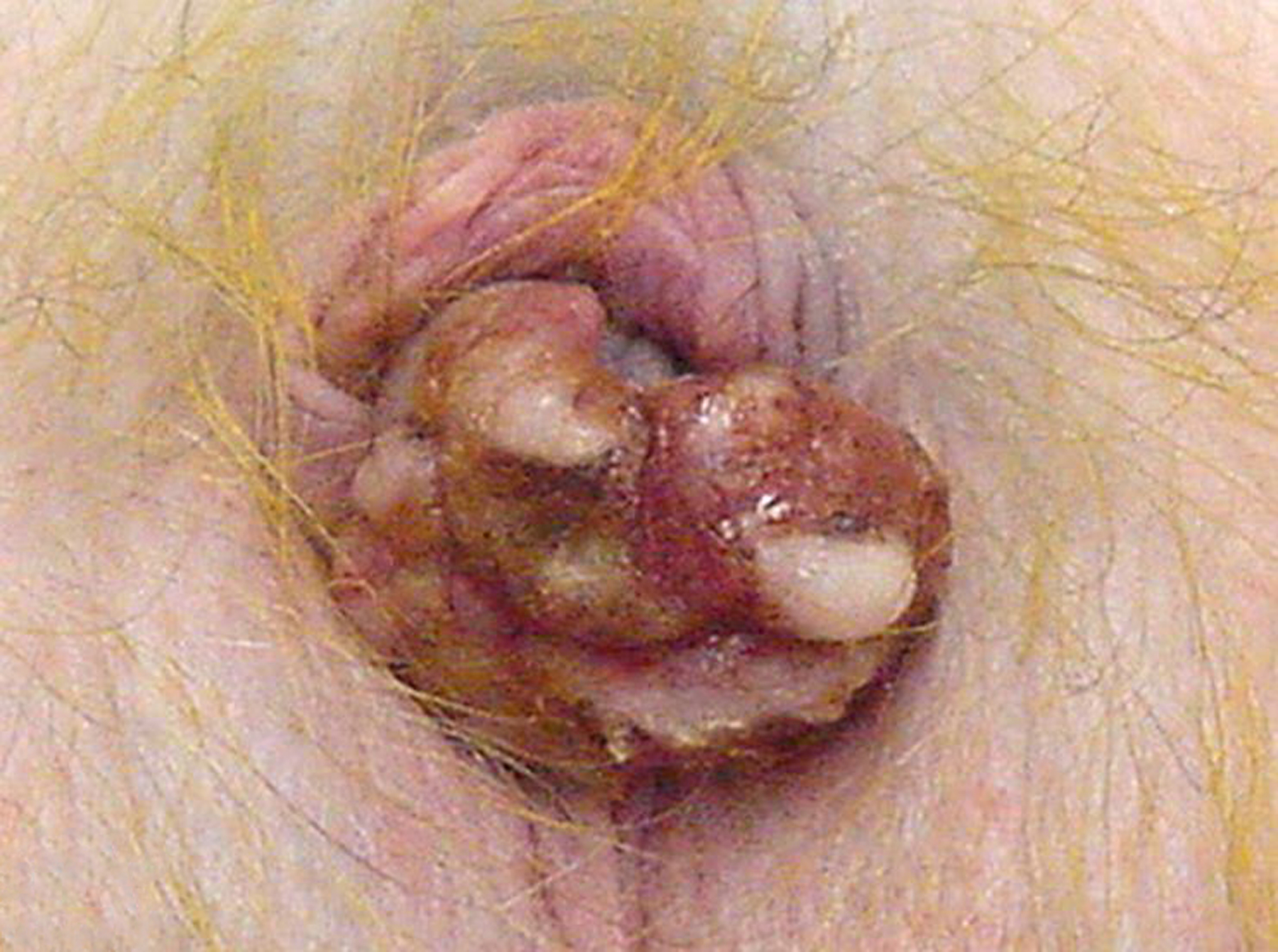
The glans penis is thickened, red to tan and multifocally ulcerated.
Figure 26. Squamous cell carcinoma, penis, 4-year-old male rhesus macaque.
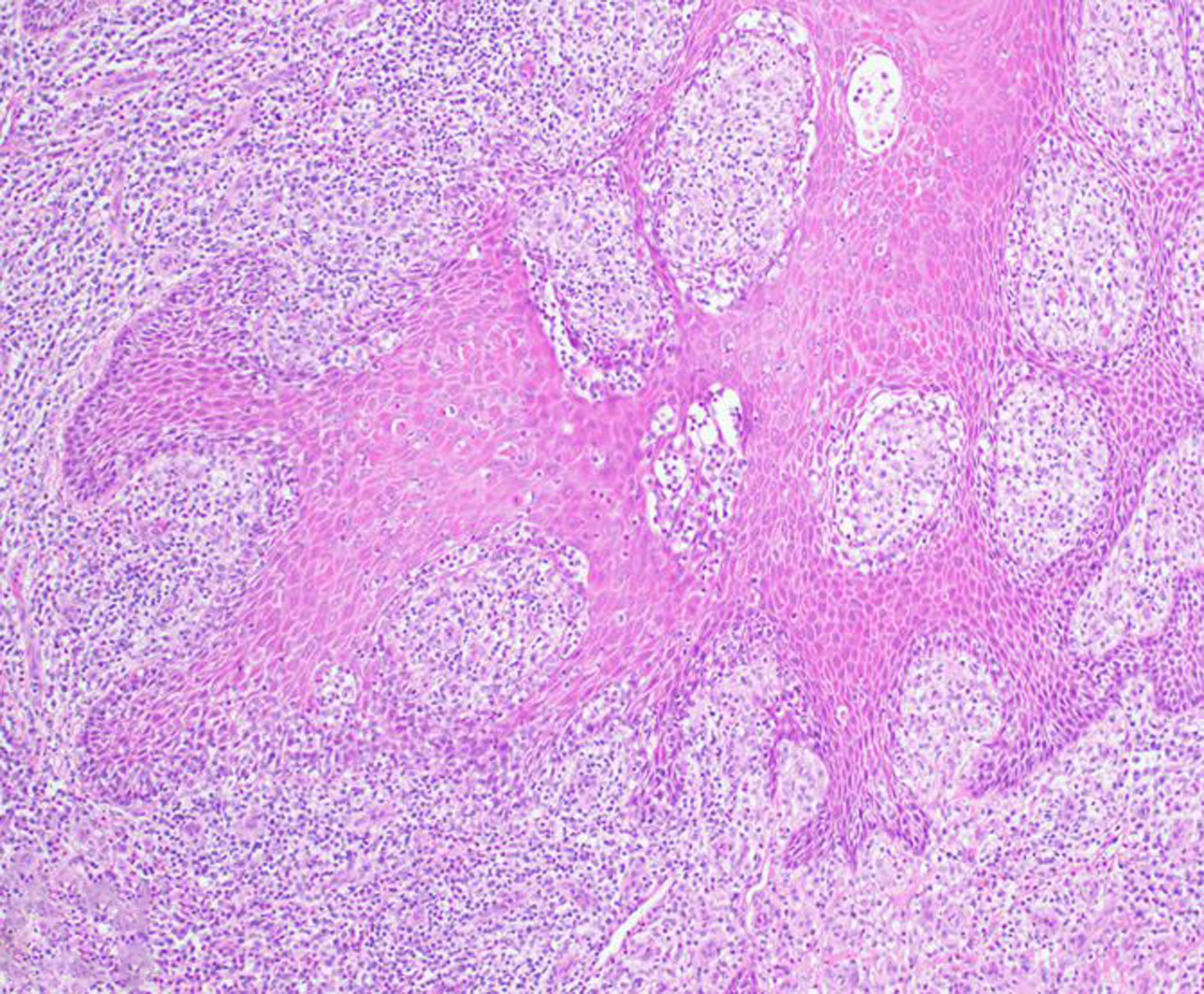
Neoplastic squamous epithelial cells invade deeply into the penile submucosa in association with a florid mononuclear inflammatory response. HE.
Figure 27. Squamous cell carcinoma, penis, 4-year-old male rhesus macaque.
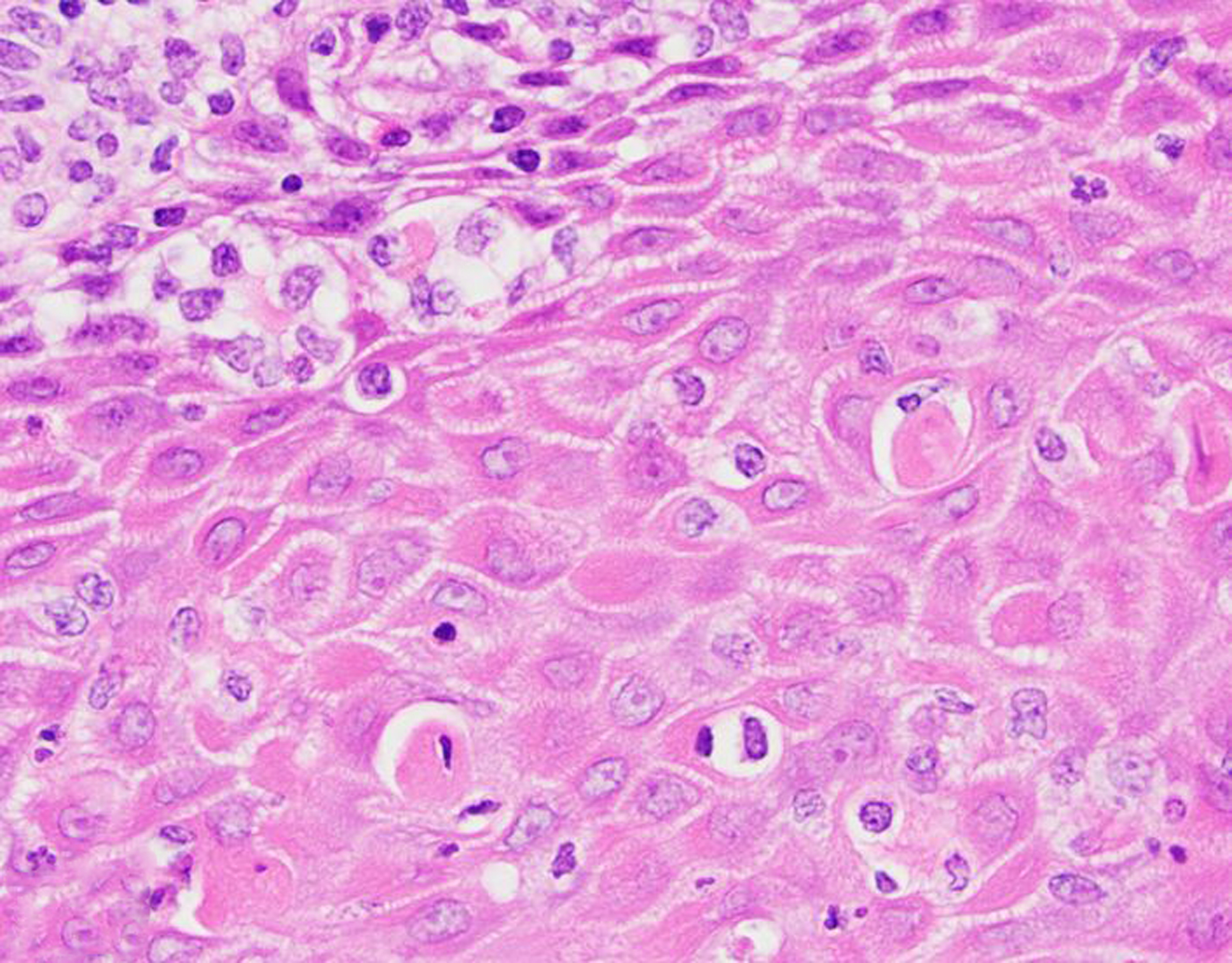
There is absence of a basement membrane, abnormal layering, anisokaryosis and dyskeratosis, and a lymphoplasmacytic and histiocytic infiltrate. HE.
Cryptorchidism (16 cases) was the most frequent congenital lesion of the male reproductive tract. Hypospadias (2 rhesus macaques), preputial orifice hypoplasia, and penile aplasia (1 baboon) were also observed.
Discussion
We conducted a comprehensive review of spontaneous genitourinary lesions occurring in NHPs over a 30-year period at two primate centers to serve as a resource for veterinarians, pathologists and researchers working with these species. Background histopathologic findings in kidneys and in male and female reproductive tracts of NHPs have been previously described in the literature.16,46,70,63,64,73,88 Data gathered from this study highlighted some differences between OWMs and NWMs. A possible limitation of this study is bias towards more florid, grossly apparent lesions such as endometriosis and lesions of the vital organs (i.e. kidney) versus other subtle microscopic lesions of the male and female reproductive tracts.
Amyloidosis was the most common renal lesion. In NHPs, amyloid often accumulates within the renal interstitium (especially in the medulla) as a result of chronic inflammation (enterocolitis, rheumatoid arthritis, chronic catheterization, viral infection, and parasitism in macaques). This finding is most often incidental, but amyloid can compress and replace tubules in some advanced cases.38,52 Compared to medullary amyloidosis, glomerular amyloidosis was rare across all NHP species. Few cases of clinically significant glomerular amyloid deposition have been described in macaques and marmosets.20,38,48
The cause of nephrosis or nephropathy was infrequently identified during the study period. Reported causes of renal tubular disease in NHPs include acute rhabdomyolysis, fatal fasting syndrome, aminoglycoside antibiotics, and nonsteroidal anti-inflammatory drugs.2,9,20,28,42,68,78 Fatal fasting syndrome is a disease entity of obese macaques following anorexia and weight loss characterized by moderate to severe hepatic and proximal renal tubular epithelial cell lipidosis; focal pancreatic necrosis and pancreatitis have also been reported.9 Azotemia is the most common laboratory abnormality.20,9 Antemortem clinicopathologic data are necessary to make a diagnosis of fatal fasting syndrome. Correlative data was not available for all cases during the study period; thus, fatal fasting syndrome may be underestimated in our data set.
Renal lesions (particularly nephritis, glomerulonephritis and glomerulonephropathy) were frequent diagnoses in NWMs (primarily marmosets and tamarins), while genital lesions were infrequent in those species. Glomerulonephritis and glomerulopathy were more frequently diagnosed in baboons and common marmosets compared to macaques. The occurrence of spontaneous glomerulopathy in young common marmosets and additional lesions in aging marmosets suggests that this species may be a useful animal model for studying spontaneous renal diseases.43,87 In laboratory chimpanzees, cardiac disease may lead to progressive renal dysfunction (i.e. “cardiorenal syndrome”), with the most common associated lesions being glomerulosclerosis and interstitial nephritis.17
Lesions of the urinary bladder were rare during the study period. Cystolithiasis was rare, with most cases attributed to retrograde ejaculation in male rhesus macaques.29 Tumors of the kidney and urinary bladder were uncommon, with 30 and 4 cases diagnosed, respectively. Lymphoma was the most common renal tumor. Lymphomas in NHPs are often associated with oncogenic viruses such as rhesus lymphocryptovirus and simian T-cell leukemia virus; oncogenic viral infection could not be ruled out in several of our cases.12,57,66 Only a single neoplasm of the urinary tract (renal hemangioma) was observed in sooty mangabeys.
Female reproductive tract lesions were more frequently encountered than male reproductive tract lesions; this observation may be related to a number of potential factors, including more limited postmortem examination of male reproductive tissues, variable demographics of the NHP colonies, and differences in age at study assignment and experimental endpoint for males versus females. These factors are potential limitations of this study.
Endometriosis was the most frequent lesion of the female reproductive tract in NHPs. Of the animals examined in this study, it was most frequent in OWMs (baboons > macaques > mangabeys), and rarely in chimpanzees and common marmosets (Supplementary Table 1), making OWMs attractive animal models for the corresponding human condition. Endometriosis, defined as the presence of ectopic endometrial tissue outside the uterus, is the leading cause of pre-senescent reproductive failure in rhesus and cynomolgus macaques.26,46,76 In baboons, the prevalence of spontaneous endometriosis has been shown to increase with the duration of captivity.22 Endometriosis may be encountered as an incidental lesion or as a clinically significant finding in NHPs. Secondary lesions of the urinary tract were occasionally observed, such as adhesions resulting in hydroureter or hydronephrosis, and are also seen in women as sequelae of endometriosis.80 Endometrial hyperplasia and uterine or cervical polyps were also diagnosed more frequently in OWMs compared to NWMs.
Leiomyoma was the most frequent neoplasm of the female genital tract in NHPs, and granulosa cell tumor was the most frequent ovarian tumor; these findings are consistent with previous reports.7,10,13,18,19,25,39,44,45,47,50,54,60,67 Uterine leiomyomas are generally considered benign tumors in NHPs, but complications may arise when the tumors become excessively large, leading to uterine torsion or compression of surrounding structures such as the ureters, aorta or vena cava.33 Leiomyoma is the most common indication for hysterectomy in women and several research groups are currently working to establish a NHP model of uterine transplantation.40,56,77,82 Neoplasia of the urinary and genital tracts was rare in the NWMs examined in this study.
Dysplastic and neoplastic lesions of the cervix are associated with species-specific papillomavirus infections in baboons and macaques making these species attractive animal models for studies of human papillomavirus oncogenesis.6,35,58,84,85,83 As in humans, benign and malignant squamous genital proliferations in NHPs are likely caused by separate viruses.19,58 In women, the target for oncogenic papillomaviruses is the squamo-columnar junction of the cervix uteri.58 High-grade cervical intraepithelial neoplasia lesions are also reported at the squamo-columnar junction in cynomolgus macaques.84 There were a small number of cervical lesions in macaques and baboons during the study period; the papillomavirus status of these animals remains undetermined.
Papillomavirus-associated vaginal papillomas and carcinomas of the vulva and sex skin have previously been reported in NHPs.5,30,84 Squamous cell carcinoma of the vulva, perineum and/or sex skin has been observed in baboons and chimpanzees; potential risk factors include prolonged sunlight exposure and repetitive trauma to the area.5,30 Chronic herpesvirus papio 2 and Histoplasma capsulatum var duboisii infections may confer additional risk in baboons.11,30,51
There are rare reports of papillomavirus infection leading to penile carcinoma in rhesus macaques as well as spontaneous squamous cell carcinoma at the mucocutaneous junction of the penis and prepuce.37 Penile squamous cell carcinoma in men is associated with human papillomavirus (predominantly human papillomavirus 16) infection.62 Chronic preputial inflammation due to phimosis or lichen sclerosis is also associated with penile squamous cell carcinoma in men.32 Recently, genital condyloma-like lesions have been described in cynomolgus monkeys originating from the island of Mauritius; using PCR, macaque lymphocryptovirus, but not papillomavirus or poxvirus, was identified in those lesions.34
Malignant ovarian epithelial tumors comprise more than 90% of ovarian neoplasms in women over 40 years of age, so there is considerable interest in developing an NHP model of ovarian adenocarcinoma.81 However, spontaneous ovarian tumors are rarely reported in NHPs, a trend that our data further supports (Table 2).19 Rhesus macaques have been evaluated as a model for the study of ovarian cancer chemopreventive drugs, but the low incidence of spontaneous ovarian neoplasia in this species may limit the utility of this model.8 Other research groups have studied hormonal regulation and epitheliectomy of the ovarian surface epithelium in macaques as means of reducing ovarian cancer risk.69,86
Oviductal neoplasia is rare in NHPs, with leiomyoma, atypical polypoid adenomyofibroma, and adenomyofibroma being previously reported.10,71,74 We identified a single malignant oviductal neoplasm during the 30-year study period. Given the minimal anisokaryosis and high degree of differentiation, the differential diagnosis was fimbrial adenomyofibroma, which has previously been reported in a cynomolgus macaque.74 However, the tumor was unencapsulated, expansile, and multifocally incited a prominent scirrhous response, which favored a diagnosis of adenocarcinoma. This tumor is of particular interest because a high proportion of high-grade serous ovarian cancers in women are now thought to be of oviductal origin.81 In contrast to benign prostatic hyperplasia (BPH), a frequent diagnosis of aging men, prostatic hyperplasia was rarely observed in all NHPs during the study period.59 In two retrospective studies of aging (25- to 29-year-old) male chimpanzees, spontaneous BPH was reported in one group, but not in the other.13,75 Hyperplastic and neoplastic lesions originating from the prostatic basal cell population, histologically similar to those reported in men, were previously reported as common findings in a group of 19 aged, male macaques.55 These lesions likely have little, if any, clinical consequence in macaques, as they are found in the middle to outer regions of the prostate and the macaque prostate gland does not completely encircle the urethra as it does in man.55 Malignant prostatic tumors were not observed during the study period.
A variety of congenital or developmental lesions were diagnosed at low frequency during the study period. Polycystic kidney disease is the most commonly reported renal anomaly of NHPs, and is common among slender lorises (Loris lydekkerianus).20,65 Infantile polycystic kidney disease has been reported in rhesus macaques although this condition was not observed during the study period.3 Other developmental renal anomalies previously reported in NHPs but not represented in our data set include renal fusion and renal ectopia.14,15,72 Uterus didelphys was diagnosed in a 23-year-old rhesus macaque as an incidental finding. A single case of uterus didelphys has previously been reported in a neonatal tamarin (Sanguinus fuscicollis).15
Nonhuman primates are among the most commonly used large preclinical animal models in drug development, primarily for safety assessment and pharmacokinetics studies. Although spontaneous clinically significant urogenital disease is uncommon in NHPs, diseases of the urinary and genital systems are common in humans and several NHP species are used as animal models of these conditions.24 Further, as gene modification technologies emerge, the epidemiology and/or phenotype of these disease entities in NHPs may change over time. Knowledge of the background lesions occurring in NHP species may assist pathologists and investigators in the evaluation of new animal models as they are developed.
Supplementary Material
Acknowledgements:
The authors would like to thank the veterinary pathologists and necropsy staff, past and present, of the Yerkes National Primate Research Center.
Funding: This investigation used resources that were supported by the Yerkes base grant P51OD11132, and Southwest National Primate Research Center grant P51OD011133 from the Office of Research Infrastructure Programs, National Institutes of Health. This investigation was conducted in facilities constructed with support from the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Numbers C06 RR014578, C06 RR016228, C06 RR015456, and C06 RR017332.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Abbott DH, Saltzman W, Schultz-Darken NJ, Smith TE Specific neuroendocrine mechanisms not involving generalized stress mediate social regulation of female reproduction in cooperatively breeding marmoset monkeys. Ann N Y Acad Sci. 1997;807: 219–238. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch RC, McConnell EE, Imes GD, Schmidt JM A review of exertional rhabdomyolysis in wild and domestic animals and man. Vet Pathol. 1977;14: 314–324. [DOI] [PubMed] [Google Scholar]
- 3.Baskin GB, Roberts JA, McAfee RD Infantile polycystic renal disease in a rhesus monkey (Macaca mulatta). Lab Anim Sci. 1981;31: 181–183. [PubMed] [Google Scholar]
- 4.Bauer C The baboon (Papio sp.) as a model for female reproduction studies. Contraception. 2015;92: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck AP, Magden ER, Buchl SJ, Baze WB Malignant Neoplasia of the Sex Skin in 2 Chimpanzees (Pan troglodytes). Comp Med 2016;66: 154–161. [PMC free article] [PubMed] [Google Scholar]
- 6.Bergin IL, Bell JD, Chen Z, Zochowski MK, Chai D, Schmidt K, Culmer DL, Aronoff DM, Patton DL, Mwenda JM, Wood CE, Burk RD Novel genital alphapapillomaviruses in baboons (Papio hamadryas anubis) with cervical dysplasia. Vet Pathol. 2013;50: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binhazim AA, Chapman WL Jr., Isaac W Multiple spontaneous lesions in an aged spider monkey. Lab Anim Sci. 1989;39: 355–357. [PubMed] [Google Scholar]
- 8.Brewer M, Baze W, Hill L, Utzinger U, Wharton JT, Follen M, Khan-Dawood F, Satterfield W Rhesus macaque model for ovarian cancer chemoprevention. Comp Med. 2001;51: 424–429. [PubMed] [Google Scholar]
- 9.Bronson RT, O’Connell M, Klepper-Kilgore N, Chalifoux LV, Sehgal P Fatal fasting syndrome of obese macaques. Lab Anim Sci. 1982;32: 187–192. [PubMed] [Google Scholar]
- 10.Brown SL, Anderson DC, Dick EJ Jr., Guardado-Mendoza R, Garcia AP, Hubbard GB Neoplasia in the Chimpanzee (Pan spp.). Journal of Medical Primatology. 2009;38: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler TM, Hubbard GB An epizootic of histoplasmosis duboisii (African histoplasmosis) in an American baboon colony. Lab Anim Sci. 1991;41: 407–410. [PubMed] [Google Scholar]
- 12.Carville A, Mansfield KG Comparative pathobiology of macaque lymphocryptoviruses. Comp Med. 2008;58: 57–67. [PMC free article] [PubMed] [Google Scholar]
- 13.Chaffee BK, Beck AP, Owston MA, Kumar S, Baze WB Magden ER, Dick EJ Jr., Lammey M, Abee CR Spontaneous Reproductive Tract Lesions in Aged Captive Chimpanzees. Vet Pathol. 2016;53: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalifoux LV. Crossed renal ectopia in a squirrel monkey (Saimiri sciureus) and an owl monkey (Aotus trivirgatus). J Med Primatol. 1986;15: 235–239. [PubMed] [Google Scholar]
- 15.Chalifoux LV, Elliott MW Congenital anomalies in two neonatal tamarins (Saguinus oedipus and Saguinus fuscicollis). J Med Primatol. 1986;15: 329–337. [PubMed] [Google Scholar]
- 16.Chamanza R, Marxfeld HA, Blanco AI, Naylor SW, Bradley AE Incidences and range of spontaneous findings in control cynomolgus monkeys (Macaca fascicularis) used in toxicity studies. Toxicol Pathol. 2010;38: 642–657. [DOI] [PubMed] [Google Scholar]
- 17.Chilton J, Wilcox A, Lammey M, Meyer D Characterization of a Cardiorenal-like Syndrome in Aged Chimpanzees (Pan troglodytes). Vet Pathol. 2016;53: 417–424. [DOI] [PubMed] [Google Scholar]
- 18.Cianciolo RE, Butler SD, Eggers JS, et al. Spontaneous neoplasia in the baboon (Papio spp.). J Med Primatol. 2007;36: 61–79. [DOI] [PubMed] [Google Scholar]
- 19.Cline J, Wood C, Vidal J, et al. Selected Background Findings and Interpretation of Common Lesions in the Female Reproductive System in Macaques. Toxicol Pathol. 2008;36: 142s–163s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cline JM, Brignolo L, Ford EW Urogenital System In: Abee CR, Mansfield K, Tardif S, Morris T, ed. Nonhuman Primates in Biomedical Research. London, UK: Elsevier Inc.; 2012:483–562. [Google Scholar]
- 21.Cooper TK, Gabrielson KL Spontaneous lesions in the reproductive tract and mammary gland of female non-human primates. Birth Defects Res B Dev Reprod Toxicol. 2007;80: 149–170. [DOI] [PubMed] [Google Scholar]
- 22.D’Hooghe TM, Bambra CS, De Jonge I, Lauweryns JM, Koninckx PR. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75: 98–101. [DOI] [PubMed] [Google Scholar]
- 23.Dalmose AL, Hvistendahl JJ, Olsen LH, Eskild-Jensen A, Djurhuus JC, Swindle MM Surgically induced urologic models in swine. J Invest Surg. 2000;13: 133–145. [DOI] [PubMed] [Google Scholar]
- 24.DiPietrantonio A, Yuenger J, Ireland K, Rao A: Nonhuman Primate Evaluation and Analysis. Part 1: Analysis of Future Demand and Supply In: Nonhuman Primate Evaluation and Analysis., ed. Programs NIoHOoRI. National Institutes of Health, Bethesda, MD, 2018 [Google Scholar]
- 25.Durkes A, Garner M, Juan-Salles C, Ramos-Vara J Immunohistochemical characterization of nonhuman primate ovarian sex cord-stromal tumors. Vet Pathol. 2012;49: 834–838. [DOI] [PubMed] [Google Scholar]
- 26.Gall AJ, Olds JE, Wunschmann A, Selmic LE, Rasmussen J, Lewis AD Lesions of the Female Reproductive Tract in Japanese Macaque (Macaca Fuscata) from Two Captive Colonies. J Zoo Wildl Med. 2018;49: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattone VH 2nd, Tian C, Zhuge W, Sahni M, Narayan O, Stephens EB. SIV-associated nephropathy in rhesus macaques infected with lymphocyte-tropic SIVmac239. AIDS Res Hum Retroviruses. 1998;14: 1163–1180. [DOI] [PubMed] [Google Scholar]
- 28.Giddens WE Jr., Boyce JT, Blakley GA, Morton WR Renal disease in the pigtailed macaque (Macaca nemestrina). Vet Pathol. 1981;18: 70–81. [DOI] [PubMed] [Google Scholar]
- 29.Gumber S, Courtney CL, Strait KR, Sharma P, Freebersyser JE, Crane MM Retrograde ejaculation associated spontaneous sperm cystolithiasis in four rhesus macaques (Macaca mulatta). Exp Toxicol Pathol. 2013;65: 1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad JL, Dick EJ Jr., Guardado-Mendoza R, Hubbard GB Spontaneous squamous cell carcinomas in 13 baboons, a first report in a spider monkey, and a review of the non-human primate literature. J Med Primatol. 2009;38: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafez ESE, Jaszczak S Comparative anatomy and histology of the cervix uteri in non-human primates. Primates. 1972;13: 297–314. [Google Scholar]
- 32.Hakenberg OW, Drager DL, Erbersdobler A, Naumann CM, Junemann KP, Protzel C. The Diagnosis and Treatment of Penile Cancer. Dtsch Arztebl Int. 2018;115: 646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanley PW, Barnhart KF, Satterfield WC, McArthur MJ, Buchl SJ, Baze WB Obstructive uropathy secondary to uterine leiomyoma in a chimpanzee (Pan troglodytes). Comp Med. 2012;62: 543–545. [PMC free article] [PubMed] [Google Scholar]
- 34.Harari A, Wood CE, Van Doorslaer K, Chen Z, Domaingue MC, Elmore D, Koenig P, Wagner JD, Jennings RN Burk RD Condylomatous genital lesions in cynomolgus macaques from Mauritius. Toxicol Pathol. 2013;41: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harbison CE, Ellis ME, Westmoreland SV Spontaneous cervicovaginal lesions and immune cell infiltrates in nonhuman primates. Toxicol Pathol. 2013;41: 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herndon JG, Bein ML, Nordmeyer DL, Turner JJ Seasonal testicular function in male rhesus monkeys. Horm Behav. 1996;30: 266–271. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard GB, Wood DH, Fanton JW. Squamous cell carcinoma with metastasis in a rhesus monkey (Macaca mulatta). Lab Anim Sci. 1983;33: 469–472. [PubMed] [Google Scholar]
- 38.Hukkanen RR, Liggitt HD, Anderson DM, Kelley ST Detection of systemic amyloidosis in the pig-tailed macaque (Macaca nemestrina). Comp Med. 2006;56: 119–127. [PubMed] [Google Scholar]
- 39.Kaspareit J, Friderichs-Gromoll S, Buse E, Habermann G Spontaneous neoplasms observed in cynomolgus monkeys (Macaca fascicularis) during a 15-year period. Exp Toxicol Pathol. 2007;59: 163–169. [DOI] [PubMed] [Google Scholar]
- 40.Kisu I, Banno K, Matoba Y, Adachi M, Aoki D Basic research on uterus transplantation in nonhuman primates in Japan. J Obstet Gynaecol Res. 2018;44: 1871–1881. [DOI] [PubMed] [Google Scholar]
- 41.Kramer R, Burns M Normal Clinical and Biological Parameters of the Common Marmoset (Callithrix jacchus) In: Medicine ACoLA, ed. The Common Marmoset in Captivity and Biomedical Research. Academic Press; 2019:93–107. [Google Scholar]
- 42.Laber-Laird KE, Jokinen MP, Lehner ND Fatal fatty liver-kidney syndrome in obese monkeys. Lab Anim Sci. 1987;37: 205–209. [PubMed] [Google Scholar]
- 43.Lee HJ, Gonzalez O, Dick EJ, Donati A, Feliers D, Choudhury GG, Ross C, Venkatachalam M, Tardif SD, Kasinath BS Marmoset as a Model to Study Kidney Changes Associated With Aging. J Gerontol A Biol Sci Med Sci. 2019;74: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long CT, Luong RH, McKeon GP, Albertelli M Uterine leiomyoma in a Guyanese squirrel monkey (Saimiri sciureus sciureus). J Am Assoc Lab Anim Sci. 2010;49: 226–230. [PMC free article] [PubMed] [Google Scholar]
- 45.Lowenstine LJ. Neoplasms and Proliferative Disorders in Nonhuman Primates In: Benirschke K, ed. Primates : the road to self-sustaining populations. New York: Springer-Verlag; 1986:781–814. [Google Scholar]
- 46.Lowenstine LJ. A primer of primate pathology: lesions and nonlesions. Toxicol Pathol. 2003;31 Suppl: 92–102. [DOI] [PubMed] [Google Scholar]
- 47.Lowenstine LJ, McManamon R, Terio KA Comparative Pathology of Aging Great Apes: Bonobos, Chimpanzees, Gorillas, and Orangutans. Vet Pathol. 2016;53: 250–276. [DOI] [PubMed] [Google Scholar]
- 48.Ludlage E, Murphy CL, Davern SM, Solomon A, Weiss DT, Glenn-Smith D, Dworkin S, Mansfield KG Systemic AA amyloidosis in the common marmoset. Vet Pathol. 2005;42: 117–124. [DOI] [PubMed] [Google Scholar]
- 49.Luyckx VA, Tonelli M, Stanifer JW . The global burden of kidney disease and the sustainable development goals. Bulletin of the World Health Organization. 2018;96: 414–422D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marr-Belvin AK, Bailey CC, Knight HL, Klumpp SA, Westmoreland SV, Miller AD Ovarian pathology in rhesus macaques: a 12-year retrospective. J Med Primatol. 2010;39: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martino MA, Hubbard GB, Butler TM, Hilliard JK Clinical disease associated with simian agent 8 infection in the baboon. Lab Anim Sci. 1998;48: 18–22. [PubMed] [Google Scholar]
- 52.Matz-Rensing KaL LJ New World and Old World Monkeys In: Terio KA MD, St. Leger J, ed. Pathology of Wildlife and Zoo Animals. London, UK: Elsevier Inc.; 2018:343–373. [Google Scholar]
- 53.McAloose DaS IH Prosimians In: Terio KA MD, and St. Leger J, ed. Pathology of Wildlife and Zoo Animals. London, UK: Elsevier Inc.; 2018:323–342. [Google Scholar]
- 54.McClure HM: Neoplastic diseases in nonhuman primates: literature review and observations in an autopsy series of 2,176 animals In: The comparative pathology of zoo animals : proceedings of a symposium held at the National Zoological Park, Smithsonian Institution, October 2–4, 1978, ed. Migaki RJMaG, pp. 549–565. Smithsonian Institution Press, Washinton, D.C., 1980 [Google Scholar]
- 55.McEntee MF, Epstein JI, Syring R, Tierney LA, Strandberg JD Characterization of prostatic basal cell hyperplasia and neoplasia in aged macaques: comparative pathology in human and nonhuman primates. Prostate. 1996;29: 51–59. [DOI] [PubMed] [Google Scholar]
- 56.Mihara M, Kisu I, Hara H, et al. Uterine autotransplantation in cynomolgus macaques: the first case of pregnancy and delivery. Hum Reprod. 2012;27: 2332–2340. [DOI] [PubMed] [Google Scholar]
- 57.Miller AD. Neoplasia and Proliferative Disorders of Nonhuman Primates In: Abee CR, Mansfield K, Tardif S, Morris T, ed. Nonhuman Primates in Biomedical Research. 2 ed London, UK: Elsevier Inc.; 2012:325–356. [Google Scholar]
- 58.Mirkovic J, Howitt BE, Roncarati P, et al. Carcinogenic HPV infection in the cervical squamo-columnar junction. J Pathol. 2015;236: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mobley D, Feibus A, Baum N Benign prostatic hyperplasia and urinary symptoms: Evaluation and treatment. Postgrad Med. 2015;127: 301–307. [DOI] [PubMed] [Google Scholar]
- 60.Moore CM, Hubbard GB, Leland MM, Dunn BG, Best RG Spontaneous ovarian tumors in twelve baboons: a review of ovarian neoplasms in non-human primates. J Med Primatol. 2003;32: 48–56. [DOI] [PubMed] [Google Scholar]
- 61.Okulicz WC, Ace CI, Scarrell R Zonal changes in proliferation in the rhesus endometrium during the late secretory phase and menses. Proc Soc Exp Biol Med. 1997;214: 132–138. [DOI] [PubMed] [Google Scholar]
- 62.Olesen TB, Sand FL, Rasmussen CL, et al. Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 2019;20: 145–158. [DOI] [PubMed] [Google Scholar]
- 63.Patrick DJ, Rebelatto MC Toxicologic Pathology and Background Lesions of Nonhuman Primates In: Joerg Bluemel SK, Schenck Emanuel, Weinbauer Gerhard F., ed. The Nonhuman Primate in Nonclinical Drug Development and Safety Assessment. Amsterdam: Academic Press; 2015:235–256. [Google Scholar]
- 64.Pereira Bacares ME, Vemireddi V, Creasy D Testicular Fibrous Hypoplasia in Cynomolgus Monkeys ( Macaca fascicularis): An Incidental, Congenital Lesion. Toxicol Pathol. 2017;45: 536–543. [DOI] [PubMed] [Google Scholar]
- 65.Plesker R, Schulze H Polycystic nephropathy in slender lorises (Loris lydekkerianus). Am J Primatol. 2006;68: 838–844. [DOI] [PubMed] [Google Scholar]
- 66.Ramer JC, Garber RL, Steele KE, Boyson JF, O’Rourke C Thomson JA Fatal lymphoproliferative disease associated with a novel gammaherpesvirus in a captive population of common marmosets. Comp Med. 2000;50: 59–68. [PubMed] [Google Scholar]
- 67.Remick AK, Van Wettere AJ, Williams CV Neoplasia in prosimians: case series from a captive prosimian population and literature review. Vet Pathol. 2009;46: 746–772. [DOI] [PubMed] [Google Scholar]
- 68.Reuter JD, Dysko RC, Chrisp CE Review of exertional rhabdomyolysis and a case in a rhesus monkey (Macaca mulatta). J Med Primatol. 1998;27: 303–309. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez GC, Walmer DK, Cline M, Krigman H, Lessey BA, Whitaker RS, Dodge R Hughes CL Effect of progestin on the ovarian epithelium of macaques: cancer prevention through apoptosis? J Soc Gynecol Investig. 1998;5: 271–276. [DOI] [PubMed] [Google Scholar]
- 70.Sato J, Doi T, Kanno T, Wako Y, Tsuchitani M, Narama I Histopathology of incidental findings in cynomolgus monkeys ( macaca fascicularis ) used in toxicity studies. J Toxicol Pathol. 2012;25: 63–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satoh H, Kai K, Yabe K, Fujii F, Furuhama K Mullerian tumor (atypical polypoid adenomyoma) with sex-cord differentiation arising from the oviduct in an adolescent cynomolgus monkey (Macaca fascicularis). Toxicol Pathol. 2003;31: 179–184. [DOI] [PubMed] [Google Scholar]
- 72.Seier JV, Fincham JE, Taljaard JJ Horseshoe kidneys in vervet monkeys. J Med Primatol. 1990;19: 595–599. [PubMed] [Google Scholar]
- 73.Shirai N, Evans MG Testicular microlithiasis in a clinically healthy cynomolgus monkey (Macaca fascicularis). J Toxicol Pathol. 2018;31: 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Starost MF. Adenomyofibroma of the fimbria in a cynomolgus monkey (Macaca fascicularis). J Vet Diagn Invest. 2009;21: 892–894. [DOI] [PubMed] [Google Scholar]
- 75.Steiner MS, Couch RC, Raghow S, Stauffer D The chimpanzee as a model of human benign prostatic hyperplasia. J Urol. 1999;162: 1454–1461. [PubMed] [Google Scholar]
- 76.Story L, Kennedy S Animal studies in endometriosis: a review. Ilar j. 2004;45: 132–138. [DOI] [PubMed] [Google Scholar]
- 77.Stouffer RL, Woodruff TK Nonhuman Primates: A Vital Model for Basic and Applied Research on Female Reproduction, Prenatal Development, and Women’s Health. Ilar j. 2017;58: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uchino H, Fujishima J, Fukuoka K, Iwakiri T,Kamikuri A, Maeda H, Nakama K Usefulness of urinary biomarkers for nephrotoxicity in cynomolgus monkeys treated with gentamicin, cisplatin, and puromycin aminonucleoside. J Toxicol Sci. 2017;42: 629–640. [DOI] [PubMed] [Google Scholar]
- 79.Walker ML, Anderson DC, Herndon JG, Walker LC Ovarian aging in squirrel monkeys (Saimiri sciureus). Reproduction. 2009;138: 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang P, Wang X, Li Y, Jun B, Xia D, Wang S, Pan H . Hydronephrosis due to ureteral endometriosis in women of reproductive age. Int J Clin Exp Med 2015;8: 1059–1065. [PMC free article] [PubMed] [Google Scholar]
- 81.Webb PM, Jordan SJ Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41: 3–14. [DOI] [PubMed] [Google Scholar]
- 82.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008;198: 34 e31–37. [DOI] [PubMed] [Google Scholar]
- 83.Wood CE. Morphologic and Immunohistochemical Features of the Cynomolgus Macaque Cervix. Toxicologic Pathology. 2008;36: 119S–129S. [Google Scholar]
- 84.Wood CE, Borgerink H, Register TC, Scott L, Cline JM Cervical and vaginal epithelial neoplasms in cynomolgus monkeys. Vet Pathol. 2004;41: 108–115. [DOI] [PubMed] [Google Scholar]
- 85.Wood CE, Chen Z, Cline JM, Miller BE, Burk RD Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. J Virol. 2007;81: 6339–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright JW, Jurevic L, Stouffer RL Dynamics of the primate ovarian surface epithelium during the ovulatory menstrual cycle. Hum Reprod. 2011;26: 1408–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada N, Sato J, Kanno T, Wako Y, Tsuchitani M Morphological study of progressive glomerulonephropathy in common marmosets (Callithrix jacchus). Toxicol Pathol. 2013;41: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 88.Zoller M, Friderichs-Gromoll S, Kaspareit J Testicular and epididymal appendages in the cynomolgus macaque (Macaca fascicularis). J Med Primatol. 2009;38: 448–454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


