Abstract
Endometriosis is a common gynecological disease defined as the growth of endometrial tissues outside the uterus. Although the mechanism underlying the progression of endometriosis has not been fully elucidated, cancer-like aerobic glycolysis is considered to mediate the elevated growth and resistance to apoptosis of endometriotic cells. The heartwood of Caesalpinia sappan L. (family Leguminosae) is a herbal medicinal product used to treat gynecological symptoms, including algomenorrhea and amenorrhea. The results of the present study revealed that endometriotic 12Z cells exhibited more rapid growth than normal endometrial cells (THES). The expression levels of pyruvate dehydrogenase kinase (PDK)1 and 3 and lactate production were higher in 12Z cells than in THES cells. In addition, the 12Z cells were more sensitive to the cytotoxicity of the aqueous extract of C. sappan heartwood (CS) than the THES cells. CS inhibited lactate production and phosphorylation of pyruvate dehydrogenase A by reducing the expression of PDK1. CS also increased mitochondrial reactive oxygen species (ROS) levels, decreased mitochondrial membrane potential and consequently stimulated the apoptosis of 12Z cells. CS-induced cell death was substantially inhibited by exogenous PDK1 expression. In conclusion, CS may be a novel drug candidate for treating endometriosis by inhibiting aerobic glycolysis and inducing ROS-mitochondria-mediated apoptotic cell death.
Keywords: endometriosis, PDK1, Caesalpinia sappan, ROS, apoptosis
Introduction
Endometriosis, one of the most common gynecological diseases is defined as the growth of endometrial tissues outside the uterus (1). The precise mechanisms underlying the progression of endometriosis are still unclear (2,3). Although endometriosis is a benign, non-cancerous disease, it exhibits cancer-like characteristics such as uncontrolled cell growth, resistance to apoptosis, enhanced angiogenesis, and immune escape (2,4). In addition, cancer-associated somatic mutations in non-cancerous endometrial tissue have recently been reported by several research groups (5,6). Thus, several anticancer drugs, including angiogenesis and cell signaling inhibitors, have been studied as potential candidates for the treatment of endometriosis (7,8).
Recently, cancer-like aerobic glycolysis and lactate production were reported to be elevated in patients with endometriosis (9,10). Cancer cells generally prefer glycolysis to oxidative phosphorylation for the generation of ATP even under high oxygen conditions (11). The Warburg's effect is a phenomenon that affects the cancer microenvironment through various pathways, such as tumor cell growth and invasion, angiogenesis, and immune avoidance (12-14). Aerobic glycolysis is considered to mediate uncontrolled cell proliferation and resistance to apoptosis (10,12) in endometriosis and malignant cancer. Recently, a well-known pyruvate dehydrogenase kinase (PDK) inhibitor, dichloroacetate (DCA), was reported to inhibit the glycolytic phenotype of endometriotic cells and reduce lesion size in mouse models (10,15). Thus, we hypothesized that Warburg-like metabolic characteristics could be a good therapeutic target for endometriosis treatment.
The heartwood of Caesalpinia sappan L. (belonging to the Leguminosae family) is a herbal medicinal product used for improving blood circulation, accelerating hemostasis, removing extravasated blood, relieving pain, and reducing swelling (16). In particular, C. sappan has been used to treat gynecological symptoms, including dysmenorrhea and amenorrhea (17,18). Several studies have already shown that C. sappan causes apoptosis of several cancer cell lines (19-22). Recently, cotreatment with cisplatin and C. sappan components was shown to arrest the cell cycle and increase apoptosis of colon cancer cells (23).
However, to our best knowledge, the effect of C. sappan on endometriosis has not been studied. Thus, in this study, we investigated whether C. sappan has an inhibitory effect on the growth of endometriotic 12Z cells. In addition, the activities and expressions of the enzymes involved in aerobic glycolysis were examined as a possible mechanism underlying the suppression of 12Z cell growth.
Materials and methods
Materials
Antibodies against caspase 3, caspase 9, and poly ADP-ribose polymerase (PARP) were purchased from Cell Signaling Technology, Inc. The antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was supplied by Santa Cruz Biotechnology, whereas those for lactate dehydrogenase A (LDHA) and phosphorylated pyruvate dehydrogenase A (p-PDHA) were provided by Abcam. Antibodies against PDK1 were provided by Enzo Life Sciences and those against PDHA were purchased from Invitrogen; Thermo Fisher Scientific, Inc. The antibody against PDK3 was provided by Signalway Antibody. All reagents, including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), were purchased from Sigma-Aldrich; Merck KGaA unless otherwise indicated.
Preparation of aqueous extract of C. sappan heartwood (CS)
Heartwood of C. sappan was purchased from Omniherb Co. (Yeongcheon, Korea). The material was imported from Indonesia in 2016, authenticated by botanical experts working for the company, and certified based on the guidelines of the Ministry of Food and Drug Safety, Korea Government. A voucher specimen was deposited at the Healthy Aging Korean Medical Research Center, Pusan National University (voucher no. HAK-037). The herbal material (100 g) was crushed, extracted with distilled water (1 liter) at 100˚C for 2 h, and then the extract was filtered using Whatman paper (3 mm; Whatman PLC) to remove the insoluble particles.
Next, the extract was concentrated using a rotary evaporator (Eyela) and lyophilized using a freeze dryer (Labconco) to yield a powder (designated as CS, Fig. 1A). The powder (10.2 g) was dissolved in dimethyl sulfoxide (DMSO) to prepare a stock solution (100 mg/ml) that was diluted with culture medium to prepare the working solutions before use in the subsequent experiments.
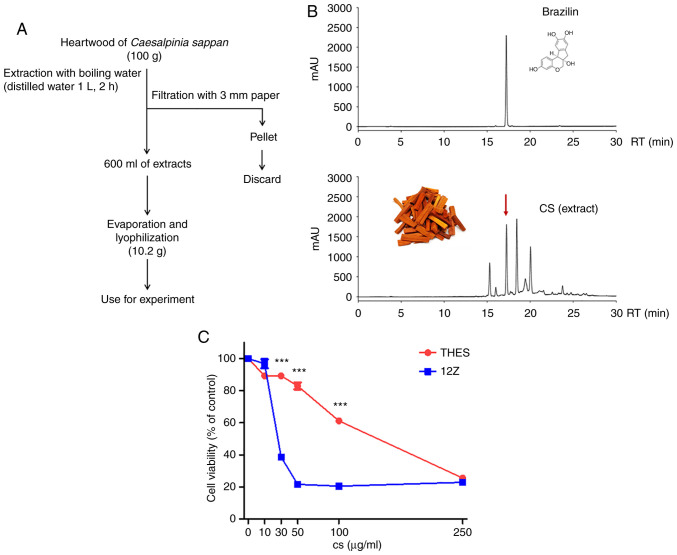
Figure 1.
Extraction, phytochemical analysis, and cytotoxicity of CS. (A) Scheme of CS extraction. (B) High-performance liquid chromatography analysis of CS using brazilin as a standard. (C) THES and 12Z cells were treated with CS for 24 h and cytotoxicity was measured using MTT assay. Results of three independent experiments are presented as mean ± standard deviation. ***P<0.001 compared with THES cells. CS, Caesalpinia sappan L heartwood extract.
High-performance liquid chromatography (HPLC) analysis
The phytochemical features of the CS were verified using HPLC with an Agilent 1200 series system (Agilent Technologies). The analytical column was a Waters Sunfire™ C18 (5 µm 4.6x250 mm) maintained at 30˚C. The mobile phases A and B consisted of water plus 0.1% formic acid and methanol (MeOH) plus 0.1% formic acid, respectively. The gradient flow was as follows: A/B=95-20/5-80 (0-30 min) → A/B=20-5/80-95 (30-40 min) → A/B=5-3/95-97 (40-50 min) → A/B=3-30/97-70 (50-55 min) → A/B=30-95/70-5 (55-60 min). The samples were analyzed chromatographically at a flow rate of 1 ml/min using ultraviolet detection at 280 nm. The volume injected into the column was 20 µl and brazilin (Cayman Chemical Company) dissolved in DMSO was used as the standard compound.
Cell culture
Immortalized normal human endometrial THES cells (ATCC® CRL-4003™) were purchased from American Type Culture Collection. Immortalized human endometriotic epithelial 12Z cells were generously provided by Dr Starzinski-Powitz (Johann-Wolfgang-Goethe-Universitaet). THES cells were cultured at 37˚C in an atmosphere containing 5% CO2/air in Dulbecco's modified Eagle's medium plus F-12 (Welgene) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA). The 12Z cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Welgene) containing 10% heat-inactivated FBS and 1% penicillin/streptomycin.
Cell viability assay
The cytotoxicity of CS was examined using an MTT assay. Briefly, the cells were incubated in 24-well culture plates with the indicated concentrations of CS for 24 h. MTT solution (2 mg/ml) was added to each well of the plates, which were then incubated for 4 h at 37˚C exposed to an atmosphere of 5% CO2. The culture medium was removed and the formazan crystals formed in the live cells were measured by determining the absorbance at 540 nm using a Spectramax M2 microplate reader (Molecular Devices, LLC).
Lactate production
Lactate production by 12Z cells was measured using a lactate fluorometric assay kit (BioVision, San Francisco, CA, USA), according to the manufacturer's instructions. Phenol red- and serum-free RPMI-1640 medium were added to the subconfluent cells in 6-well plates, which were incubated for 1 h at 37˚C and then 1 µl of the medium from each well was assessed using a lactate assay kit with a Spectramax M2 spectrofluorometer (Molecular Devices).
RNA isolation and quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from THES and 12Z cells using a GeneJET RNA purification kit (Thermo Fisher Scientific, Inc.). An equal amount of total RNA (1 µg) from each sample was then reverse transcribed using oligo-dT primers with M-MLV reverse transcriptase (RT, Thermo Fisher Scientific, Inc.). The cDNA was amplified using qPCR with AccuPower® PCR PreMix (Bioneer Co.). Real-time qPCR was performed using the StepOnePlus real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the RealHelix qPCR kit (NanoHelix). Relative mRNA levels were normalized to 18S rRNA and the primers used in this study are shown in Table S1.
Transfection and viral infection
The PDK1 PCR product was ligated using pMX-IRES-puromycin vector with EcoRI and XhoI as restriction enzyme sites. The primers used for cloning are shown in Table S1. The pMX-IRES-puromycin vector (EV) and pMX-IRES-puromycin PDK1 vector (PDK1) were transfected into Plat-A cells using Lipofectamine™ 2000 (Invitrogen), the supernatant was collected after 24 h, and then it was replaced with the filtered viral supernatant and 5 µg/ml polybrene (sc-134220; Santa Cruz Biotechnology) instead of the 12Z cell culture medium. Twenty-four hours after the infection, the cells were selected using 2 µg/ml puromycin (CAS58-58-2; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and cultured for 2 weeks.
Western blot analysis
Total protein was extracted from the cells using 1% NP-40 lysis buffer [150 mM sodium chloride (NaCl), 10 mM HEPES (pH 7.45), 1% NP-40, 5 mM sodium pyrophosphate (Na4P2O7), 5 mM sodium fluoride (NaF), and 2 mM sodium vanadate (Na3VO4)] containing a protease inhibitor cocktail (Roche Applied Science, Penzburg, Germany). Equal amounts (10 µg) of protein from each sample were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The fractionated proteins were electrophoretically transferred onto nitrocellulose filters (HYBOND ECL; GE Healthcare), which were blocked with 5% nonfat dry milk at room temperature for 1 h and incubated with primary antibodies against caspase 3, caspase 9, PARP, p-PDHA, PDHA, LDHA, PDK1, PDK3, or GAPDH at 4˚C overnight. The membranes were washed three times and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies. Bands of the target proteins were detected using an enhanced chemiluminescence Plus kit (Thermo Fisher Scientific) and visualized using the ImageQuant LAS 4000 imaging system (GE Healthcare).
Measurement of intracellular and mitochondrial reactive oxygen species (ROS)
The production of intracellular and mitochondrial ROS was determined using 5-(and-6)-carboxy-2,7-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) and MitoSOX™ red (both from Thermo Fisher Scientific), respectively. Briefly, 100 µM carboxy-H2DCFDA or 5 µM MitoSOX™ red was added to cells cultured in conditioned medium, followed by incubation at 37˚C for 30 min (carboxy-H2DCFDA) or 10 min (MitoSOX™ red). The cells were washed twice with phosphate-buffered saline and the fluorescence intensity was analyzed using a BD FACS Canto II flow cytometer (BD Biosciences) by measuring excitation/emission at a wavelength of 488/525 nm (carboxy-H2DCFDA) or 510/580 nm (MitoSOX™ red).
Detection of apoptotic cells
The 12Z cells were treated with the indicated concentration of CS for 24 h. Apoptotic cells were examined using the Annexin V-FITC Apoptosis Detection kit (Thermo Fisher Scientific). The cells (1x106) were resuspended in 500 µl of binding buffer and incubated with 5 µl of Annexin V-FITC. The fluorescence intensities of the samples were examined using a BD FACS CANTO II measuring excitation/emission at a wavelength of 494/525 nm.
LDHA activity assay
LDHA activity was determined by measuring the decrease of absorbance at 340 nm from the oxidation of NADH in 20 mM HEPES-K+, pH 7.2, 0.05% BSA, 20 µM NADH and 2 mM pyruvate using a Spectramax M2 spectrofluorometer at excitation/emission wavelengths of 340/460 nm, as previously described. For the in vitro LDHA activity assay, 10 ng of recombinant LDHA protein was mixed with various concentrations of CS and the reaction solution containing 20 µM NADH, 20 mM HEPES (pH 7.2), 2 mM pyruvate and 0.05% BSA. In the case of the intracellular assay, 1 µg of total protein from cell lysates treated with indicated concentrations of CS were used. The amount of NADH was measured using the Spectramax M2 spectrofluorometer with 340 nm/360 nm of excitation/emission wavelengths. The activity of LDHA was inversely calculated from the amount of NADH consumption.
In vitro pyruvate dehydrogenase kinase (PDK) assay
The kinase activity of PDK was confirmed as described previously. Briefly, the GST-PDK1 construct (obtained from Jing Chen, Emory University) was transfected into 293T cells using polyethylenimine (PEI, Sigma-Aldrich). At 24 h after transfection, cells were harvested and lysed using buffer [150 mM NaCl, 10 mM HEPES (pH 7.45), 1% NP-40, 5 mM NaPyrophosphate, 5 mM NaF, 2 mM Na3VO4]. GST-PDK1 was pulled down by Glutathione Sepharose 4B beads (GE Healthcare). The beads bound with GST-PDK1 were washed with PBS, followed by application to PDK1 kinase assay. The beads were incubated with 50 ng of recombinant PDHA (E1p clone obtained from David Chuang, University of Texas) as a substrate of PDK1 for 5 min at 30˚C in PDK1 kinase buffer containing 20 mM potassium phosphate buffer pH 7.5, 0.1 mM EDTA, 1 mM MgCl2, 2 mM DTT, and 250 µM ATP. The samples were applied to SDS-PAGE, followed by immunoblotting using antibodies against GST, PDHA, and phosphor-PDHA.
Statistical analysis
The results are expressed as mean ± standard deviation (SD). Statistical analysis was performed using an unpaired Student's t-test (for comparison of lactate production between THES and 12Z cells) or a one-way analysis of variance with Tukey's post-hoc test using GraphPad Prism software (GraphPad Software). The minimum significance level was set at P-value of 0.05 and at least three independent replications were performed for each experiment.
Results
CS differentially inhibited the growth of normal endometrial and endometriotic cells
The hot water CS extract (Fig. 1A) prepared in this study was characterized using HPLC, which confirmed it was identical to an extract investigated in a previous study (24), and its standard compound brazilin (Fig. 1B). The inhibitory effect of CS on the growth of normal endometrial THES cells and ectopic endometrium originating from 12Z endometrial cells was examined. The growth of both cell lines was reduced by CS treatment in a dose-dependent manner (Fig. 1C). The half-maximal inhibitory concentration of CS on the growth of THES and 12Z cells was 100.1 and 29.41 µg/ml, respectively, indicating that the endometriotic 12Z cells were 3.4 times more sensitive to CS-induced cytotoxicity than the normal endometrial THES cells were.
PDK expression was increased in endometriotic cells
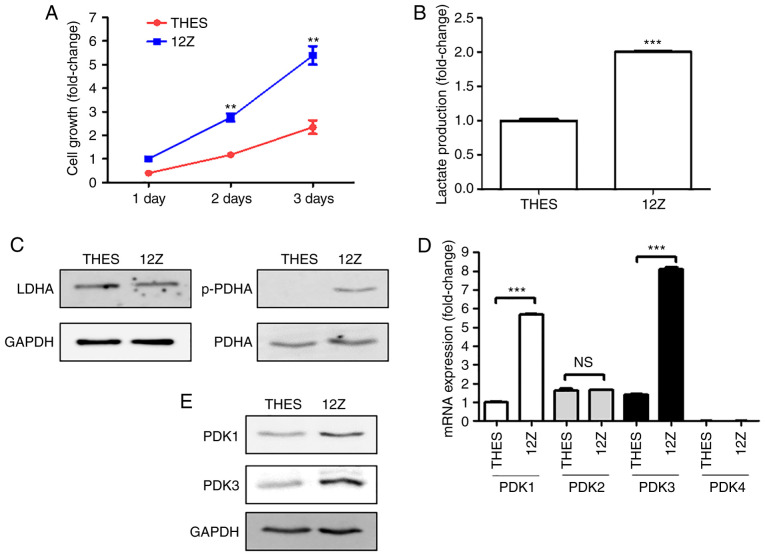
To explain the differential cytotoxicity of CS, its effects on the growth rates of normal THES and ectopic 12Z endometrial cells were compared and the 12Z cells grew approximately two-fold faster than the THES cells (Fig. 2A). The endometriotic 12Z cells also produced twice as much lactate as the normal THES cells (Fig. 2B). Although there was no change in LDHA expression between THES and 12Z cells, the phosphorylation of PDH was considerably higher in 12Z cells than in THES cells (Fig. 2C). The qPCR and western blot analysis results demonstrated that the expression of PDK1 and PDK3 was much higher in 12Z cells than it was in normal endometrial cells (Fig. 2D and E). Similar to previous studies (9,10), these results suggest that PDK1 and PDK3 might be responsible for the Warburg-like phenotype of ectopic endometriotic cells.
Figure 2.
Endometriotic cells are more glycolytic than normal endometrial cells via the expression of PDKs. (A) Normal endometrial THES and endometriotic 12Z cells were incubated for 1 to 3 days and the growth rates were measured using cell counting. (B) Cells were seeded in six-well culture plates, cultured for 24 h, and production of lactate in THES and 12Z cell culture media was measured using a commercially available lactate assay kit. Cells were seeded in six-well culture plates and cultured for 24 h. Expression levels of pyruvate metabolism-related enzymes (C) LDHA and p-PDH, and (E) PDK1 and 3 were examined using western blot analysis. GAPDH expression was used as internal control. mRNA expression level of cells was determined using quantitative polymerase chain reaction. (D) mRNA level of PDKs was confirmed in both cell lines. Results of three independent experiments are presented as mean ± standard deviation. **P<0.05 and ***P<0.001 compared with THES cell. PDKs, pyruvate dehydrogenase kinases; LDHA, lactate dehydrogenase A; p-PDHA, phosphorylated-PDHA; PDHA, pyruvate dehydrogenase E1α.
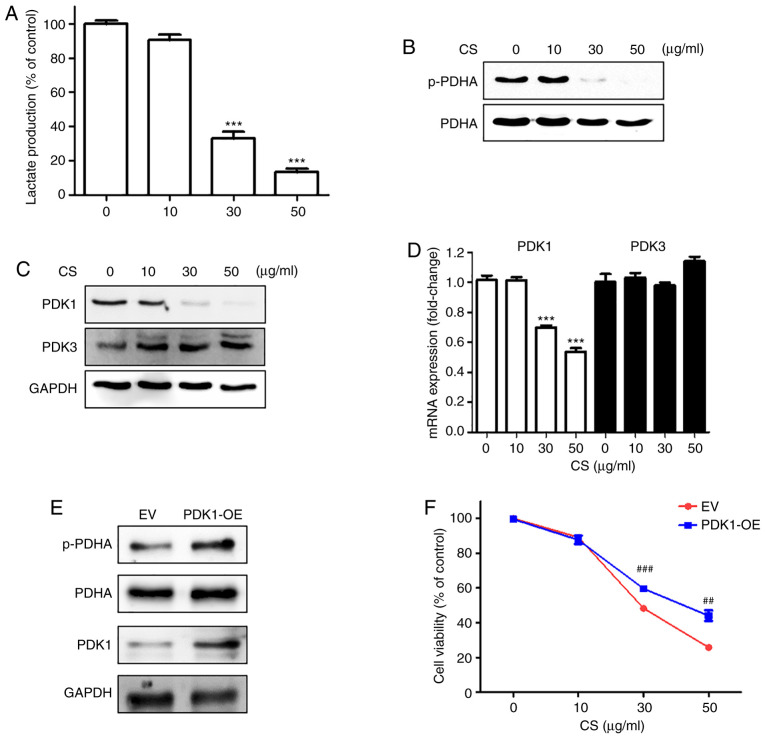
CS reduced lactate production by suppressing PDK expression
To elucidate whether regulation of the Warburg-like phenotype is related to the differential cytotoxicity of CS, we first examined lactate production of CS-treated cells, which was decreased in a dose-dependent manner (Fig. 3A). However, the in vitro enzymatic activity of LDHA, which converts pyruvate to lactate, was not regulated by CS treatment (Fig. S1A). In addition, treatment of 12Z cells with CS did not markedly affect the intracellular activity and expression of LDHA (Fig. S1B and C), which was only slightly reduced by the high concentration. However, the data do not explain the prominent reduction of lactate production by CS at the same dose. The results suggest that the reduction in lactate production by CS might be mediated through another mechanism that does not involve the suppression of LDHA.
Figure 3.
CS inhibited PDK1 expression in endometriotic cells. The 12Z cells were treated with CS at the indicated concentrations for 12 h. (A) Lactate was measured using a commercially available lactate assay kit. (B) Phosphorylation of PDHA was examined using western blot analysis. (C) Expression levels of PDK1 and PDK3 were examined using western blot analysis with GAPDH as the internal control. (D) Expression levels of PDK1 and 3 were examined using qPCR. (E) Overexpression of PDK1 in 12Z cells was confirmed through western blot analysis. (F) 12Z cells were treated with CS for 24 h. Cell death was measured using the MTT assay. Results of three independent experiments are presented as mean ± standard deviation. ***P<0.001 compared to control (first lane); ##P<0.05 and ###P<0.001 compared to EV. CS, Caesalpinia sappan L heartwood extract; PDK, pyruvate dehydrogenase kinase; p-PDHA, phosphorylated-PDHA; PDHA, pyruvate dehydrogenase E1α; OE, overexpression; EV, empty vector.
Lactate production can be diminished by activating PDH activity. The activity of the PDH complex is regulated by phosphorylation of its E1α subunit (PDHA) through the kinase action of PDKs (25). Treatment of 12Z cells with CS reduced the phosphorylation of PDHA in a dose-dependent manner (Fig. 3B), similar to the effects of DCA, a well-known PDK inhibitor (Fig. S2A and B). DCA also suppressed the viability of 12Z cells (Fig. S2C). However, unlike DCA, CS did not directly affect the activity of PDK1 in the in vitro kinase activity assay (Fig. S3). In addition, the investigation of the effect of brazilin, a major component of CS, showed that in contrast to CS, brazilin alone did not reduce the phosphorylation of PDHA (Fig. S4).
The protein and mRNA expression levels of PDK1 were significantly decreased by CS treatment in a concentration-dependent manner (Fig. 3C and D). Although PDK1 and PDK3 were elevated in endometriotic 12Z cells, the expression of PDK3 was not altered in a CS-dependent manner. Thus, we confirmed the cytotoxicity of CS against 12Z cells overexpressing PDK1. The efficiency of PDK1 overexpression using viral infection with its expressing vector (PDK1-OE) was confirmed through the phosphorylation of PDHA and PDK1 expression (Fig. 3E). In addition, CS-induced cytotoxicity was inhibited by approximately two-fold in the 12Z cells infected with the PDK1-expressing vector (Fig. 3F). These results suggested that the differential cytotoxicity of CS on endometriotic 12Z cells might be, at least partially, due to the decreased expression of PDK1.
CS induced apoptosis of endometriotic cells
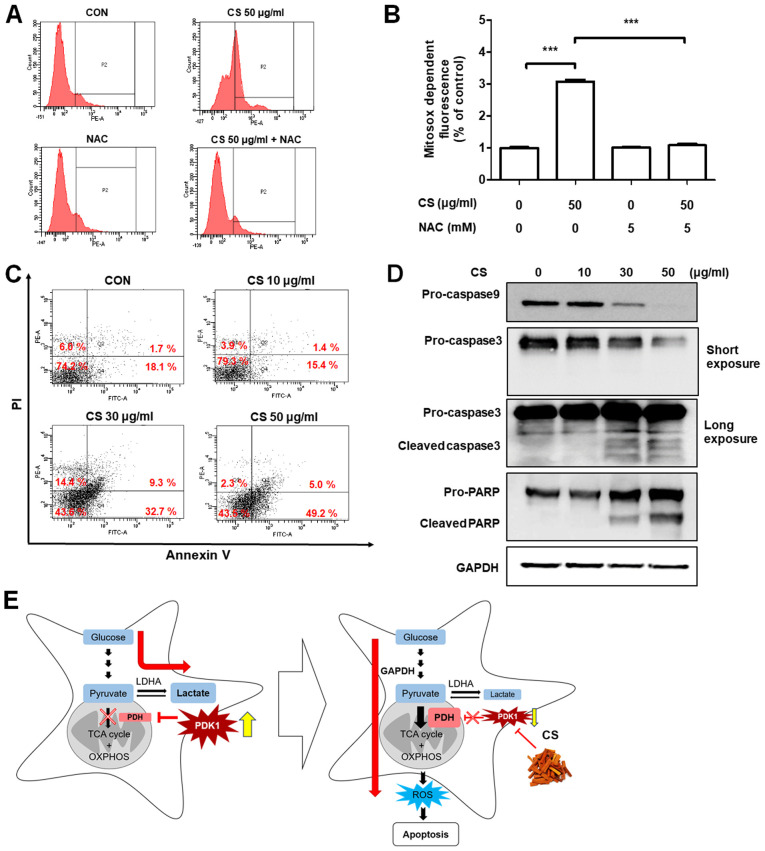
Metabolic reprogramming of cancer cells from aerobic glycolysis to OXPHOS has been shown to increase mitochondrial ROS-dependent apoptosis (26,27). Thus, mitochondrial ROS production and apoptotic cell death were examined in CS-treated endometriotic 12Z cells. The production of mitochondrial ROS, measured using MitoSOX staining, was significantly increased following CS treatment, and this effect was inhibited by n-acetylcysteine (NAC) treatment (Fig. 4A and B). In addition, similar results were obtained with carboxy-H2DCFDA staining, a marker for total ROS (Fig. S5A and B). However, the change in total ROS was not as high as that of mitochondrial ROS. Next, we found that the apoptosis of CS-treated 12Z cells was significantly increased in a dose-dependent fashion (Fig. 4C). The expression levels of proteins related to apoptotic signaling, such as cleaved caspase 3 and PARP, were also increased in CS-treated 12Z cells (Fig. 4D). The pro-form of caspase 9 was also reduced following CS treatment. However, caspase 8, a marker of extrinsic apoptosis signaling, was not activated (data not shown). These results suggest that the induction of a large quantity of mitochondrial ROS by the metabolic shift from glycolysis to OXPHOS, through the suppression of PDK1 expression, plays an important role in CS-induced apoptosis of endometriotic 12Z cells (Fig. 4E).
Figure 4.
CS-induced apoptosis of endometriotic cells. (A) 12Z cells were treated with CS at indicated concentrations for 12 h and mitochondrial reactive oxygen species levels were measured using fluorescence-activated cell sorting analysis using MitoSOX™ red. (B) MitoSOX-dependent fluorescence results are shown as mean ± standard deviation of three independent experiments. ***P<0.001. (C) 12Z cells were treated with CS at indicated concentrations for 24 h and number of apoptotic cells was measured using FACS analysis with PI-Annexin V double staining. (D) Apoptosis signaling activation of caspase 9, caspase 3, and PARP was examined using western blot analysis with GAPDH as the internal control. The 12Z cells were treated with CS at indicated concentrations for 12 h. (E) Schematic diagrams illustrating the action of CS in endometriosis. CS, Caesalpinia sappan L heartwood extract; PI, propidium iodide; PARP, poly ADP-ribose polymerase.
Discussion
Although endometriosis is an important gynecological disorder, current treatments show very limited efficacy. First-line treatment of endometriosis is mainly surgical removal of the lesion (3,28). Medications for endometriosis consist of anti-inflammatory agents and hormonal therapy, such as gonadotropin-releasing hormone agonist, progesterone analogs, and aromatase inhibitors (29). However, recurrence following conservative surgery was 40-75% within 5 to 6 years, and hormonal therapy is difficult to continue for long periods because of the considerable side effects (30). Therefore, novel therapeutic options with targets other than hormones are needed to treat endometriosis. Several non-hormonal strategies, including suppression of angiogenesis, induction of apoptosis, and regulation of non-coding RNAs and immune cells, have been proposed for endometriosis treatment (31-33). In addition, herbal medicines may be a particularly good alternative to hormonal therapy because they have long been used to treat endometriosis-related dysmenorrhea, pelvic inflammation, and cysts (34). Previous clinical and animal studies have shown that several herbal medicinal formulas are effective in suppressing the growth of endometriotic lesions (35-38). However, the mechanism underlying the efficacy of these herbal medicines has not been fully elucidated.
Previous studies have shown that transforming growth factor (TGF)-β and hypoxia induce Warburg-like metabolic reprogramming by activating PDK1 expression in endometriotic cells (9). In this study, we showed that rapidly growing endometriotic 12Z cells expressed high levels of PDK1 and PDK3. The high expression of PDKs is related to resistance to apoptosis and uncontrolled cell survival in cancer and endometriosis (10,39). Thus, metabolic reprogramming from glycolysis to OXPHOS might be an alternative strategy for endometriosis treatment. The results of a previous study (15) and our present findings also showed that DCA, an inhibitor of PDK activity, reduced the viability of endometriotic 12Z cells. CS induced the selective death of a higher number of endometriotic cells than of normal endometrial cells. The lactate production of 12Z cells was reduced following CS treatment by the inhibition of PDK1 expression but not activity. In addition, the exogenous overexpression of PDK1 partially protected against CS-induced cytotoxicity. These results led us to assume that the selective cytotoxicity of CS on glycolytic 12Z cells might be, at least partially, due to the metabolic reprogramming of endometriotic cells from glycolysis to OXPHOS.
In endometriosis, TGF-β and hypoxia were suggested as the primary causes of elevated glycolysis and PDK1 expression (9,10,40). However, TGF-β has been reported to regulate the expression of PDK2 and PDK4, but not PDK1(41). In addition, a previous study demonstrated that CS treatment increased the expression of TGF-β in a murine allograft rejection model. Thus, we hypothesized that TGF-β signaling is not related to suppression of PDK1 expression by CS treatment. In addition to TGF-β, it was reported that PDK1 and PDK3 genes are regulated by hypoxia through stabilization of HIF1α (42,43). However, in this study, CS suppressed the expression of PDK1 in 12Z cells, but not PDK3. Thus, we assume that CS does not affect the hypoxia-HIF1α axis. Several studies have presented differential regulation of PDK isoforms, especially PDK1 and PDK3, through histone acetylation or miRNA (44-46). Therefore, to elucidate the precise mechanism underlying the suppression of PDK1 mRNA expression by CS treatment, further extensive studies should be conducted. The epigenetic regulation of gene transcription, such as histone modification or mRNA stability, might be a good explanation of the differential regulatory mechanism of PDK1 expression by CS treatment.
Differential apoptosis between the endometrium of women with and without endometriosis has been reported (47). In patients with endometriosis, the resistance to apoptosis and expression of anti-apoptotic genes such as B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein is increased (48-50). Resistance to apoptotic cell death in endometriosis causes differences in endometrial structure, proliferation, immune response, adhesion molecules, and cytokines expression compared to the normal endometrium (51). Generally, resistance to physiological apoptosis is a characteristic of malignant tumors (11). Although endometriosis is a benign disease, endometriotic tissue has been reported to show similar characteristics to those of cancerous tissue (52,53). Thus, the acceleration of apoptotic cell death is a recognized strategy for endometriosis treatment (54). Reprogramming cellular metabolism from glycolysis to OXPHOS is well known to lead to the generation of excessive mitochondrial ROS with consequent mitochondria-dependent apoptosis of cancer cells (26,27). Our previous studies also demonstrated that inhibiting aerobic glycolysis by suppressing LDHA or PDK activity using phytochemicals enhances ROS production and mitochondria-mediated apoptosis of cancer cells (55,56). In this study, we confirmed that CS increased mitochondrial ROS and apoptotic death of endometriotic 12Z cells via the intrinsic pathway. Although CS is known to induce cancer cell death through apoptosis (20), we elucidated the precise mechanism underlying this phenomenon, especially in endometriosis.
Considering the importance of the metabolic shift in endometriosis, the suppression of Warburg-like metabolic characteristics might be an alternative therapeutic target for endometriosis. In particular, DCA is now under development as a non-contraceptive treatment for women with endometriosis (15) and 100 mg/kg DCA was found to reduce the size of endometrial lesions in a murine model. However, the concentration of DCA that reduced the growth of endometrial cells was not determined in the previous study. In this study, we showed that 40 mM DCA reduced the growth of 12Z cells by approximately 30% compared to untreated cells, although lactate production and phosphorylation of PDHA were successfully suppressed at that concentration. Furthermore, 40 mM DCA is equivalent to 5.157 mg/ml, which is a considerably higher concentration than that of CS used in this study, which was approximately 30 to 50 µg/ml. These results indicate that CS might be effective for the treatment of endometriosis at a considerably lower dose than that used for DCA.
In this study, CS also exerted a selective cytotoxicity on endometriotic cells with a relatively low cytotoxicity on normal endometrial THES cells. However, these results do not guarantee a low in vivo toxicity with CS. A previous safety study showed that CS did not exhibit severe acute or subacute toxicity in rats (57). Although brazilin, the major component of CS showed reproductive toxicity (58), it is generally accepted as a safe compound for use in food, beverages, and cosmetics (24). To confirm the safety and in vivo efficacy of CS in endometriosis, further extensive animal studies should be performed. In addition, the effect of brazilin on the phosphorylation of PDHA was investigated and in contrast to CS, brazilin alone did not reduce the phosphorylation of PDHA. CS has numerous other components in addition to brazilin and, therefore, the possible effect of other components and their combinations should be examined in further studies.
In conclusion, in this study, we demonstrated that CS possesses a selective cytotoxicity against glycolytic endometriotic cells as opposed to normal THES cells. The underlying mechanism of the CS-induced toxicity on endometriotic cells was probably mediated by the suppression of PDK1 expression and subsequent ROS-mediated apoptosis. Thus, our findings suggest that CS could be a potent candidate for the development of novel anti-endometriosis drugs and its effects are meditated through inhibition of the Warburg-like metabolic phenotype.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was supported by Biomedical Research Institute Grant (grant no. 2018B027), Pusan National University Hospital (to JKJ).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BSK, JKJ and KTH designed the experiment. BSK and SJB performed the main experiment. HRC and SOL conducted the HPLC analysis of the plant materials. TWC and HJC designed and conducted the in vitro PDK assay. JHC and SJB statistically analyzed the data. BSK, SJB, JKJ and KTH interpreted the results. BSK prepared a draft of the manuscript. JHC, JKJ, and KTH revised the manuscript. JKJ acquired funding and contributed to resources. JKJ and KTH supervised the study and confirmed the authenticity of all the raw data. All authors have read and approved the final manuscript. All authors have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ping S, Ma C, Liu P, Yang L, Yang X, Wu Q, Zhao X, Gong B. Molecular mechanisms underlying endometriosis pathogenesis revealed by bioinformatics analysis of microarray data. Arch Gynecol Obstet. 2016;293:797–804. doi: 10.1007/s00404-015-3875-y. [DOI] [PubMed] [Google Scholar]
- 3.Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7:349–357. [PMC free article] [PubMed] [Google Scholar]
- 4.Nothnick W, Alali Z. Recent advances in the understanding of endometriosis: The role of inflammatory mediators in disease pathogenesis and treatment. F1000Res. 2016;5(186) doi: 10.12688/f1000research.7504.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, Yamawaki K, Adachi S, Takahashi T, Kase H, et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 2018;24:1777–1789. doi: 10.1016/j.celrep.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376:1835–1848. doi: 10.1056/NEJMoa1614814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Xin X, Hua T, Shi R, Chi S, Jin Z, Wang H. Efficacy of anti-VEGF/VEGFR agents on animal models of endometriosis: A systematic review and meta-analysis. PLoS One. 2016;11(e0166658) doi: 10.1371/journal.pone.0166658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD. Kinase signalling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Hum Reprod Update. 2016;22:382–403. doi: 10.1093/humupd/dmv060. [DOI] [PubMed] [Google Scholar]
- 9.Young VJ, Brown JK, Maybin J, Saunders PT, Duncan WC, Horne AW. Transforming growth factor-β induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J Clin Endocrinol Metab. 2014;99:3450–3459. doi: 10.1210/jc.2014-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HC, Lin SC, Wu MH, Tsai SJ. Induction of pyruvate dehydrogenase kinase 1 by hypoxia alters cellular metabolism and inhibits apoptosis in endometriotic stromal cells. Reprod Sci. 2019;26:734–744. doi: 10.1177/1933719118789513. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Yang Z, Chen Z, Chen R, Zhao D, Zhou Y, Qiao L. Effects of the suppression of lactate dehydrogenase A on the growth and invasion of human gastric cancer cells. Oncol Rep. 2015;33:157–162. doi: 10.3892/or.2014.3600. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-Garcia FJ. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front Immunol. 2016;7(52) doi: 10.3389/fimmu.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne AW, Ahmad SF, Carter R, Simitsidellis I, Greaves E, Hogg C, Morton NM, Saunders PTK. Repurposing dichloroacetate for the treatment of women with endometriosis. Proc Natl Acad Sci USA. 2019;116:25389–25391. doi: 10.1073/pnas.1916144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heo J. Donguibogam. Namsandang, 1994. [Google Scholar]
- 17.Lee CB. Coloured Flora of Korea. Hangmunsa, 1982. [Google Scholar]
- 18.Kim JG. Illustrated natural drug encyclopedia. Namsandang, 1984. [Google Scholar]
- 19.Cao R, Zhang H, Guo J, Liu XH, Liu C, Zhu CH, Wu XZ. A novel pharmacological method to study the chinese medicinal formula Hua-Zheng-Hui-Sheng-Dan. Evid Based Complement Alternat Med. 2015;2015(436807) doi: 10.1155/2015/436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung TM, Dang NH, Dat NT. Methanol extract from vietnamese caesalpinia sappan induces apoptosis in HeLa cells. Biol Res. 2014;47(20) doi: 10.1186/0717-6287-47-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim EC, Hwang YS, Lee HJ, Lee SK, Park MH, Jeon BH, Jeon CD, Lee SK, Yu HH, You YO. Caesalpinia sappan induces cell death by increasing the expression of p53 and p21WAF1/CIP1 in head and neck cancer cells. Am J Chin Med. 2005;33:405–414. doi: 10.1142/S0192415X05003016. [DOI] [PubMed] [Google Scholar]
- 22.Zhong X, Wu B, Pan YJ, Zheng S. Brazilein inhibits survivin protein and mRNA expression and induces apoptosis in hepatocellular carcinoma HepG2 cells. Neoplasma. 2009;56:387–392. doi: 10.4149/neo_2009_05_387. [DOI] [PubMed] [Google Scholar]
- 23.Handayani S, Susidarti RA, Jenie RI, Meiyanto E. Two active compounds from Caesalpinia sappan L. in combination with cisplatin synergistically induce apoptosis and cell cycle arrest on WiDr cells. Adv Pharm Bull. 2017;7:375–380. doi: 10.15171/apb.2017.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nirmal NP, Rajput MS, Prasad RG, Ahmad M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: A review. Asian Pac J Trop Med. 2015;8:421–430. doi: 10.1016/j.apjtm.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav N, Kumar S, Marlowe T, Chaudhary AK, Kumar R, Wang J, O'Malley J, Boland PM, Jayanthi S, Kumar TK, et al. Oxidative phosphorylation-dependent regulation of cancer cell apoptosis in response to anticancer agents. Cell Death Dis. 2015;6(e1969) doi: 10.1038/cddis.2015.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–1299. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 29.Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother. 2018;19:1109–1125. doi: 10.1080/14656566.2018.1494154. [DOI] [PubMed] [Google Scholar]
- 30.Kuohung W, Jones GL, Vitonis AF, Cramer DW, Kennedy SH, Thomas D, Hornstein MD. Characteristics of patients with endometriosis in the United States and the United Kingdom. Fertil Steril. 2002;78:767–772. doi: 10.1016/s0015-0282(02)03342-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang T, De Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun Rev. 2018;17:945–955. doi: 10.1016/j.autrev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Egorova A, Petrosyan M, Maretina M, Balashova N, Polyanskih L, Baranov V, Kiselev A. Anti-angiogenic treatment of endometriosis via anti-VEGFA siRNA delivery by means of peptide-based carrier in a rat subcutaneous model. Gene Ther. 2018;25:548–555. doi: 10.1038/s41434-018-0042-7. [DOI] [PubMed] [Google Scholar]
- 33.Sahin C, Mamillapalli R, Yi KW, Taylor HS. microRNA Let-7b: A novel treatment for endometriosis. J Cell Mol Med. 2018;22:5346–5353. doi: 10.1111/jcmm.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flower A, Liu JP, Lewith G, Little P, Li Q. doi: 10.1002/14651858.CD006568.pub3. Chinese herbal medicine for endometriosis. Cochrane Database Syst Rev: CD006568, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Su SY, Muo CH, Sung FC, Morisky DE. Reduction of surgery rate in endometriosis patients who take Chinese medicine: A population-based retrospective cohort study. Complement Ther Med. 2014;22:632–639. doi: 10.1016/j.ctim.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Flower A, Lewith GT, Little P. A feasibility study exploring the role of Chinese herbal medicine in the treatment of endometriosis. J Altern Complement Med. 2011;17:691–699. doi: 10.1089/acm.2010.0073. [DOI] [PubMed] [Google Scholar]
- 37.Hu C, Wang Z, Pang Z, Lu W, Cai X, Yang J, Wang D, Cao P. Guizhi fuling capsule, an ancient Chinese formula, attenuates endometriosis in rats via induction of apoptosis. Climacteric. 2014;17:410–416. doi: 10.3109/13697137.2013.876618. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Hu C, Tang W, Gui T, Qian R, Xing Y, Cao P, Wan G. Therapeutic potential of Wenshen Xiaozheng tang, a traditional Chinese medicine prescription, for treating endometriosis. Reprod Sci. 2013;20:1215–1223. doi: 10.1177/1933719113483008. [DOI] [PubMed] [Google Scholar]
- 39.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin J, Choi H, Hsieh MH, Neugent ML, Ahn JM, Hayenga HN, Singh PK, Shackelford DB, Lee IK, Shulaev V, et al. Targeting hypoxia-inducible factor-1α/pyruvate dehydrogenase kinase 1 axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018;58:216–231. doi: 10.1165/rcmb.2016-0186OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soukupova J, Malfettone A, Hyroššová P, Hernández-Alvarez MI, Peñuelas-Haro I, Bertran E, Junza A, Capellades J, Giannelli G, Yanes O, et al. Role of the transforming growth factor-β in regulating hepatocellular carcinoma oxidative metabolism. Sci Rep. 2017;7(12486) doi: 10.1038/s41598-017-12837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, Giaccia AJ. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- 44.Naia L, Cunha-Oliveira T, Rodrigues J, Rosenstock TR, Oliveira A, Ribeiro M, Carmo C, Oliveira-Sousa SI, Duarte AI, Hayden MR, Rego AC. Histone deacetylase inhibitors protect against pyruvate dehydrogenase dysfunction in Huntington's disease. J Neurosci. 2017;37:2776–2794. doi: 10.1523/JNEUROSCI.2006-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu C, Yan C, Cao W, Li F, Qu Y, Guan K, Si C, Yu Z, Qu Z. miR-128-3p contributes to mitochondrial dysfunction and induces apoptosis in glioma cells via targeting pyruvate dehydrogenase kinase 1. IUBMB Life. 2020;72:465–475. doi: 10.1002/iub.2212. [DOI] [PubMed] [Google Scholar]
- 46.Feng L, Cheng K, Zang R, Wang Q, Wang J. miR-497-5p inhibits gastric cancer cell proliferation and growth through targeting PDK3. Biosci Rep. 2019;39(BSR20190654) doi: 10.1042/BSR20190654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun DP, Ding J, Shaheen F, Willey JC, Rana N, Dmowski WP. Quantitative expression of apoptosis-regulating genes in endometrium from women with and without endometriosis. Fertil Steril. 2007;87:263–268. doi: 10.1016/j.fertnstert.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 48.Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69:1042–1047. doi: 10.1016/s0015-0282(98)00073-9. [DOI] [PubMed] [Google Scholar]
- 49.Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000;74:760–766. doi: 10.1016/s0015-0282(00)01522-3. [DOI] [PubMed] [Google Scholar]
- 50.Salmassi A, Acar-Perk B, Schmutzler AG, Koch K, Püngel F, Jonat W, Mettler L. Apoptosis resistance in endometriosis. Bioimpacts. 2011;1:129–134. doi: 10.5681/bi.2011.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–147. doi: 10.1111/j.1749-6632.2001.tb03797.x. [DOI] [PubMed] [Google Scholar]
- 52.Lu Y, Cuellar-Partida G, Painter JN, Nyholt DR. Morris AP, Fasching PA, Hein A, Burghaus S, et al. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum Mol Genet. 2015;24:5955–5964. doi: 10.1093/hmg/ddv306. Australian Ovarian Cancer Study; International Endogene Consortium (IEC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machado DE, Berardo PT, Palmero CY, Nasciutti LE. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J Exp Clin Cancer Res. 2010;29(4) doi: 10.1186/1756-9966-29-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vetvicka V, Laganà AS, Salmeri FM, Triolo O, Palmara VI, Vitale SG, Sofo V, Králíčková M. Regulation of apoptotic pathways during endometriosis: From the molecular basis to the future perspectives. Arch Gynecol Obstet. 2016;294:897–904. doi: 10.1007/s00404-016-4195-6. [DOI] [PubMed] [Google Scholar]
- 55.Chung TW, Kim EY, Han CW, Park SY, Jeong MS, Yoon D, Choi HJ, Jin L, Park MJ, Kwon YJ, et al. Machilin a inhibits tumor growth and macrophage M2 polarization through the reduction of lactic acid. Cancers (Basel) 2019;11(963) doi: 10.3390/cancers11070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwak CH, Lee JH, Kim EY, Han CW, Kim KJ, Lee H, Cho M, Jang SB, Kim CH, Chung TW, Ha KT. Huzhangoside a suppresses tumor growth through inhibition of pyruvate dehydrogenase kinase activity. Cancers (Basel) 2019;11(712) doi: 10.3390/cancers11050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sireeratawong S, Piyabhan P, Singhalak T, Wongkrajang Y, Temsiririrkkul R, Punsrirat J, Ruangwises N, Saraya S, Lerdvuthisopon N, Jaijoy K. Toxicity evaluation of sappan wood extract in rats. J Med Assoc Thai. 2010;93 (Suppl 7):S50–S57. [PubMed] [Google Scholar]
- 58.Yuan ZY, Lei F, Chai YS, Wu H, Zhao S, Wang YG, Feng TS, Li HY, Li HY, Zhan HL, et al. Reproductive toxicity of brazilein in ICR mice. Chin J Nat Med. 2016;14:441–448. doi: 10.1016/S1875-5364(16)30041-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.