Abstract
Canadian expert guidelines recommend low-risk women to consume a daily multivitamin supplement containing 400 µg of folic acid (FA) to prevent neural tube defects. Mandatory food fortification coupled with intake of prenatal vitamin/mineral supplements (PVS), most of which contain ≥ 1000 µg-FA, has resulted in an unprecedented shift in Canadian pregnant women folate status. This study assessed the knowledge, attitude and practice (KAP) of physicians regarding periconceptional FA recommendations, intake and health related outcomes, since they play an essential role in promoting appropriate FA intake. Seventy-seven physicians answered the self-administered KAP survey. Only half of physicians knew the correct dose and duration of FA for low-risk women. Approximately 70% were unsure of, or unfamiliar with the most recent guidelines and 60% of physicians most often recommend a ≥ 1000 µg-FA supplement. Knowledge score 1 (KS1), which related to low-risk women, was associated with physicians’ attitude toward believing that most PVS contain the recommended amount of FA (p = 0.004). Significant correlations were also found between KS1 and the total practice score (TPS) (r = 0.45, p < 0.0001) as well as between the total knowledge score and TPS (r = 0.38, p = 0.0007). Our findings show that physicians lacking knowledge regarding periconceptional FA is associated with their attitude and practice. Despite a vast majority of physicians being unsure or uncomfortable recommending PVS that are not in line with recommendations, a lack of knowledge and a widely accessible 400 µg-FA PVS, enables a contradictory practice in reality.
Keywords: Attitude, Folic Acid, Knowledge, Physicians, Practice, Pregnancy, Periconception, Guidelines
1. Introduction
Neural tube defects (NTDs), including spina bifida and anencephaly, result from failure of neural tube closure during the third and fourth weeks of gestation (Zaganjor et al., 2016). They are among the most common, yet preventable, birth defects and an important health issue because of their detrimental physical, psychological and social impact on Canadian children and their families (Ami et al., 2016, Yi et al., 2011). Worldwide, it is estimated that approximately 260,100 neonates are affected with NTDs each year, and 1 out of 2,500 births in Canada; thus, becoming a major cause of morbidity and mortality among fetuses and babies (Blencowe et al., 2018, Irvine et al., 2015). Strong evidence from observational and randomized controlled trials has convincingly shown that folic acid (FA) can prevent the primary and secondary occurrence of NTDs (Czeizel and Dudas, 1992, Berry et al., 1999, Smithells et al., 1983, Mulinare et al., 1988, Medical Research Council Vitamin Study Research Group, 1991). FA provides the universal methyl-group required for one-carbon transfer pathways, which are fundamental in amino acid metabolism, as well as DNA synthesis, repair and methylation, all of which are imperative to fetal development during the periconception window (Bailey, 2010, Fox and Stover, 2008, Lucock, 2000, Shah and Al-Wassia, 2010). This critical time preceding, including and immediately following human conception constitutes periods of fast cellular division and replication (Steegers-Theunissen et al., 2013). NTD-prevention with optimal FA intake is thus a major public health concern.
Internationally, periconceptional supplementation with 400-μg-FA is recommended for the prevention of NTDs (WHO, 2019, Institute of Medicine (US), 1998, Canada, 2009). Today, at least 82 countries, including Canada, have legislation to mandate nationwide mandatory fortification programs in which at least one industrially milled cereal grain product (e.g., wheat flour, maize, or rice) is fortified with FA (Garrett and Chapter, 2018, Global Progress, 2019). This initiative has significantly decreased the prevalence of NTDs in those countries (Zaganjor et al., 2016, Blencowe et al., 2018, De Wals et al., 2007). The Government of Canada and the Society of Obstetricians and Gynaecologists of Canada (SOGC) both recommend a daily multivitamin supplement containing 400-μg of FA for the primary prevention of NTDs among low-risk women from before conception and throughout lactation (Wilson et al., 2015). Despite these recommendations, a majority of prenatal vitamin supplements (PVS) on the Canadian market contain an amount of FA that is equivalent to or exceeds the tolerable upper intake level (UL) of 1000-µg-FA (Canada, 2007). However, there is no strong evidence to support the UL threshold for pregnancy, since it was primarily based on case reports of neurological damage of high intakes of folate in vitamin B12-deficient non-pregnant individuals (Institute of Medicine (US), 1998). Mandatory food fortification, coupled with high supplemental-FA intake, has resulted in intake levels that exceed the UL and consequently high red blood cell folate concentrations among pregnant women and women of childbearing age (WCBA) across the country (Colapinto et al., 2011 Feb, Colapinto et al., 2012, Fayyaz et al., 2014, Plumptre et al., 2015, Dubois et al., 2017, Savard et al., 2018).
More recently, there is growing concern about this upward shift in FA intake and status in the pregnant population, due to it’s role in epigenetic programming (Bailey, 2010, Lucock, 2000). Evidence from animal studies and some human epidemiologic data suggest that alterations in methylation patterns due to suboptimal FA intake can affect epigenetic regulation of gene expression with the capacity to adversely influence pregnancy outcomes (Yajnik and Deshmukh, 2008, Huot et al., 2016, Kiefte-de Jong et al., 2012, McStay et al., 2017). Though inconclusive, the association between high FA-intake and increased risk of disease susceptibility in offspring has been raised (Lillycrop and Burdge, 2012, Lamers et al., 2018). The long-term consequences of high blood folate concentrations on fetal development due to epigenetic programming or other mechanisms are not yet clear.
Given that there is no documented additional benefit of higher than recommended doses of FA, the precautionary principle can be applied in clinical practice to anticipate unintended adverse consequences that may arise (Field and Stover, 2018, Canada, 2000). Physicians play an important role in promoting FA intake; however, as women’s preferred source for preconception information (Frey and Files, 2006), it is imperative that physicians are kept up to date to best inform their patients. The misalignment between current Canadian expert guidelines and the FA-content in prenatal supplements prevents both physicians and women from adhering to clinical practice guidelines. Currently, very little is known about physicians’ knowledge regarding periconceptional and perinatal FA-related issues and their stance on the misalignment between FA intake and SOGC recommendations. Although some studies have addressed physicians’ awareness and practicing behaviours regarding periconceptional FA, we are the first to do so in a Canadian context. In addition, this is the first study to have analyzed the link between physicians’ knowledge, attitude and practice (KAP) while addressing the currently existing misalignment between FA-content in PVS and expert guidelines. The goal of the present study was to assess the knowledge, attitude and practice of physicians regarding periconceptional FA recommendations, intakes, and health-related outcomes for women at low risk of an NTD-affected pregnancy.
2. Methods
2.1. Study design
A cross-sectional survey was administered to physicians from August 2018 to May 2019, in Canada’s National Capital Region, i.e. the cities of Ottawa (Ontario) and Gatineau (Quebec).
2.2. Participants
A list of family physicians practicing in the city of Ottawa (Ontario) was first obtained from the online College of Family Physicians of Canada directory. Physicians who were less likely to see WCBA patients and pregnant women in their practice were excluded (n = 150; e.g., emergency or sports medicine, palliative care), leaving a sample of 1050 physicians. A total of 112 institutions (family health teams (FHTs), family health organizations (FHO), clinics and hospitals) were contacted by telephone, 99 answered and 17 agreed to schedule in-person visits to distribute the self-administered anonymous questionnaires. Initially, random sampling targeted 66 institutions; however, due to recruitment challenges, an additional 46 institutions were identified and contacted through convenience and snowball sampling. To be included, physicians had to be practising at one of the aforementioned medical institutions and be fluent in English or French. Ethics approval was obtained from the University of Ottawa and participating hospitals visited during Grand Rounds. In total, A total of 17 institutions agreed to participate and 77 physicians completed the survey.
3. Data collection
The questionnaire was available in English and French, consisting of both open and closed questions, including 20 multiple-choice questions, eight Likert-scale or yes/no answers, and four open-ended questions. A portion of the questionnaire’s knowledge section, pertaining to FA-recommendations and NTD-risk factors, was developed directly from the 2015 SOGC guideline (Wilson et al., 2015). Sociodemographic and professional characteristics of the respondents such as sex, age, and number of years of practice were also collected. The knowledge, attitude and practice sections of the questionnaire were developed proceeding a 2017 workshop held in Ottawa that convened key stakeholders from academia, industry, government and health professional groups with the overall goal to identify challenges and solutions to aligning supplemental FA intakes with current evidence-based recommendations (Lamers et al., 2018). The attitude and practice sections were designed to capture physicians’ familiarity, and attitude toward the most recent SOGC guidelines and to understand both their current and future practice in relation to the guideline recommendations. After being presented with the current and correct recommendation statement, three open-ended questions (qualitative component) enabled physicians to express how they perceive the misalignment and guidelines. The questionnaire was reviewed through consultations with a committee of non-participating physicians and experts for content validation and to eliminate ambiguity. The questionnaire was translated to French using the linguistic validation methodology described by Vallerand (Vallerand, 1989).
The survey’s questions were categorized as knowledge, attitude or practice items (correct/incorrect answers) and summed up to obtain knowledge, attitude and practice scores. Eight questions worth one point each related to general knowledge of FA and low-risk recommendations, composing the first sub-knowledge score (KS1) and those relating to knowledge of moderate and high-risk factors for NTDs made up the second sub-knowledge score (KS2), where each risk factor was worth one point. The total knowledge score (TKS) encompasses the sum of the two sub-scores while items pertaining to physicians’ attitude and practice accounted for the total attitude and practice scores, TAS and TPS, respectively. Six 5-point Likert scale questions captured physicians’ attitudes (strongly agree = 5, agree = 4, unsure = 3, disagree = 2 and strongly disagree = 1); however, then merged and regrouped into agree, unsure or disagree attitude subgroups for comparison analyses. A total of four questions assessed practice, where two were multiple choice and two were yes/no, carrying a potential weight of one point each, alike.
4. Statistical analyses
All data was analyzed using Statistical Analysis Software (SAS version 9.4). Descriptive statistics (i.e. means ± standard deviation (SD) or frequencies – n (%)) were used to describe the study’s sample characteristics and to summarize the total knowledge, attitude and practice scores (TKS, TAS and TPS, respectively) as well as the two sub-knowledge scores (KS1 and KS2). General linear models were used to assess the associations between the attitude statements and sub and total knowledge scores. Subsequently, Tukey’s post hoc tests were conducted to compare differences among attitude subgroups, categorized as those who responded agree, unsure or disagree. Similarly, general linear models were used to assess differences in sociodemographic or professional practice characteristics and KAP scores, with Tukey’s post hoc tests to explore differences between subgroups. Bonferroni correction for multiple hypothesis testing was also performed as appropriate to correct for multiple hypotheses testing issues. Since all scores were continuous and normally distributed, Pearson correlation tests were performed to examine the association between all sub and total KAP scores. Results were considered as significant when p-value < 0.05.
5. Results
5.1. Study population
Of the study sample, 81.8% were female family physicians practicing in a hospital or FHT/FHO setting as their primary place of employment (Table 1). More than half of respondents were still in various stages of their professional training (e.g., medical students or residents). Approximately one-fifth of physicians were distributed in each category of years of practice, while one-tenth of the sample had 16–25 years of experience. A majority of the surveyed physicians saw ≤ 30 of both non-pregnant WCBA and pregnant women per week.
Table 1.
Sociodemographic and professional practice characteristics of study participants (n = 77).
| Variables | |
|---|---|
| Age, Mean ± SD | 41.3 ± 13.3 |
| Gender n (%) | 63 (81.8) 14 (18.2) |
| Females | |
| Males | |
| Type of practice/ specialization, n (%) | |
| Family physician | 48 (62.3) 16 (20.8) 7 (9.1) 6 (7.8) |
| Obstetrician-Gynecologist (OB-GYN) | |
| Medical Resident | |
| Medical Student | |
| Place of employment, n (%) | |
| Hospital | 25 (32.5) 13 (16.9) 21 (27.3) |
| Private practice (PP) | |
| Family Health Team (FHT) or Family Health Organization (FHO) | |
| Mixed Hospital & PP Hospital & FHT/FHO PP & FHT/FHO |
18 (23.4) 11 (14.3) 4 (5.2)4 (5.2) |
| Number of years in professional practice, n (%) | |
| Still in training | 18 (23.4) 17 (22.1) 16 (20.8) 8 (10.4) 18 (23.4) |
| <5 years | |
| 5–15 years | |
| 16–25 years | |
| >25 years | |
| Average number of nonpregnant, childbearing-aged women seen per week, n (%) | |
| 0–15 | 35 (45.5) 28 (36.4) 9 (11.7) 5 (6.5) |
| 16–30 | |
| 31–50 | |
| > 50 | |
| Average number of pregnant women seen per week, n (%) | |
| 0–15 | 51 (66.2) 14 (18.2) 9 (11.7) 3 (3.9) |
| 16–30 | |
| 31–50 | |
| > 50 |
Data presented are means ± SD or frequencies as appropriate. *2 missing age values
5.2. Knowledge, attitude and practice scores
The total and sub KAP scores are presented in Table 2. With a possible maximum TKS score of 17, a majority of physicians answered more than half of the knowledge items incorrectly and no physician achieved a perfect score. With a potential total attitude score of 30, results show that most physicians were unsure or had positive attitudes in regard to FA recommendations and health-related outcomes. In relation to the guidelines and physicians’ practice, approximately half of physicians had good practice behaviours or answered the practice questions correctly.
Table 2.
Knowledge, attitude and practice (KAP) scores of physicians regarding periconceptional folic acid recommendations, intake and health related outcomes.
| Knowledge, Attitude and Practice (KAP) Scores | Mean ± SD | Maximum Potential Score |
|---|---|---|
| Total knowledge score (TKS)a | 6.4 ± 1.7 | 17 |
| Knowledge score of general and low risk factors for NTDs (KS1) | 1.8 ± 1.2 | 8 |
| Knowledge score of moderate and high risk factors for NTDs (KS2) | 4.6 ± 1.2 | 9 |
| Total attitude score (TAS) | 18.0 ± 2.5 | 30 |
| Total practice score (TPS) | 2.04 ± 1.2 | 4 |
TKS is the sum of KS1 and KS2. NTD = neural tube defects.
5.3. Knowledge statements
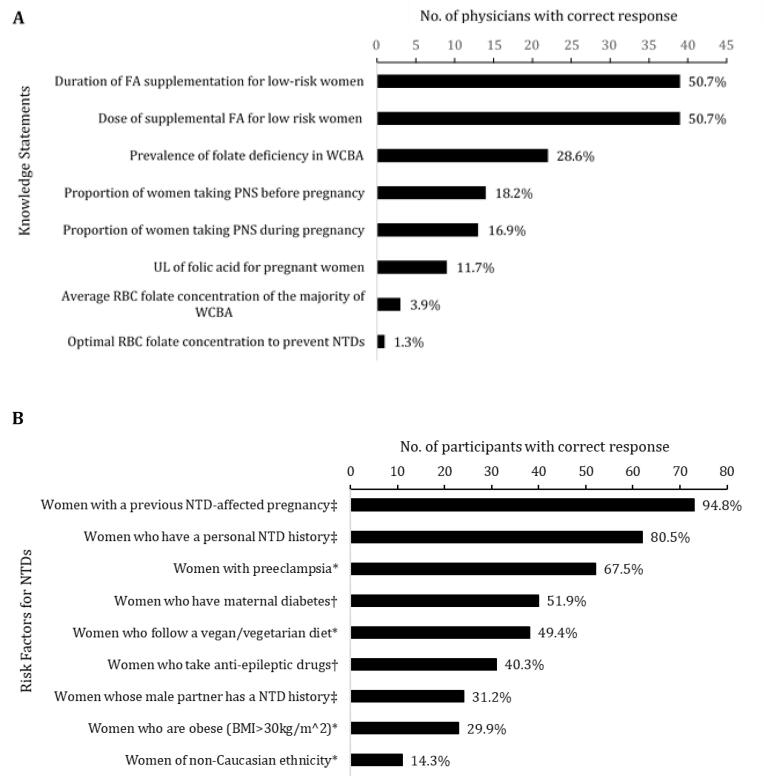
Approximately half of physicians knew the correct dose and duration of supplemental FA recommended by the SOGC (Fig. 1 – panel A). Half of physicians could identify the recommended 400-µg dose of FA for low-risk women from a multiple-choice list. However, when physicians were asked to fill in the blank and provide the recommended dose for moderate-risk women, only one respondent had the correct answer and no physician knew the recommendation for high-risk women. Only one physician knew the correct optimal blood folate concentration for NTD prevention while a few had knowledge on the current folate status of Canadian WCBA. Among a set of potential personal or comorbid conditions, physicians selected which ones were not a risk factor, a moderate-risk factor or a high-risk factor for having a pregnancy affected by a NTD. Almost all physicians were aware that women with a previous NTD-affected pregnancy and most knew that women who have a personal NTD history, constitute high-risk factors (Fig. 1 – panel B). Knowledge of other risk factors from the SOGC guideline, and distractor options, was much lower. Further, only one-third of physicians knew that women whose male partner with a NTD history is also a high-risk factor.
Fig. 1.
Physicians’ knowledge regarding (A) periconceptional and perinatal FA recommendations, pregnant Canadian women’s status, and health-related outcomes; and (B) moderate and high risk factors for neural tube defects (NTDs). Percentages indicate the correct response rate. WCBA = women of childbearing age; PVS = prenatal vitamin/mineral supplements; UL = tolerable upper intake level; RBC = red blood cell; BMI = body mass index expressed in units of kg/m2, resulting from mass in kilograms and height in meters.* Not a risk factor. † Moderate risk factor. ‡ High risk factor.
5.4. Attitude statements
Attitudes toward current prenatal vitamin/mineral supplement recommendations were quite heterogeneous regarding the SOGC guidelines (Table 3). More than two-thirds of physicians were unsure or not familiar with the most recent guidelines. Although close to 90% of physicians agreed or were unsure whether most PVS contained the recommended dose of FA, almost all of them thought that their recommendations were in line with the most recent guidelines. Despite this misalignment, close to 85% were unsure or not comfortable recommending a PVS that was not in line with the guidelines. Overall, these finding emphasize the misalignment and contradiction between physicians’ attitude toward the recommendations and the reality of their professional practice.
Table 3.
Relationship between knowledge and attitude scores regarding folic acid supplement recommendations during pregnancy.
| Statement | Attitude | n (%) | KS1 | p-value | KS2 | p-value | TKS | p-value |
|---|---|---|---|---|---|---|---|---|
| I am familiar with the most recent | Agree | 24 (31.2) | 2.0 ± 0.24 | 0.42 | 4.8 ± 0.25 | 0.58 | 6.8 ± 0.34 | 0.35 |
| guideline | Unsure | 25 (32.5) | 1.6 ± 0.23 | 4.6 ± 0.25 | 6.2 ± 0.34 | |||
| Disagree | 28 (36.4) | 1.8 ± 0.22 | 4.4 ± 0.24 | 6.3 ± 0.32 | ||||
| There could be potential adverse effects | Agree | 21 (27.3) | 2.0 ± 0.26 | 0.45 | 4.7 ± 0.27 | 0.57 | 6.6 ± 0.37 | 0.26 |
| due to high FA intake during pregnancy | Unsure | 31 (40.3) | 1.6 ± 0.21 | 4.4 ± 0.22 | 6.0 ± 0.30 | |||
| Disagree | 25 (32.5) | 2.0 ± 0.23 | 4.8 ± 0.25 | 6.7 ± 0.34 | ||||
| High FA intake may negatively modify fetal | Agree | 15 (19.5) | 1.9 ± 0.30 | 0.87 | 4.6 ± 0.32 | 0.21 | 6.5 ± 0.44 | 0.54 |
| development | Unsure | 36 (46.8) | 1.75 ± 0.2 | 4.8 ± 0.21 | 6.6 ± 0.28 | |||
| Disagree | 26 (33.8) | 1.8 ± 0.23 | 4.3 ± 0.24 | 6.2 ± 0.33 | ||||
| Most PVS contain the recommended | Agree | 58 (75.3) | 2.0 ± 0.14a,c | 0.004 | 4.5 ± 0.16 | 0.42 | 6.6 ± 0.22 | 0.40 |
| amount of FA | Unsure | 9 (11.7) | 0.7 ± 0.37a | 5.1 ± 0.41 | 5.8 ± 0.56 | |||
| Disagree | 10 (13.0) | 1.7 ± 0.35b,c | 4.5 ± 0.4 | 6.2 ± 0 0.53 | ||||
| My recommendations are in line with the | Agree | 47 (61.0) | 2.0 ± 0.17 | 0.32 | 4.6 ± 0.18 | 0.57 | 6.6 ± 0.25 | 0.65 |
| most recent guideline | Unsure | 28 (36.4) | 1.6 ± 0.22 | 4.5 ± 0.24 | 6.2 ± 0.32 | |||
| Disagree | 2 (2.6) | 1.0 ± 0.82 | 5.5 ± 0.88 | 6.5 ± 1.20 | ||||
| I’m comfortable recommending PVS that | Agree | 12 (15.6) | 1.9 ± 0.34 | 0.82 | 5.2 ± 0.35 | 0.19 | 7.1 ± 0.48 | 0.26 |
| are not in line with the guideline | Unsure | 23 (29.9) | 1.7 ± 0.25 | 4.4 ± 0.26 | 6.1 ± 0.35 | |||
| Disagree | 42 (54.6) | 1.9 ± 0.18 | 4.5 ± 0.19 | 6.4 ± 0.26 |
P values were estimated using general linear models with Tukey’s post hoc test for subgroup comparisons. The letters indicate which attitude subgroups in response to statements were found to differ significantly. Subgroups with the same letters do not differ significantly while those with different letters do. FA = folic acid; PVS = prenatal vitamin/mineral supplement.
*The total knowledge score (TKS) is the sum of knowledge sub-scores one and two (KS1 and KS2, respectively) and they are presented as means ± SE.
5.5. Practice statements
In terms of physicians’ clinical practice, more than half believed that they followed the guidelines, which were aligned with most available PVS FA content (Table 4). More than one-third simply did not know whether their practice was in line with recommendations and available PVS on the market, confirming the attitude findings. However, only one-third in fact recommend a supplement in line with the recommendations of 400 μg-FA, and more than half recommend a PVS containing ≥ 1 000 μg-FA, again reflecting a lack of knowledge and awareness of the guidelines. When asked about the reasoning behind the supplement they most often recommend, almost half of physicians responded that it was “for no particular reason” while a quarter of them based their decision on women’s preference.
Table 4.
Physicians’ practice regarding folic acid supplement recommendations for low-risk women.
| Practice Statement | n (%) |
|---|---|
| Which option best describes your clinical practice? | |
| I follow the guidelines, which are in line with most available PVS | 41 (53.3) |
| I do not follow the guidelines because most PVS are not in line | 2 (2.6) |
| I follow the guidelines and recommend non-PVS with correct FA-content | 4 (5.2) |
| I do not follow the guidelines and recommend non-PVS regular supplements | 1 (1.3) |
| I don’t know | 29 (37.7) |
| Supplement I most often recommend | |
| 400 µg | 23 (29.9) |
| 1,000 µg | 42 (54.6) |
| >1,000 µg | 4 (5.2) |
| Any prenatal multivitamin supplement | 8 (10.4) |
| Reason for recommendation | |
| I recommend a well-known brand name | 2 (2.6) |
| Because of supplement composition/content | 5 (6.5) |
| Because it is the women’s preference | 19 (24.7) |
| Because it is covered by many insurance companies | 4 (5.2) |
| Because of lower cost | 12 (15.6) |
| For no particular reason | 35 (45.5) |
*PVS = prenatal vitamin/mineral supplements; FA = folic acid.
5.6. Associations between knowledge, attitude and practice
None of the attitude statements were significantly associated with any of the knowledge scores except for one (Table 3). A significant relationship was found between the following attitude statement “most PNS contain the recommended amount of FA” and KS1 (p = 0.004). Tukey’s post hoc test revealed that the KS1 score of physicians who were unsure about the statement was significantly lower than that of those who disagreed. This result remained significant after Bonferroni correction for multiple comparisons (p = 0.05/6 statements = 0.008). Pearson correlations were also performed to assess the relationships between all sub and total KAP scores. Both sub-knowledge scores were significantly associated with the TKS (r = 0.68, p < 0.0001 for KS1 and TKS; and r = 0.72, p < 0.0001 for KS2 and TKS). Pearson’s correlation test also confirmed that KS1 and KS2 are not interrelated (r = −0.015, p = 0.89) and thus, physicians’ knowledge of low-risk recommendations does not seem to be associated with their knowledge of moderate and high risk recommendations. Significant associations were also found between KS1 and TPS; r = 0.45, p < 0.0001, as well as between TKS and the TPS; r = 0.38, p = 0.0007. However, no significant correlations were observed between the other scores (r = -0.09, p = 0.39 for TKS and TAS and r = -0.13, p = 0.26 for TAS and TPS). In summary, possessing more knowledge of FA-related topics and guideline recommendations appears to be associated with an improved practice score.
5.7. Qualitative assessment of physicians’ perceptions and barriers
The questionnaire’s open-ended questions were an opportunity to capture physicians’ perceptions and barriers regarding the misalignment between FA supplement content and recommendations. Physicians expressed that the guidelines were lengthy and confusing and approximately a quarter of respondents mentioned that they need to update themselves on the latest guidelines and intend to do so as a follow up to the survey. Furthermore, multiple physicians noted that indeed a majority of available PVS in the marketplace contain 1 mg of FA, limiting their recommendations which may be a deterrence to staying up to date with them.
5.8. Associations between KAP scores and participant characteristics
Differences in total (and sub) scores of knowledge, attitude and practice and socio-demographic characteristics of participants are shown in Table 5. We found that older physicians had a significantly higher knowledge score regarding moderate and high-risk factors for NTDs (KS2). Physicians with 16–25 years of practice had a significantly higher KS2 (p = 0.004) as well as TKS (p = 0.002) compared with those with less or more years of practice. These two results remained significant after Bonferroni correction for multiple comparisons (threshold p = 0.008). No other sociodemographic or professional practice-related characteristics were associated with any of the KAP scores.
Table 5.
Comparison between mean total scores of knowledge, attitude and practice (KAP) by socio-demographic and professional practice characteristics of participants.
| Variable | Groups of responses | Sub-knowledge score 1(KS1) | P | Sub-knowledge score 2(KS2) | P | Total knowledge score(TKS) | P | Attitude score(TAS) | P | Practice score(TPS) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | ≤35 years | 1.9 ± 0.2 | 0.51 | 4.3 ± 0.2 | 0.04 | 6.2 ± 0.3 | 0.31 | 18.3 ± 0.4 | 0.49 | 1.9 ± 0.2 | 0.66 |
| >35 years | 1.7 ± 0.2 | 4.9 ± 0.2 | 6.6 ± 0.3 | 17.9 ± 0.4 | 2.1 ± 0.2 | ||||||
| Type of | Family physician | 1.8 ± 0.2 | 0.18 | 4.8 ± 0.2 | 0.08 | 6.7 ± 0.2 | 0.21 | 17.9 ± 0.4 | 0.42 | 1.9 ± 0.2 | 0.52 |
| practice | OB-GYN | 1.3 ± 0.3 | 4.4 ± 0.3 | 5.8 ± 0.4 | 18.1 ± 0.6 | 2.2 ± 0.3 | |||||
| Medical resident | 2.1 ± 0.4 | 4.4 ± 0.5 | 6.6 ± 0.6 | 17.7 ± 1.0 | 1.9 ± 0.5 | ||||||
| Medical student | 2.3 ± 0.5 | 3.5 ± 0.5 | 5.8 ± 0.7 | 19.7 ± 1.0 | 2.7 ± 0.5 | ||||||
| Place of | Hospital | 1.9 ± 0.2 | 0.61 | 4.6 ± 0.3 | 0.71 | 6.5 ± 0.3 | 0.39 | 17.5 ± 0.5 | 0.13 | 1.9 ± 0.3 | 0.69 |
| employment | Private practice | 2.0 ± 0.3 | 4.9 ± 0.3 | 7.0 ± 0.5 | 19.3 ± 0.7 | 2.0 ± 0.3 | |||||
| FHT/FHO | 1.9 ± 0.3 | 4.6 ± 0.3 | 6.4 ± 0.4 | 17.6 ± 0.5 | 2.0 ± 0.3 | ||||||
| Mixed | 1.5 ± 0.3 | 4.4 ± 0.3 | 5.9 ± 0.4 | 18.5 ± 0.6 | 2.3 ± 0.3 | ||||||
| Years of | Still in training | 2.0 ± 0.3 | 0.14 | 4.1 ± 0.3 a | 0.004 | 6.1 ± 0.4 a | 0.002 | 19.0 ± 0.6 | 0.38 | 2.0 ± 0.3 | 0.66 |
| practiceb | <5 years | 1.4 ± 0.3 | 4.5 ± 0.3 a | 5.9 ± 0.4 a | 17.8 ± 0.6 | 1.8 ± 0.3 | |||||
| 5–15 years | 1.4 ± 0.3 | 4.3 ± 0.3 a | 5.8 ± 0.4 a | 17.8 ± 0.6 | 1.9 ± 0.3 | ||||||
| 16–25 years | 2.1 ± 0.4 | 5.9 ± 0.4b | 8.0 ± 0.5b | 18.4 ± 0.9 | 2.5 ± 0.4 | ||||||
| >25 years | 2.2 ± 0.3 | 4.9 ± 0.3 a | 7.2 ± 0.4 a | 17.4 ± 0.6 | 2.2 ± 0.3 | ||||||
| Non-pregnant | 0–15 | 1.9 ± 0.2 | 0.86 | 4.6 ± 0.2 | 0.54 | 6.5 ± 0.3 | 0.95 | 18.4 ± 0.4 | 0.31 | 2.0 ± 0.2 | 0.91 |
| women seen | 16–30 | 1.9 ± 0.2 | 4.4 ± 0.2 | 6.3 ± 0.3 | 17.7 ± 0.5 | 1.9 ± 0.2 | |||||
| per week | 31–50 | 1.6 ± 0.4 | 5.1 ± 0.4 | 6.7 ± 0.6 | 18.4 ± 0.8 | 2.1 ± 0.4 | |||||
| > 50 | 1.6 ± 0.5 | 4.8 ± 0.6 | 6.4 ± 0.8 | 16.4 ± 1.1 | 2.4 ± 0.6 | ||||||
| Pregnant | 0–15 | 1.9 ± 0.2 | 0.32 | 4.6 ± 0.2 | 0.56 | 6.6 ± 0.2 | 0.60 | 18.3 ± 0.4 | 0.60 | 1.9 ± 0.2 | 0.06 |
| women seen | 16–30 | 1.4 ± 0.3 | 4.9 ± 0.3 | 6.4 ± 0.5 | 17.4 ± 0.7 | 2.5 ± 0.3 | |||||
| per week | 31–50 | 1.4 ± 0.4 | 4.3 ± 04 | 5.8 ± 0.6 | 17.4 ± 0.8 | 1.6 ± 0.4 | |||||
| > 50 | 2.0 ± 0.7 | 4.0 ± 0.7 | 6.0 ± 1.0 | 18.0 ± 1.5 | 3.3 ± 0.7 | ||||||
P values were estimated using general linear model analyses and subgroup comparisons by Tukey’s post hoc tests. The letters indicate which sociodemographic or professional practice were found to differ significantly. Subgroups with the same letters do not differ significantly while those with different letters do. OB-GYN = obstetrician-gynecologist; FHT = family health team; FHO = family health organization.
6. Discussion
This study aimed to assess physicians’ knowledge, attitude and practice regarding the SOGC guidelines, FA intake and its health-related outcomes. Results obtained in the present study showed that physicians had some knowledge of FA, including periconceptional PVS recommendations and health related topics, however, the lack of some knowledge possibly led to the relatively low rate of correct practicing behaviours. Regardless of the current state of KAP scores, a vast majority of physicians are either unsure or would not feel comfortable recommending a PVS that is not in line with current guideline recommendations.
Knowledge and awareness of FA in WCBA or pregnant women has been assessed in other studies, but physicians are rarely the targeted population (Sayers et al., 1997, Perlow, 2001, Morin et al., 2001, de Jong-Van den Berg, 2005, Brandenburg et al., 1999, Amitai et al., 2004, Nelson et al., 2014). Although some studies have addressed physicians’ awareness and practicing behaviours regarding periconceptional FA, we are the first to do so in a Canadian context. Furthermore, this is the first study to have analyzed the link between physicians’ KAP while addressing the currently existing misalignment between FA-content in PVS and expert guidelines. Most surveys reported in the literature reveal that physicians lack knowledge regarding FA recommendations during pregnancy (Aggarwal et al., 2010, Auriel et al., 2011, Williams et al., 2006, Li et al., 2011, Demilew and Asres, 2017). Aggarwal et al. assessed 48 pediatricians, 54 obstetricians and 100 recently qualified medical graduates in India regarding their awareness of FA dose, timing of supplementation and knowledge about its role in prevention of NTDs. Although a majority were aware that FA has a role in NTD prevention, knowledge about dose and timing of supplementation was lacking (Aggarwal et al., 2010). Auriel et al. found that 88% of Israeli gynecologists, but only 60% of physicians always recommend FA prior to conception (Auriel et al., 2011). In a similar study, Williams et al. also assessed US health care providers’ knowledge and practice regarding FA for NTD prevention, and similarly to our findings, approximately half of the family physicians were knowledgeable about the correct low-risk dose, while dramatically fewer physicians knew the correct dose to prevent recurrence of a NTD (Williams et al., 2006). Regardless of their diverse geographic, economic and social settings, findings from the present study are in line with other findings, particularly concerning FA-recommendations for moderate and high-risk women. Present findings support the literature indicating that poor preconception practice behaviours are primarily due to a lack of knowledge or awareness, and the need for more educational resources to address this topic with physicians (Lefebvre et al., 2007, Temple, 1999).
Physicians from the present study possessed some knowledge of FA; however, insufficient knowledge may have led to a lower rate of correct practicing behaviours. Despite half of physicians knowing the correct low-risk dose (400 µg); in reality, a majority most often recommend a PVS containing ≥ 1 000 μg-FA, reflecting physicians’ perception of the current misalignment between the guidelines and FA-content in PVS. However, most physicians were either unsure or uncomfortable recommending a PVS that is in disagreement with the guidelines, in contrast to their current practice. The two main factors that were associated with the rate of correct knowledge, mainly for moderate and higher-risk women (KS2), were older age of physicians and the number of years of professional practice (>15, but <25). Almost half of the sample was made up of physicians with less than five years of professional experience and thus, may be indicative of how beneficial it could be to update medical undergraduate and resident curricula to more formally address the current guidelines.
Preconception care (PCC) is crucial since women have poor knowledge of FA (Sayers et al., 1997, Brandenburg et al., 1999, Lolowa et al., 2019) and this is particularly true among women with a low socioeconomic status (Perlow, 2001, de Jong-Van den Berg, 2005, Chacko et al., 2003, Hisam et al., 2014). This is especially concerning since approximately half of pregnancies in Canada are unplanned (Canada, 2017). Perinatal counselling is an approach that requires an open dialogue between the physician and the woman, to allow a better understanding of the user’s knowledge, perceptions and lifestyle (Braspenningx et al., 2013). Although women’s understanding and prioritization of optimizing their health prior to conception varies, primary care physicians are women’s continued preferred source for preconception information (Frey and Files, 2006). Physicians’ lack of knowledge is reflected in their poor prescribing practice of FA during the periconceptional period. Several studies, including the present one, have observed this trend (Auriel et al., 2011, Li et al., 2011, Demilew and Asres, 2017, Curtis et al., 2006). Although many physicians expressed a positive attitude toward their practice and perinatal recommendations, the reality is that there is a lack of routine prescribing of FA, particularly a lack in prescribing the correct recommended dose.
The SOGC guideline was first published in 1993 and has since undergone three revisions, which largely involve modifications to the three risk-category definitions. The evolution of the guidelines over time has led to some confusion; however, it is the discrepancy regarding supplemental doses of FA found in PVS that most notably explains why almost half of physicians chose “for no particular reason” when asked about the reason for the supplement they most often recommend. A majority of PVS are over-the-counter and thus, not covered nor reimbursed by insurance companies. Prescribed supplements are only available at the 1.1 mg and 5 mg FA doses (Lamers et al., 2018), which may reflect the pressure felt by some physicians to recommend higher than recommended doses in order to take into consideration women’s preference and lower cost; both of which were the next most popular options. Finally, physicians may be hesitant to recommend something that is not widely available nor labelled as prenatal. Most recently, Health Canada’s multi-vitamin/mineral supplemental monograph was updated (September 25, 2018) to include an optional statement which aligns the allowable FA content with the recommended dose of FA which may lead to promising improvements in the future (Government of Canada HC. Ingredient Search [Internet], 2004).
7. Strengths and limitations
In light of concern over the unprecedented shift of Canadian pregnant women’s folate status, our study is the first to assess the knowledge, attitude and practice of Canadian physicians regarding periconceptional FA. We are also the first to correlate physicians’ KAP in addition to taking into consideration the misalignment concerning FA content in PVS. Undertaken in the National Capital Region, findings from the present study may not necessarily reflect a nationally representative sample of all Canadian physicians since it was a small sample with a large majority of females in their early to mid-career practicing in a mixed environment. Based on our literature search, gender is not predictive of periconception FA knowledge in other studies; however, it may be correlated with recommending multivitamin use to WCBA (Williams et al., 2006). It is possible that female physicians are more willing to participate in research studies pertaining to pregnancy and related topics. There may be other underlying factors relating to female physicians’ practice that could skew the resulting observed levels of KAP. Interestingly, however, a majority of studies assessing physicians, whether it be their knowledge, attitude and/or practice, regardless of geographical location, possess an interesting commonality. A majority of respondents tend to be female (Li et al., 2018), which is unexpected given the fact that it has been historically reported that a majority of Canadian family physicians generally remains male (Physicians in Canada, 2017). The Physicians in Canada 2016 Summary Report highlighted that more recently, the number of female physicians continues to rise, with a 21% increase seen between 2012 and 2016 (Institute, 2016). One possible explanation for the distribution seen in the present study is that in 2018, the distribution of sex by neonatal-perinatal medicine specialty in Canada was 62.2% female vs 37.8% male (Canadian Medical Association, 2018).
Almost half of our sample was made up of physicians with less than five years of professional experience. Their chosen answers likely depict an accurate reality of their upcoming practicing behaviour and is indicative of the necessity to update medical undergraduate and resident curricula to more formally address the current guidelines. The primary limitation to the generalization of the results is the sample being a small, convenience sample. The number of questions we were able to include due to completion time constraints to capture physicians’ KAP limited the depth of data collected. On the other hand, a shorter completion time encouraged physicians to participate in the study, who otherwise expressed little interest or time to participate. The questionnaire was reviewed through consultations with a committee of non-participating physicians and experts for content validation and to eliminate ambiguity. However, further validation and reliability procedures would be warranted for a larger-scale follow-up study.
8. Conclusion
Physicians lack knowledge regarding periconceptional FA, which is associated with their attitude and practice, prevents most physicians from adhering to Canadian guidelines. Physicians are the linking bodies between expert guidelines and women, playing a large role in promoting appropriate use of FA supplements. It is imperative that medical training and continued education programs are informed of the lack of knowledge and be based on current evidence and clinical practice guidelines to ensure optimal maternal FA intake and offspring health. In the future, sampling physicians across the country using larger, random sampling methods would enable a more representative picture of KAP among Canadian physicians as well as how it differs by personal and professional characteristics.
CRediT authorship contribution statement
Liana Arielle Mida: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing - original draft, Writing - review & editing. Vincent della Zazzera: Resources. Bénédicte Fontaine-Bisson: Conceptualization, Methodology, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would also like to acknowledge Katrina El Asmar (KEA) and Myriam Beaudry (MB)’s assistance in recruitment as well as Carolina Soto (CS)’s aid in data entry. KEA received scholarships from the “Consortium national de formation en santé (CNFS)” and CS from the Undergraduate Research Opportunity Program (UROP) from the University of Ottawa.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Aggarwal A., Kumhar G.D., Harit D., Faridi M.M.A. Role of folic acid supplementation in prevention of neural tube defects: physicians yet unaware! J. Prevent. Med. 2010;51(3):131–132. [PubMed] [Google Scholar]
- Ami N., Bernstein M., Boucher F., Rieder M., Parker L. Folate and neural tube defects: The role of supplements and food fortification | Canadian Paediatric Society. Paediatr Child Health. 2016;21(3):145–149. doi: 10.1093/pch/21.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai Y., Fisher N., Haringman M., Meiraz H., Baram N., Leventhal A. Increased awareness, knowledge and utilization of preconceptional folic acid in Israel following a national campaign. Prev. Med. 2004;39(4):731–737. doi: 10.1016/j.ypmed.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Auriel E., Biderman A., Belmaker I., Freud T., Peleg R. Knowledge, Attitudes, and Practice among Women and Doctors Concerning the Use of Folic Acid. ISRN Obstet. Gynecol. 2011;2011:1–5. doi: 10.5402/2011/946041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey L.B., editor. Folate in health and disease. 2nd ed. Taylor & Francis; Boca Raton: 2010. p. 583 p.. [Google Scholar]
- Berry R.J., Li Z., Erickson J.D., Li S., Moore C.A., Wang H. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. J. Med. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Blencowe H., Kancherla V., Moorthie S., Darlison M.W., Modell B. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann. NY Acad. Sci. 2018;1414(1):31–46. doi: 10.1111/nyas.13548. [DOI] [PubMed] [Google Scholar]
- Brandenburg H., Traas M.A., Laudy J., Ursem N., Westerveld A.M., Wladimiroff J.W. Periconceptional use of folic acid amongst women of advanced maternal age. Prenat Diagn. 1999;19(2):132–135. [PubMed] [Google Scholar]
- Braspenningx S., Haagdorens M., Blaumeiser B., Jacquemyn Y., Mortier G. Preconceptional care: a systematic review of the current situation and recommendations for the future. Facts Views Vis Obgyn. 2013;5(1):13–25. [PMC free article] [PubMed] [Google Scholar]
- Canada H. Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks - August 1, 2000 [Internet]. aem. 2000 [cited 2019 Jun 18]. Available from: https://www.canada.ca/en/health-canada/corporate/about-health-canada/reports-publications/health-products-food-branch/health-canada-decision-making-framework-identifying-assessing-managing-health-risks.html.
- Canada H. Licensed Natural Health Products Database (LNHPD) [Internet]. aem. 2007 [cited 2020 Dec 7]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/applications-submissions/product-licensing/licensed-natural-health-products-database.html#wb-cont.
- Canada, Health Canada. Prenatal nutrition guidelines for health professionals: folate. [Internet]. Ottawa: Health Canada; 2009 [cited 2019 Jun 18]. Available from: http://epe.lac-bac.gc.ca/100/200/301/hcan-scan/prenatal_nutrition_guidelines-ef/H164-109-4-2009E.pdf.
- Canada PHA of. Chapter 2: Preconception care [Internet]. aem. 2017 [cited 2019 Jun 20]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/maternity-newborn-care-guidelines-chapter-2.html.
- Canadian Medical Association. Number and percent distribution of physicians by specialty and sex, Canada 2018. 2018; 2.
- Chacko M.R., Anding R., Kozinetz C.A., Grover J.L., Smith P.B. Neural tube defects: knowledge and preconceptional prevention practices in minority young women. Pediatrics. 2003;112(3 Pt 1):536–542. doi: 10.1542/peds.112.3.536. [DOI] [PubMed] [Google Scholar]
- Colapinto C.K., O’Connor D.L., Tremblay M.S. Folate status of the population in the Canadian Health Measures Survey. Can. Med. Assoc. J. 2011;183(2):7. doi: 10.1503/cmaj.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colapinto C.K., O’Connor D.L., Dubois L., Tremblay M.S. Folic acid supplement use is the most significant predictor of folate concentrations in Canadian women of childbearing age. Appl. Physiol. Nutr. Metab. 2012;37(2):284–292. doi: 10.1139/h11-161. [DOI] [PubMed] [Google Scholar]
- Curtis M., Abelman S., Schulkin J., Williams J.L., Fassett E.M. Do We Practice What We Preach? A Review of Actual Clinical Practice with Regards to Preconception Care Guidelines. Matern Child. Health J. 2006;10(1):53. doi: 10.1007/s10995-006-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel A.E., Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. J. Med. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- de Jong-Van den Berg LTW, Hernandez-Diaz S, Werler MM, Louik C, Mitchell AA. Trends and predictors of folic acid awareness and periconceptional use in pregnant women. Am. J. Obstet. Gynecol. 2005 Jan;192(1):121–8. [DOI] [PubMed]
- De Wals P., Tairou F., Van Allen M.I., Uh S.-H., Lowry R.B., Sibbald B. Reduction in neural-tube defects after folic acid fortification in Canada. J. Med. 2007;357(2):135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- Demilew Y.M., Asres Nigussie A. Knowledge of Health Professionals on Folic Acid Use and Their Prescribing Practice in Bahir Dar City Administration, Northwest Ethiopia: Cross-Sectional Study. PLoS ONE [Electronic Resource]. 2017;12(1) doi: 10.1371/journal.pone.0170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L., Diasparra M., Bédard B., Colapinto C.K., Fontaine-Bisson B., Morisset A.-S. Adequacy of nutritional intake from food and supplements in a cohort of pregnant women in Québec, Canada: the 3D Cohort Study (Design, Develop, Discover) Am. J. Clin. Nutrit. 2017;106(2):541–548. doi: 10.3945/ajcn.117.155499. [DOI] [PubMed] [Google Scholar]
- Fayyaz F., Wang F., Jacobs R.L., O’Connor D.L., Bell R.C., Field C.J. Folate, vitamin B12, and vitamin B6 status of a group of high socioeconomic status women in the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Appl. Physiol., Nutrit. Metabol. = Physiol. Appl., Nutrition et Metabol. 2014;39(12):1402–1408. doi: 10.1139/apnm-2014-0181. [DOI] [PubMed] [Google Scholar]
- Field M.S., Stover P.J. Safety of folic acid. Ann. NY Acad. Sci. 2018;1414(1):59–71. doi: 10.1111/nyas.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.T., Stover P.J. Folate-mediated one-carbon metabolism. Vitam. Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- Frey K.A., Files J.A. Preconception healthcare: what women know and believe. Matern. Child Health J. 2006;10:S73–S77. doi: 10.1007/s10995-006-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett GS, Bailey LB. Chapter 23 - Global Status of Folic Acid Fortification—Progress and Gaps11This chapter has been abridged from Garrett, G. S., Bailey, L. A Public Health Approach for Preventing Neural Tube Defects: Folic Acid Fortification and Beyond. Annals of the New York Academy of Sciences, February 2018. In: Mannar MGV, Hurrell RF, editors. Food Fortification in a Globalized World [Internet]. Academic Press; 2018 [cited 2019 Jun 11]. p. 231–9. Available from: http://www.sciencedirect.com/science/article/pii/B9780128028612000237. [DOI] [PubMed]
- Global Progress of Industrially Milled Cereal Grains- Food Fortification Initiative [Internet]. [cited 2019 Jun 11]. Available from: http://www.ffinetwork.org/global_progress/.
- Government of Canada HC. Ingredient Search [Internet]. 2004 [cited 2019 Aug 18]. Available from: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=multi_vitmin_suppl.
- Hisam A., Rahman M.U., Mashhadi S.F. Knowledge, attitude and practice regarding folic acid deficiency; A hidden hunger. J. Med. Sci. 2014;30(3):583–588. doi: 10.12669/pjms.303.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P.S.P., Ly A., Szeto I.M.Y., Reza-Lopez S.A., Cho D., Kim Y.-I. Maternal and postweaning folic acid supplementation interact to influence body weight, insulin resistance, and food intake regulatory gene expression in rat offspring in a sex-specific manner. Appl. Physiol., Nutri. Metabol. = Physiol. Appl., Nutrit. Metabol. 2016;41(4):411–420. doi: 10.1139/apnm-2015-0503. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline [Internet]. Washington (DC): National Academies Press (US); 1998 [cited 2019 Jun 4]. (The National Academies Collection: Reports funded by National Institutes of Health). Available from: http://www.ncbi.nlm.nih.gov/books/NBK114310/. [PubMed]
- Canadian Institute for Health Information. Physicians in Canada, 2016: Summary Report. 2017;27.
- Irvine B., Luo W., León J.A. Congenital Anomalies in Canada 2013: A Perinatal Health Surveillance Report by the Public Health Agency of Canada’s Canadian Perinatal Surveillance System. Health Promot Chronic Dis. Prev. Can. 2015;35(1):21–22. doi: 10.24095/hpcdp.35.1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefte-de Jong J.C., Timmermans S., Jaddoe V.W.V., Hofman A., Tiemeier H., Steegers E.A. High Circulating Folate and Vitamin B-12 Concentrations in Women During Pregnancy Are Associated with Increased Prevalence of Atopic Dermatitis in Their Offspring. J. Nutri. 2012;142(4):731–738. doi: 10.3945/jn.111.154948. [DOI] [PubMed] [Google Scholar]
- Lamers Y., MacFarlane A.J., O’Connor D.L., Fontaine-Bisson B. Periconceptional intake of folic acid among low-risk women in Canada: summary of a workshop aiming to align prenatal folic acid supplement composition with current expert guidelines. Am. J. Clin. Nutrit. 2018;108(6):1357–1368. doi: 10.1093/ajcn/nqy212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L.G., Ordean A., Midmer D., Kahan M., Tolomiczenko G. Physicians’ knowledge of alcohol, tobacco and folic acid in pregnancy. Substance Abuse. 2007;28(1):3–9. doi: 10.1300/J465v28n01_02. [DOI] [PubMed] [Google Scholar]
- Li A., Cronin S., Bai Y.Q., Walker K., Ammi M., Hogg W. Assessing the representativeness of physician and patient respondents to a primary care survey using administrative data. BMC Family Pract. 2018;19(1):77. doi: 10.1186/s12875-018-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Zhu J., Zeng Z., Wang Y., Liang J., Yuan P. Study of KAP with regard to taking folic acid supplements and factors affecting the recommendation and prescription of those supplements among obstetricians and specialists in women's health in six provinces of Northern China, 2009. BioScience Trends. 2011;5(3):104–110. doi: 10.5582/bst.2011.v5.3.104. [DOI] [PubMed] [Google Scholar]
- Lillycrop K.A., Burdge G.C. Epigenetic mechanisms linking early nutrition to long term health. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26(5):667–676. doi: 10.1016/j.beem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Lolowa A.M., Selim N., Alkuwari M., Salem Ismail M. Knowledge and intake of folic acid among teachers of childbearing age in the State of Qatar: a cross-sectional study. BMJ Open. 2019;9(4) doi: 10.1136/bmjopen-2018-025005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. [Review] [134 refs] Mol. Genet. Metab. 2000;71(1–2):121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- McStay C., Prescott S., Bower C., Palmer D. Maternal Folic Acid Supplementation during Pregnancy and Childhood Allergic Disease Outcomes: A Question of Timing? Nutrients. 2017;9(2):123. [Google Scholar]
- Medical Research Council Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991 Jul;338(8760):131–7. [PubMed]
- Morin V.I., Mondor M., Wilson R.D. Knowledge on periconceptional use of folic acid in women of British Columbia. Fetal Diagn. Ther. 2001;16(2):111–115. doi: 10.1159/000053892. [DOI] [PubMed] [Google Scholar]
- Mulinare J., Cordero J.F., Erickson J.D., Berry R.J. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA. 1988;260(21):3141–3145. [PubMed] [Google Scholar]
- Nelson C.R.M., Leon J.A., Evans J. The relationship between awareness and supplementation: Which Canadian women know about folic acid and how does that translate into use? Can J Public Health. 2014;105(1):40–46. doi: 10.17269/cjph.105.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlow J.H. Comparative use and knowledge of preconceptional folic acid among Spanish- and English-speaking patient populations in Phoenix and Yuma, Arizona. Am. J. Obstet. Gynecol. 2001;184(6):1263–1266. doi: 10.1067/mob.2001.112974. [DOI] [PubMed] [Google Scholar]
- Physicians in Canada, 2017: Summary Report. [Internet]. 2019 [cited 2020 Dec 7]. Available from: https://www.deslibris.ca/ID/10100535.
- Plumptre L., Masih S.P., Ly A., Aufreiter S., Sohn K.-J., Croxford R. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015;102(4):848–857. doi: 10.3945/ajcn.115.110783. [DOI] [PubMed] [Google Scholar]
- Savard C., Lemieux S., Weisnagel S., Fontaine-Bisson B., Gagnon C., Robitaille J. Trimester-Specific Dietary Intakes in a Sample of French-Canadian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients. 2018;10(6):768. doi: 10.3390/nu10060768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers G.M., Hughes N., Scallan E., Johnson Z. A survey of knowledge and use of folic acid among women of child-bearing age in Dublin. J. Public Health. 1997;19(3):328–332. doi: 10.1093/oxfordjournals.pubmed.a024639. [DOI] [PubMed] [Google Scholar]
- Shah P, Al-Wassia, Haydi. Folic acid supplementation for the prevention of neural tube defects: promotion and use. Nutrition and Dietary Supplements. 2010 Sep;105.
- Smithells R.W., Seller M.J., Harris R., Fielding D.W., Schorah C.J., Nevin N.C. Further experience of vitamin supplementation for prevention of neural tube defect recurrences. Lancet. 1983;321(8332):1027–1031. doi: 10.1016/s0140-6736(83)92654-5. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen R.P.M., Twigt J., Pestinger V., Sinclair K.D. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. [Review] Human Reproduction Update. 2013;19(6):640–655. doi: 10.1093/humupd/dmt041. [DOI] [PubMed] [Google Scholar]
- Temple N.J. Survey of nutrition knowledge of Canadian physicians. J. Am. Coll. Nutr. 1999;18(1):26–29. doi: 10.1080/07315724.1999.10718823. [DOI] [PubMed] [Google Scholar]
- Vallerand R.J. Vers une méthodologie de validation trans-culturelle de questionnaires psychologiques: Implications pour la recherche en langue française. Canad. Psychol./Psychologie canadienne. 1989;30(4):662–680. [PubMed] [Google Scholar]
- WHO | Periconceptional folic acid supplementation to prevent neural tube defects [Internet]. WHO. [cited 2019 Jun 18]. Available from: http://www.who.int/elena/titles/folate_periconceptional/en/.
- Williams J.L., Abelman S.M., Fassett E.M., Stone C.E., Petrini J.R., Damus K. Health care provider knowledge and practices regarding folic acid, United States, 2002–2003. Matern. Child Health J. 2006;1(5 Suppl):S67–S72. doi: 10.1007/s10995-006-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.D., Committee G., Wilson R.D., Audibert F., Brock J.-A., Carroll J. Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies. J. Obstet. 2015;37(6):534–552. doi: 10.1016/s1701-2163(15)30230-9. [DOI] [PubMed] [Google Scholar]
- Yajnik C.S., Deshmukh U.S. Maternal nutrition, intrauterine programming and consequential risks in the offspring. [Review] [51 refs] Rev. Endocr. Metabol. Disorders. 2008;9(3):203–211. doi: 10.1007/s11154-008-9087-z. [DOI] [PubMed] [Google Scholar]
- Yi Y., Lindemann M., Colligs A., Snowball C. Economic burden of neural tube defects and impact of prevention with folic acid: a literature review. Eur. J. Pediatr. 2011;170(11):1391–1400. doi: 10.1007/s00431-011-1492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaganjor I., Sekkarie A., Tsang B.L., Williams J., Razzaghi H., Mulinare J. Describing the Prevalence of Neural Tube Defects Worldwide: A Systematic Literature Review. PLoS One [Internet]. 2016 doi: 10.1371/journal.pone.0151586. Apr 11 [cited 2019 Jun 10];11(4). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]