Abstract
BACKGROUND:
Changes in sexual behaviors in frontotemporal dementia (FTD) are common and multifaceted, but not well characterized.
OBJECTIVE:
To characterize changes in sexual behaviors and intimacy in frontotemporal dementia (FTD) compared to corticobasal syndrome (CBS) and normal controls (NC), and to evaluate the neuroanatomical associations of these changes.
METHODS:
Spouses of 30 FTD patients, 20 CBS patients, and 35 NC completed the Sexual Symptoms in Neurological Illness and Injury Questionnaire (SNIQ), which captures changes in sexual interest, inappropriate sexual behaviors, and prosocial sexual behaviors. 25 patients with FTD and 14 patients with CBS also received 18-flouorodeoxyglucose positron-emission topography (18-FDG-PET) scans to determine the metabolic changes associated with these symptoms.
RESULTS:
FTD patients showed a greater increase in inappropriate sexual behaviors than CBS patients [p=0.009] and NC [p<0.001] and a greater decrease in prosocial sexual behaviors than CBS patients [p=0.026] and NC [p<0.001]. Groups did not differ in change in sexual interest. Among both patient groups, the most common change was decreased prosocial sexual behaviors [p<0.01]. Hypometabolism in Brodmann’s Area 10 (BA10), within the right frontal pole, correlated with decreased prosocial sexual behaviors [p(FWE-corr)<0.05, k=44]. No anatomical associations were found with other sexual changes.
CONCLUSION:
Decreased prosocial sexual behavior was associated with hypometabolism in BA 10, an area tied to social knowledge and theory of mind, supporting the idea that changes reflect social-cognitive deficits due to frontal dysfunction.
Keywords: neurodegenerative disorders, frontotemporal dementia, sexual behavior, prefrontal cortex, frontal lobe
INTRODUCTION
Frontotemporal dementia (FTD) is a neurodegenerative disease that causes progressive changes in personality and behavior, which can include apathy, socially inappropriate behaviors, and impaired social cognition [1]. FTD has been associated with inappropriate sexual behaviors [2–4], although these behaviors are not uniformly defined [5]. In one study, FTD patients displayed more “disinhibited” sexual behaviors (e.g., groping) than “intimacy-seeking” behaviors (e.g., attempts to initiate sexual contact with a spouse) than patients with other types of dementia [6]. In a second study, “hypersexual” behaviors, including increased sexual desire, public masturbation, and attempts at sexual encounters in inappropriate contexts, were observed in 6 of 47 patients with behavioral-variant FTD (bvFTD) and no patients with Alzheimer’s disease (AD) [3]. Complaints about such changes in sexual behavior are frequently reported by family members, and may tilt clinicians toward a diagnosis of FTD.
In spite of these characterizations, other research associates FTD with decreased sexual and romantic interest [7–9]. In one study, 90% of patients with bvFTD showed decreased initiation of and receptiveness to sexual advances, according to their partners [8]. In addition, 80% showed decreased affection towards their partners, even among the minority who displayed inappropriate sexual behaviors (e.g. childish sexual behaviors or increased interest in pornography) [8]. Thus, behaviors typically termed hypersexual co-occurred with hyposexual changes.
Our understanding of the neuroanatomy associated with sexual symptoms in dementia is predominantly speculative [10, 11]. Several hypotheses exist regarding the regions and networks that underlie specific sexual changes in neurodegenerative disease. According to one theory, inappropriate sexual behaviors can stem from dysfunction in four distinct neural systems: the frontal system, causing executive failures often described as disinhibition; the temporolimbic and hypothalamic systems, leading to changes in arousal; and the striatum, associated with obsessive-compulsive sexual behaviors [12]. Others further hypothesize that damage to the temporolimbic region, involved in inhibiting sexual arousal, could be associated with hypersexual behaviors, and that damage to the frontal lobe, as well as to the hypothalamus, could be associated with hyposexuality [8, 10]. Visual inspections of structural MRI, SPECT, and FDG-PET scans in patients with FTD and AD showed possible associations between dysfunction of the right anterior temporolimbic area and increased sexual arousal, and between dysfunction of the right prefrontal, orbitofrontal, and anterior cingulate cortices and hypersexual, not hyposexual, behaviors [3].
To our knowledge, only one study has experimentally investigated the neuroanatomical associations of sexual changes in FTD-spectrum disorders. Voxel-based morphometry of structural MR scans revealed that in 59 patients with bvFTD, semantic-variant primary progressive aphasia (svPPA) and AD, hypersexual behavior (increased frequency of sexual behaviors) was associated with bilateral cerebellar atrophy, and hyposexual behavior (decreased frequency of sexual behaviors) was associated with atrophy of the right posterior supramarginal gyrus, middle frontal gyrus, and bilateral posterior thalamus [13]. Interestingly, no associations with the temporolimbic region were found. The findings highlight the uncertainty of our framework for describing the anatomical underpinnings of sexual changes due to FTD and motivate further exploration through research.
The aim of the current study was to characterize changes in sexual behaviors in patients with FTD, measured by the Sexual Symptoms in Neurological Illness and Injury Questionnaire (SNIQ) [9], a new scale that allows for a systematic investigation of specific changes in sexual behaviors due to neurodegenerative illness, and to investigate associations of these changes with regional changes in brain functioning, measured by 18-fluorodeoxyglucose positron emission tomography (18F-FDG-PET).
MATERIALS AND METHODS
Participants
30 patients with FTD, 20 patients with corticobasal syndrome (CBS) and 35 normal controls (NC) participated in this study. Demographic and clinical data are presented in Table 1. CBS patients were used as clinical controls, as CBS is also neurodegenerative, but is associated with fewer neuropsychiatric and behavioral changes than FTD [14] and is predominantly characterized by atrophy in the posterior frontal lobes and basal ganglia [15, 16], regions hypothesized to have fewer associations with sexual symptoms than anterior frontal and temporolimbic regions, as described above.
Table 1.
Patient demographics and clinical characteristics.
| Diagnosis (n) | Age (years) | Sex (M/F) | Ethnicity (Caucasian/ African American/ Asian) | Years of education | Years since first symptom | FTD subtype (count) | NPI total | FrSBe total change (raw score) | MDRS-2 total |
|---|---|---|---|---|---|---|---|---|---|
| FTD (30) | 60.43 (8.95) | 12/18 | 30/0/0 | 15.57 (2.94) | 4.86 (3.17) | bvFTD (24) svPPA (1) nfPPA (3) FTD-MND (2) |
32.11 (15.97) | 77.04 (31.42) | 107.68 (23.38) |
| CBS (20) | 62.70 (5.89) | 9/11 | 19/0/1 | 15.00 (2.47) | 4.82 (2.14) | N/A | 13.53 (15.82) | 41.44 (24.82) | 110.71 (18.95) |
| Controls (35) | 59.20 (10.89) | 17/18 | 27/4/2 | 15.64 (2.57) | N/A | N/A | --- | --- | --- |
bvFTD: behavioral variant frontotemporal dementia; svPPA: semantic variant primary progressive aphasia; nfPPA: non-fluent variant primary progressive aphasia; FTD-MND: FTD with motor neuron disease
NPI: Neuropsychiatric Inventory; FrSBe: Frontal Systems Behavioral Interview; MDRS-2: Mattis Dementia Rating Scale-2
Patients were evaluated sequentially within an ongoing research study on FTD and CBS in the Cognitive Neuroscience Section of the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) in Bethesda, MD. Inclusion criteria were a diagnosis of possible or probable FTD or CBS and a caregiver willing and able to accept the responsibilities involved in the study. All FTD subtypes [bvFTD, semantic and non-fluent variants of primary progressive aphasia (svPPA & nfPPA), and FTD with motor neuron disease (FTD-MND)] were included in the study, as behavioral symptoms have been reported in all phenotypes, many of which overlap in content, although they may vary in severity [17–20]. Participants were excluded if they were pregnant, or if they had behavioral symptoms or other medical or social condition that would preclude the gathering of data for the study.
Patients and caregivers spent nine days participating in a clinical evaluation, neuropsychological and neurological testing, and imaging studies. Their diagnoses were re-evaluated by a neurologist, psychiatrist, and neuropsychologist using criteria that were current at the time of evaluation [21, 22]. All patients had study partners with Durable Power of Attorney who consented to research participation, along with patient assent. The study was approved by the NIH IRB.
The SNIQ was completed by caregivers. Caregivers were heterosexual spouses, with the exception of four (an ex-girlfriend, a friend, and two children of the patients). Non-spouse informants estimated changes in sexual behavior based on reports from other family members, including telephone consultation with patients’ spouses.
Control subjects were recruited online through ResearchMatch.org. Interested volunteers completed the survey as informants about their spouses or partners using Qualtrics (www.qualtrics.com). Inclusion criteria were: 1) Age 40 to 84, 2) in a partner relationship for at least five years, and 3) no neurological or psychiatric diagnoses. Informants indicated consent to the study before completing the questionnaire. All procedures were approved by the Columbia IRB.
Questionnaires
The Sexual Symptoms in Neurological Illness and Injury Questionnaire (SNIQ) is a validated, caregiver-completed questionnaire [9]. It measures changes in sexual interest (e.g. “Views internet pornography”), inappropriate sexual behaviors (e.g. “Performs sexually childish behavior”), and prosocial sexual behaviors, a term used to describe romance and intimacy within a relationship (e.g. “Expresses love for partner”). Using a 5-point Likert-type scale, informants rated patients on the frequency of 24 behaviors (from “Never” to “All of the time or almost all of the time”) both before the onset of illness and at the present time. Informants for healthy controls rated the frequency of their partners’ behaviors as compared to 5 years ago. The SNIQ also includes two open response items about changes in objects of sexual arousal and other sexual changes. The questionnaire covers a wide range of behaviors, provides information on pre-morbid behaviors (important for characterizing change, given the large range of normal sexual behaviors [23]), and uses frequency measures that allow for quantification of behaviors.
To characterize other behavioral symptoms of the FTD and CBS patients, we administered the Neuropsychiatric Inventory (NPI) [24] and the Frontal Systems Behavior Scale (FrSBe) [25]. To assess general cognition, we administered the Mattis Dementia Rating Scale-2 (MDRS-2) [26]. See Table 1.
Imaging
MRI
Patients were included in the imaging analyses if they had MRI and FDG-PET scans of acceptable quality. The imaging analyses included 25 FTD and 14 CBS patients. Structural T1-weighted MR imaging was performed on a General Electric 1.5 or Phillips 3T scanner (3D-T1-SPGR sequence, 120 contiguous slices, slice thickness = 1.5 mm, in- plane resolution = 0.9375 mm by 0.9375 mm, flip angle = 20 degrees). One participant received T2-weighted instead of T1-weighted imaging.
18F-FDG-PET
18F-FDG-PET scans were acquired using a General Electric Advance PET Scanner. Subjects fasted for eight hours prior to the scan. Subjects were given an intravenous injection of 5 millicuries of 18F-FDG. A transmission scan was used to correct the emission data. At the end of the 40-minute uptake period, the emission (PET) scan was performed (15 minutes per set of 35 brain slices).
Data Analysis
Demographics
Two-tailed independent t-tests were performed to compare cognitive and behavioral symptoms in patients with FTD and CBS. One-way ANOVAs were used to compare groups on age and education, and chi-square tests were performed to compare groups on sex and race. Because sexual behaviors are known to vary by age and sex in older adults [23], Pearson’s correlations were performed to test associations between SNIQ domain scores and subject age, and two-tailed independent t-tests were performed to test sex differences in domain scores.
Behavioral
A one-way ANOVA was performed on the three subject groups (FTD, CBS, and NC) using mean change score for each SNIQ domain as the dependent variable. Post-hoc Tukey HSD tests were also performed. A categorical, dichotomous variable (change vs. no-change) was created for each domain, to evaluate how common changes in each domain were within the groups. Increases and decreases in sexual interest were considered separately. Pearson correlations between sexual and non-sexual behavioral symptoms, measured by the FrSBe and NPI, were performed in the FTD patient group only.
Imaging
Imaging data were analyzed using statistical parametric mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12). The structural MRI and 18F-FDG PET scans were co-registered using Normalized Mutual Information, normalized to the MNI template, and smoothed (FWHM=6 mm) using the SPM12 software. Normalization was manually checked to ensure alignment. The default values in SPM12 were used in the analyses. Smoothed, normalized 18F-FDG PET volumes were entered into group analyses.
To restrict analyses to the hypothesized regions, ROIs were chosen based on established regions of atrophy in FTD (all phenotypes) [27–31] and CBS [15, 16]. A mask of these regions was created using the Harvard Oxford Cortical and Subcortical atlases, available through the Functional MRI of the Brain Software Library [32] (Supplemental Materials). For each voxel within this mask, separate General Linear Models were tested on the total change score for each SNIQ domain, with the intensity value of FDG uptake at each voxel as an explanatory variable. To control for overall cognition, MDRS-2 t-scores were added as a covariate. One subject with CBS who did not complete the MDRS-2 was removed from analyses. We also included diagnosis (CBS or FTD) as a covariate. Only clusters that survived Family-Wise Error (FWE)-correction for multiple comparisons at p < 0.05 and had a spatial extent threshold of at least 20 voxels were considered significant in these analyses.
RESULTS
Demographics
Patients with FTD and CBS did not differ significantly in cognitive dysfunction, as measured by the MDRS-2 (see Table 1), [t (45) = −0.78, p = 0.443], but FTD patients had significantly more behavioral symptoms than CBS patients, as measured by the NPI [t (44) = 5.70, p < 0.001] and the total FrSBe [t (46) = 4.43, p < 0.001]. There were no significant differences in age [F (2,82) = .911, p = 0.406], education [F (2,82) = 1.10, p = 0.338], sex [χ2 (2) = .94, p = 0.626], or race [χ2 (4) = 8.40, p = 0.078] across the three subject groups.
No significant correlations were found between subject age and any of the SNIQ domain scores, p > 0.05. There was a significant difference between men and women in change in prosocial sexual behaviors [t (83) = 2.13, p = 0.036], with men showing a greater decrease in prosocial behaviors [M = −0.80] than women [M = 0.42]. Change scores in inappropriate sexual behaviors and in sexual interest did not differ significantly by sex.
SNIQ
Results from the SNIQ analyses are reported in Table 2. Significant group differences were seen in prosocial sexual behaviors [F (2,82) = 8.70, p < 0.001] and inappropriate sexual behaviors [F (2,82) = 9.31, p < 0.001]. Post-hoc comparisons using the Tukey HSD test revealed that FTD patients had a greater increase in inappropriate sexual behaviors and a greater decrease in prosocial sexual behaviors than CBS patients and NC. Patients with CBS also had a significant decrease in prosocial sexual behaviors compared to NC. There was no significant difference between groups in sexual interest [F (2,82) = 0.080, p = 0.92]. Because change in prosocial sexual behaviors differed by sex, this ANOVA was re-run with sex as a covariate. The effect of diagnostic group remained significant [p < 0.001].1
Table 2.
SNIQ Comparisons.
| FTD | CBS | Control | FTD vs CBS p value |
FTD vs NC p value |
CBS vs NC p value |
|
|---|---|---|---|---|---|---|
| Sexual Interest | −0.03 (0.28) | −0.04 (0.17) | −0.02 (0.10) | 0.971 | 0.982 | 0.916 |
| Inappropriate Sexual Behaviors | 0.18 (0.30) | 0.00 (0.13) | −0.02 (0.11) | 0.009† | <0.001† | 0.858 |
| Prosocial Sexual Behaviors | −1.18 (0.91) | −0.64 (0.77) | −0.14 (0.42) | 0.026* | <0.001† | 0.040* |
Mean changes (“At the Present Time” minus “Before Illness”/”Five Years Ago”) in Sexual interest, Inappropriate sexual behaviors, and Prosocial sexual behaviors domain scores. Comparisons made using the Tukey HSD test. Numbers in parentheses represent standard deviations.
p < 0.05
p < 0.01
Within both the FTD and CBS groups, the most common change was a decrease in prosocial sexual behaviors (observed in 25 of 30 FTD patients and 13 of 20 CBS patients), followed by an increase in inappropriate sexual behaviors (14 of 30 FTD and 4 of 20 CBS), a decrease in sexual interest (11 of 30 FTD and 2 of 20 CBS), and an increase in sexual interest (3 of 30 FTD and 2 of 20 CBS), see Figure 1.
Figure 1:
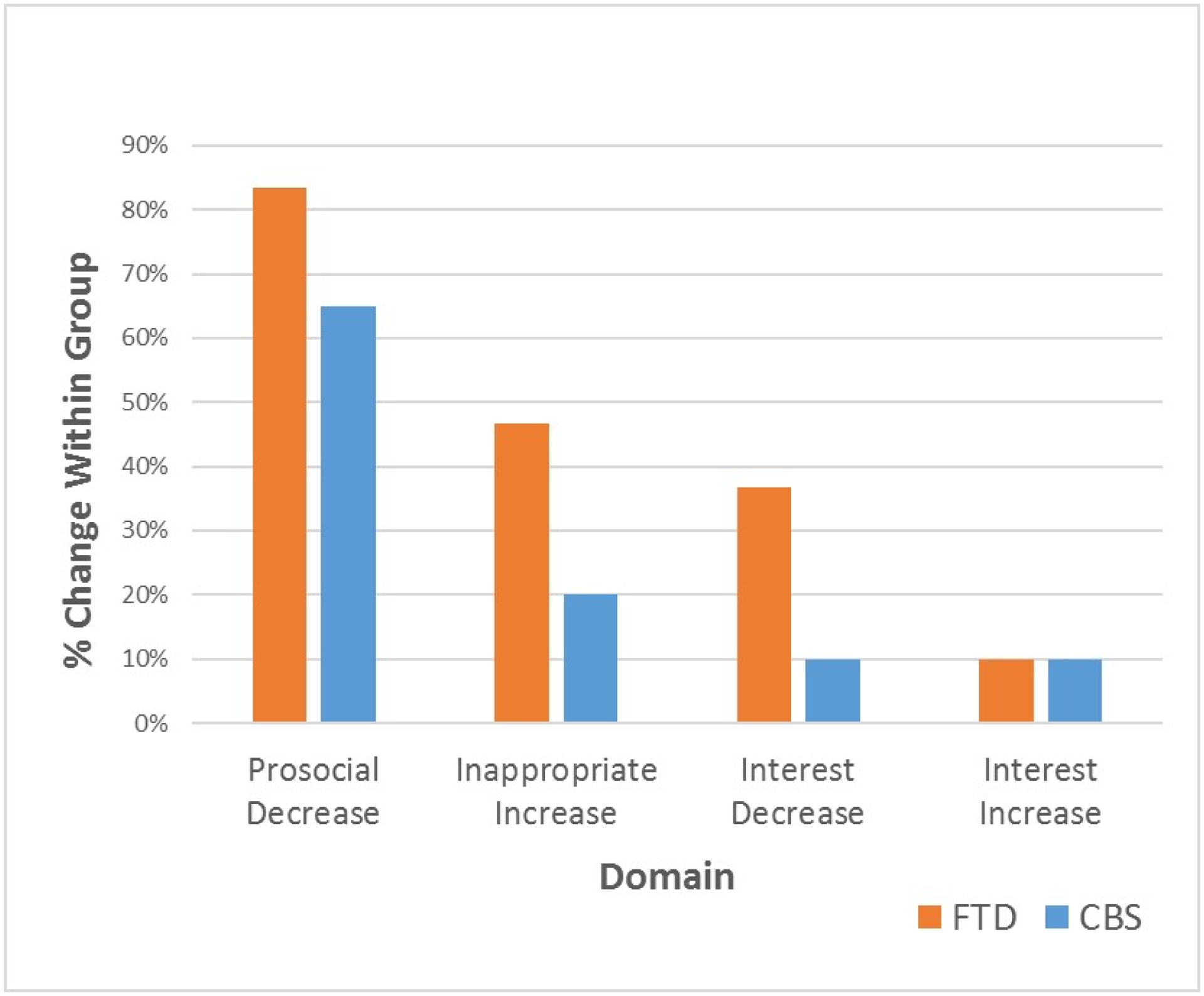
Frequency of Sexual Changes in FTD and CBS Patients
* Percentage based on the number of patients per group (FTD: N = 30; CBS: N=20) who showed a change in the indicated direction.
In FTD patients, changes in inappropriate sexual behaviors were negatively correlated with overall behavioral symptoms, as measured by both the total FrSBe change score, (r = −0.42, p = 0.02), and the total NPI score (r = −0.45, p = 0.02). No other significant correlations were found.
FDG-PET
Of the domains analyzed, only prosocial sexual behaviors significantly correlated with regional metabolism on FDG-PET scans. Specifically, hypometabolism of Brodmann’s Area 10 (BA10), within the right frontal pole, was associated with a decrease in prosocial sexual behaviors (single cluster size=44 voxels, Talairach Daemon coordinates for cluster max voxel at [18, 62, 4] mm, max T value = 4.45, FWE-corrected p < 0.05), see Figure 2 & Figure 3. To confirm that our findings were not confounded by atrophy in the frontal areas, analyses were re-run using estimated total intracranial volume (eTIV) based on Free-Surfer analysis [33] of T1 scans as an additional covariate, which resulted in the same region of significant correlation. We did not include eTIV as a covariate in the final analysis, in order to avoid excluding a participant who had only received a T2-weighted MRI scan.
Figure 2:
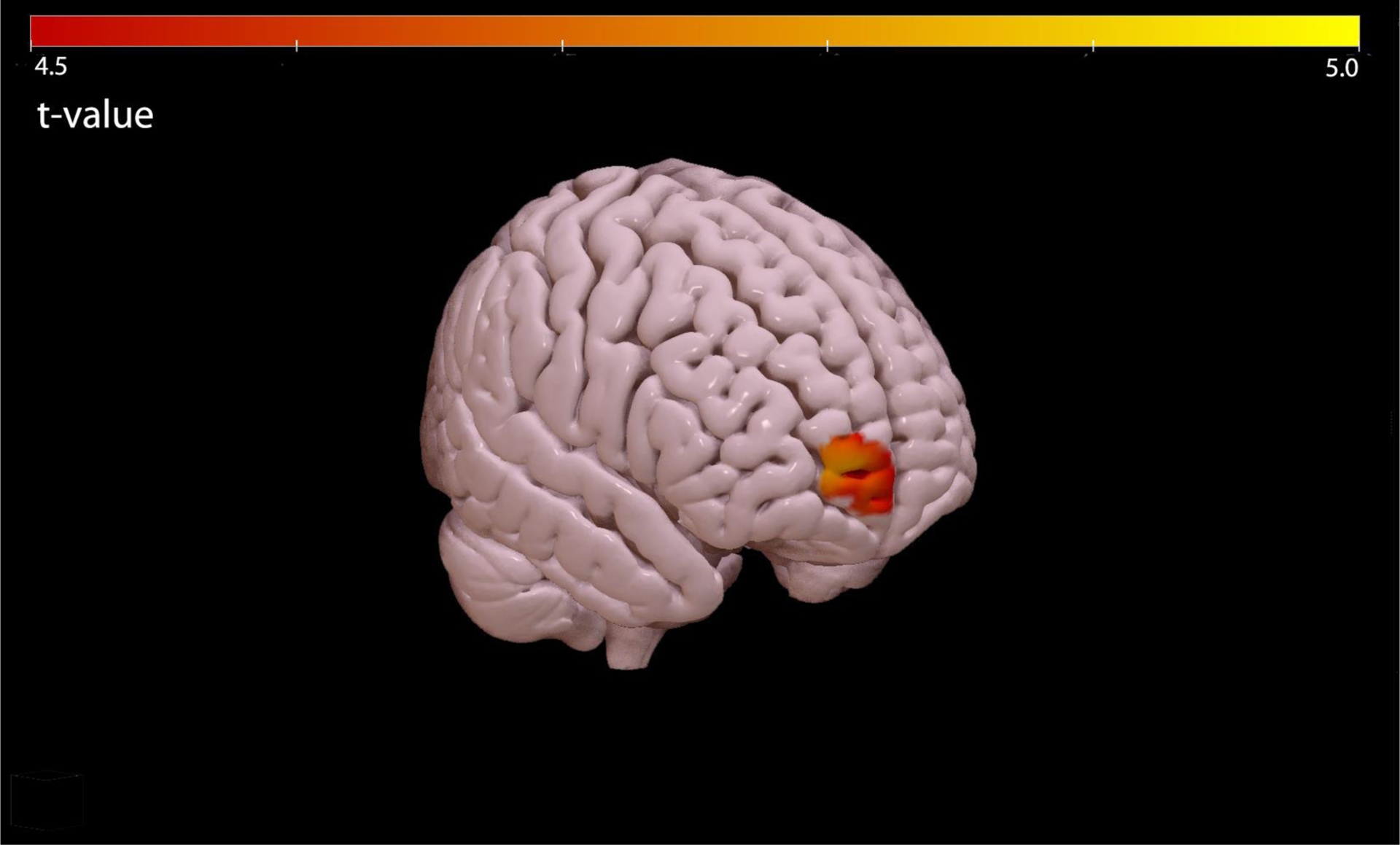
Region within BA10 associated with reduced prosocial sexual behaviors in patients with FTD and CBS.
Figure 3:
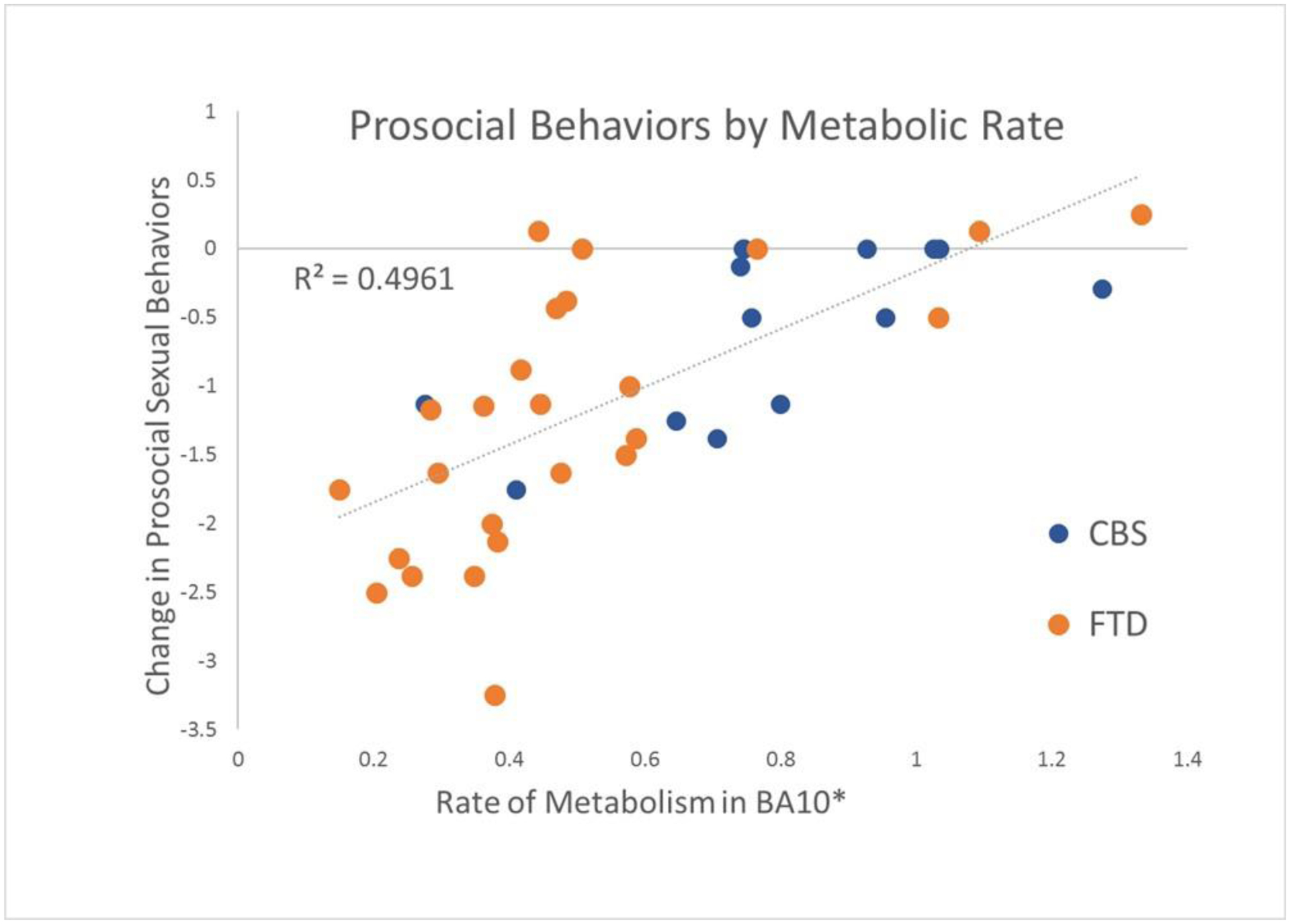
Prosocial Behaviors by Metabolic Rate
*Voxel within BA10 most strongly associated with scores on the Prosocial Sexual Behaviors subscale (Cluster max voxel, MNI coordinates 18–4, 720, −4 mm). Rates shown above are multiplied by 10−4.
† Negative change scores indicate a reduction in prosocial sexual behaviors
DISCUSSION
The goal of this study was to characterize sexual changes in patients with FTD and to determine whether these changes correlated with regional neural metabolism. We found that decreased prosocial sexual behaviors, including expressing love and performing romantic actions, were significantly reduced in FTD patients as compared to other groups, that this was the most common change in FTD patients, and that it was associated with hypometabolism in the right frontal pole within BA 10. We also found that patients with FTD had increased inappropriate sexual behaviors compared to the other groups, although this change did not correlate with regional metabolism.
SNIQ Group Differences
Patients with FTD had a greater reduction in prosocial sexual behaviors than patients with CBS and NC, consistent with prior reports [8, 13]. It is interesting that the change was greater in men than women, a distinction not previously reported. In our open response questions, multiple informants elaborated on the nature of these prosocial changes, stating that their partners were less attentive to their sexual satisfaction and less interested in foreplay. One wife writes, “Changes in sexual behavior are consistent with other behavior changes, i.e., he is interested in fulfilling his own needs only and isn’t able to consider another person’s needs/wants.” Patients with CBS also showed reduced prosocial sexual behaviors compared to NC.
Of the changes examined, reduced prosocial sexual behaviors were by far the most common in our FTD sample, reported in 25 of 30 patients. This is notable given the relative lack of emphasis on these symptoms in FTD literature. It may be that private changes within a relationship garner less clinical attention than outwardly inappropriate behaviors, even if the former are more common, severe, or distressing. Caregivers may be reluctant to volunteer the details of their sexual and romantic lives without explicit querying.
As expected, patients with FTD showed increased inappropriate sexual behaviors compared to other groups. However, the increase in inappropriate sexual behaviors was found in the absence of a group difference in change in sexual interest. In fact, a decrease in sexual interest was more prevalent among FTD patients than an increase. As others have suggested, inappropriate sexual behaviors may be due not to an increase in sexual interest, but rather to social-cognitive deficits [7, 8, 13]. For example, patients could engage in sexually inappropriate behaviors for reasons including but not limited to loss of the knowledge that certain behaviors are socially prohibited, inability to differentiate situations in which behaviors are or are not appropriate, or failure to perceive the disapproval of others and adjust behaviors accordingly [34].
It is important to note however that three patients did have increased sexual interest, and two experienced a past period of increased sex drive prior to a decrease. Future research might investigate whether this early increase in sexual interest represents a prodromal or unique phase of the disease in a subgroup of patients, as has been suggested [3]. Similarly, it is interesting that more severe behavioral changes as reported on the FrSBe and NPI was associated with fewer inappropriate sexual behaviors. It is possible that with disease progression, patients become incapable of enacting such behaviors, due to either functional decline or external intervention.
PET Findings
Reduced prosocial sexual behaviors were associated with hypometabolism within BA 10, specifically within the right frontal pole. Research has indicated that BA 10 [35, 36], and the frontal pole specifically [37, 38], is activated during tasks of “social knowledge,” including mentalizing and theory of mind (i.e. the ability to infer the knowledge, motives, and mental states of others). In addition, the right hemisphere has been preferentially associated with behavioral symptoms in FTD [2, 39–42]. Romantic actions, such as taking a spouse to dinner, expressing love, or being physically affectionate outside of sex, necessitate consideration of the feelings of one’s partner, and an understanding of what he or she might want—both of which require theory of mind. Damage to BA 10 has also been associated with apathy and emotional blunting, characteristic early symptoms of FTD [43, 44]. It is thus possible that changes in sexual behaviors are related to other symptoms of FTD. Patients whose symptoms include apathy and emotional blunting may no longer have the same need for intimacy, or be able to recognize and empathize with the emotional needs of their partners [11].
Our findings are partially consistent with previous findings associating hyposexuality in FTD with reduced gray matter volume in the right frontal cortex, specifically the posterior supramarginal and middle frontal gyri, although associations were also found with subcortical areas, including the thalamus [13]. It is possible that all regions are involved in prosocial sexual behaviors, or alternatively that the different scales used in each study were sensitive to overlapping but distinct constructs.
Interestingly, reduced prosocial sexual behaviors were identified in patients with CBS as well as with FTD, and visual inspection of the scatterplot indicates that the correlation was present across both groups (Figure 3). This raises the possibility of a common neuroanatomical driver of this change across neurodegenerative disorders. Future research into neuroanatomical associations of sexual symptoms in neurodegenerative disease may benefit from taking a cross-diagnosis approach.
Limitations
There were several limitations to our study. In general, we had few endorsements of items pertaining to inappropriate sexual behaviors and increased sexual interest, a result seen in other studies [8, 9, 13]. While of interest, this may have limited our ability to find group differences and uncover functional associations with these domains. In addition, nearly all respondents were white and heterosexual, which limits the generalizability of our results.
Another concern was that the anatomical correlate identified during our imaging analysis was close to the edge of the brain, and therefore might be an artifact of atrophy in frontal areas. To correct for this possibility, we used an anatomical mask to limit findings to expected ROIs within cortical and subcortical regions, and we re-ran our analysis with eTIV as a covariate. The additional covariate did not change our outcome. Figure 3 also shows a correlation (R2=0.50) within both patient groups between metabolic activity in the cluster maximum voxel and change in prosocial sexual behaviors. These additional analyses support the validity of our finding.
It should also be noted that while SNIQ scores were adjusted for baseline sexual behaviors, due to the nature of our study we were not able to control for baseline FDG uptake in members of our sample.
An additional limitation is that many patients in our sample were taking psychotropic medications for behavioral symptoms, including SSRIs and antipsychotics such as olanzapine and risperidone, which are known to cause sexual dysfunction [45, 46]. Additionally, a small number of patients with CBS (n=7) were taking either Sinemet or Mirapex, dopaminergic drugs which in rare cases may cause hypersexual behaviors [47, 48]. We did not account for the possible interference of patient medication in our analyses.
Finally, while the results of our analyses raise the possibility of impaired theory of mind in our patient group, we did not administer any tests to directly measure this construct. Future studies may more directly investigate the hypothesis that patients with FTD experience impaired theory of mind in conjunction with apathy and emotional withdrawal.
Clinical Implications
Our research associates reduced prosocial sexual behaviors with decreased activity in the prefrontal cortex. In our sample, these so-called “negative” symptoms occurred in more patients with FTD than inappropriate sexual behaviors or changes in sexual interest. Reduced prosocial sexual behaviors and inappropriate sexual behaviors were not mutually exclusive: all but two of the 14 FTD patients who showed increased inappropriate sexual behaviors also had decreased prosocial sexual behaviors. These symptoms appeared to be present in many patients without an increase in sexual interest.
Our results have important clinical implications. Clinicians should be aware of the possibility of overlapping sexual symptoms. The terms “hypersexual” and “disinhibited” may not accurately describe common sexual symptoms of FTD--the term “inappropriate sexual behavior”, or simply a description of the behavior, may be more accurate and clinically useful. Clinicians should ask and educate patients with FTD and their spouses about sexual symptoms, with particular attention given to negative changes, such as reduced ability for intimacy and affection in the context of relationships.
Supplementary Material
ACKNOWLEDGMENTS
This work was previously presented at the 2019 International Neuropsychological Conference, and was published as a conference abstract in the Journal of the International Neuropsychological Society. We thank Karen Detucci and Alyson Cavanagh for patient testing. We thank Luke Hearne for imaging consultation. We thank the patients and caregivers who participated in this study, without whom this work would not be possible.
FUNDING
This work was supported by the Intramural Program of The National Institutes of Health / The National Institute of Neurological Disorders and Stroke, by a grant from the Division of Extramural Research of The National Institutes of Health / The National Institute of Neurological Disorders and Stroke to EDH [R00 NS060766], and by a grant from The National Institutes of Health / The National Institute of Neurological Disorders and Stroke to EDH and SC [4R01 NS076837-05].
Footnotes
Note: given the heterogeneity of our FTD sample, we ran the between-groups ANOVAs with only bvFTD patients included in the FTD group. The significance of all results was unchanged. Therefore, in order to retain maximum power, all FTD phenotypes were included in the main analyses.
CONFLICT OF INTEREST/DISCLOSURE STATEMENT
The authors report no conflicts of interest.
References
- [1].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mendez MF, Chen AK, Shapira JS, Miller BL (2005) Acquired sociopathy and frontotemporal dementia. Dementia and geriatric cognitive disorders 20, 99–104. [DOI] [PubMed] [Google Scholar]
- [3].Mendez MF, Shapira JS (2013) Hypersexual behavior in frontotemporal dementia: a comparison with early-onset Alzheimer’s disease. Arch Sex Behav 42, 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cipriani G, Ulivi M, Danti S, Lucetti C, Nuti A (2016) Sexual disinhibition and dementia. Psychogeriatrics 16, 145–153. [DOI] [PubMed] [Google Scholar]
- [5].Torrisi M, Cacciola A, Marra A, De Luca R, Bramanti P, Calabrò RS (2017) Inappropriate behaviors and hypersexuality in individuals with dementia: an overview of a neglected issue. Geriatrics & gerontology international 17, 865–874. [DOI] [PubMed] [Google Scholar]
- [6].De Medeiros K, Rosenberg PB, Baker AS, Onyike CU (2008) Improper sexual behaviours in elders with dementia living in residential care. Dementia and geriatric cognitive disorders 26, 370–377. [DOI] [PubMed] [Google Scholar]
- [7].Miller BL, Darby AL, Swartz JR, Yener GG, Mena I (1995) Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia 6, 195–199. [DOI] [PubMed] [Google Scholar]
- [8].Ahmed RM, Kaizik C, Irish M, Mioshi E, Dermody N, Kiernan MC, Piguet O, Hodges JR (2015) Characterizing sexual behavior in frontotemporal dementia. Journal of Alzheimer’s Disease 46, 677–686. [DOI] [PubMed] [Google Scholar]
- [9].Fieo RA, Silverman H, O’Shea D, Manoochehri M, Grafman J, Huey ED (2018) Establishing dimensionality of sexual behaviours in patients with regional brain dysfunction. Brain Injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rees PM, Fowler CJ, Maas CP (2007) Sexual function in men and women with neurological disorders. The Lancet 369, 512–525. [DOI] [PubMed] [Google Scholar]
- [11].Nordvig AS, Goldberg DJ, Huey ED, Miller BLJN (2019) The cognitive aspects of sexual intimacy in dementia patients: a neurophysiological review. 1–9. [DOI] [PubMed] [Google Scholar]
- [12].Black B, Muralee S, Tampi RR (2005) Inappropriate sexual behaviours in dementia. Journal of geriatric psychiatry and neurology 18, 155–162. [DOI] [PubMed] [Google Scholar]
- [13].Ahmed RM, Goldberg ZL, Kaizik C, Kiernan MC, Hodges JR, Piguet O, & Irish M (2018) Neural correlates of changes in sexual function in frontotemporal dementia: implications for reward and physiological functioning. Journal of Neurology 265, 2562–2572. [DOI] [PubMed] [Google Scholar]
- [14].Zamboni G, Grafman J, Krueger F, Knutson K, Huey E (2010) Anosognosia for behavioral disturbances in frontotemporal dementia and corticobasal syndrome: a voxel-based morphometry study. Dementia and geriatric cognitive disorders 29, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY (2011) Clinicopathological correlations in corticobasal degeneration. Annals of neurology 70, 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Albrecht F, Bisenius S, Schaack RM, Neumann J, Schroeter ML (2017) Disentangling the neural correlates of corticobasal syndrome and corticobasal degeneration with systematic and quantitative ALE meta-analyses. npj Parkinson’s Disease 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lillo P, Garcin B, Hornberger M, Bak TH, Hodges JR (2010) Neurobehavioral features in frontotemporal dementia with amyotrophic lateral sclerosis. Archives of Neurology 67, 826–830. [DOI] [PubMed] [Google Scholar]
- [18].Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J (2009) Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain 132, 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cosseddu M, Benussi A, Gazzina S, Alberici A, Dell’Era V, Manes M, Cristillo V, Borroni B, Padovani A (2019) Progression of behavioural disturbances in frontotemporal dementia: a longitudinal observational study. European journal of neurology. [DOI] [PubMed] [Google Scholar]
- [20].Murley AG, Coyle-Gilchrist I, Rouse M, Jones PS, Li W, Wiggins J, Lansdall C, Vázquez PR, Wilcox A, Tsvetanov KA (2019) Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boeve BF (2005) Corticobasal Degeneration: The syndrome and the disease In Atypical Parkinsonian Disorders: Clinical and research aspects, Litvan I, ed. Humana Press Inc., Totowa, NJ, pp. 309–334. [Google Scholar]
- [22].Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert P, Albert M (1998) Frontotemporal lobar degeneration A consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. [DOI] [PubMed] [Google Scholar]
- [23].Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ (2007) A study of sexuality and health among older adults in the United States. N Engl J Med 357, 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- [25].Grace J, Malloy P (2001) Frontal Systems Behavior Scale (FrSBe): Professional Manual, Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- [26].Mattis S (1976) Mental status examination for organic mental syndrome in the elderly patient. Geriatric psychiatry: A handbook for psychiatrists and primary care physicians. [Google Scholar]
- [27].Schroeter ML, Raczka K, Neumann J, Von Cramon DY (2008) Neural networks in frontotemporal dementia—a meta-analysis. Neurobiology of aging 29, 418–426. [DOI] [PubMed] [Google Scholar]
- [28].Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML (2008) Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of neurology 65, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, Rossor AM, Stevens JM, Cipolotti L, Rossor MN (2001) Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Annals of neurology 49, 433–442. [PubMed] [Google Scholar]
- [30].Miller BL, Gearhart R (1999) Neuroimaging in the diagnosis of frontotemporal dementia. Dementia and geriatric cognitive disorders 10, 71–74. [DOI] [PubMed] [Google Scholar]
- [31].Schroeter ML, Raczka K, Neumann J, Von Cramon DY (2007) Towards a nosology for frontotemporal lobar degenerations—a meta-analysis involving 267 subjects. Neuroimage 36, 497–510. [DOI] [PubMed] [Google Scholar]
- [32].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- [33].Fischl BJN (2012) FreeSurfer. 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huey ED, Zahn R, Grafman J (2007) “[H] E IS NO MORE A PERSON NOW BUT A WHOLE CLIMATE OF OPINION”(). Cortex; a journal devoted to the study of the nervous system and behavior 43, 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nature reviews. Neuroscience 7, 268. [DOI] [PubMed] [Google Scholar]
- [36].Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW (2006) Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of cognitive neuroscience 18, 932–948. [DOI] [PubMed] [Google Scholar]
- [37].Bludau S, Eickhoff SB, Mohlberg H, Caspers S, Laird AR, Fox PT, Schleicher A, Zilles K, Amunts KJN (2014) Cytoarchitecture, probability maps and functions of the human frontal pole. 93, 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Orr JM, Smolker HR, Banich MTJPO (2015) Organization of the human frontal pole revealed by large-scale DTI-based connectivity: implications for control of behavior. 10, e0124797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J (2008) Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology 71, 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Eslinger PJ, Moore P, Troiani V, Antani S, Cross K, Kwok S, Grossman M (2007) Oops! Resolving social dilemmas in frontotemporal dementia. Journal of Neurology, Neurosurgery & Psychiatry 78, 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mychack P, Kramer J, Boone K, Miller B (2001) The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology 56, S11–S15. [DOI] [PubMed] [Google Scholar]
- [42].Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL (2005) Neuroanatomical correlates of behavioural disorders in dementia. Brain 128, 2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kipps CM, Hodges JR (2006) Theory of mind in frontotemporal dementia. Social Neuroscience 1, 235–244. [DOI] [PubMed] [Google Scholar]
- [44].Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL (2006) Structural anatomy of empathy in neurodegenerative disease. Brain 129, 2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bella AJ, Shamloul R (2013) Psychotropics and sexual dysfunction. Central European journal of urology 66, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F (2001) Incidence or sexual dysfunction associated with antidepressant agents: A prospective multicenter study of 1022 outpatients. The Journal of clinical psychiatry. [PubMed] [Google Scholar]
- [47].Oei NY, Rombouts SA, Soeter RP, Van Gerven JM, Both S (2012) Dopamine modulates reward system activity during subconscious processing of sexual stimuli. Neuropsychopharmacology 37, 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Both S, Everaerd W, Laan E, Gooren L (2005) Effect of a single dose of levodopa on sexual response in men and women. Neuropsychopharmacology 30, 173–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


