Abstract
TRMU is a nuclear gene crucial for mitochondrial DNA translation by encoding tRNA 5-methylaminomethyl-2-thiouridylate methyltransferase, which thiolates mitochondrial tRNA. Biallelic pathogenic variants in TRMU are associated with transient infantile liver failure. Other less common presentations such as Leigh syndrome, myopathy, and cardiomyopathy have been reported. Recent studies suggested that provision of exogenous L-cysteine or N-acetylcysteine may ameliorate the effects of disease-causing variants and improve the natural history of the disease. Here, we report six infants with biallelic TRMU variants, including four previously unpublished patients, all treated with exogenous cysteine. We highlight the first report of an affected patient undergoing orthotopic liver transplantation, the long-term effects of cysteine supplementation, and the ability of the initial presentation to mimic multiple inborn errors of metabolism. We propose that TRMU deficiency should be suspected in all children presenting with persistent lactic acidosis and hypoglycemia, and that combined N-acetylcysteine and L-cysteine supplementation should be considered prior to molecular diagnosis, as this is a low-risk approach that may increase survival and mitigate the severity of the disease course.
Keywords: TRMU, liver failure, cysteine, mitochondrial disorder, orthotopic liver transplant
Introduction
The mitochondrial hepatopathies comprise several nuclear-encoded mitochondrial disorders that classically present with liver failure, often accompanied by neurodevelopmental delay. Most commonly, these disorders are attributed to biallelic pathogenic variants in POLG, MPV17, and DGUOK. The molecular defect in these cases is a disorder of mitochondrial DNA (mtDNA) maintenance, resulting in depletion of mtDNA. Affected patients typically present in infancy with progressive liver failure and risk of imminent death secondary to severe lactic acidosis and hypoglycemia. [1–3].
Zeharia et al described the first cohort of patients with TRMU deficiency in 2009 (OMIM #613070) [4]. The patients received supportive care for coagulopathy, gastrointestinal bleeding, hyperlactatemia, and failure to thrive. Although hepatic failure was fatal in some cases, the majority had spontaneous clinical improvement within two to three weeks of symptom onset. Nine patients survived the initial insult without need for liver transplantation. On long-term follow-up, the patients who survived were alive and well without recurrence of liver failure.
To date, over twenty patients with biallelic pathogenic variants in TRMU have been described in the literature. These patients have presented in infancy with growth failure, elevated liver enzymes, hyperlactatemia, hypoglycemia, and a variety of neurodevelopmental abnormalities. The majority of patients exhibited spontaneous recovery of liver function, although some survivors developed cirrhosis, liver nodules, and persistent hepatomegaly [4–10]. Notably, despite the transient nature of the liver dysfunction in many patients, several patients with TRMU deficiency have died: 4/13 in the Zeharia cohort and roughly one in three in most case series [4,5,11]. Orthotopic liver transplantation (OLT) has been suggested as a therapeutic option for these patients, but to this date there are no reports of patients with TRMU deficiency undergoing OLT [8].
The mitochondrial transfer RNA (mt-tRNA) thiouridylase encoded by TRMU utilizes cysteine as the substrate for thiolation, a critical post-transcriptional modification to mt-tRNAs to allow for “wobble” during translation. During the first months of life, cysteine is a conditionally essential amino acid due to the physiologic nadir in activity of the cystathionase enzyme, which maintains the endogenous cysteine supply [12,13]. Recent reports suggested that supplementation of L-cysteine and/or N-acetylcysteine can mitigate the effects of disease-causing variants in TRMU, both in vitro and in vivo [11,14,15]. The long-term effects of cysteine supplementation have not yet been reported.
In this report, we describe the clinical course of six infants diagnosed with TRMU deficiency at two different large medical centers. We emphasize the first infant reported to undergo OLT, multiple patients whose initial presentations lacked features of liver failure, and the effects of cysteine supplementation, both long-term and pre-symptomatically.
Materials and Methods
Patients with genetically confirmed TRMU deficiency were seen in the inpatient and outpatient departments of either Children’s Hospital of Philadelphia or Texas Children’s Hospital between June 2016 and December 2019.
All patients provided written informed consent under a research protocol reviewed and approved by the institutional review board of each institution.
Molecular testing for TRMU deficiency was performed commercially either through trio whole exome sequencing (WES) in four patients, or targeted known familial mutation analysis for two patients, through GeneDx laboratories (Patients 1–4) and Baylor Genetics (Patients 5 and 6). Variant analysis and interpretation were performed by the commercial laboratories using the guidelines for standard interpretation of sequence variants published by the American College of Medical Genetics and Genomics [16].
Results
Six patients with genetically confirmed TRMU deficiency were identified at Children’s Hospital of Philadelphia or Texas Children’s Hospital. 2/6 patients (Patients 5 and 6) have been previously published [11].
Patient 1.
Patient 1 was a full-term female born after an uncomplicated pregnancy. Birth weight was appropriate for gestational age. The postnatal course was complicated by prolonged time to regain birthweight (two weeks). At six weeks of life, she stopped gaining weight and subsequently exhibited decreased suck strength and loss of head control. Initial work-up revealed hypoglycemia (value not available from outside hospital medical record) and lactic acidosis (Table 1), prompting admission to the local hospital with a working diagnosis of glycogen storage disease (GSD) type Ia. However, hypoglycemia persisted and lactic acidosis worsened with increasing glucose infusion rate, and liver ultrasound was normal, all pointing away from this working diagnosis.
Table 1.
Patient lab values at initial presentation (I), peak of illness (P), and most recent (R). Age at initial, peak, and most recent lab values provided in parentheses.
| Phase | Bicarbonate | Lactate | Ammonia | CK | AST | ALT | PT/INR | PTT | Conjugated bilirubin | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ref. range | 18–29 mmol/L | 0.5–1.6 mmol/L | 9–33 umol/L | 60–305 U/L | 20–64 U/L Patients 1–4 35–140 U/L Patients 5–6 |
12–42 U/L Patients 1–4 6–50 U/L Patients 5–6 | 10.9–13.4s / 0.8–1.2 | 22–36s | 0–0.3 mg/dL | |
| Patient 1 | I (3m) | 11 | 12.7 | 11 | 60 | 151 | 87 | 16/1.32 | 34.7 | 0.2 |
| P (3m) | 18.5 | 38 | 1383 (15m) | 376 | 165 | 21.4/1.86 | 46.6 | 0.1 | ||
| R (41m) | 3.0 | <9 | 682 | 98 | 40 | 12.6/1.04 | 27.9 | 0.6 | ||
| Patient 2 | I (2m) | 24 | 2.5 | 33 | 91 (1m) | 48 (1m) | 15.8/1.2 | 38 | 1.5 (1m) | |
| P (6m) | 9.7 | 83 | 142 (10m) | 1322 | 918 | 16.2/1.6 | 38 (2m) | 1.5 (1m) | ||
| R (10m) | 4.8 | 14 | 142 | 161 | 121 | 14.3/1.2 | 33.3 | 0.8 (10m) | ||
| Patient 3 | I (2m) | 3.6 | 10.7 | <9 | 711 | 190 | 324 | 19.3/1.79 | 60.6 | 1.3 |
| P (3m) | 19.3 (9w) | 393 | 4099 | 2615 | 503 | 43/4.26 | 192.4 | 20.9 | ||
| R (3y) | 1.4 (19m) | 17 (13m) | 67 | 67 | 12.6/1.1 | 33 | 0 | |||
| Patient 4 | I (2w) | 22 (3w) | 10.8 | 51 | 79 | 64 | 16.5/1.3 (3w) | 40.6 (3w) | <0.1 | |
| P (3m) | 24.8 | 393 | 158 (2m) | 367 | 65 (5d) | 38.4/3.77 | 199.2 | 0.7 | ||
| R (N/A) | ||||||||||
| Patient 5 | I (<1w) | 7 | 14.8 | 165 | 221 | 89 | 24 | 15.1 / 1.2 (1m) | 32.0 (1m) | 0.0 |
| P (2m) | >19.9 | 344 (<1w) | 1228 | 309 | 62.1 / 7.2 | 53.5 | 8.3 | |||
| R (N/A) | ||||||||||
| Patient 6 | I (<1w) | 16 | 5.7 | 17 | 64 | 24 | 15.7 / 1.2 | 35.7 | 0.0 | |
| P (3m) | 41.9 | 90 (2m) | 73 (2m) | 540 | 347 | 19.7 / 1.6 | 34.8 (2m) | 8.5 | ||
| R (15m) | 5.5 | 117 | 72 | 15.4 / 1.2 | 0.0 |
m: months, w: weeks. Reference ranges for PT/INR and PTT are approximate, as normal reference values vary in infancy.
There was persistent, mild liver dysfunction (Table 1). The disease process peaked with severe lactic acidosis (Table 1). The patient required markedly high intravenous glucose infusion rate (peak 13) and exogenous bicarbonate supplementation, all suggestive of a mitochondrial disorder or disorder of fatty acid oxidation, with relatively preserved liver function. A gastrostomy tube was placed for enteral feedings and enteral 20% dextrose administration. Laboratory values gradually improved and she was discharged home at the age of four months after a months-long ICU stay on continuous enteral 20% dextrose, enteral bolus feeds, and enteral bicarbonate supplementation (28 mEq/kg/day). By the time of discharge, she no longer had hypotonia, and she was able to sit with support and roll from stomach to back, appropriate development for age.
Family history was notable for a clinical diagnosis of Leigh syndrome in a maternal first cousin once removed (no molecular testing performed), Duchenne muscular dystrophy in three maternal first cousins once removed, and father with multiple endocrine neoplasia type IIA.
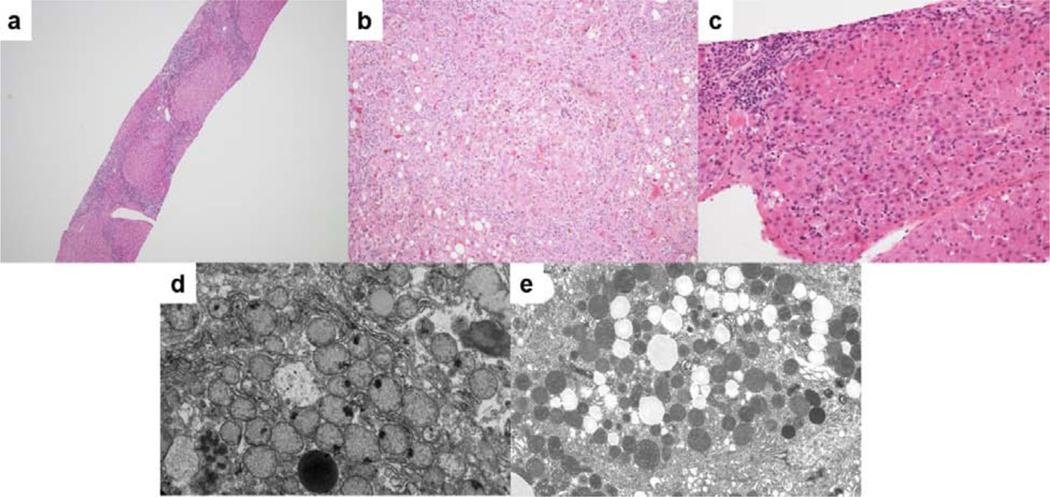
Liver histology showed bridging fibrosis and focal eosinophilic ground-glass appearance without necrosis, fatty change, or giant cell transformation (Supplemental File 1, Figure 1). Liver mtDNA quantification was above the range associated with mtDNA depletion. Muscle histology showed round muscle fibers with severe variation in size and shape, fibrosis, rimmed vacuoles, and numerous ragged red fibers on trichrome staining (Supplemental File 1, Supplemental Figure 1). ETC enzymology showed very low activities of complexes I, III, and IV in the liver, and low activity of complex IV in the muscle, with normal complex II and citrate synthase activity in both. Fibroblast ETC enzymology was normal.
Figure 1.
Liver histology images for Patient 1. A) Portal inflammation with bridging fibrosis and nodularity. B) Foci of oncocytic hepatocytes. C) Parenchymal collapse with steatosis, cholestasis, and hepatocellular multinucleation. D) Numerous mitochondria with loss of cristae and focal densities. E) Fat droplets and increased numbers of mitochondria with dense internal structure.
During the initial hospitalization, clinical trio WES with mitochondrial genome sequencing revealed no pathogenic variants. Chromosomal microarray analysis revealed a novel 1.9kb deletion of uncertain significance on 7p13.1. Approximately two years after the initial exome report, formal WES re-analysis was ordered, with special attention to TRMU. This uncovered a likely pathogenic missense variant and a pathogenic one and a half exon deletion in TRMU, in trans with confirmed parental heterozygous state (Table 2).
Table 2.
Patient genotypes and treatment.
| TRMU variants | Therapeutic Interventions | ||
|---|---|---|---|
| Patient | |||
| At diagnosis | Current | ||
| 1 | -c.1084G>A/p.A362T (LP) -deletion 22q13.31 46730453–4673227 (P) |
Biotin Citrulline Arginine Niacinamide B50 Bicarbonate |
Ubiquinol Citrulline N-acetylcysteine |
| 2 | -c.1084G>A/p.A362T (LP) -deletion 22q13.31 46730453–4673227 (P) |
Bicarbonate N-acetylcysteine L-cysteine |
N-acetylcysteine L-cysteine |
| 3 | -c.246C>G/p.F82L (LP) -c.525_527delCTT/p.F176del (LP) |
Carnitine Thiamine Folate Biotin |
Bicarbonate N-acetylcysteine |
| 4 | c.1142G>A/p.G381E (LP) c.37_48dup12/p.G13_D16dup (LP) |
Levocarnitine Riboflavin Thiamine Biotin Bicarbonate |
Deceased |
| 5 | c.117G>A/p.W39X (P) c.680G>C/p.R227T (VUS) |
Carnitine Carglumic acid Ketogenic diet |
Deceased |
| 6 | c.117G>A/p.W39X (P) c.680G>C/p.R227T (VUS) |
Hominex-1™ N-acetylcysteine Selenium Vitamin C Vitamin E |
N-acetylcysteine L-cysteine Selenium Vitamin C |
P: pathogenic variant, VUS: variant of unknown clinical significance, LP: likely pathogenic variant
At her most recent follow-up evaluation at 45 months of age she was doing well in preschool, with normal cognition and development, able to climb stairs and speak in full sentences. She had a history of subtle expressive speech delay, which resolved by age 42 months. The hypoglycemia resolved spontaneously around six months and lactic acidosis began improving at 12 months. Clinically, she was taking all nutrition by mouth and growing well (55%ile for weight, 32%ile for height). She had no hypoglycemia and acidosis had resolved. She had mildly elevated lactate (baseline 2.5 mmol/L) and mild elevation of transaminases with no evidence of synthetic liver dysfunction. She had persistently elevated creatine kinase (CK) levels (Table 1) with no clinical symptoms of myopathy. Cardiology, ophthalmology, and audiology evaluations were all within normal limits.
During her initial presentation, she received a number of vitamins and supplements, including biotin, citrulline, arginine, niacinamide, and B50. After muscle and liver biopsy, she was started on N-acetylcysteine (110mg/kg/day). At this time, she remains on ubiquinol (5 mg/kg/day), citrulline (40 mg/kg/day), and N-acetylcysteine (90 mg/kg/day). Growth Differentiation Factor 15 (GDF15) level was >6000 at 16 months and 1644 and three years.
Patient 2.
Patient 2, the younger brother of Patient 1, was born full term after an uncomplicated pregnancy. Based on the family history, he was prenatally diagnosed with TRMU deficiency via amniocentesis. L-cysteine supplementation was started prenatally, during the third trimester, at a dose of 500mg twice a day.
In the immediate postnatal period, he received supplementation with bicarbonate (1.2 mEq/kg/day), N-acetylcysteine (150mg/kg/day), and L-cysteine (140 mg/kg/day) with a goal of providing a total of 300mg/kg/day of cysteine among the two supplements and dietary intake. Bicarbonate supplementation was quickly discontinued due to metabolic alkalosis. His lactate levels were monitored closely and remained in the normal or slightly above normal range. At six months, he was hospitalized with metabolic acidosis, hyperammonemia, and transaminitis in the setting of a viral infection (Table 1). In contrast to his sister, he did not require a protracted critical care hospitalization. Between six and 12 months of age, his lactate rose to 4–6 mmol/L, where it stabilized. He did not require long-term bicarbonate supplementation. He has normal development. He had delay in eating solid foods beyond puree-texture, requiring feeding therapy at 12 months. However, he has been nourished exclusively by mouth. He walked at 12 months and had first words at 15 months. At 24 months of age he participates in tumbling classes and has normal expressive and receptive language.
He was last seen at 12 months old, when he was doing well with no clinical symptoms. Growth was normal (43%ile weight, 5%ile length, 44%ile head circumference). Labwork revealed persistent lactic acidosis, mildly elevated transaminases, and slightly low fibrinogen levels (157, ref. 172–471 mg/dL), but normal coagulation studies, normal CK levels, and normal ammonia level (Table 1). He remains on N-acetylcysteine (110 mg/kg/day) and L-cysteine (85 mg/kg/day) supplementation. GDF15 level was 2984 at five weeks and >6000 at nine months.
Patient 3.
Patient 3 was born full term via repeat cesarean section after an uncomplicated pregnancy. At birth, he was noted to be tachypneic, but was discharged home in the usual time frame without need for respiratory support. Around two months of age, he presented with feeding intolerance and dehydration. On initial presentation, he exhibited severe lactic acidosis and ketotic hypoglycemia (Table 1). Further investigation revealed coagulopathy, transaminitis, and elevated CK (Table 1).
His hospital course was complicated by progressively worsening liver function resulting in hyperbilirubinemia, hyperammonemia, hepatomegaly, transaminitis, coagulopathy, and ascites (Table 1). He underwent OLT. Post-transplant course was complicated by chronic respiratory failure requiring placement of a tracheostomy. MRI and MR spectroscopy of the brain were normal. Clinical trio WES revealed biallelic likely pathogenic variants in TRMU (Table 2).
In December 2019, he was 37 months old with tracheostomy receiving continuous positive airway pressure (CPAP). He had chronic lung disease and upper airway obstruction. Due to oropharyngeal dysphagia, he received feeds through a gastrostomy tube. Post-transplant course was also complicated by acute cellular rejection and EBV-associated post-transplant lymphoproliferative disease (PTLD) requiring rituximab, with resultant hypogammaglobulinemia. Recent brain MRI, performed due to abnormal gait and frequent falls, revealed symmetric signal abnormalities in deep white matter, consistent with mitochondrial encephalopathy. He had short stature (length <1%ile) with spared weight (18%ile). Because he has been lost to follow-up at our center, we do not have ages at achieving developmental milestones. Outside documentation reports global developmental delay and developmental regression.
Initially, his supplements included levocarnitine, thiamine, folate, and biotin, but he is now maintained on bicarbonate (4.4 mEq/kg/day) and N-acetylcysteine (70 mg/kg/day). He is lost to follow-up at our center but remains on his medications at the initial dosage.
Patient 4.
Patient 4 was born full term after an uncomplicated pregnancy and delivery. The neonatal period was complicated by hypoglycemia at 20 mg/dL requiring intravenous dextrose for two days. At two weeks of life, he presented with lethargy and elevated temperature. Due to the presence of hypoglycemia, hepatomegaly, and elevated lactate (13.3, ref. 0.6–2.0 mmol/L), he received a working diagnosis of GSD type Ia. Liver ultrasound was not completed. He was discharged home on continuous enteral feeds to maintain euglycemia. Genetic testing for GSD type Ia returned negative.
At two months, Patient 4 was admitted for further evaluation of persistent hypoglycemia, accompanied by transaminitis, lactatemia, coagulopathy, and moderate volume ascites (Table 1). Brain MRI revealed symmetric restricted diffusion within the thalami and putamen. The diagnosis of compound heterozygous likely pathogenic TRMU variants was made on clinical trio WES during the initial admission (Table 2). At initial presentation, he was placed on levocarnitine, riboflavin, thiamine, biotin, and bicarbonate supplementation. N-acetylcysteine (150 mg/kg/day) was added upon the diagnosis of TRMU deficiency. GDF15 at four months was >6000.
Over the course of admission, liver function stabilized, with improvement in INR to 1.7, and ascites was well controlled on oral diuretics. Feeding regimen was adjusted to stabilize blood glucose levels. He was discharged at 11 weeks of age, on a regimen of supplements, continuous nasogastric feeds, diuretics for ascites, and close monitoring.
Around three months, he was readmitted with worsening ascites, respiratory failure, hyperammonemia and encephalopathy. Paracentesis was completed twice. Hyperammonemia initially responded to IV ammonia scavenging medications, but later recrudesced, accompanied by worsening coagulopathy, lactic acidosis, progressive respiratory failure, and refractory hypotension (Table 1). Ultimately, life-sustaining measures were withdrawn, and he expired.
Patient 5.
Patient 5 was born full term and received 48 hours of phototherapy for hyperbilirubinemia in the neonatal period. At four days of life she presented to the emergency department for tachypnea and poor feeding. Laboratory evaluations revealed hypoglycemia and acidosis (Table 1). An infectious workup was negative. She remained with persistently elevated lactate and ammonia (Table 1), raising concern for an organic acidemia. She was treated with IV dextrose, carnitine, and carglumic acid. The newborn screen returned normal and treatment for an organic acidemia was discontinued.
Metabolic laboratory work-up included urine organic acid analysis which revealed elevated lactate, plasma acylcarnitine profile with nonspecific elevations, and plasma amino acid analysis with elevated citrulline and significantly elevated alanine (Table 1, Supplemental Table 1), which led to concern for mitochondrial dysfunction. A pyruvate metabolism defect was highly suspected and a ketogenic diet was initiated, but lactate levels remained elevated. At two weeks of age, she again developed tachypnea, metabolic acidosis, hypoglycemia, and further increase in lactate levels (17 mmol/L ref. 0.2–1.7 mmol/L), prompting another infectious work-up, which was again negative. The metabolic decompensation resolved with provision of more calories in the diet. Liver dysfunction, evidenced by elevated transaminases and bilirubin, steadily worsened, and coagulopathy developed (Table 1).
Trio WES revealed compound heterozygous variants in TRMU, one pathogenic and one variant of unknown significance (VUS) (Table 2). Following the diagnosis, the ketogenic diet was discontinued. Provision of exogenous L-cysteine was initiated upon worsening of lactic acidosis. She developed epileptiform activity on electroencephalogram, prompting treatment with anti-epileptic drugs. At the age of 8 weeks, she succumbed to sepsis due to a central line infection. By the time of death, she was on N-acetylcysteine (105 mg/kg/day), as well as vitamin E (200 IU daily), vitamin C (25mg daily), and selenium (15 μg daily) for their antioxidant properties.
Patient 6.
Patient 6, the younger brother of Patient 5, was prenatally diagnosed with TRMU deficiency and treated with cysteine from birth, pre-symptomatically. He was born full term and started on a diet of Similac Advance® with Hominex-1® formula due to its higher concentration of L-cysteine compared to breastmilk or other term infant formulas. In addition to the customized diet, he was started on N-acetylcysteine (105 mg/kg/day), selenium (15 μg/day), vitamin C (25 mg daily), and vitamin E (200 IU daily). Plasma amino acid analysis revealed low methionine levels, thus Hominex-1 was discontinued and L-cysteine supplementation (70 mg/kg/day) was initiated (Supplemental Table 1). He began to grow and develop well with normal liver function, and was discharged home at five weeks of age.
He was readmitted at two months due to increasing transaminases in the setting of emesis. Over a one-month hospitalization, he exhibited coagulopathy requiring blood products, lactic acidosis requiring IV bicarbonate, and hyperammonemia requiring lactulose. Liver dysfunction and overall clinical picture were severe enough to warrant listing for liver transplant (Table 1). He clinically stabilized and was able to be discharged home on oral sodium citrate/citric acid, N-acetylcysteine (70 mg/kg/day), and L-cysteine (300 mg/kg/day).
He had three more admissions in the setting of emesis, hypoglycemia, and difficulty with enteral feeds. By the age of six months, he was tolerating enteral bolus feeds without hypoglycemia. Around 14 months, he had a sudden increase in liver enzymes, prompting a liver biopsy which showed stage 4 micronodular cirrhosis with mitochondrial proliferation. By 15 months, he had stably elevated transaminases and lactate, as well as hepatomegaly (Table 1). He was cruising and babbling, but not yet walking or speaking words. He was last seen at 26 months old and he was able to walk independently with normal fine motor skills, able to feed himself and carry out other activities of daily living. He was able to say a few words. He had normal weight (8%ile) and head circumference (25%ile), but short stature (<0.01%ile). His oral sodium citrate/citric acid supplements have been weaned off. He continues on N-acetylcysteine, L-cysteine, selenium, and vitamin C at the same doses.
Discussion
TRMU deficiency is a rare nuclear-encoded mitochondrial disorder classically characterized by transient, reversible infantile liver failure secondary to a combined respiratory chain defect [4,10]. Although the classical presentation is infantile liver failure, there are reports of patients presenting with other symptoms, including poor feeding, hypoglycemia, myopathy, Leigh syndrome, and cardiomyopathy [4,9,11]. Recent literature has supported the use of exogenous cysteine to ameliorate the effects of disease-causing TRMU variants, but the in vivo data has been limited to two patients [11,14,15].
In this series we have presented six cases of TRMU deficiency, four previously unpublished, with several unique elements. First, all of our patients received cysteine supplementation, in the form of N-acetylcysteine and/or L-cysteine. Second, we present two prenatally diagnosed patients who received pre-symptomatic cysteine supplementation. Third, we present the first report of a child with TRMU deficiency undergoing OLT. Fourth, these cases demonstrate that TRMU deficiency can have heterogeneous presentations, mimicking other inborn errors of metabolism, most commonly glycogen storage disease type IA in our cohort (Table 3).
Table 3.
Clinical presentation, metabolic differential diagnosis, age of cysteine supplementation, and presence/absence of liver failure of patients with TRMU deficiency.
| Patient | Initial Presentation | Differential Diagnosis | Age of onset of cysteine supplementation | Liver Failure* |
|---|---|---|---|---|
| 1 | Failure to thrive, developmental regression, lactic acidosis, hypoglycemia |
GSD type IA Fatty acid oxidation disorder |
17 months | No |
| 2 |
*Prenatal diagnosis Hypoglycemia, metabolic acidosis, hyperammonemia, transaminitis with viral infection |
- | 3rd trimester (Prenatal) | No |
| 3 | Dehydration, lactic acidosis, ketotic hypoglycemia, coagulopathy, transaminitis |
- | 2.5 months | Yes |
| 4 | Hypoglycemia, lactic acidosis, hepatomegaly, coagulopathy | GSD type Ia | 2 months | Yes |
| 5 | Poor feeding, lactic acidosis, hyperammonemia |
Organic acidopathy Pyruvate metabolism defect |
6 weeks | Yes |
| 6 |
*Prenatal diagnosis Emesis, transaminitis |
- | Birth (Presymptomatic) | No |
Liver failure defined by failure of synthetic liver function as defined by INR >2.
The biochemical defect in patients with TRMU deficiency is that of impaired mitochondrial translation. The protein product of TRMU, tRNA 5-methylaminomethyl-2-thiouridylate methyltransferase, modifies a nucleotide in the wobble position of mt-tRNAGlu, mt-tRNALys, and mt-tRNAGln. Thiolation of this nucleotide stabilizes the relevant mt-tRNAs and promotes efficient mitochondrial translation [17]. This thiolation requires cysteine as a sulfur donor. The endogenous production of L-cysteine from L-methionine is catalyzed by the hepatic cystathionase enzyme. The activity of this enzyme increases exponentially with postnatal age, and thus cysteine is considered a conditional essential amino acid in the neonatal period, especially in preterm infants [13]. For infants with TRMU deficiency, the reversibility of the liver failure may reflect increasing physiologic activity of hepatic cystathionase resulting in increased endogenous L-cysteine production and bioavailability [12].
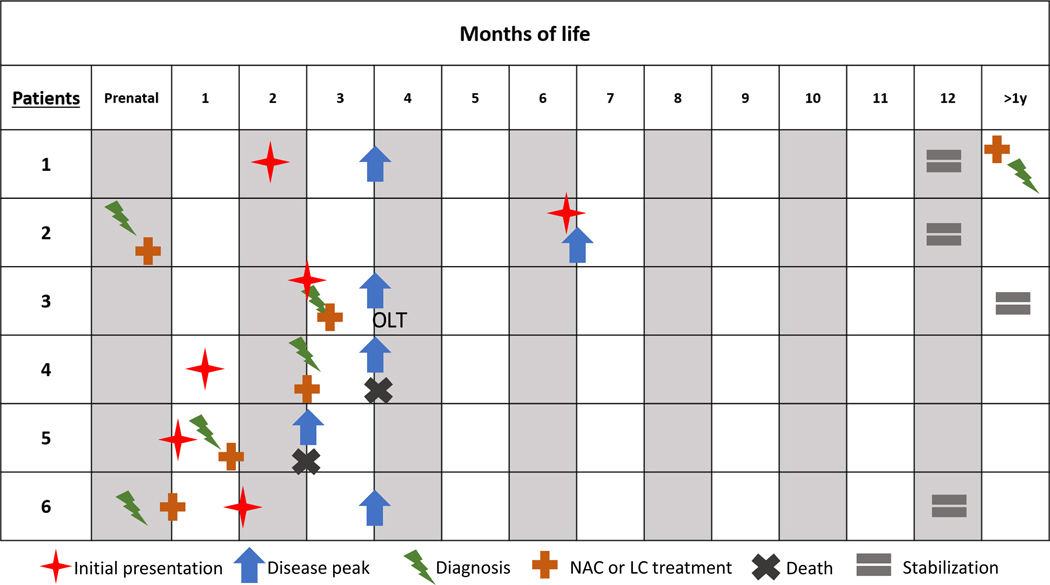
Previously, our group published the first reports of in vivo L-cysteine and N-acetylcysteine supplementation in two siblings (Patients 5 and 6) with TRMU deficiency [11]. Here, we expand this population to six patients, with clinical, laboratory, and developmental features summarized in Table 4. A diagram of each patient’s clinical course is shown in Figure 2. Notably, the two patients in this study who were diagnosed and treated presymptomatically (2 & 6) had milder clinical courses than their affected siblings. Of our six patients, one patient expired from overwhelming liver failure, and the other required liver transplant for survival. These outcomes underscore that provision of exogenous N-acetylcysteine alone is insufficient to ameliorate this condition and may suggest that additional cysteine in the form of L-cysteine is crucial to shorten liver dysfunction and improve overall survival. Additionally, the natural history of these two patients may reflect an as-yet uncharacterized genotype-phenotype correlation. Most of our patients have received long-term supplementation with N-acetylcysteine, since this compound plays a role as a reduced glutathione precursor and it may also improve the redox potential in children with mitochondrial disease, and specifically mitochondrial hepatopathies. Therefore, there are two mechanisms for the observed benefit of N-acetylcysteine supplementation [18]. Of the patients who survived the initial insult (Patients 1, 2, 3, 6), all remain on exogenous cysteine supplementation, whether N-acetylcysteine, L-cysteine, or both. Three have normal or near-normal development, all showing evidence of ongoing hepatopathy. Continued long-term follow-up of TRMU deficiency patients on exogenous cysteine supplementation will be important to determine the natural history.
Table 4.
Patients’ age in December 2019, along with clinical, laboratory, and developmental features, and developmental therapies. Patient 3 lost to follow-up at our center, thus limited information is available.
| Patient | Age in December 2019 | Clinical Features | Laboratory Features | Developmental Features | Therapies |
|---|---|---|---|---|---|
| 1 | 45 months | No symptoms | ↑ AST ↑ CK ↑ Lactate |
Resolved speech delay | None |
| 2 | 12 months | No symptoms | ↑ Lactate ↑ AST ↑ ALT ↑ Conjugated bilirubin |
Normal development | None |
| 3 | 37 months | Short stature | ↑ AST (mild) ↑ ALT (mild) ↑ CK |
Global delay Regression | Not known |
| 4 | N/A, deceased | N/A | N/A | N/A | N/A |
| 5 | N/A, deceased | N/A | N/A | N/A | N/A |
| 6 | 26 months | Stage 4 micronodular cirrhosis Hepatomegaly Short stature |
↑ Lactate ↑ AST ↑ALT |
Speech delay | Physical Occupational Speech |
Figure 2.
Clinical disease course for Patients 1–6 over the first year of life and beyond. Stabilization – stable laboratory lactate and non-recurrent hospitalizations. NAC – N-acetylcysteine. LC – L-cysteine.
One previous report has suggested OLT as a potential therapeutic option for infants with TRMU-related liver failure [8]. As the neurological development in most patients is normal or near-normal, and the liver failure is transient as long as patients survive the first year of life, the health of the graft would not be jeopardized by the underlying genetic defect. Our report is the first to describe a patient with TRMU deficiency to undergo OLT (Patient 3). His long-term survival has been accompanied by several complications; while rejection and PTLD could occur in any child who undergoes OLT, chronic respiratory failure and deep white matter signal abnormalities are distinct features, which may reflect the natural history of the mitochondrial disease. It is possible that the respiratory failure is a symptom of myopathy as this patient had a mildly elevated CK level, which was also a persistent finding in Patient 1. Primary myopathy has previously been described alongside Leigh syndrome in a patient with TRMU deficiency [9].
The unique presentations of our patients underscore the need to maintain a high index of suspicion for this diagnosis (Table 3). For multiple patients, the initial metabolic differential diagnosis did not include mitochondrial hepatopathies. Indeed, our patients were clinically suspected to have inborn errors of metabolism as diverse as GSD (due to hepatomegaly, hypoglycemia, and lactic acidosis), organic acidemias (due to metabolic acidosis and hyperammonemia), and fatty acid oxidation disorders and pyruvate metabolism defects (due to lactic acidosis and hypoglycemia). Thus, we propose that in cases of infantile hypoglycemia and lactic acidosis, TRMU deficiency should be on the differential diagnosis. We further propose that exogenous cysteine supplementation should be empirically initiated as soon as the diagnosis is suspected, as it is a low-risk medication with potentially life-saving effects. The dosing range varied in our patients, from 70 to 150 mg/kg/day for N-acetylcysteine and 85 to 300 mg/kg/day for L-cysteine. Moving forward, prospective studies to determine the optimal dosing ranges in TRMU deficiency should be considered, but our clinical experience suggests a goal of 300 mg/kg/day, with 150 mg/kg/day of N-acetylcysteine and the remainder made up from L-cysteine and diet. We did not assess specific laboratory markers in our study, but potential markers include GDF15, FGF21, blood cysteine levels, and resolution of lactatemia and liver failure.
Conclusion.
In any infant with lactic acidosis and hypoglycemia of unknown etiology, with or without hepatopathy, TRMU deficiency should be considered, and exogenous cysteine furnished, until this condition is ruled out. OLT can prolong survival in children with liver failure secondary to TRMU deficiency, but long-term complications may persist in some cases. In children who do not undergo OLT and survive the initial insult, long-term liver dysfunction and myopathy may be present, but development is normal or near-normal. Provision of exogenous cysteine, especially pre-symptomatically, may be lifesaving and alter the natural history of the disease.
Supplementary Material
Highlights.
Transient neonatal liver failure is common but not universal in TRMU deficiency.
Presentation in affected patients can mimic many other inborn errors of metabolism.
Exogenous L-cysteine and N-acetylcysteine supplements may ameliorate disease course.
Liver transplantation can be lifesaving and the disease does not harm the graft.
Long-term complications may persist after the acute presentation.
Acknowledgments
While engaged in this work, CNM was funded by the T32 GM07526-42 Medical Genetics Research Fellowship Program. RDG was funded by K08-DK113250. The authors would like to thank Dr. Pierre Russo and Dr. Mariarita Santi-Vicini for provision of histology images.
Competing Interests Statement
Dr. Loomes declares consulting relationships with Albireo Pharma, Mirum Pharmaceuticals and Retrophin, and grant funding for clinical trials from Albireo Pharma and Mirum Pharmaceuticals.
Dr. Monteil declares that the views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. I am a military service member. This work was prepared as part of my official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
Dr. Scaglia declares grant funding for clinical trials from NIH-5U54-NS078059-09, PTC Therapeutics, Stealth BioTherapeutics, and Entrada Therapeutics, and is an investigator in the North American Mitochondrial Disease Consortium.
Dr. Ganetzky declares consulting relationships with Minovia therapeutics.
Abbreviations
- TRMU
tRNA 5-methylaminomethyl-2-thiouridylate methyltransferase
- OLT
orthotopic liver transplantation
- GSD
glycogen storage disease
- GDF15
Growth Differentiation Factor 15
- FGF21
Fibroblast Growth Factor 21
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cohen BH, Chinnery PF, Copeland WC, POLG-Related Disorders, University of Washington, Seattle, 1993. http://www.ncbi.nlm.nih.gov/pubmed/20301791 (accessed March 18, 2020). [Google Scholar]
- [2].El-Hattab AW, Scaglia F, Wong L-J, Deoxyguanosine Kinase Deficiency, University of Washington, Seattle, 1993. http://www.ncbi.nlm.nih.gov/pubmed/20301766 (accessed March 18, 2020). [Google Scholar]
- [3].El-Hattab AW, Wang J, Dai H, Almannai M, Scaglia F, Craigen WJ, Wong L-JC, MPV17-Related Mitochondrial DNA Maintenance Defect, University of Washington, Seattle, 1993. http://www.ncbi.nlm.nih.gov/pubmed/22593919 (accessed March 18, 2020). [Google Scholar]
- [4].Zeharia A, Shaag A, Pappo O, Mager-Heckel AM, Saada A, Beinat M, Karicheva O, Mandel H, Ofek N, Segel R, Marom D, Rötig A, Tarassov I, Elpeleg O, Acute Infantile Liver Failure Due to Mutations in the TRMU Gene, Am. J. Hum. Genet 85 (2009) 401–407. 10.1016/j.ajhg.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gaignard P, Gonzales E, Ackermann O, Labrune P, Correia I, Therond P, Jacquemin E, Slama A, Mitochondrial infantile liver disease due to trmu gene mutations: three new cases, in: JIMD Rep., Springer, 2013: pp. 117–123. 10.1007/8904_2013_230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gil-Margolis M, Mozer-Glassberg Y, Tobar A, Ashkenazi S, Zeharia A, Marom D, [TRMU MUTATIONS - REVERSIBLE INFANTILE LIVER FAILURE OR MULTISYSTEM DISORDER?], Harefuah. 157 (2018). [PubMed] [Google Scholar]
- [7].Grover Z, Lewindon P, Clousten A, Shaag A, Elpeleg O, Coman D, Hepatic copper accumulation: A novel feature in transient infantile liver failure due to trmu mutations?, in: JIMD Rep., Springer, 2015: pp. 109–113. 10.1007/8904_2014_402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schara U, Von Kleist-Retzow JC, Lainka E, Gerner P, Pyle A, Smith PM, Lochmüller H, Czermin B, Abicht A, Holinski-Feder E, Horvath R, Acute liver failure with subsequent cirrhosis as the primary manifestation of TRMU mutations, J. Inherit. Metab. Dis 34 (2011) 197–201. 10.1007/s10545-010-9250-z. [DOI] [PubMed] [Google Scholar]
- [9].Taylor RW, Pyle A, Griffin H, Blakely EL, Duff J, He L, Smertenko T, Alston CL, Neeve VC, Best A, Yarham JW, Kirschner J, Schara U, Talim B, Topaloglu H, Baric I, Holinski-Feder E, Abicht A, Czermin B, Kleinle S, Morris AAM, Vassallo G, Gorman GS, Ramesh V, Turnbull DM, Santibanez-Koref M, McFarland R, Horvath R, Chinnery PF, Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies, JAMA - J. Am. Med. Assoc 312 (2014) 68–77. 10.1001/jama.2014.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Uusimaa J, Jungbluth H, Fratter C, Crisponi G, Feng L, Zeviani M, Hughes I, Treacy EP, Birks J, Brown GK, Sewry CA, McDermott M, Muntoni F, Poulton J, Reversible infantile respiratory chain deficiency is a unique, genetically heterogenous mitochondrial disease, J. Med. Genet 48 (2011) 660–668. 10.1136/jmg.2011.089995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Soler-Alfonso C, Pillai N, Cooney E, Mysore KR, Boyer S, Scaglia F, L-Cysteine supplementation prevents liver transplantation in a patient with TRMU deficiency, Mol. Genet. Metab. Reports 19 (2019) 100453. 10.1016/j.ymgmr.2019.100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boczonadi V, Bansagi B, Horvath R, Reversible infantile mitochondrial diseases, J. Inherit. Metab. Dis 38 (2015) 427–435. 10.1007/s10545-014-9784-6. [DOI] [PubMed] [Google Scholar]
- [13].Zlotkin SH, Zlotkin SH, The development of cystathionase activity during the first year of life, Pediatr. Res 16 (1982) 65–68. 10.1203/00006450-198201001-00013. [DOI] [PubMed] [Google Scholar]
- [14].Bartsakoulia M, Mller JS, Gomez-Duran A, Yu-Wai-Man P, Boczonadi V, Horvath R, Cysteine Supplementation May be Beneficial in a Subgroup of Mitochondrial Translation Deficiencies, J. Neuromuscul. Dis 3 (2016) 363–379. 10.3233/JND-160178. [DOI] [PubMed] [Google Scholar]
- [15].Boczonadi V, Smith PM, Pyle A, Gomez-Duran A, Schara U, Tulinius M, Chinnery PF, Horvath R, Altered 2-thiouridylation impairs mitochondrial translation in reversible infantile respiratory chain deficiency, Hum. Mol. Genet 22 (2013) 4602–4615. 10.1093/hmg/ddt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology, Genet. Med 17 (2015) 405–423. 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guan MX, Yan Q, Li X, Bykhovskaya Y, Gallo-Teran J, Hajek P, Umeda N, Zhao H, Garrido G, Mengesha E, Suzuki T, Del Castillo I, Peters JL, Li R, Qian Y, Wang X, Ballana E, Shohat M, Lu J, Estivill X, Watanabe K, Fischel-Ghodsian N, Mutation in TRMU related to transfer RNA modification modulates the phenotypic expression of the deafness-associated mitochondrial 12S ribosomal RNA mutations, Am. J. Hum. Genet 79 (2006) 291–302. 10.1086/506389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Glutathione as a Redox Biomarker in Mitochondrial Disease—Implications for Therapy, J. Clin. Med 6 (2017) 50 10.3390/jcm6050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.