Abstract
Troxerutin (TRX), a semi-synthetic bioflavonoid derived from rutin, has been reported to exert several pharmacological effects including antioxidant, anti-inflammatory, antihyperlipidemic, and nephroprotective. However, the related molecular details and its mechanisms remain poorly understood. In the present review, we presented evidences from the diversity in vitro and in vivo studies on the therapeutic potential of TRX against neurodegenerative, diabetes, cancer and cardiovascular diseases with the purpose to find molecular pathways related to the treatment efficacy. TRX has a beneficial role in many diseases through multiple mechanisms including, increasing antioxidant enzymes and reducing oxidative damage, decreasing in proapoptotic proteins (APAF-1, BAX, caspases-9 and-3) and increasing the antiapoptotic BCL-2, increasing the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) and downregulating the nuclear factor κB (NFκ). TRX also reduces acetylcholinesterase activity and upregulates phosphoinositide 3-kinase/Akt signaling pathway in Alzheimer’s disease models. Natural products such as TRX may develop numerous and intracellular pathways at several steps in the treatment of many diseases. Molecular mechanisms of action are revealing novel, possible combinational beneficial approaches to treat multiple pathological conditions.
Keywords: Troxerutin, flavonoids, inflammation, antioxidant, natural product, neurodegeneration
1. Introduction
Troxerutin (TRX) also known as vitamin P4 is a naturally occurring flavonoid derived from rutin (3’,4’,7’-Tris[O-(2- hydroxyethyl)] rutin) that has recently attracted the attention of many studies due to its pharmacological properties [1, 2]. TRX is mainly found in tea, coffee, cereals, fruits and vegetables. Due to its high water solubility, it is easily absorbed in the gastrointestinal tract (GIT) and presents low tissue toxicity [3, 4]. It has been indicated that TRX has interesting pharmacological effects including antioxidant, anti-inflammatory, antihyperlipidemic, antineoplastic, antithrombotic, antifibrotic and nephroprotective [1, 5, 6].
Previous review studies have shown that some polyphenolic natural products such as resveratrol, rutin and ellagic acid play an important role in the prevention and treatment of chronic diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), type 2 diabetes, cardiovascular diseases (CVDs) and cancer [7-10]. According to the findings of these studies, these antioxidants mainly exert their protective and therapeutic effects with anti-oxidant and anti-inflammatory properties on vital organs.
Similarly, TRX has a protective role in many tissues such as brain, kidney, heart, vascular and liver [3]. In addition, TRX inhibits testicular toxicity induced by nickel in rats [6]. TRX also has the capability to improve learning and memory impairments induced by amyloid-beta (Aβ) in AD models [1, 5, 6].
Moreover, TRX has beneficial activity against insulin resistance and diabetes and improves testicular function and sperm production in prepubertal type 1 diabetic male rats via reducing oxidative stress [11].
Oxidative stress is an imbalance between free radicals such as ROS and RNS species and the antioxidant systems. Free radicals with an uneven electron can lead to large chain chemical reactions with important macromolecules like proteins, nucleic acids and membrane fatty acids. Therefore, oxidative stress can disrupt the function of these macromolecules and induce apoptotic processes [12]. Oxidative stress is directly involved in many pathological conditions such as cancer, Parkinson disease, AD, heart failure, diabetes and depression [13, 14]. A study on Wistar rats reported that TRX exerts a protective effect on hippocampal neurons against oxidative stress and apoptosis induced by Aβ, associated with a decrease in malondialdehyde (MDA) levels, the major product of lipid peroxidation, and an increase in SOD and GPx activities [1]. Similarly, TRX improves oxidative stress in the blood of streptozotocin (STZ)_induced type-1 diabetic evidenced by a decrease in MDA level and an increase in the activity of antioxidant enzymes SOD, GPX, and CAT compared with diabetic groups with no significant effect on non-diabetic rats [15]. An investigation on mice fed calorie-rich diet has illustrated that TRX prevented mitochondrial oxidative stress and myocardial apoptosis, related to decrease in ROS production, lipid peroxidation, proapoptotic proteins (APAF-1, BAX, caspases-9 and-3 and increase in the antiapoptotic protein (BCL-2) [16]. TRX also prevented BDE-47-induced kidney cell apoptosis through an antioxidant and antiapoptotic activity [3]. TRX showed anticancer properties in hepatocarcinoma cell line via increasing nuclear translocation of Nrf2, a key regulator of the antioxidant response, decreasing oxidative stress and suppressing the expression of IKKβ, and downregulating NF-κB mediated inflammation and proliferation [17-19]. Similarly, TRX protects against diabetic cardiomyopathy in a rat model of type 2 diabetes by reducing ROS levels, NF-κB protein expression and Akt activation [20].
The purpose of this review was to review recent scientific reports on TRX, analyzing its antioxidant effect and its potential role as a therapeutic agent, providing a picture of the chemistry, mechanisms of action and elucidating the effects on patients.
The present review focuses and summarizes the important findings regarding the health impacts of TRX and the diversity of its pharmacological effects of TRX on chronic diseases, including AD, PD, metabolic syndrome, diabetes, cancer, cardiovascular diseases as well as its related molecular mechanisms.
2. Background of Troxerutin
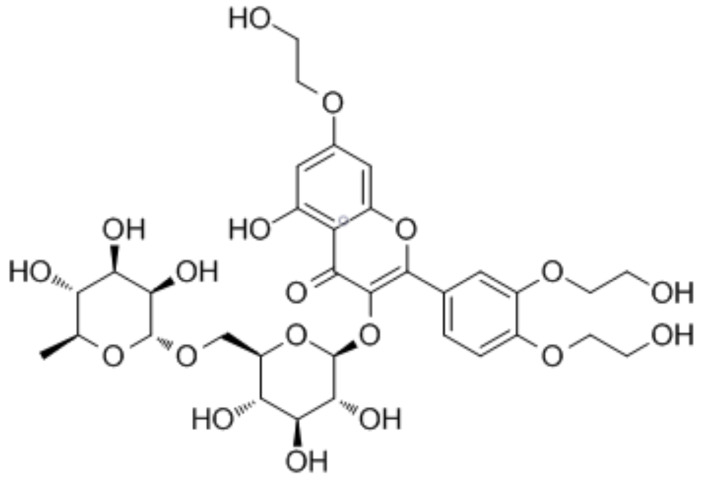
TRX is a flavonol obtained from rutoside, a natural flavonoid, which is hydroxylated at the 3', 4' and 7th positions in the rutin structure. TRX is a yellow powder and comfortably soluble in water [15]. It was isolated from Styphnolobium japonicum Styphnolobium japonicum (L.) Schott, 1830, and also is found in significant amounts in tea, coffee, some fruits and plants [21]. TRX should be stored at 2-8°C and protected from air and light to avoid its degradation. TRX belongs to the class of organic compounds identified as flavonoid-3-o-glycosides. These are phenolic compounds, including a flavonoid moiety that is O-glycosidically linked to a carbohydrate moiety at the C3-position (Fig. 1) [22]. TRX constitutes approximately 80% of tris rutin as the main compound, whereas bis rutin, tetrakis rutin, and mono rutin are occurring in a negligible extent [23]. A study indicated that synthesizing troxerutin-acylated derivatives, significantly increases the bioavailability and antioxidant activities of TXR in cells via improving its lipophilicity. Indeed, acylation of TRX by P. aeruginosa and P. stutzeri allow obtaining two products, troxerutin monoester and troxerutin diester, which showed better bioavailability, absorption and antioxidant activities than native TRX [24].
Fig. (1).
The structure of TRX. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. Troxerutin in the treatment of Neurodegenerative diseases
Millions of people around the world suffer from neurodegenerative diseases [25]. Neurodegenerative diseases comprise a chronic and heterogeneous group of central nervous system disorders characterized by the functional and structural damage of neurons or neuronal cell death in the specific brain and spinal cord regions [26, 27]. The common pathological mechanisms underlying the development of neurodegenerative diseases include dysfunction of neuronal excitability, oxidative stress, aggregation of abnormal protein, mitochondrial dysfunction, induction of apoptosis, dysfunction in the function of proteases, and neuroinflammation [27, 28]. Common neurological diseases such as AD, PD, ALS, HD and MS are the most important medical topics. These disorders are mainly associated with increased mortality in individuals, and their prevalence around the world is increased by aging [29]. AD is the most prevalent form of age-related progressive neurodegenerative disease and, while the PD is recognized as the second most prevalent degenerative neurological movement disorder of adult-onset. Currently, available treatments are not completely effective to reduce the progress of these diseases; therefore, therapeutic interventions and successful preventions are emphasized [30, 31]. AD as escalating dementia is characterized by the neuronal and synaptic loss in the cerebral cortex and certain subcortical regions, including degeneration in the temporal lobe, regions of the frontal cortex, the cingulate gyrus and in brainstem nuclei like the locus coeruleus [32]. Accumulation of the extracellular Aβ resembling plaques senile, tau deposition and intracellular NFTs in the brains of AD patients are the main hallmarks of AD [33]. The brain is one of the tissues which is most susceptible to oxidative stress and damage due to its high oxygen consumption, poor antioxidant defense and high content of unsaturated fats with high susceptibility to lipid peroxidation [34]. The damage caused by oxidative stress in the brain is harmful since it is a post-mitotic tissue formed by nerve cells with a poor capacity for self-renewal due to their low proliferative capacity. Increasing evidences show that oxidative stress is implicated in the pathogenesis of AD [35-37].
Some studies have shown that the generation of free radicals such as ROS leads to mitochondrial dysfunction and increased Aβ peptide accumulation in the brain [38-40]. Several studies have been designed to determine the possible effect of TRX as a neuroprotective agent (Table 1). For example, in in vivo and in vitro experimental models, TRX has been shown to reduce ischemic damage by reducing oxidative stress in a process mediated by increased SOD activity and decreased MDA levels in the rat cerebral tissues [41]. In addition to the neurovascular unit (NVU) model for oxygen-glucose deprivation and reoxygenation (OGD/R) damage, TRX has protective effects on neurons after cerebral ischemia/reperfusion (I/R) injury via reducing the levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α, proapoptotic markers (Bax, p53, and caspase-3), and improving blood-brain barrier maintenance [42]. Lu and colleagues demonstrated the neuroprotective effects of TRX in the D-galactose-treated mouse model induced by subcutaneous injections of D-galactose for 8 weeks. In this study, TRX effectively improved learning and memory through decreasing advanced AGEs, ROS and protein carbonyl levels in the basal forebrain, hippocampus and frontal cortex. The results evidenced that TRX reduced the activity of AchE, increased neuronal acetylcholine receptor subunit alpha-7(nAchRa7) expression and interaction between nAchRa7 and the memory-related proteins either postsynaptic density protein 95 (PSD95) or NMDAR1 in the basal forebrain, hippocampus and front cortex of mice [43]. In addition, Lu and colleagues reported that oral administration of TRX improved the spatial learning, memory deficits and cognitive performance and reduced oxidative stress via NGF-dependent activation of the TrkA pathway in D-galactose-treated 8-week-old male Kunming strain mice [44]. It has been reported that TRX possesses neuroprotective properties and reduces oxidative stress via decreasing the levels of neurotoxic ROS, protein carbonyl and AGEs in the hippocampus of mice fed a high cholesterol-induced diet for 20 weeks. In addition, TRX significantly reduced the cognitive deficits by increasing phosphoinositide 3-kinase/Akt signaling pathway activity and inhibiting the endoplasmic reticulum stress pathway in the mouse hippocampus [45]. Babri and colleagues demonstrated the effectiveness of TRX in a mouse model of AD induced by intracerebroventricular injection of Aβ1-42. In this study, the oral administration of TRX (300 mg/kg) for a period of 8 days, significantly attenuated the impairments in learning and improved memory. This ability is probably related to its anti-inflammatory and antioxidant properties, which enhance hippocampal LTP, improve the functionality of the cholinergic system, and reduce the AChE activity and/or levels of AGEs in different regions of the brain, especially, in the hippocampus [46]. Lu and colleagues showed that DA (2 mg/kg, intraperitoneal injection) administration for 3 weeks induced a significant impairment in mitochondrial function, increased ROS generation, GFAP and Cox-2 levels and induced the release of inflammatory cytokines such as IL-1β and TNF-α, leading to memory impairment via the PKC-z–dependent K-ras/Raf/MEK/ERK1/2 signaling pathway in the hippocampus of mice. They also found that oral administration of TRX significantly improved learning and memory by inhibiting Cdk1 expression [47]. In an electrophysiological study on male Wistar rats, it was observed that intracerebroventricularly (i.c.v.) administration of Aβ1-42 in to right lateral ventricle dramatically reduced the LTP of the DG. Continuous administration of the TRX for two weeks evidenced an improvement in LTP and prevented the hippocampal synaptic failure induced by Aβ peptide [48]. Additionally, intraperitoneal injection of TRX for 6 weeks, improved cognitive performance through reducing oxidative stress, decreeing MDA level and increasing the levels of GSH and the activity of SOD and in STZ-induced diabetic rats. The principal mechanism of the effects of TRX, as mentioned above, probably involves glutamate-cysteine ligase expression, especially glutamate-cysteine ligase catalytic subunit in hippocampal tissues [49].
Table 1.
Some of the studies on TRX.
| Type of Study /Model | Dosage of TRX | Duration | Results | Refs. |
|---|---|---|---|---|
| Animal (rat)/ MCAO model |
40 mg/ml | NIL | TRX and cerebroprotein hydrolysate injection reduced cerebral ischemic damage via the reduce of oxidative stress and amelioration of angiogenesis. | [36] |
| In vitro/ NVU | TRX and cerebroprotein hydrolysate injections 10, 100 and 1000 µM |
NIL | TRX has protective effects on neurons after cerebral ischemia/reperfusion injury via reducing the levels of inflammatory cytokines, proapoptotic markers, and improving blood-brain barrier maintenance. | [42] |
| Animal (mice)/ D-gal-treated model | 150 mg/kg/day | 8 weeks | TRX improved learning and memory through decreasing AGEs, ROS and protein carbonyl levels in the basal forebrain, hippocampus and front cortex |
[44] |
| Animal (mice)/ D-gal-treated model | 150 mg/kg/day | 8 weeks | TRX reduced cognitive impairment and brain oxidative stress via NGF-dependent activation of TrkA pathway | [43] |
| Animal (mice)/ cholesterol-induced cognitive deficits model | 150 mg/kg/day | 20 weeks | TRX reduced oxidative stress and the cognitive deficits by increasing PI3K/Akt signaling pathway activity in the hippocampus | [45] |
| (6-OHDA)- induced rat model of PD | 150 mg/kg/day | 1 week | TRX inhibiting astrogliosis, apoptosis and oxidative stress | [55] |
Farajdokht et al., demonstrated that intracerebroventricular injection of Aβ1-42 (300 mg/kg) enhanced the levels of MDA and AChE and also reduced the activities of SOD and GPx in the hippocampus of rats [1]. According to this study, chronic administration of TRX also exerts significant anti-apoptotic and neuroprotective effects on hippocampal neurons probably due to its antioxidant and AChE-inhibitory effects. In a STZ-induced diabetic rat model, TRX improved cognitive dysfunction by enhancing SOD activity and increasing the content of Nrf2 as a core transcription factor of antioxidant proteins in the hippocampus [50]. Diba et al., investigated the effect of chronic TRX treatment on high-fat diet-induced in pregnant rats. In this study, TRX improved learning and spatial memory impairment by enhancing serum and hippocampal apelin levels in the offspring and decreasing the pro-inflammatory cytokines in the hippocampus [51].
In LPS-induced rat inflammation and oxidative damage model, intraperitoneal TRX administration for 6 consecutive days improved memory impairment via reducing ROS production and MDA levels and ameliorating the release of inflammatory mediators such as TNFα and NF-κB [52]. The mechanism of action of the TRX is mediated by the SIRT1 / SIRT3 signaling pathway. PD is a progressive and chronic neurodegenerative disorder caused by degeneration dopaminergic neurons in the substantia nigra and the progressive aggregation of α-synuclein protein and accumulation of Lewy bodies and Lewy neurites [53, 54]. Baluchnejadmojarada et al., studied the neuroprotective effects of TRX (150 mg/kg/day) for 1 week in 6-hydroxydopamine (6-OHDA)-induced rat model of PD. TRX treatment reduced apomorphine-induced rotational behavior and improved the performance of rats in the narrow beam test by inhibiting astrogliosis, apoptosis and also reduced oxidative stress in the striatum in a process probably mediated by PI3K / ERβ signaling cascade [55].
4. Troxerutin in the treatment of chronic metabolic diseases
Today, chronic non-communicable metabolic diseases, such as obesity, T2DM and NAFLD are increasing dramatically in developed and developing countries [56, 57].
The International Diabetes Federation (IDF) reported that 451 million people between the ages of 18 to 99 years were affected by diabetes in 2017. The number of patients is expected to increase to 693 million by 2045 [58]. T2DM is a metabolic disease characterized by an elevated level of blood glucose, arising due to a decline in pancreatic β-cell function and insulin resistance in peripheral tissues [59, 60]. The prevalence of diabetes has increased over the past decade, with obesity being one of the main causes in 90% of cases [61, 62]. T2DM has been strongly associated with micro- and macrovascular diseases such as coronary artery diseases, peripheral arterial disease, retinopathy, chronic kidney disease and end-stage renal disease [63]. In T2DM, it is well established that abnormalities such as hyperglycemia, insulin resistance, hyperinsulinemia and dyslipidemia increase oxidative stress, which is ultimately associated with damage to endothelial cells in large and small vessels [64]. The pancreas contains lower antioxidant defense enzymes such as SOD, catalase, and GPx compared to other tissues. Thus, chronic hyperglycemia makes the pancreatic β cells more vulnerable to oxidative stress through increasing ROS generation [65, 66]. Type 1 diabetes is a heterogeneous autoimmune disease in which the activity of T lymphocytes and inflammatory cytokines leads to the destruction of insulin-producing pancreatic islet β cells and chronic hyperglycemia [67, 68].
Ranjith et al., studied the antidiabetic effects of the TRX in monosodium glutamate -induced insulin resistance by mediating PPARs in rats. The administration of TRX reduced the concentrations of plasma glucose and insulin and also improved insulin sensitivity. In addition, TRX had protective effects against liver damage by decreasing the activity of aminotransferases (AST and ALT) enzymes. TRX reduced oxidative stress via increasing the activities of enzymatic antioxidants (SOD, CAT and GPx) and non-enzymatic antioxidants (reduced GSH, vitamin C and E) in plasma and liver tissue. The treatment also increased the mRNA expression of adiponectin in the liver, which has anti-fibrotic activity via an inhibitory effect on hepatic stellate cell-mediated adenosine monophosphate-activated protein kinase and PPAR-α pathways [69]. In an in vitro study, it has been demonstrated that TRX prevented protein glycation such as albumin through hydrophobic interactions and also reduced the development of complications in hyperglycemia [70]. Administration of TRX for a period of 4 weeks in high fat and sucrose-induced diabetic adult male rats reduced fasting serum glucose, lipid profile and insulin levels, probably through improving insulin sensitivity resulting from glucose tolerance. TRX increased the mRNA and protein levels of the insulin receptor in the gastrocnemius muscle of the diabetic group. It also, increased b-arrestin-2 and c-Src protein levels, glucose uptake, oxidation, glycogen level and increased expression of GLUT4 in the gastrocnemius muscle of T2DM group [71]. At the early stage of diabetic retinopathy induced by STZ, the oral administration of TRX has been shown to significantly reduce the concentration of VEGF protein compared to the diabetic control group, and this effect might be related to its antioxidant properties [72]. Vascular damage in aorta from a rat model of diabetes, TRX administration once daily for 4 weeks significantly reduced lipid aggregation in tunica intimae and tunica media of the aorta in diabetic rats. According to this study, the mechanism of action of the TRX is related to the reduction in MDA level and the increase in the activity of SOD and GPx, which ultimately leads to a decrease in oxidative stress [73]. In an in vivo study on diabetic rats, TRX administration once daily for 4 weeks significantly reduced the NF-κB protein expression and ROS levels, and also activated the AKT/IRS/JNK signaling pathway, in myocardial tissue [20]. Additionally, it has been shown that other damaging effects of diabetes include myocardial apoptosis [74]. Mokhtari et al., in STZ-induced diabetic rats, evidenced that TRX administration once daily for 4 weeks reduced cardiac troponin levels in the myocardium and protected from myocardial I/R via increasing the phosphorylation and inactivation of GSK-3β, a protein kinase with a critical role in mitochondrial dysfunction and apoptosis. The authors assumed that the TRX probably exerts its anti-apoptotic effects by increasing the phosphorylation of GSK-3β via activation of PI3K/Akt signaling pathway in the myocardial tissue [75]. Dietary administration of TRX for 4 weeks significantly reduced the mRNA levels of NF-κB, IRAK-1, and TRAF-6 in the hippocampus tissue of healthy and diabetic rats. The protective effects of TRX have been suggested to be related to its ability to inhibit the NF-κB mRNA expression and its adaptor proteins TRAF-6 and IRAK-1 via regulation of their targets such as miR-146a [76]. The administration of TRX after induction of T1DM in rats for 6 weeks significantly attenuated the oxidative stress via reducing MDA levels and increasing the antioxidant defenses, including GSH and SOD in the hippocampus tissue. It has been suggested that the mechanism of action might involve GCLC [49]. Badalzadehb et al., indicated that TRX improved the oxidative stress in the blood of STZ-induced T1DM rats. The results of their study indicated that the antidiabetic effects of TRX derive from the reduction of oxidative stress by decreasing lipid peroxidation and increasing the activities of antioxidant enzymes in plasma of diabetic rats [15]. In another study, the effect of TRX on diabetic male Sprague-Dawley rats significantly increased the SOD activity in the hippocampus [77]. The administration TRX was suggested to delay the development of diabetes-induced cognitive dysfunction by increasing the expression level of Nrf2 in the hippocampus [77]. It has been shown that diabetes mellitus can cause male infertility in pre-testis, testis and post-testis associated with the excessive production of free radicals which can damage the sperm DNA [78, 79]. A study by Oskuye et al., reported that the administration of TRX in STZ-induced diabetic rats for 4 weeks significantly reduced the damage to testicular tissue and improved the quantity and quality parameters of sperm. The protective effects of the TRX have been suggested to be related to its ability to reduce oxidative stress and serum glucose in diabetic rats [11]. Additionally, it was investigated the protective effects of TXR on testicular function and structure in T1DM adult rats by reduction of apoptosis. It has been shown TRX significantly reduced plasma glucose levels, improved the number of spermatogonia, spermatocyte, round and elongated spermatid, and Sertoli and Leydig cells due to its antiapoptotic and antioxidant activity [80].
In mice fed a high-fat, high-fructose diet (HFFD), TRX has been demonstrated to ameliorate glucose tolerance, insulin sensitivity, regulate electrocardiogram patterns and reduce cardiomyocyte hypertrophy [81]. It was shown that TRX downregulated superoxide production, NADPH oxidase (NOX) p22phox subunit and reduced the levels of TGF-β1 and α–SMA, which have a key role in fibrogenesis. In addition, TRX decreased the levels of matrix metalloproteinase (MMP)-9 and -2 with increasing in tissue inhibitors of metalloproteinases (TIMP)-1 and -2 in the heart of mice. The authors found that TRX was an effective compound to manage T2DM and cardiac dysfunction linked to metabolic syndrome [81]. Malinska and colleagues, in a hereditary hypertriglyceridemic (HHTg) rat model of metabolic syndrome treated with TRX (150 mg/kg, for 4 weeks), found that TRX was able to reduce non-fasting blood glucose, hyperinsulinemia and hepatic cholesterol accumulation owing to its effects on genes related to cholesterol synthesis and lipid oxidation. The researchers also found that TRX reduced the expression of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), involved in cholesterol synthesis, and increased the expression of PPARα, a key regulator of lipid and glucose metabolism, in the hepatic tissue of HHTg rats. Moreover, TRX significantly reduced lipogenic enzymes such as stearoyl-CoA desaturase (SCD1), which might be associated with the increase in the activity of CYP1A1 (cytochrome P450, family 1, subfamily A, polypeptide 1) in the liver [82].
The antioxidant activity of TRX has been studied by Geetha and colleagues using mice fed HFFD for 45 days. TRX administration (150 mg/kg) has been found to significantly ameliorate whole-body insulin sensitivity and oxidative stress by increasing the activity of enzymatic and non-enzymatic antioxidants and reducing the lipid content in the heart tissue. Moreover, TRX increased the expression of genes involved in fatty acid oxidation (PPAR-α, PGC-1 α, and CPT-1β), and decreased the expression of genes involving in fatty acid synthesis such as SREBP-1c and fatty acid transport (CD 36 and FATP-1) in the heart tissue [83]. In another study, it has been found that TRX (150 mg/kg) has protective effects in male offspring of high-fat diet-fed rats via reducing the hippocampus and serum levels of TNF-α and IL-6 and increasing brain-derived neurotrophic factor (BDNF) levels [84]. In addition, the administration of TRX (150 mg/kg) during pregnancy significantly increased the serum levels of apelin-13 and down-regulated the expression of apelin-13 receptor mRNA in the ovarian tissue of the offspring of HFD fed mothers. Additionally, TRX increased the number of primary, secondary, and graph follicles, and reduced atretic follicles [85]. Zhang and colleagues studied the anti-obesity effects of TRX (150 mg/kg, orally, for 20 weeks) in HFD mice. They established that TRX significantly ameliorated obesity and reduced the mass of epididymal adipose tissue, and increased the levels of serum adiponectin. The authors also showed that TRX inhibited inflammatory response via suppressing the nuclear translocation of NF-κB p65 and reduced oxidative stress via decreasing the levels of 4-hydroxynonenal (4-HNE), ROS generation and increasing GSH levels in the liver tissue. TRX also reduced the expression of the nucleotide oligomerization domain (NOD), as well as interaction between NOD1/2 with interacting protein-2 (RIP2), by reducing oxidative stress-induced endoplasmic reticulum in the liver tissue [86].
An investigation on mice fed with HFD has been indicated that TRX (150 mg/kg, orally) prevented obesity, liver steatosis and injury via increasing GSH content and SOD1 protein expression. TRX also modulated NAD+ metabolism by increasing NAD+ level and sirtuin 1 (Sirt1) activity, and promoting the nuclear entry of lipin 1 (which modulates fatty acid oxidation gene expression) and reducing the ratio of Lpin 1b/a. Finally, TRX improved lipid homeostasis in the liver by increasing fatty acid oxidation and triglyceride secretion and inhibiting lipogenesis [87].
5. Nephroprotective effects of TRX
The nephroprotective effects of TRX have been reported by different authors. For example, Fan and colleagues studied the effect of TRX (150 mg/kg, orally) for 4 weeks on the renal injury induced by D-galactose in the male Kunming strain mice [88]. The authors found that TRX significantly reduced the renal injury induced by D-gal via three different molecular mechanisms: increasing antioxidant enzyme activities such as SOD, CAT and GPx, reducing MDA content and, inhibiting the expression of NF-κB, iNOS and COX-2 [88]. In another study on D-galactose-treated mice have been found that chronic administration of TRX (150 mg/kg) by oral gavage for a period of 8 weeks reduced renal dysfunction and histopathologic changes. It also reduced the NADPH oxidase activity, increased the activities of SOD, CAT and GPX, down-regulated the levels of ROS and 8-hydroxy-2' -deoxyguanosine (8-OHdG), and reduced the number TUNEL-positive cells in the and DNA damage in the kidney cells. Thereafter, the nephroprotective effects of TRX in the D-galactose-induced DNA damage model have been suggested to be related to its antioxidant activity [89]. Shan et al., studied the nephroprotective effect of TRX (100 mg/kg) against 2,2',4,4'-tetrabromodiphenyl ether (BDE-47)-induced apoptosis in male C57BL/6J mice. The administration of TRX reduced the ubiquitination of Nrf2, increased Nrf2 activity and improved oxidative stress of kidney cells via increasing the activities of SOD, GPx, and CAT, and also reduced release of mitochondrial cytochrome c. The results of this study indicated that TRX inhibited the expression of FAS, FAS ligand, and caspase-8 as well as prevented cytotoxicity induced by BDE-47 in the kidney [3]. In another study by Shan and colleagues, it was found that administration of TRX (100 mg/kg, orally) in male C57BL/6J mice for 8 weeks regulated the deleterious effects of inflammation through CXCR4-TXNIP/NLRP3 inflammasome in the kidney of mice treated by BDE-47 [50]. The nephroprotective activity of TRX was studied on liposoluble toxin ochratoxin A (OTA)-induced nephrotic mice. TRX at 150 mg/kg dose for 12 weeks improved the weight loss of kidney and the inflammatory response via reducing the expression of proinflammatory cytokines such as IL-6, Kim1, TGF-α, and TGF-β in the cortex and medulla of the kidney. In addition, TRX decreased renal lipotoxicity by elevating the mRNA levels of sphingomyelinase (SMase) and activated chloride channels (CLCs) in the damaged kidney [90]. Dehnamaki and colleagues described the beneficial effects of TRX on kidney injury induced by cisplatin in mice. In this model, TRX (150 mg/kg, orally) for 3 days significantly reduced the levels of blood urea nitrogen (BUN), creatinine and improved oxidative stress by increasing SOD activity and reducing MDA levels in renal tissue [91]. In another study, the administration of TRX (100 mg/kg, orally) for 20 days in rats significantly reduced the renal toxicity of nickel (20 mg/kg body weight) and protected the normal histological structure of the renal tissue. These findings suggest that the nephroprotective effect of TRX occurs via reducing oxidative stress [92]. Similarly, the treatment with TRX (150 mg/ kg, orally) for 15 days against gentamicin-induced acute kidney injury rat model showed that TRX significantly prevented the reduction in glomerular filtration rate (GFR) induced by gentamycin as well as normalized the levels of serum creatinine, blood urea nitrogen, urinary albumin, and the urinary albumin to creatinine ratio. In addition, TRX significantly decreased lipid peroxidation, protein oxidation and kidney injury molecule-1 (KIM-1) protein abundance. TRX protective effects were related to an attenuation of oxidative stress, anti-inflammatory activity through reducing the levels of inflammatory cytokines such as TNF-α, IL-6 and IL-10, anti-apoptotic effects through increasing the proliferating cell nuclear antigen (PCNA) protein and inhibition of the phosphorylation of p38 mitogen activated protein kinase (MAPK) and c-Fos [93].
6. Hepatoprotective Effects of TRX
Several studies have investigated the therapeutic potential of TRX against liver damage. For example, Adam and colleagues have shown the beneficial hepatoprotective of TRX on coumarin –induced liver injury in rats. The results of their study indicated that TRX promoted liver repair through reducing lipid peroxidation, ALT and LDH activities induced by coumarin. TRX reduced the metabolism of coumarin and its metabolite levels in bile, such as 3-hydroxycoumarin and 7-hydroxycoumarin [94]. Similarly, Zhang et al., demonstrated the positive effects of TRX (150 mg/kg, orally, 4 weeks) on D-Galactose-induced liver injury in rats. The hepatoprotective effects of TRX in this model were related to a decrease in the lipid peroxidation levels and oxidative stress through elevating the activities of antioxidant enzymes as well as ameliorating the upregulation of NF-κB, iNOS, and COX-2 in the liver of D-galactose treated rats [95]. In another report, it has been found that the treatment with TRX (5 and 10 mg/kg) for 6 days before gamma irradiation significantly reduced the oxidative stress via increasing the activity of SOD and reducing the MDA level in mouse liver. TRX (10 mg/kg) also decreased the irradiation-induced pathological changes such as edema and necrosis in livers of affected mice [96]. Zamanian et al., suggested that TRX (300 mg/kg, orally) had hepatoprotective and antifatigue effects in rats, evidenced by a delayed muscle fatigue during exhausting swimming exercise. The mechanism responsible for its effects was related to a reduction in ALT, apoptotic markers such as Bax and increasing SOD activity in the hepatic tissue as well as the Bcl-2/Bax ratio [4].
7. Troxerutin in the treatment of cancer
Several studies reported that TRX treatment leads to apoptosis and anticancer effects. In this sense, Thomas and colleagues found that TRX administration (50 mg/kg, orally) in rats with preneoplastic liver induced by N-nitrosodiethylamine (NDEA) protected against the development of NAFLD to NASH and HCC. TRX enhanced the antioxidant defenses and reduced oxidative damage and the generation of ROS. In addition, the treatment reduced the levels of CYP enzymes (CYP450 and CYP2E1), inhibited cell proliferation and inflammatory processes, reduced fibrosis and formation of nodules, modulated the imbalance in the MDM2–p53 interaction and also modulated apoptosis by reducing expression of MDM2 and Bcl-2, and increasing the expression of p53 and Bax [97]. Another in vitro study has been shown that TRX inhibited the cell viability, cell migration and induced apoptosis in HuH-7 hepatocarcinoma cells in a time-dependent manner. Subsequently, the suppression of the oxidative stress in the hepatocarcinoma cell line by TRX was mediated by triggering the keap-1/Nrf-2/HO-1 signaling pathway. The results of this study showed that the TRX anti-inflammatory activity was related to the inhibition of the NF-κB pathway and its downstream targets in order to induce apoptosis. Therefore, it was suggested that the anti-apoptotic effect of the TRX related to NF-κB inhibition and Nrf2 activation might be related to the simultaneous regulation of both molecular pathways [19].
Panat and colleagues explored the effect of TRX (0.5-5 mM) against both PC3 (radiosensitive) and DU145 (a radioresistant) prostate cancer cell lines using the MTT assay. Pretreatment with TRX before γ-radiation considerably increased the generation of ROS and DNA strand breaks in DU145 cells and had a synergistic effect with γ-radiation. TRX also showed a cytotoxic effect in PC3 cells due to its binding to the DNA and inducing DNA strand breaks [98]. Xu and colleagues have evaluated the inhibitory effects of TRX on human gastric cancer cells resistant to 5-Fluorouracil (5-Fu). They found that TRX and 5-FU co-treatment in a dose-dependent manner reduced cell proliferation. TRX, in combination with 5-FU, reduced the phosphorylation and the activity of the signal transducer and activator of transcription 3 (STAT3) which plays a key role in the suppression of p65. Additionally, TRX induced apoptosis in gastric cancer cells resistant to 5-FU by suppressing Bcl-2 and regulating proapoptotic proteins such as Bax and Bid. The mechanism responsible for the anticancer effects of TRX could derive from the suppression of p-STAT3/NF-κB (p65 and p50) and Bcl-2 [99]. In an in vitro study, Subastri and coworkers reported that TRX has pro-oxidant activity against Huh-7 cells in complex with copper (Cu), via generation of free radicals such as superoxide and hydroxyl radicals, DNA damage, and induction of apoptosis by reducing Bcl-2 and Bcl-xL. In addition, TRX significantly protected rat liver tissue from diethylnitrosamine (DEN)-induced HCC by reducing the levels of AST, LDH, ALP, GST and lipid peroxidation and reducing the preneoplastic lesion formation [100]. Rajamanickam and colleagues reported that supplementation with TRX inhibited the activities of phase I enzymes such as cytochrome P450 and b5 and increased the activities of phase II enzymes such as GST, DT-diaphorase (DTD) and uridine diphospho glucuronyl transferase (UDPGT) in 1,2-dimethylhydrazine (DMH) induced experimental rat colon carcinogenesis. The treatment with TRX also reduced the activity of bacterial enzymes such as the mucinase, β-glucosidase and β-galactosidase when compared with the DMH-treated group, and thus, increased mucin content in the colon. TRX, at the dose of 25 mg/kg, dramatically reduced the formation of aberrant crypt foci (ACF) and its total number via suppressing the progression of preneoplasia to malignant neoplasia. In addition, TRX reversed the histological changes in hepatic tissue and inhibited colon inflammation through decreasing of lymphoid aggregation and penetration of the inflammatory cells into the mucosal and submucosal layers [101]. A study on embryonic fibroblast cells 3T3-L1 and breast cancer cells MDA-MB-231 has been illustrated. Fluorescence spectroscopy showed that the pretreatment with TRX prevented the interaction of 2-aminoanthracene (2-AA, a carcinogenic agent) with DNA as well as gel-electrophoresis demonstrated that TRX inhibited the 2-AA + UVA radiation-induced DNA injury [102].
8. Troxerutin in the treatment of cardiovascular diseases
Cardiovascular diseases (CVD) comprise a range of situations that affects the heart and blood vessels, including coronary artery disease, heart failure, hypertensive heart disease, cardiomyopathy, peripheral artery disease and etc. [103, 104]. In 2008, 30% of all worldwide deaths was attributed to CVD. It is estimated that by 2030, above 23 million people will die from CVD each year [105]. Pharmacological interventions in people with CVD may cause potential side effects and, consequently, numerous natural products have been assessed for pharmacological therapy [82]. Shu et al., have shown that TXR has cardioprotective effects in in vivo and in vitro models via decreasing levels of TNF-α, IL-10 and some apoptosis markers such as Bax and caspase 3 as well as activating PI3K/Akt signaling pathway and markedly reducing myocardial infarct size [106]. In another study in rats, TRX alleviated myocardial I/R damage by inhibiting miR‐146a‐5p, apoptotic factors such as Bcl-2, attenuated the insufficiency of hemodynamic factors of the heart induced by I/R, creatine kinase (CK), LDH and proinflammatory cytokines [107]. TRX also showed a beneficial role as a cardioprotective agent on arrhythmias induced by I/R. The treatment significantly decreased the number of premature ventricular complexes and duration and frequency of ventricular fibrillation as well as markedly decreased myocardial proinflammatory cytokine levels of TNF-α and IL-1β respect to the untreated group [108]. Another investigation suggested that TRX had an inhibitory effect on GSK-3β via increasing its phosphorylation form, thus, attenuating the apoptotic index after I/R in diabetic myocardium of rats [75]. Badalzadeh et al., demonstrated that TRX has protective effects on diabetes-induced vascular injuries in rat aorta and reduced vascular histopathological injuries when compared with untreated diabetic rats. They have also shown that TXR elevated the activity of antioxidant enzymes and reduced the levels of MDA [73]. Mitochondrial dysfunction plays an important role in the establishment of heart disease associated with metabolic syndrome. In a mouse model of HFFD-induced metabolic syndrome, a decrease in respiratory chain complex activity mtDNA content and mitochondrial biogenesis as well as an increase in oxidative stress factors and ROS generation occurred [109]. TRX administration was capable to reverse these effects and showing beneficial roles in a mouse model of metabolic syndrome.
9. Other properties of interest of Troxerutin
Raja and colleagues evaluated the effect of TRX on Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME)-induced hypertension, oxidative stress and dysregulation of lipid metabolism in male albino Wistar rats. TRX (100 mg/kg) treatment significantly reduced systolic blood pressure (SBP), thiobarbituric acid reactive substances (TBARS) levels, lipid hydroperoxides (LOOH), liver and kidney lipid content (total cholesterol, triglycerides, free fatty acids), and increased the activities of enzymatic antioxidants and the levels of non-enzymatic antioxidants [110].
Another in vivo study with mice treated with a high-fat diet indicated that chronic administration of TRX (150 mg/kg) during the pregnancy improved anxiety- and depressive-like behaviors probably via reducing the serum levels of cortisol, glucose and cholesterol. The results suggest that these effects of TRX may be related to its anti-inflammatory and anti-oxidant properties [111].
Ma and coworkers demonstrated that the administration of TRX (40 mg/ml) and cerebroprotein hydrolysate (TCHI) in a rat model of cerebral ischemia injury increased HDL levels and reduced LDL levels. It also significantly attenuated oxidative stress via increasing SOD activity and reducing MDA levels. Moreover, TCHI treatment improved angiogenesis by increasing proliferation and enhancing endothelial cell function, including adhesion, migration, and capillary formation [41].
In an in vitro investigation, Xue and colleagues showed that TRX (20 µM) has an interesting potential for the improvement of osteoarthritis. Pretreatment with TRX inhibited the AGEs-induced production of pro-inflammatory factors in chondrocytes, such as COX-2, iNOS, nitric oxide, prostaglandin E2 (PGE2), TNF-α, and IL-6, and suppressed the MAPK activity. On the other hand, the treatment with TRX reduced the damaging effect of AGEs on extracellular matrix (ECM) in chondrocytes [22]. In Wistar rats with nickel-induced testicular toxicity, the treatment with TRX (100 mg/kg/day) for 30 days reduced oxidative stress via increasing in SOD, CAT, GPx, GST and GR. TRX also reduced glucose-6-phosphate dehydrogenase (G6PD) activity, GSH, ascorbate, total sulphydryl groups, and testis-organ weight. In addition, nickel accumulation, lipid peroxidation products and carbonyl protein concentrations were significantly decreased in TRX-treated animals [112].
Zamanian and coworkers studied the antifatigue effects of TRX (75, 150 and 300 mg/kg, for 30 days) in trained male rats. The TRX administration at all three doses of delayed muscle fatigue during exhausting swimming exercise. TRX significantly increased the serum levels of glucose and reduced CK activity. In addition, TRX (300 mg/kg) reduced the oxidative stress process by increasing the activity of SOD, and also reduced ALP and LDH activities and MMP-9 and BUN serum levels [2]. In another study by Zamanian and colleagues, the short-term effects of TRX on muscle fatigue and Bcl-2 and Bax gene expression were evaluated in the hepatic tissue of rats. TRX (300 mg/kg) treatment dramatically delayed swimming muscle fatigue. The results of this study indicated that TRX (300 mg/kg) attenuated oxidative stress via increasing the activity of SOD in the liver tissue, and reduced the serum ALT activity. It also reduced apoptosis presses by reducing mRNA expressions of Bax and increasing the Bcl-2/Bax ratio [4].
Conclusion
We summarized the major beneficial effects of the TRX in Figs. 2 & 3. Based on several in vitro and in vivo studies, TRX, a natural flavonol, had a significant role in the prevention and treatment of diseases, emphasizing the important use of plant compounds. TRX acts through several mechanisms primarily associated with its antioxidant and anti-inflammatory capacity that show beneficial effects against various pathological conditions, including neurogenerative, liver diseases, diabetes or cancer. TRX has the ability to activate both enzymatic and non-enzymatic antioxidant defense mechanisms and decrease the degree of oxidative damage, especially in the lipids. The signaling pathways involved in the beneficial effects of TRX include the activation of the Nrf2 pathway and the inhibition of the NF-κB pathway. Although in vitro studies or in preclinical models have shown promising positive effects on several diseases, future clinical trials using the TRX and/or its derivatives are necessary to find new candidates for therapeutic medications in the future.
Fig. (2).
Some of the main effects of TRX. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
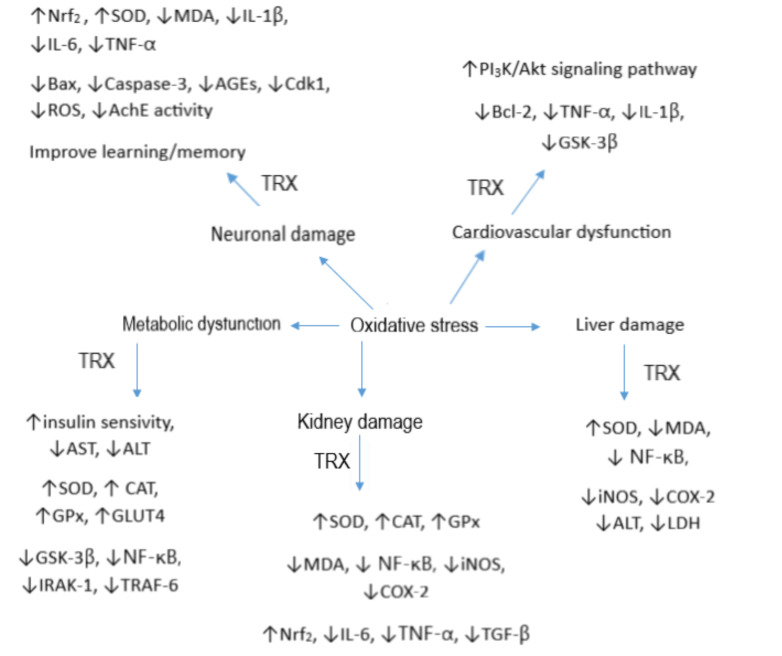
Fig. (3).
Role of oxidative stress in some chronic diseases and TRX was able to decrease oxidative stress through several molecular mechanisms. (A higher resolution / colour version of this figure is available in the electronic copy of the article). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
The authors are grateful to Kermanshah University of Medical Sciences that supported us.
list of Abbreviations
- AChE
Acetylcholinesterase
- AGE
Advanced Glycosylated End products
- ALS
Amyotrophic lateral sclerosis
- ALT
Alanine transaminase
- APAF-1
Apoptotic protease activating factor 1
- AST
Aspartate transaminase
- Aβ
Amyloid-β peptide
- BAX
Bcl-2-like protein 4
- CAT
Catalase
- Cdk1
Cyclin-dependent kinase 1
- CNS
Central nervous system
- COX-2
Cyclooxygenase-2
- DA
Domoic acid
- DG
Dentate gyrus
- GFAP
Glial fibrillary acidic protein
- GIT
Gastrointestinal tract
- GPx
Glutathione peroxidase
- HCC
Hepatocellular carcinoma.
- HD
Huntington's disease
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- iNOS
inducible nitric oxide synthase
- LPS
Lipopolysaccharides
- LTP
Long-term potentiation
- MDA
Malondialdehyde
- MS
Multiple sclerosis
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- NFTs
Neurofibrillary tangles
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDAR
N-methyl-D-aspartate receptor
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PI3K
Phosphoinositide 3-kinase
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- T2DM
Type 2 diabetes mellitus
- TNF-α
Tumor necrosis factor alpha
- TRX
Troxerutin
- VEGF
Vascular endothelial growth factor
- α-SMA
α-smooth muscle actin
Consent for Publication
Not applicable.
Funding
Sureda was granted by Spanish government, Instituto de Salud Carlos III (CIBEROBN, CB12/03/30038).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Farajdokht F., Amani M., Mirzaei B.F., Alihemmati A., Mohaddes G., Babri S. Troxerutin protects hippocampal neurons against amyloid beta-induced oxidative stress and apoptosis. EXCLI J. 2017;16:1081–1089. doi: 10.17179/excli2017-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamanian M., Hajizadeh M.R., Esmaeili N.A., Shamsizadeh A., Allahtavakoli M. Antifatigue effects of troxerutin on exercise endurance capacity, oxidative stress and matrix metalloproteinase-9 levels in trained male rats. Fundam. Clin. Pharmacol. 2017;31(4):447–455. doi: 10.1111/fcp.12280. [DOI] [PubMed] [Google Scholar]
- 3.Shan Q., Zhuang J., Zheng G., Zhang Z., Zhang Y., Lu J., Zheng Y. Troxerutin reduces kidney damage against BDE-47-induced apoptosis via inhibiting NOX2 activity and increasing nrf2 activity. Oxid. Med. Cell. Longev. 2017;2017:6034692. doi: 10.1155/2017/6034692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamanian M., Shamsizadeh A., Esmaeili N. A.; Hajizadeh, M.; Allahtavakoli, F.; Rahmani, M.; Kaeidi, A.; Safari Khalegh, H.; Allahtavakoli, M. Short-term effects of troxerutin (vitamin P4) on muscle fatigue and gene expression of Bcl-2 and Bax in the hepatic tissue of rats. Can. J. Physiol. Pharmacol. 2017;95(6):708–713. doi: 10.1139/cjpp-2016-0653. [DOI] [PubMed] [Google Scholar]
- 5.Azarfarin M., Farajdokht F., Babri S., Salehpour F., Taghizadeh M., Mohaddes G. Effects of troxerutin on anxiety- and depressive-like behaviors induced by chronic mild stress in adult male rats. Iran. J. Basic Med. Sci. 2018;21(8):781–786. doi: 10.22038/IJBMS.2018.26915.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elangovan P., Pari L. Ameliorating effects of troxerutin on nickel-induced oxidative stress in rats. Redox Rep. 2013;18(6):224–232. doi: 10.1179/1351000213Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng X. Health benefits and molecular mechanisms of resveratrol: a narrative review. 2020. [DOI] [PMC free article] [PubMed]
- 8.Ríos J.-L. A pharmacological update of ellagic acid. 2018. [DOI] [PubMed]
- 9.Budzynska B. Rutin as neuroprotective agent: from bench to bedside. 2019. [DOI] [PubMed]
- 10.Khushboo S.B., Sharma B. Antidepressants: mechanism of action, toxicity and possible amelioration. J. Appl. Biotechnol. Bioeng. 2017;3:1–13. [Google Scholar]
- 11.Zavvari O.Z., Mirzaei B.F., Hamidian G.R., Mehri K., Qadiri A., Ahmadi M., Oghbaei H., Vatankhah A.M., Keyhanmanesh R. Troxerutin affects the male fertility in prepubertal type 1 diabetic male rats. Iran. J. Basic Med. Sci. 2019;22(2):197–205. doi: 10.22038/ijbms.2018.32678.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousefi-Manesh H., Shirooie S., Partoazar A., Nikoui V., Estakhri M.R.A., Bakhtiarian A. Hepatoprotective effects of phosphatidylserine liposomes on carbon tetrachloride-induced hepatotoxicity in rats. 2019. [DOI] [PubMed]
- 13.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta V., Sharma B. Role of phytochemicals in neurotrophins mediated regulation of Alzheimer’s disease. Int. J. Complement. Alt. Med. 2017;7(4):00231. [Google Scholar]
- 15.Badalzadeh R., Chodari L., Ghorbanzadeh V. Troxerutin, a bioflavonoid, improves oxidative stress in blood of streptozotocin-induced type-1 diabetic rats. Indian J. Pharm. Sci. 2017;13(2):75–86. [Google Scholar]
- 16.Geetha R., Sathiya P.C., Anuradha C.V. Troxerutin abrogates mitochondrial oxidative stress and myocardial apoptosis in mice fed calorie-rich diet. Chem. Biol. Interact. 2017;278:74–83. doi: 10.1016/j.cbi.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Battino M., Giampieri F., Pistollato F., Sureda A., de Oliveira M.R., Pittalà V., Fallarino F., Nabavi S.F., Atanasov A.G., Nabavi S.M. Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnol. Adv. 2018;36(2):358–370. doi: 10.1016/j.biotechadv.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Rashidian A., Muhammadnejad A., Dehpour A.R., Mehr S.E., Akhavan M.M., Shirkoohi R., Chamanara M., Mousavi S.E., Rezayat S.M. Atorvastatin attenuates TNBS-induced rat colitis: the involvement of the TLR4/NF-kB signaling pathway. Inflammopharmacology. 2016;24(2-3):109–118. doi: 10.1007/s10787-016-0263-6. [DOI] [PubMed] [Google Scholar]
- 19.Thomas N.S., George K., Selvam A.A.A. Anticancer mechanism of troxerutin via targeting Nrf2 and NF-κB signalling pathways in hepatocarcinoma cell line. Toxicol. In Vitro. 2019;54:317–329. doi: 10.1016/j.tiv.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y., Zheng G. Troxerutin protects against diabetic cardiomyopathy through NF-κB/AKT/IRS1 in a rat model of type 2 diabetes. Mol. Med. Rep. 2017;15(6):3473–3478. doi: 10.3892/mmr.2017.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Pablo-Fernández E., Lees A.J., Holton J.L., Warner T.T. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. prognosis and neuropathologic correlation of clinical subtypes of parkinson disease. JAMA Neurol. 2019;76(4):470–479. doi: 10.1001/jamaneurol.2018.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue X., Chen Y., Wang Y., Zhan J., Chen B., Wang X., Pan X. Troxerutin suppresses the inflammatory response in advanced glycation end-product-administered chondrocytes and attenuates mouse osteoarthritis development. Food Funct. 2019;10(8):5059–5069. doi: 10.1039/C9FO01089K. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi M., Canavesi R., Aprile S., Grosa G., Del Grosso E. Troxerutin, a mixture of O-hydroxyethyl derivatives of the natural flavonoid rutin: Chemical stability and analytical aspects. J. Pharm. Biomed. Anal. 2018;150:248–257. doi: 10.1016/j.jpba.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Xin X., Zhang M., Li X., Lai F., Zhao G. Biocatalytic synthesis of acylated derivatives of troxerutin: their bioavailability and antioxidant properties in vitro. Microb. Cell Fact. 2018;17(1):130. doi: 10.1186/s12934-018-0976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson C.T., Sharma V., Iablokov S.N., Albayrak L., Khanipov K., Uchitel S., Chopra D., Mills P.J., Fofanov Y., Rodionov D.A., Peterson S.N. 16S rRNA gene profiling and genome reconstruction reveal community metabolic interactions and prebiotic potential of medicinal herbs used in neurodegenerative disease and as nootropics. PLoS One. 2019;14(3):e0213869. doi: 10.1371/journal.pone.0213869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Compta Y., Parkkinen L., Kempster P., Selikhova M., Lashley T., Holton J.L., Lees A.J., Revesz T. The significance of α-synuclein, amyloid-β and tau pathologies in Parkinson’s disease progression and related dementia. Neurodegener. Dis. 2014;13(2-3):154–156. doi: 10.1159/000354670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang X., Wang J., Jiang H., Shi L., Xie J. Hyperpolarization-Activated cyclic nucleotide-gated channels: an emerging role in neurodegenerative diseases. Front. Mol. Neurosci. 2019;12:141. doi: 10.3389/fnmol.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarozzi A., Angeloni C., Malaguti M., Morroni F., Hrelia S., Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013;2013:415078. doi: 10.1155/2013/415078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizek P., Kumar N., Jog M.S. An update on the diagnosis and treatment of Parkinson disease. CMAJ. 2016;188(16):1157–1165. doi: 10.1503/cmaj.151179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang S., Yao B., Li N., Lin S., Huang Z. Association of dopamine beta-hydroxylase polymorphisms with Alzheimer’s disease, parkinson’s disease and schizophrenia: evidence based on currently available loci. Cell. Physiol. Biochem. 2018;51(1):411–428. doi: 10.1159/000495238. [DOI] [PubMed] [Google Scholar]
- 33.Sergeant N., Vingtdeux V., Eddarkaoui S., Gay M., Evrard C., Le Fur N., Laurent C., Caillierez R., Obriot H., Larchanché P.E., Farce A., Coevoet M., Carato P., Kouach M., Descat A., Dallemagne P., Buée-Scherrer V., Blum D., Hamdane M., Buée L., Melnyk P. New piperazine multi-effect drugs prevent neurofibrillary degeneration and amyloid deposition, and preserve memory in animal models of Alzheimer’s disease. Neurobiol. Dis. 2019;129:217–233. doi: 10.1016/j.nbd.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Hulbert A.J., Pamplona R., Buffenstein R., Buttemer W.A. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007;87(4):1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 35.Rosini M., Simoni E., Caporaso R., Basagni F., Catanzaro M., Abu I.F., Fagiani F., Fusco F., Masuzzo S., Albani D., Lanni C., Mellor I.R., Minarini A. Merging memantine and ferulic acid to probe connections between NMDA receptors, oxidative stress and amyloid-β peptide in Alzheimer’s disease. Eur. J. Med. Chem. 2019;180:111–120. doi: 10.1016/j.ejmech.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Dastan Z., Pouramir M., Ghasemi-Kasman M., Ghasemzadeh Z., Dadgar M., Gol M., Ashrafpour M., Pourghasem M., Moghadamnia A.A., Khafri S. Arbutin reduces cognitive deficit and oxidative stress in animal model of Alzheimer’s disease. Int. J. Neurosci. 2019;129(11):1145–1153. doi: 10.1080/00207454.2019.1638376. [DOI] [PubMed] [Google Scholar]
- 37.Tobore T.O. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer’s disease. Neurol. Sci. 2019;40(8):1527–1540. doi: 10.1007/s10072-019-03863-x. [DOI] [PubMed] [Google Scholar]
- 38.Luca M., Luca A., Calandra C. The role of oxidative damage in the pathogenesis and progression of Alzheimer’s Disease and vascular dementia. Oxid. Med. Cell. Longev. 2015;2015:504678. doi: 10.1155/2015/504678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy P.H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 2009;218(2):286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan M.H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma W., Wang S., Liu X., Tang F., Zhao P., Cheng K., Zheng Q., Zhuo Y., Zhao X., Li X., Feng W. Protective effect of troxerutin and cerebroprotein hydrolysate injection on cerebral ischemia through inhibition of oxidative stress and promotion of angiogenesis in rats. Mol. Med. Rep. 2019;19(4):3148–3158. doi: 10.3892/mmr.2019.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhào H., Liu Y., Zeng J., Li D., Zhang W., Huang Y. Troxerutin and cerebroprotein hydrolysate injection protects neurovascular units from oxygen-glucose deprivation and reoxygenation-induced injury In Vitro. Evid. Based Complement. Alternat. Med. 2018;2018:9859672. doi: 10.1155/2018/9859672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J., Wu D.M., Hu B., Cheng W., Zheng Y.L., Zhang Z.F., Ye Q., Fan S.H., Shan Q., Wang Y.J. Chronic administration of troxerutin protects mouse brain against D-galactose-induced impairment of cholinergic system. Neurobiol. Learn. Mem. 2010;93(2):157–164. doi: 10.1016/j.nlm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Lu J., Wu D.M., Hu B., Zheng Y.L., Zhang Z.F., Wang Y.J. NGF-Dependent activation of TrkA pathway: A mechanism for the neuroprotective effect of troxerutin in D-galactose-treated mice. Brain Pathol. 2010;20(5):952–965. doi: 10.1111/j.1750-3639.2010.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu J., Wu D.M., Zheng Z.H., Zheng Y.L., Hu B., Zhang Z.F. Troxerutin protects against high cholesterol-induced cognitive deficits in mice. Brain. 2011;134(Pt 3):783–797. doi: 10.1093/brain/awq376. [DOI] [PubMed] [Google Scholar]
- 46.Qin L., Zhang J., Qin M. Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci. Lett. 2013;534:285–288. doi: 10.1016/j.neulet.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Lu J., Wu D.M., Zheng Y.L., Hu B., Cheng W., Zhang Z.F., Li M.Q. Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein β-mediated inflammatory response and oxidative stress. J. Immunol. 2013;190(7):3466–3479. doi: 10.4049/jimmunol.1202862. [DOI] [PubMed] [Google Scholar]
- 48.Babri S., Mohaddes G., Feizi I., Mohammadnia A., Niapour A., Alihemmati A., Amani M. Effect of troxerutin on synaptic plasticity of hippocampal dentate gyrus neurons in a β-amyloid model of Alzheimer׳s disease: an electrophysiological study. Eur. J. Pharmacol. 2014;732:19–25. doi: 10.1016/j.ejphar.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S., Li H., Zhang L., Li J., Wang R., Wang M. Effects of troxerutin on cognitive deficits and glutamate cysteine ligase subunits in the hippocampus of streptozotocin-induced type 1 diabetes mellitus rats. Brain Res. 2017;1657:355–360. doi: 10.1016/j.brainres.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Shan Q., Zheng G.H., Han X.R., Wen X., Wang S., Li M.Q., Zhuang J., Zhang Z.F., Hu B., Zhang Y., Zheng Y.L. Troxerutin Protects kidney tissue against bde-47-induced inflammatory damage through CXCR4-TXNIP/NLRP3 signaling. Oxid. Med. Cell. Longev. 2018;2018:9865495. doi: 10.1155/2018/9865495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diba R., Mohaddes G., Mirzaie Bavil F., Farajdokht F., Bayandor P., Hosseindoost M., Mehri K., Zavvari O.Z., Babri S. Protective effects of troxerutin on maternal high-fat diet-induced impairments of spatial memory and apelin in the male offspring. Iran. J. Basic Med. Sci. 2018;21(7):682–687. doi: 10.22038/IJBMS.2018.28170.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamali-Raeufy N., Kardgar S., Baluchnejadmojarad T., Roghani M., Goudarzi M. Troxerutin exerts neuroprotection against lipopolysaccharide (LPS) induced oxidative stress and neuroinflammation through targeting SIRT1/SIRT3 signaling pathway. Metab. Brain Dis. 2019;34(5):1505–1513. doi: 10.1007/s11011-019-00454-9. [DOI] [PubMed] [Google Scholar]
- 53.Rangasamy S.B., Dasarathi S., Pahan P., Jana M., Pahan K. Low-Dose aspirin upregulates tyrosine hydroxylase and increases dopamine production in dopaminergic neurons: implications for Parkinson’s Disease. J. Neuroimmune Pharmacol. 2019;14(2):173–187. doi: 10.1007/s11481-018-9808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L., Pu J. Alpha-Synuclein in Parkinson’s Disease: from pathogenetic dysfunction to potential clinical application. Parkinsons Dis. 2016;2016:1720621. doi: 10.1155/2016/1720621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMillan P.J., White S.S., Franklin A., Greenup J.L., Leverenz J.B., Raskind M.A., Szot P. Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson’s disease and Alzheimer’s disease. Brain Res. 2011;1373:240–252. doi: 10.1016/j.brainres.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song P., Rudan D., Zhu Y., Fowkes F.J.I., Rahimi K., Fowkes F.G.R., Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob. Health. 2019;7(8):e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 58.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 59.Yang Q., Zhou L., Liu C., Liu D., Zhang Y., Li C., Shang Y., Wei X., Li C., Wang J. Brain iron deposition in type 2 diabetes mellitus with and without mild cognitive impairment-an in vivo susceptibility mapping study. Brain Imaging Behav. 2018;12(5):1479–1487. doi: 10.1007/s11682-017-9815-7. [DOI] [PubMed] [Google Scholar]
- 60.Zang L., Shimada Y., Nakayama H., Chen W., Okamoto A., Koide H., Oku N., Dewa T., Shiota M., Nishimura N. Therapeutic silencing of centromere protein x ameliorates hyperglycemia in zebrafish and mouse models of type 2 diabetes mellitus. Front. Genet. 2019;10:693. doi: 10.3389/fgene.2019.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hossain P., Kawar B., El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N. Engl. J. Med. 2007;356(3):213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 62.Stenlöf K., Cefalu W.T., Kim K.A., Alba M., Usiskin K., Tong C., Canovatchel W., Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes. Metab. 2013;15(4):372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rheinberger M., Jung B., Segiet T., Nusser J., Kreisel G., Andreae A., Manz J., Haas G., Banas B., Stark K., Lammert A., Gorski M., Heid I.M., Krämer B.K., Böger C.A. Poor risk factor control in outpatients with diabetes mellitus type 2 in Germany: The DIAbetes COhoRtE (DIACORE) study. PLoS One. 2019;14(3):e0213157. doi: 10.1371/journal.pone.0213157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Folli F., Corradi D., Fanti P., Davalli A., Paez A., Giaccari A., Perego C., Muscogiuri G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011;7(5):313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 65.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996;20(3):463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 66.Grankvist K., Marklund S.L., Täljedal I.B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981;199(2):393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santos A.S., Cunha Neto E., Fukui R.T., Ferreira L.R.P., Silva M.E.R. Increased expression of circulating microrna 101-3p in type 1 diabetes patients: new insights into mirna-regulated pathophysiological pathways for Type 1 Diabetes. Front. Immunol. 2019;10:1637. doi: 10.3389/fimmu.2019.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranjith V., Radika M., Anuradha C. Effect of troxerutin on insulin resistance induced by post-natal administration of monosodium glutamate: a comparative study with rosiglitazone. J Clin Lab Investiga Updat. 2013;1:36–47. [Google Scholar]
- 70.Awasthi S., Ravi A., Saraswathi N.T. Troxerutin imparts preservative effects on albumin by preventing Maillard reaction-mediated early and advanced glycation modification. J. Biomol. Struct. Dyn. 2017;35(12):2681–2687. doi: 10.1080/07391102.2016.1229218. [DOI] [PubMed] [Google Scholar]
- 71.Sampath S., Karundevi B. Effect of troxerutin on insulin signaling molecules in the gastrocnemius muscle of high fat and sucrose-induced type-2 diabetic adult male rat. Mol. Cell. Biochem. 2014;395(1-2):11–27. doi: 10.1007/s11010-014-2107-2. [DOI] [PubMed] [Google Scholar]
- 72.Chung H.K., Choi S.M., Ahn B.O., Kwak H.H., Kim J.H., Kim W.B. Efficacy of troxerutin on streptozotocin-induced rat model in the early stage of diabetic retinopathy. Arzneimittelforschung. 2005;55(10):573–580. doi: 10.1055/s-0031-1296907. [DOI] [PubMed] [Google Scholar]
- 73.Badalzadeh R., Layeghzadeh N., Alihemmati A., Mohammadi M. Beneficial effect of troxerutin on diabetes-induced vascular damages in rat aorta: histopathological alterations and antioxidation mechanism. Int. J. Endocrinol. Metab. 2015;13(2):e25969. doi: 10.5812/ijem.25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Badalzadeh R., Mokhtari B., Yavari R. Contribution of apoptosis in myocardial reperfusion injury and loss of cardioprotection in diabetes mellitus. J. Physiol. Sci. 2015;65(3):201–215. doi: 10.1007/s12576-015-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mokhtari B., Badalzadeh R., Alihemmati A., Mohammadi M. Phosphorylation of GSK-3β and reduction of apoptosis as targets of troxerutin effect on reperfusion injury of diabetic myocardium. Eur. J. Pharmacol. 2015;765:316–321. doi: 10.1016/j.ejphar.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 76.Yavari R., Badalzadeh R., Alipour M.R., Tabatabaei S.M. Modulation of hippocampal gene expression of microRNA-146a/microRNA-155-nuclear factor-kappa B inflammatory signaling by troxerutin in healthy and diabetic rats. Indian J. Pharmacol. 2016;48(6):675–680. doi: 10.4103/0253-7613.194847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S., Yuan L., Zhang L., Li C., Li J. Prophylactic use of troxerutin can delay the development of diabetic cognitive dysfunction and improve the expression of nrf2 in the hippocampus on stz diabetic rats. Behav. Neurol. 2018;2018:8678539. doi: 10.1155/2018/8678539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan J.L., Mantzoros C.S. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001;4(1-2):87–92. doi: 10.1023/A:1012947113197. [DOI] [PubMed] [Google Scholar]
- 79.Condorelli R.A., La Vignera S., Mongioì L.M., Alamo A., Calogero A.E. Diabetes mellitus and infertility: Different pathophysiological effects in type 1 and type 2 on sperm function. Front. Endocrinol. (Lausanne) 2018;9:268. doi: 10.3389/fendo.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qadiri A., Mirzaei Bavil F., Hamidian G., Zavvari Oskuye Z., Ahmadi M., Oghbaei H., Mehri K., Vatankhah A.M., Keyhanmanesh R. Administration of troxerutin improves testicular function and structure in type-1 diabetic adult rats by reduction of apoptosis. Avicenna J. Phytomed. 2019;9(4):374–385. [PMC free article] [PubMed] [Google Scholar]
- 81.Geetha R., Radika M.K., Priyadarshini E., Bhavani K., Anuradha C.V. Troxerutin reverses fibrotic changes in the myocardium of high-fat high-fructose diet-fed mice. Mol. Cell. Biochem. 2015;407(1-2):263–279. doi: 10.1007/s11010-015-2474-3. [DOI] [PubMed] [Google Scholar]
- 82.Malinska H., Hüttl M., Oliyarnyk O., Markova I., Poruba M., Racova Z., Kazdova L., Vecera R. Beneficial effects of troxerutin on metabolic disorders in non-obese model of metabolic syndrome. PLoS One. 2019;14(8):e0220377. doi: 10.1371/journal.pone.0220377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geetha R., Yogalakshmi B., Sreeja S., Bhavani K., Anuradha C.V. Troxerutin suppresses lipid abnormalities in the heart of high-fat-high-fructose diet-fed mice. Mol. Cell. Biochem. 2014;387(1-2):123–134. doi: 10.1007/s11010-013-1877-2. [DOI] [PubMed] [Google Scholar]
- 84.Hoseindoost M., Alipour M.R., Farajdokht F., Diba R., Bayandor P., Mehri K., Nayebi Rad S., Babri S. Effects of troxerutin on inflammatory cytokines and BDNF levels in male offspring of high-fat diet fed rats. Avicenna J. Phytomed. 2019;9(6):597–605. doi: 10.22038/AJP.2019.13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehri K., Banan Khojasteh S.M., Seyed M.B.K., Fereshteh F., Zavvari O.Z., Ebrahimi H., Diba R., Bayandor P., Hosseindoost M., Babri S. Effect of troxerutin on apelin-13, apelin receptors (APJ), and ovarian histological changes in the offspring of high-fat diet fed rats. Iran. J. Basic Med. Sci. 2019;22(6):637–642. doi: 10.22038/ijbms.2019.34158.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Z., Wang X., Zheng G., Shan Q., Lu J., Fan S., Sun C., Wu D., Zhang C., Su W., Sui J., Zheng Y. Troxerutin attenuates enhancement of hepatic gluconeogenesis by inhibiting NOD Activation-mediated inflammation in high-fat diet-treated mice. Int. J. Mol. Sci. 2016;18(1):E31. doi: 10.3390/ijms18010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z.F., Fan S.H., Zheng Y.L., Lu J., Wu D.M., Shan Q., Hu B. Troxerutin improves hepatic lipid homeostasis by restoring NAD(+)-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem. Pharmacol. 2014;91(1):74–86. doi: 10.1016/j.bcp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Fan S.H., Zhang Z.F., Zheng Y.L., Lu J., Wu D.M., Shan Q., Hu B., Wang Y.Y. Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int. Immunopharmacol. 2009;9(1):91–96. doi: 10.1016/j.intimp.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Liu C.M., Ma J.Q., Lou Y. Chronic administration of troxerutin protects mouse kidney against D-galactose-induced oxidative DNA damage. Food Chem. Toxicol. 2010;48(10):2809–2817. doi: 10.1016/j.fct.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 90.Yang X., Xu W., Huang K., Zhang B., Wang H., Zhang X., Gong L., Luo Y., He X. Precision toxicology shows that troxerutin alleviates ochratoxin A-induced renal lipotoxicity. FASEB J. 2019;33(2):2212–2227. doi: 10.1096/fj.201800742R. [DOI] [PubMed] [Google Scholar]
- 91.Dehnamaki F., Karimi A., Pilevarian A.A., Fatemi I., Hakimizadeh E., Kaeidi A., Allahtavakoli M., Rahmani M.R., Khademalhosseini M., Bazmandegan G. Treatment with troxerutin protects against cisplatin-induced kidney injury in mice. Acta Chir. Belg. 2019;119(1):31–37. doi: 10.1080/00015458.2018.1455418. [DOI] [PubMed] [Google Scholar]
- 92.Elangovan P., Ramakrishnan R., Amudha K., Jalaludeen A.M., Sagaran G.K., Babu F.R., Pari L. Beneficial protective effect of troxerutin on nickel-induced renal dysfunction in wistar rats. J. Environ. Pathol. Toxicol. Oncol. 2018;37(1):1–14. doi: 10.1615/JEnvironPatholToxicolOncol.2017025087. [DOI] [PubMed] [Google Scholar]
- 93.Salama S.A., Arab H.H., Maghrabi I.A. Troxerutin down-regulates KIM-1, modulates p38 MAPK signaling, and enhances renal regenerative capacity in a rat model of gentamycin-induced acute kidney injury. Food Funct. 2018;9(12):6632–6642. doi: 10.1039/C8FO01086B. [DOI] [PubMed] [Google Scholar]
- 94.Adam B.S., Pentz R., Siegers C.P., Strubelt O., Tegtmeier M. Troxerutin protects the isolated perfused rat liver from a possible lipid peroxidation by coumarin. Phytomedicine. 2005;12(1-2):52–61. doi: 10.1016/j.phymed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Z.F., Fan S.H., Zheng Y.L., Lu J., Wu D.M., Shan Q., Hu B. Troxerutin protects the mouse liver against oxidative stress-mediated injury induced by D-galactose. J. Agric. Food Chem. 2009;57(17):7731–7736. doi: 10.1021/jf9012357. [DOI] [PubMed] [Google Scholar]
- 96.Ping X., Junqing J., Junfeng J., Enjin J. Radioprotective effects of troxerutin against gamma irradiation in mice liver. Int. J. Radiat. Biol. 2012;88(8):607–612. doi: 10.3109/09553002.2012.692494. [DOI] [PubMed] [Google Scholar]
- 97.Thomas N.S., George K., Arivalagan S., Mani V., Siddique A.I., Namasivayam N. The in vivo antineoplastic and therapeutic efficacy of troxerutin on rat preneoplastic liver: biochemical, histological and cellular aspects. Eur. J. Nutr. 2017;56(7):2353–2366. doi: 10.1007/s00394-016-1275-0. [DOI] [PubMed] [Google Scholar]
- 98.Panat N.A., Singh B.G., Maurya D.K., Sandur S.K., Ghaskadbi S.S. Troxerutin, a natural flavonoid binds to DNA minor groove and enhances cancer cell killing in response to radiation. Chem. Biol. Interact. 2016;251:34–44. doi: 10.1016/j.cbi.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 99.Xu G.Y., Tang X.J. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-κB and Bcl-2 signaling pathways. Biomed. Pharmacother. 2017;92:95–107. doi: 10.1016/j.biopha.2017.04.059. [DOI] [PubMed] [Google Scholar]
- 100.Subastri A., Suyavaran A., Preedia B.E., Nithyananthan S., Barathidasan R., Thirunavukkarasu C. Troxerutin with copper generates oxidative stress in cancer cells: Its possible chemotherapeutic mechanism against hepatocellular carcinoma. J. Cell. Physiol. 2018;233(3):1775–1790. doi: 10.1002/jcp.26061. [DOI] [PubMed] [Google Scholar]
- 101.Vinothkumar R., Vinoth K.R., Sudha M., Viswanathan P., Balasubramanian T., Nalini N. Modulatory effect of troxerutin on biotransforming enzymes and preneoplasic lesions induced by 1,2-dimethylhydrazine in rat colon carcinogenesis. Exp. Mol. Pathol. 2014;96(1):15–26. doi: 10.1016/j.yexmp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 102.Subastri A., Harikrishna K., Sureshkumar M., Alshammari G.M., Aristatile B., Thirunavukkarasu C. Effect of troxerutin on 2-aminoanthracene and DNA interaction and its anti-mutagenic property. Biomed. Pharmacother. 2017;88:325–334. doi: 10.1016/j.biopha.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 103.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Huffman M.D., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Magid D., Marcus G.M., Marelli A., Matchar D.B., McGuire D.K., Mohler E.R., Moy C.S., Mussolino M.E., Nichol G., Paynter N.P., Schreiner P.J., Sorlie P.D., Stein J., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Enas E.A., Singh V., Munjal Y.P., Gupta R., Patel K.C., Bhandari S., Agarwal A.K., Joshi S.R., Anoop M., Prabhakaran D., Shah B., Reddy S., Sharma B., Trehan N., Yavagal S.T., Kasliwal R.R. Second Indo-US Health Summit Expert Panel. Recommendations of the second Indo-U.S. health summit on prevention and control of cardiovascular disease among Asian Indians. Indian Heart J. 2009;61(3):265–274. [PubMed] [Google Scholar]
- 106.Shu L., Zhang W., Huang C., Huang G., Su G. Troxerutin protects against myocardial ischemia/reperfusion injury via pi3k/akt pathway in rats. Cell. Physiol. Biochem. 2017;44(5):1939–1948. doi: 10.1159/000485884. [DOI] [PubMed] [Google Scholar]
- 107.Shu L., Zhang W., Huang G., Huang C., Zhu X., Su G., Xu J. Troxerutin attenuates myocardial cell apoptosis following myocardial ischemia-reperfusion injury through inhibition of miR-146a-5p expression. J. Cell. Physiol. 2019;234(6):9274–9282. doi: 10.1002/jcp.27607. [DOI] [PubMed] [Google Scholar]
- 108.Najafi M., Noroozi E., Javadi A., Badalzadeh R. Anti-arrhythmogenic and anti-inflammatory effects of troxerutin in ischemia/reperfusion injury of diabetic myocardium. Biomed. Pharmacother. 2018;102:385–391. doi: 10.1016/j.biopha.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 109.Rajagopalan G., Chandrasekaran S.P., Carani V.A. Troxerutin attenuates diet-induced oxidative stress, impairment of mitochondrial biogenesis and respiratory chain complexes in mice heart. Clin. Exp. Pharmacol. Physiol. 2017;44(1):103–113. doi: 10.1111/1440-1681.12671. [DOI] [PubMed] [Google Scholar]
- 110.Raja B., Saranya D., Prabhu R. Role of flavonoid troxerutin on blood pressure, oxidative stress and regulation of lipid metabolism. Front. Biosci. (Elite Ed.) 2019;11:121–129. doi: 10.2741/e851. [DOI] [PubMed] [Google Scholar]
- 111.Bayandor P., Farajdokht F., Mohaddes G., Diba R., Hosseindoost M., Mehri K., Zavvari O.Z., Babri S. The effect of troxerutin on anxiety- and depressive-like behaviours in the offspring of high-fat diet fed dams. Arch. Physiol. Biochem. 2019;125(2):156–162. doi: 10.1080/13813455.2018.1443142. [DOI] [PubMed] [Google Scholar]
- 112.Elangovan P., Jalaludeen A.M., Ramakrishnan R., Pari L. Protective effect of troxerutin on nickel-induced testicular toxicity in Wistar Rats. J. Environ. Pathol. Toxicol. Oncol. 2016;35(2):133–146. doi: 10.1615/JEnvironPatholToxicolOncol.2016015384. [DOI] [PubMed] [Google Scholar]