Abstract
Background
A grave complication of thyrotoxicosis, or thyroid storm, is the development of heart failure and cardiomyopathy. Recognizing this condition is imperative in preventing further left ventricular dysfunction and cardiogenic shock. This manuscript aims to review the literature on cardiogenic shock associated with thyrotoxicosis and present management recommendations on this rare condition.
Methods
A literature search was performed in December of 2018, using the PubMed medical search engine. A systematic search was carried out using the keywords Thyroid Storm AND Cardiogenic Shock and Thyrotoxicosis AND Shock.
Management
Decrease of thyroid hormone levels using therapeutic plasma exchange LV Unloading and ventilation by Impella and Extracorporeal Mechanical Ventilation (ECMO).
Conclusion
Patients presenting with thyroid storm-induced shock may not be suitable candidates for traditional management with β-adrenergic blockers (β-blockers). The use of β-blockers could exasperate their condition. Through extensive literature review on this rare condition, the most effective management was found to be therapeutic plasma exchange in order to decrease thyroid hormone levels, which have direct toxic effect on the heart. Furthermore, the use of ECMO and Impella is advised to reduce pressure on the heart and ensure the patient’s organs are well oxygenated and perfused while the left ventricle is recovering.
Keywords: Thyrotoxicosis, cardiogenic shock, shock, thyroid storm, ECMO, impella, therapeutic plasma exchange
1. Introduction
1.1. Rationale
A grave complication of thyrotoxicosis or thyroid storm is the development of heart failure and cardiomyopathy. Recognizing this condition is imperative in preventing further left ventricular dysfunction and cardiogenic shock. As noted in the literature, this condition is exceptionally rare; only six percent of patients develop heart failure and cardiomyopathy as a result of thyrotoxicosis with an even smaller percentage having left ventricular dysfunction [1]. Although the incidence rate for this condition is low, the mortality rate is close to thirty percent because of the association between cardiogenic shock and hypotension [2].
1.2. Objectives
This manuscript aims to review the literature on cardiogenic shock associated with thyrotoxicosis and present management recommendations on this rare condition.
2. Methods
2.1. Protocol and Registration
This manuscript’s protocol was not sent for review. For the purpose of this manuscript, Prisma guidelines were followed.
2.2. Eligibility Criteria
After the use of keywords, exclusion criteria were applied to the findings. These included papers focused on right heart failure and heart failure but no cardiogenic shock, pregnancy, Iodine or Amiodarone-induced thyrotoxicosis. The also included review articles on thyrotoxicosis and heart failure, and papers in languages other than English (as shown in Flow Chart). The choice of having these exclusions was to simply remove any possible etiology for cardiogenic shock other than thyrotoxicosis.
Flow Chart. Showing the method for choosing articles. Table 1 shows the keywords used in PubMed Central. a Other exclusions are mentioned in Eligibility Criteria. b
Table 1.
Search Key Words and results.
| Number | Search | Result |
|---|---|---|
| 1 | Thyrotoxicosis/Thyroid Storm + Cardiogenic Shock | 13 |
| 2 | Thyrotoxicosis/Thyroid Storm + Cardiogenic Shock + ECMO | 2ŧ |
| 3 | Thyrotoxicosis/Thyroid Storm + Cardiogenic Shock + Impella | 0 |
| 4 | Thyrotoxicosis/Thyroid Storm + Cardiogenic Shock + Plasmapheresis | 0 |
| 5 | Thyrotoxicosis/Thyroid Storm + Pulmonary Edema + Heart Failure | 16 |
| 6 | Thyrotoxicosis/Thyroid Storm + ECMO | 9 |
| 7 | Thyrotoxicosis/Thyroid Storm + Impella | 0 |
| 8 | Thyrotoxicosis/Thyroid Storm + Plasmapheresis | 85 |
Note: ŧ: repeat articles.
2.3. Information Sources/Search
A literature search was performed in December of 2018 using the PubMed medical search engine [3].
2.4. Study Selection
A systematic search was carried out using keyword combinations that are mentioned in Table 1. A total of 123 articles were identified with the oldest dating back to 1974 and the most recent being published in 2018.
2.5. Date Collection Process
Three researchers reviewed these articles independently and applied the exclusion criteria.
2.6. Data Items
Reviewers as well as the rest of the team used no funding for collection of data, analysis and providing the manuscript.
2.7. Risk of Bias in Individual Studies
Risk of bias at individual level for studies is extremely low. As mentioned in the introduction, this is a very rare condition and as a result, all studies were case reports, merely reporting patient management rather than structured studies.
2.8. Summary Measures
Principal summary is based on the method of management and the outcome (live/death) as well as average hospital length of stay.
2.9. Synthesis of Results
Descriptive analysis, such as mean or median and percentage, was performed for continuous variable proportions. For categorical patient variables, frequencies were used. Comparisons were made using Kaplan Meir estimator which was used for survival analysis. A p level of <0.05 was used to determine statistical significance. Prism software 8.0 was used for calculations.
2.10. Risk of Bias Across Studies
The only bias identified across studies is at the level of the provider who executed management. This is based on their level of knowledge on the subject matter and their preferred methods.
3. Results
A total of twelve articles, shown in Table 2, were found after applying the exclusion criteria. The majority of articles were case reports due to the rarity of the condition. Data on age, sex, ejection fraction, the methodology of treatment (use of β-blocker, ECMO, Impella, or TPE) were extracted from all of the articles as presented in Table 3. All patients presented here had cardiogenic shock. Some presented with hemodynamic instability and some developed cardiogenic shock shortly after use of β-blockers. One patient from the fourth article cited was excluded since the patient was managed for congestive heart failure by the use of diuretics and was later found to have thyrotoxic-induced cardiogenic shock.
Table 2.
Articles that met criteria for use in this review paper.
| Number | Author | Year of Publication |
|---|---|---|
| 1 | Abubakr et al. [10] | 2017 |
| 2 | Eyadiel et al. [9] | 2018 |
| 3 | Allencherril et al. [24] | 2015 |
| 4 | NGO et al. [7] | 2007 |
| 5 | Kim et al. [6] | 2018 |
| 6 | Dahl et al. [1] | 2008 |
| 7 | Chao et al. [25] | 2015 |
| 8 | Kiriyama et al. [34] | 2017 |
| 9 | White et al. [32] | 2018 |
| 10 | Koball et al. [35] | 2010 |
| 11 | Hsu et al. [36] | 2011 |
| 12 | Palkar et al. [37] | 2012 |
Table 3.
Descriptive analysis of papers reviewed. EF is Ejection Fraction of Left Ventricle at time of presentation. U/S is result of ultrasound showing either Left Ventricular failure (LVF) or Right Ventricular Failure (RVF). BB is if β-blocker was used or not. HLS is hospital length of stay in days. Outcome is if patient stayed alive or expired. A fib/Flutter is presence of these abnormal rhythms on admission.
| Author | Year | Age | Sex | A-Fib/Flutter | EF | U/S | BB | Pressor | ECMO | IMPELA | TPE | ECMO Decannulation | HLS | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abubakr et al. | 2017 | 39 | M | Yes | 15-20% | LVF | Y | Y | N | N | Y | - | 11 | Expired |
| Eyadiel et al. | 2018 | 27 | F | No | <10% | LVF | Y | Y | Y | Y | Y | 6 d | - | Live |
| Allencherril et al. | 2015 | 29 | M | Yes | <20% | LVF | Y | Y | Y | N | N | 7 d | - | Live |
| NGO et al. | 2007 | 32 | M | Yes | 25% | LVF | Y | Y | N | N | N | - | 9 | Live |
| 28 | M | Yes | 20% | LVF | Y | Y | N | N | N | - | 9 | Expired | ||
| Kim et al. | 2018 | 52 | M | Yes | <20% | LVF | Y | Y | Y | N | N | 6 d | 14 | Live |
| Dahl et al. | 2008 | 32 | F | No | - | - | Y | - | N | N | N | - | - | Live |
| Chao et al. | 2015 | 47 | M | - | 24% (20-24) | LVF | N | Y | Y | N | N | 3.4 d | - | Expired |
| 43 | M | - | Expired | |||||||||||
| 37 | F | - | Live | |||||||||||
| 42 | M | - | Live | |||||||||||
| 33 | F | - | Live | |||||||||||
| Kiriyama et al. | 2017 | 54 | F | Yes | <20% | LVF | N | N | Y | N | N | 18 d | 18 | Live |
| White et al. | 2018 | 57 | F | Yes | <10% | LVF | N | Y | Y | N | N | 10 d | 53 | Live |
| Koball et al. | 2010 | 68 | F | - | - | - | Y | Y | N | N | Y | - | 18 | Live |
| Hsu et al. | 2011 | 47 | M | Yes | 32% | LVF | N | Y | Y | N | N | - | 5 | Expired |
| 43 | M | No | 20% | 5 d | 20 | Live | ||||||||
| 37 | F | Yes | 32% | 5 d | 5 | Live | ||||||||
| 42 | M | No | 29% | 5 d | 5 | Live | ||||||||
| Palkar et al. | 2012 | 27 | F | No | 40% | LVF | Y | - | N | N | N | - | - | Live |
A descriptive analysis of the data is shown in Table 4. A total of 20 patients were identified in 12 case reports. These patients were divided into two groups based on the medical management they received. The case group was categorized by the use of mechanical support (ECMO and/or Impella), and the control group comprised of patients who were administered medical therapy (β-blocker and inotropes) only. Propranolol was the β-blocker used in all papers that used βB, except for the case presented by Eyadiel et al. Days of de-cannulation from ECMO are included in Table 3 in order to find if the use of TPE shortened the time to de-cannulation from ECMO. Left ventricular ejection fraction of patients was divided into four groups (a: <10%, b: 10-20%, c: 21-30%, d: >30%) as shown in Table 4.
Table 4.
Descriptive analysis of patients in the two groups of ECMO and/or Impella vs. beta-blocker (BB) and/or therapeutic plasma exchange (TPE). For age mean is presented. For sex, percentage of male is presented. For ejection fraction (EF), results were divided into four categories and the number of patients in each sub category is presented as a percentage. Group a: EF <10%, b: 10-20%, c: 21-30%, d: >30%.
| - | Mechanical Support (N=14) | Medical Therapy (N=6) |
|---|---|---|
| Age (yo) | 42 | 38 |
| Sex (Male) | 57% | 67% |
| Ejection Fraction | a: 14.3% | a: 0% |
| - | b: 28.6% | b: 50% |
| - | c: 42.9% | c: 25% |
| - | d: 14.3% | d: 25% |
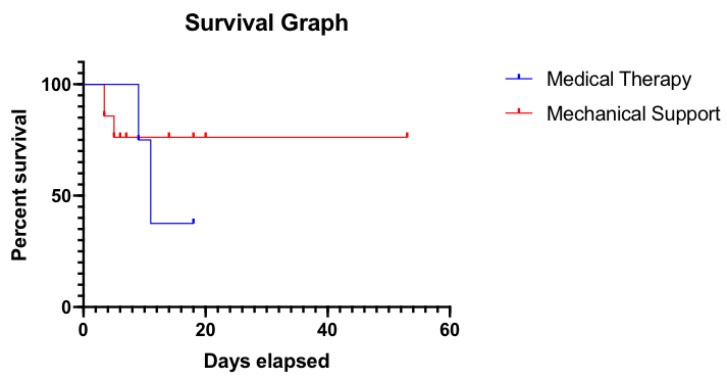
Statistical analysis was performed for survival study. Length of hospital stay was used as survival days since the diagnosis of the condition. For Mechanical Support group, in patients with no HLS documented, days to de-cannulation from ECMO were used instead. In the medical therapy group, two patients had no HLS and therefore were removed from analysis. As demonstrated in Survival Graph (Fig. 1), survival rate difference was not statistically significant between the groups with P-value of 0.68. Mean days to de-cannulation from ECMO were calculated for live patients and were found to be 6.5 days.
Fig. (1).
Survival graph. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. Discussion
4.1. Presentation and Pathophysiology
The direct and indirect actions of T3 are contributing to the main effects of thryrotoxicosis on the heart and cardiovascular system. Heart rate and left ventricular contractility increase as a result of hyperthyroidism while systemic vascular resistance decreases [1]. The decrease in vascular resistance will lead to a decrease in renal perfusion, which in turn activates the renin-angiotensin-aldosterone system. This will lead to an increased preload and as a result, increased cardiac output [4]. All of these changes lead to decreased myocardial contractile reserve, predisposing the patient to heart failure [1, 4]. This progression can be seen in patients with no prior cardiac injury.
Anywhere from 10-25% of patients with hyperthyroidism are known to have atrial fibrillation (AF) [4]. From the reviewed literature, AF along with rapid ventricular response (RVR) is one of the main electrical abnormalities observed in the patients with thyrotoxicosis at the time of presentation (TSH <0.5 mU/L or non-detectable) [5-8]. Tachycardia is a known leading factor of heart failure in these patients due to their low myocardial contractile reserve [4].
4.2. Management
β-adrenergic blockers, along with anti-thyroid hormone medications, are traditionally used as first-line management for patients presenting with thyrotoxicosis [1, 4]. Propranolol is a prominent medication utilized for thyrotoxicosis management since it decreases the conversion of T4 to the more active T3 form. However, development of cardiogenic shock after administration of β-blockers was found in many of the articles reviewed [5, 6, 9, 10]. Abubakr et al. pointed to the fact that in a hyperthyroid state, with the patient experiencing high cardiac output, β-blockers will not place the patient at risk of hemodynamic instability [9]. Patients that have decreased left ventricular ejection fraction (LVEF), propranolol is not recommended due to its risk of cardiogenic shock; this, in turn, would mandate the use of volume resuscitation and pressors drugs. Ikram et al. invasively monitored hyperthyroid and cardiac failure patients and were able to demonstrate that decreased cardiac response with the use of β-adrenergic blocker was due to decreased stroke volume and increased pulmonary artery diastolic pressure [11]. This was observed in all cases of cardiogenic shock chosen in our literature review.
For this reason, β-blockers should be prescribed with caution. Several articles recommend using ultra-short-acting variants (i.e. esmolol with a 9-minute half-life) [12, 13]. The rationale is that if there is evidence of decreased myocardial function, like worsening congestive heart failure or cardiogenic shock, the ultra-short-acting β-blockers can be discontinued and the effects reversed. Additionally if propranolol is used, a low intravenous dose (i.e. 0.5 mg) should be administered first. Choudhury & MacDermot mentioned if cardiac failure in a thyrotoxicosis patient is found to be truly secondary to congestion (decompensating due to an increase in intravascular volume), negative inotropic β-blockers should be avoided in patients with underlying ischemic, hypertensive, or valvular heart disease [14].
A study published by Mohananey et al. examined the Nationwide Inpatient Sample and found the rate of cardiogenic shock in patients who present with thyroid storm to have increased from 0.5% in 2003 to 3% in 2011. [15] They further revealed a 40% decrease in mortality for this patient population. The researchers attributed the decreased mortality rate to the increased use of intra-aortic balloon pump (IABP) and extracorporeal mechanical ventilation (ECMO) for the patients in intensive care unit.
4.3. Management of Cardiogenic Shock in Thyrotoxicosis
4.3.1. Decrease of Thyroid Hormone Levels Using Therapeutic Plasma Exchange
Use of anti-thyroid medications has shown to improve cardiac function, but this result could take weeks to observe [4]. For recovery of cardiac function, radioactive iodine ablation or thyroidectomy have been shown to be effective in the past [16, 17]. However, in light of cardiogenic shock and hemodynamic instability, these methods are not feasible. Use of therapeutic plasma exchange (TPE) for management of thyrotoxicosis has been documented since 1974 [18]. A recent publication by Eyadiel et al. demonstrated the use of therapeutic plasma exchange to decrease the thyroid hormone levels in a patient with cardiogenic shock secondary to thyrotoxicosis [8]. It has been well documented that in patients with acute thyrotoxicosis, therapeutic plasma exchange can remove thyroid hormones as well as thyroid gland autoantibodies, catecholamines and cytokines [19, 20, 30, 36]. In research by Subahi et al., a four-step algorithm was created for the management of thyrotoxic crisis [21]. Early use of plasma exchange was one of the proposals made in their algorithm for clearing the thyroid hormone. Another area where therapeutic plasma exchange (TPE) might be used is prior to urgent surgery. In a retrospective study, Ezer et al. demonstrated the successful use of TPE in patients with thyrotoxicosis not responding to classic medical therapy and in need of urgent surgery [22]. These surgeries were either on the thyroid or urgent orthopedic surgeries, and all patients experienced outstanding results with fresh frozen plasma and albumin exchange transfusion. The same results were noted by Simsir et al. who confirmed the effectiveness of TPE as an alternative to anti-thyroid medications, especially in patients who experience adverse effects or are being prepared for urgent surgery [19]. Our study found an average of 6.5 days to de-cannulation from ECMO. Eyadiel et al. demonstrated that the use of TPE along with mechanical support was shown to decrease de-cannulation time from ECMO in their report [8]. More studies are needed to prove a shortened time to de-cannulation from mechanical support with the use of TPE.
4.3.2. LV Unloading by Extracorporeal Mechanical Ventilation (ECMO) and Impella or IABP
Mohananey et al. showed a 40% improvement in survival with mechanical support [15]. Maintaining blood oxygenation is of great importance in patients with cardiogenic shock. This task was performed by the use of ECMO in the reviewed articles [5, 8, 15, 23-24] ECMO is a temporary measure used in order to replace the function of the heart and/or lungs. This, in turn, allows time for organ recovery. ECMO has been shown to be useful in conditions like respiratory distress syndrome, acute myocardial infarction, post cardiotomy shock, and severe cardiomyopathy [25-29].
The patients mentioned in the reviewed articles had compromised cardiac contraction and hypotension despite the use of pressers. Schrage et al. have shown the successful use of venoarterial ECMO in patients who present with cardiogenic shock [30]. White et al. performed a systematic review in which there were a 78.5% survival rate and near complete recovery of left ventricular function; these patients were managed with VA-ECMO [31]. The main drawback observed by the researchers was elevated after load on the left ventricle. This can be overcome by use of Impella, as used by Eyadiel et al. [8] In a retrospective study performed by Colombier et al., it was observed that the utilization of Impella was incredibly beneficial in patients presenting with refractory cardiogenic shock and were not responding well to the simultaneous use of VA-ECMO and intra-aortic balloon pump [32]. There was a higher 30-day survival rate in these patients. Implementing Impella would take pressure off of the left ventricle and provide time for recovery.
In several of the studies reviewed, IABP was another device used to supplement ECMO; these devices are known to increase myocardial oxygen perfusion and indirectly increase cardiac output by decreasing the afterload of the heart [23-24, 33, 35].
Conclusion
The most important factor in patients presenting with thyroid storm-induced shock is to determine if left ventricular ejection fraction is affected. These patients might not be candidates for traditional management with β-adrenergic blockers. Use of β-blockers could worsen their condition by causing hemodynamic instability and necessitating inotropes. By reviewing the literature on this rare condition, the most effective management was found to be therapeutic plasma exchange which decreased thyroid hormone levels and have direct toxic effect on the heart. Further use of mechanical support (ECMO and Impella) is advised to take pressure away from the heart and ensures that patient’s organs are well oxygenated and perfused while the left ventricle is recovering.
This quasi-meta analysis failed to attain statistical significance in length of survival between the mechanical support and medical therapy groups. Statistical difference in our study could be attributed to several factors including the low number of studies reviewed due to the rarity of the case; this small sample size leads to low power. One should keep in mind when researching the topic that not many articles are written on negative results, especially in cases of mortality.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
STANDARD OF REPORTING:
PRISMA guidelines and methodologies were followed.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Dahl P., Danzi S., Klein I. Thyrotoxic cardiac disease. Curr. Heart Fail. Rep. 2008;5(3):170–176. doi: 10.1007/s11897-008-0026-9. [DOI] [PubMed] [Google Scholar]
- 2.Nayak B., Burman K. Thyrotoxicosis and thyroid storm. Endocrinol. Metab. Clin. North Am. 2006;35(4):663–686. doi: 10.1016/j.ecl.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 3. https://www.ncbi.nlm.nih.gov/pubmed/
- 4.Osuna P.M., Udovcic M., Sharma M.D. Hyperthyroidism and the Heart. Methodist DeBakey Cardiovasc. J. 2017;13(2):60–63. doi: 10.14797/mdcj-13-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S., Seol S-H., Kim Y-S., Kim D-K., Kim K-H., Kim D-I. Thyrotoxicosis induced cardiogenic shock rescued by extracorporeal membrane oxygenation. J. Geriatr. Cardiol. 2018;15(2):203–204. doi: 10.11909/j.issn.1671-5411.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngo S.Y.A., Chew H.C. When the storm passes unnoticed--a case series of thyroid storm. Resuscitation. 2007;73(3):485–490. doi: 10.1016/j.resuscitation.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Riaz K., Forker A.D., Isley W.L., Hamburg M.S., McCullough P.A. Hyperthyroidism: a “curable” cause of congestive heart failure--three case reports and a review of the literature. Congest. Heart Fail. 2003;9(1):40–46. doi: 10.1111/j.1527-5299.2003.01124.x. [DOI] [PubMed] [Google Scholar]
- 8.Eyadiel L., Amjad A., Pisani B., Miller P., Jain R. Use of therapeutic plasma exchange and ECMO support with impella for LV vent as treatment for cardiogenic shock in acute thyrotoxicosis/thyroid storm. J. Card. Fail. 2018;24(8) Suppl.:S84–S85. [Google Scholar]
- 9.Abubakar H., Singh V., Arora A., Alsunaid S. Propranolol-induced circulatory collapse in a patient with thyroid crisis and underlying thyrocardiac disease: A word of caution. J. Investig. Med. High Impact Case Rep. 2017;5(4):2324709617747903. doi: 10.1177/2324709617747903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngo A.S-Y., Lung Tan D.C. Thyrotoxic heart disease. Resuscitation. 2006;70(2):287–290. doi: 10.1016/j.resuscitation.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Ikram H. Haemodynamic effects of beta-adrenergic blockade in hyperthyroid patients with and without heart failure. BMJ. 1977;1(6075):1505–1507. doi: 10.1136/bmj.1.6075.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbier G.H., Shettigar U.R., Appunn D.O. Clinical rationale for the use of an ultra-short acting beta-blocker: Esmolol. Int. J. Clin. Pharmacol. Ther. 1995;33(4):212–218. [PubMed] [Google Scholar]
- 13.Isley W.L., Dahl S., Gibbs H. Use of esmolol in managing a thyrotoxic patient needing emergency surgery. Am. J. Med. 1990;89(1):122–123. doi: 10.1016/0002-9343(90)90114-S. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury R.P., MacDermot J. Heart failure in thyrotoxicosis, an approach to management. Br. J. Clin. Pharmacol. 1998;46(5):421–424. doi: 10.1046/j.1365-2125.1998.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohananey D., Smilowitz N., Villablanca P.A., et al. Trends in the incidence and in-hospital outcomes of cardiogenic shock complicating thyroid storm. Am. J. Med. Sci. 2017;354(2):159–164. doi: 10.1016/j.amjms.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Biondi B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur. J. Endocrinol. 2012;167(5):609–618. doi: 10.1530/EJE-12-0627. [DOI] [PubMed] [Google Scholar]
- 17.Tomisti L., Materazzi G., Bartalena L., et al. Total thyroidectomy in patients with amiodarone-induced thyrotoxicosis and severe left ventricular systolic dysfunction. J. Clin. Endocrinol. Metab. 2012;97(10):3515–3521. doi: 10.1210/jc.2012-1797. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann J., Hilger P., Rusche H.J., Krüskemper H.L. Plasmapheresis in the treatment of thyrotoxic crisis (author’s transl). Dtsch. Med. Wochenschr. 1974;99(17):888–892. doi: 10.1055/s-0028-1107858. [DOI] [PubMed] [Google Scholar]
- 19.Simsir I.Y., Ozdemir M., Duman S., Erdogan M., Donmez A., Ozgen A.G. Therapeutic plasmapheresis in thyrotoxic patients. Endocrine. 2018;62(1):144–148. doi: 10.1007/s12020-018-1661-x. [DOI] [PubMed] [Google Scholar]
- 20.Wyble A.J., Moore S.C., Yates S.G. Weathering the storm: A case of thyroid storm refractory to conventional treatment benefiting from therapeutic plasma exchange. J. Clin. Apher. 2018;33(6):678–681. doi: 10.1002/jca.21658. [DOI] [PubMed] [Google Scholar]
- 21.Subahi A., Ibrahim W., Abugroun A. Diltiazem-associated cardiogenic shock in thyrotoxic crisis. Am. J. Ther. 2018;25(6):e666–e669. doi: 10.1097/MJT.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 22.Ezer A., Caliskan K., Parlakgumus A., Belli S., Kozanoglu I., Yildirim S. Preoperative therapeutic plasma exchange in patients with thyrotoxicosis. J. Clin. Apher. 2009;24(3):111–114. doi: 10.1002/jca.20200. [DOI] [PubMed] [Google Scholar]
- 23.Allencherril J., Birnbaum I. Heart failure in thyrotoxic cardiomopathy: Extracorporeal membrane oxygenation treatment for graves’ disease. J. Extra Corpor. Technol. 2015;47(4):231–232. [PMC free article] [PubMed] [Google Scholar]
- 24.Chao A., Wang C-H., You H-C., et al. Highlighting Indication of extracorporeal membrane oxygenation in endocrine emergencies. Sci. Rep. 2015;5:13361. doi: 10.1038/srep13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y-S., Chao A., Yu H-Y., et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J. Am. Coll. Cardiol. 2003;41(2):197–203. doi: 10.1016/S0735-1097(02)02716-X. [DOI] [PubMed] [Google Scholar]
- 26.Litwiński P., Dębski A., Tyczyński P., et al. Rescue extracorporeal membrane oxygenation for refractory cardiogenic shock. Postepy Kardiol. Interwencyjnej. 2015;11(4):327–329. doi: 10.5114/pwki.2015.55605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y-S., Lin J-W., Yu H-Y., et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support vs. conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet. 2008;372(9638):554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 28.Ko W-J., Lin C-Y., Chen R.J., Wang S-S., Lin F-Y., Chen Y-S. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann. Thorac. Surg. 2002;73(2):538–545. doi: 10.1016/S0003-4975(01)03330-6. [DOI] [PubMed] [Google Scholar]
- 29.Peek G.J., Mugford M., Tiruvoipati R., et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support vs. extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 30.Schrage B., Burkhoff D., Rübsamen N., et al. Unloading of the left ventricle during venoarterial extracorporeal membrane oxygenation therapy in cardiogenic shock. JACC Heart Fail. 2018;6(12):1035–1043. doi: 10.1016/j.jchf.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 31.White A., Bozso S.J., Moon M.C. Thyrotoxicosis induced cardiomyopathy requiring support with extracorporeal membrane oxygenation. J. Crit. Care. 2018;45:140–143. doi: 10.1016/j.jcrc.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Colombier S., Quessard A., Mastroianni C., et al. Benefits of impella and peripheral veno-arterial extra corporeal life support alliance. ASAIO J. 2018;••• doi: 10.1097/MAT.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 33.Kiriyama H., Amiya E., Hatano M., et al. Rapid Improvement of thyroid storm-related hemodynamic collapse by aggressive anti-thyroid therapy including steroid pulse: A case report. Medicine (Baltimore) 2017;96(22):e7053. doi: 10.1097/MD.0000000000007053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koball S., Hickstein H., Gloger M., et al. Treatment of thyrotoxic crisis with plasmapheresis and single pass albumin dialysis: A case report. Artif. Organs. 2010;34(2):E55–E58. doi: 10.1111/j.1525-1594.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 35.Hsu L-M., Ko W-J., Wang C-H. Extracorporeal membrane oxygenation rescues thyrotoxicosis-related circulatory collapse. Thyroid. 2011;21(4):439–441. doi: 10.1089/thy.2010.0230. [DOI] [PubMed] [Google Scholar]
- 36.Palkar AV, Shrivastava MS, Moulick ND. An unusual cause of flash pulmonary oedema. 2012. [DOI] [PMC free article] [PubMed]