Abstract
Background
Systemic Mastocytosis (SM) is a disorder of excessive mast cell infiltration in multiple organ tissues. Atherosclerosis is a major risk factor for developing acute coronary syndrome. In addition to lipid accumulation in the arterial wall, inflammation plays an important role in the pathogenesis of plaque rupture and activating the thrombosis cascade. The Mast cells contribution to plaque destabilization has been well established in multiple animal and human studies. In a recent study, SM has been proven to be associated with a higher incidence of acute coronary syndrome even with lower plasma lipids levels. The study showed that 20% of patients with SM had cardiovascular events compared to only 6% in the control group with adjustment to all cardiac risk factors.
Case
We presented a patient with no risk factors for heart disease other than old age and history of SM who developed acute myocardial infarction.
Conclusion
SM can be life-threatening and can result in ACS, anaphylactic reaction, syncope, or cardiac arrest. Clinicians should have a high index of suspicion of acute coronary syndrome (ACS) occurrence in the setting of inflammatory conditions, such as SM and KS, and vice versa, where SM should be considered or ruled out in patients who suffer from anaphylaxis and cardiac arrest or myocardial infarction.
Keywords: SM, electrocardiogram, subclinical hypothyroidism, patient, myocardial infarction, acute coronary syndrome
1. Introduction
Systemic Mastocytosis (SM) is a disorder of excessive mast cell infiltration in multiple organ tissues. Atherosclerosis is a major risk factor for developing acute coronary syndrome [1]. In addition to lipid accumulation in the arterial wall, inflammation plays an important role in the pathogenesis of plaque rupture and activating the thrombosis cascade [2]. The Mast cells’ contribution to plaque destabilization has been well established in multiple animal and human studies [3]. In a recent study, SM has been proven to be associated with a higher incidence of acute coronary syndrome even with lower plasma lipids levels [4]. The study showed that 20% of patients with SM had cardiovascular events compared to only 6% in the control group with adjustment to all cardiac risk factors. Here, we present a case of acute myocardial infarction in a patient with SM with limited risk factors other than age.
2. Case presentation
We present a case of a 78-year-old female with a past medical history of gastroesophageal reflux disease, subclinical hypothyroidism and recently diagnosed SM who was transferred to our institution for ST-elevation myocardial infarction. She was diagnosed with SM two years ago after she had multiple anaphylactic reactions and cardiac arrest secondary to anaphylactic shock. She was found to have elevated tryptase 119 ug/L and the diagnosis was confirmed with bone marrow biopsy. Since then, she has been on anti-histamine.
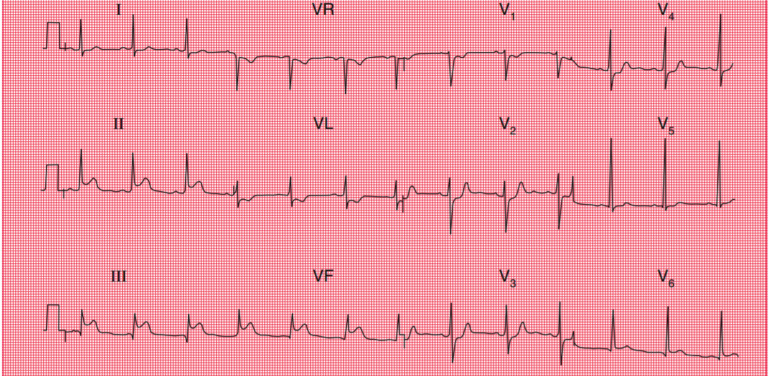
She presented to the emergency department at an outside hospital with substernal chest pain that radiates to both shoulders and elbows. She described it as pressure-like and was severe with associated symptoms of nausea, vomiting, and diaphoresis. Laboratory analysis revealed troponin T 0.24 and a normal lipid panel and hemoglobin A1C. Electrocardiogram (EKG) was notable for ST elevation in Lead II, Lead III and AvF (Fig. 1). The patient initially refused to take medications due to her concerns for anaphylactic reaction and agreed after she was premedicated with methylprednisolone and famotidine. She was treated with aspirin 326mg and Plavix 300mg. She refused thrombolytics. Once arrived at our institution, her symptoms resolved and repeated EKG showed resolution of ST-segment elevation and small Q waves in the inferolateral leads. Echocardiogram demonstrated mild hypokinesis of the basal to mid inferior wall. The ejection fraction was 60-65%.
Fig. (1).
EKG with ST elevation in Lead III, Lead II and AvF. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
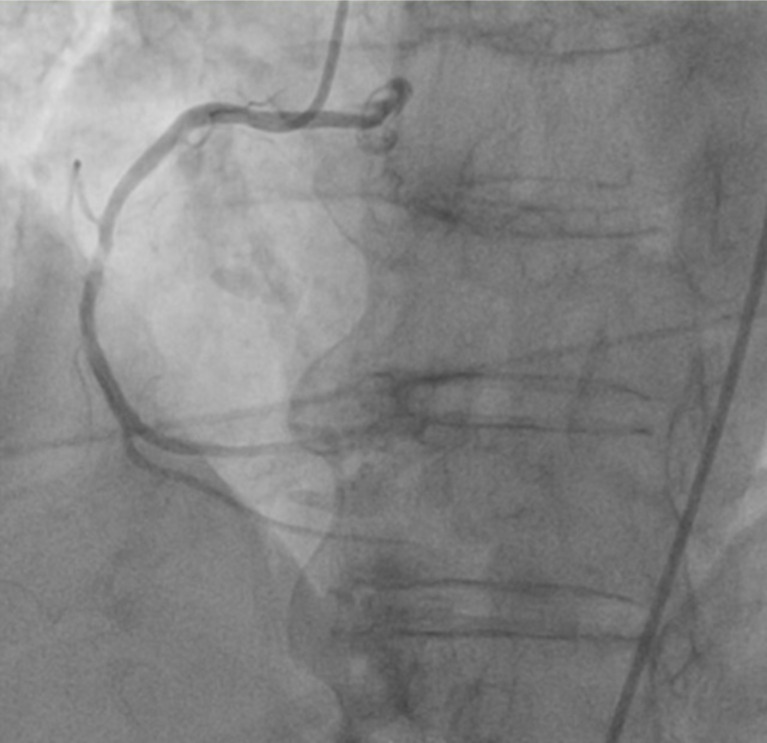
Her case was discussed with her allergist in a large academic medical center in the area and we decided to do an oral challenge test for the dual antiplatelet therapy which she tolerated without any premedication. The patient was taken to the catheterization suite with premedication for assumed contrast allergy. Angiography revealed 95% stenosis in the mid-right coronary artery (RCA) which was believed to be the culprit lesion. There were no significant obstructive lesions noted in other coronary arteries (Fig. 2). She underwent percutaneous coronary intervention (PCI) with successful drug-eluting stent placement to the mid-RCA. She was started on guideline-directed medical therapy which she tolerated without any allergic reaction. She did not have any chest pain after the procedure and her cardiac enzymes down trended. She was discharged home in a stable condition 2 days later.
Fig. (2).
Coronary angiogram showing 95% stenosis in right coronary artery. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. Discussion
Systemic mastocytosis (SM) is a pathological buildup of mast cells in the body organs. Systemic mastocytosis has been associated with a wide variety of cardiovascular diseases which can be the first presentation of the disease. Cardiac arrest of unknown etiology was found to be the initial manifestation of SM in 26% of the cases, which likely is a result of coronary vasospasm or acute coronary syndrome. There are 23 cases reported in the literature on the relation between SM and cardiac diseases which were summarized in a meta-analysis published in 2017 by Paratz, E [5]. The incidence of cardiac diseases in SM patients is not well known because of the under-diagnosis of the disease. Acute coronary syndrome and cardiac arrest are the most common cardiac complications of SM that have been reported in the literature [5]. The level of mast cells in the myocardium is low compared to the rest of body organs, however the number of cells increases in systemic diseases or inflammation. Cardiac complications can be due to anaphylactic reaction in the setting of rapid mast cell degranulation or can present in stable SM disease due to chronic higher levels of mast cells in coronary vessels, myocardium and atherosclerotic plaques [6].
Mast cells have been implicated in the development of atherosclerotic cardiovascular disease. Patients with SM have a higher prevalence of the cardiovascular disease, regardless of total and LDL cholesterol levels [4]. Moreover, we found in the literature that SM patients had higher rates of coronary artery disease (CAD) and cerebrovascular accidents (CVA) based on a large population study in Europe [7]. As atherosclerosis advances in stage, the number of mast cells increases, which translates to an amplified risk of plaque rupture due to mast cell degranulation.
The mast cell is also the only inflammatory cell type to have independent predictability of future cardiovascular events. Two mast cell substances essential to plaque instability are chymase and tryptase. Our patient had elevated tryptase in the setting of STEMI. This central role of mast cells in atherosclerotic disease sheds light on potentially newer treatment approaches that can target leukocyte infiltration, lipid buildup, and matrix disintegration [8, 9].
Vascular inflammation plays a major role in the development of acute coronary syndrome by its effect on plaque stabilization. During inflammation, immune cells accumulate in large numbers around an atherosclerotic plaque and degranulate their contents, which can cause rupture of the plaque’s fibrous cap. Such rupture leads to the release of thrombogenic factors that lead to stimulation of the platelet and coagulation pathways [2]. A state of hypercoagulability can be associated with SM and increase the risk of thrombus formation and acute coronary syndrome [10].
Furthermore, the role of inflammation in myocardial remodeling has been established after infarction. In a randomized control trial using the histamine-1 receptor antagonist Loratadine on patients on patients with recent myocardial infarction, there has been more improvement in exercise tests compared to patients who were on placebo. Targeting inflammation has been the main focus of current research for primary and secondary prevention for coronary disease [11].
Other reported cardiac conditions with SM were arrhythmia [12, 13], AV block, and pericardial effusion secondary to the involvement of myopericardium [14]. Another commonly mentioned presentation is allergic angina or Kounis syndrome which occurs acutely due to coronary vasospasm [15]. KS is characterized by an acute event triggered by an allergen that induces vasospasm. KS is categorized into three types: I, II, and III. The underlying mechanism is excessive mast cell stimulation provoked by an allergic reaction. In type I KS, coronary arteries are spastic with no obstruction. Type II KS has non-obstructive atherosclerosis. In both types I and II, inflammatory mediators are released in large quantities leading to plaque rupture [16].
Type III KS, however, occurs in the setting of intracoronary thrombosis. These patients can have inflammatory cell infiltration and inflammatory cell release as a direct result of intracoronary stent placement. Our case had SM with myocardial infarction due to plaque rupture and thrombosis of the RCA without KS because the inflammatory process was chronic and systemic. Our patient did not have any allergic symptoms and did not have anaphylactic reaction.
4. Role of inflammation in myocardial infarction
Inflammation increases the risk of plaque rupture. The site of plaque rupture was found to be where coronary thrombi occurred in autopsy studies. The entire process of plaque rupture with the release of thrombogenic substances is called plaque remodeling. This remodeling involves the influx of inflammatory cells, reduction in smooth muscle cells, and destruction of matrix wall by proteinases. Mast cells are found in larger numbers near the shoulder or adventitia of a plaque compared to its intima. The adventitia is most affected by mast cell degranulation, leading to loss of matrix integrity and increased risk of plaque rupture that can manifest as an acute coronary event [17].
Inflammation plays an important role in the development of atherosclerosis and acute coronary syndromes. The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) showed that inhibiting interleukin (IL)-1β can lower both C-reactive protein (CRP) and acute coronary events in a population of patients who experienced an MI within the previous 30 days [18].
The Global Utilization of Strategies To Open occluded arteries (GUSTO-IV) study found CRP to be an independent prognostic factor for mortality at 1 year, but CRP did not have a relation to a recurrence of myocardial infarction within 30 days of the initial infarction [19]. There is a direct proportional relationship between statin benefit and degree of inflammation, where more benefit is gained from statins when there is more inflammation [20]. A study called SECURE-PCI (Statins Evaluation in Coronary Procedures and Revascularization) trial showed that high-dose atorvastatin caused a 50% reduction in MACE (major adverse cardiovascular events) in patients who underwent primary PCI (percutaneous coronary intervention) [21]. LV function was more difficult to recover at 6 weeks post-MI with higher Interleukin (IL) 8 levels [22]. Compared to other inflammatory markers, Il-8 was the most specific cytokine for worsened LV systolic function, larger infarctions, and adverse outcomes for up to 1 year of follow-up [23].
Rymer et al. demonstrated that multiple anti-inflammatory agents failed to demonstrate an improvement in clinical outcomes in ACS possibly due to insufficient understanding of the pathophysiology [24]. Other research showed some promise with the use of anti-inflammatory agents in preclinical studies, but the agents had to be administered either before or during the acute cardiovascular event. However, in clinical practice, multiple anti-inflammatory agents are not used until a patient has an acute coronary event. However, statins have shown to have anti-inflammatory effect, and experts argue about its effect on mortality after MI is related to its anti-inflammatory effect. Therefore, further studies are needed to clarify the role of different anti-inflammatory agents in ACS. Tardif et al. demonstrated that low-dose colchicine significantly reduced the risk of ischemic cardiovascular events in patients with recent MI [25]. Methotrexate failed to reduce cardiovascular events [26].
Also, there are multiple ongoing clinical trials about using monoclonal antibodies in CAD, but the outcomes will be available in the next few years [27].
Conclusion
SM can be life-threatening and present for the first time as ACS, anaphylactic reaction, syncope, or cardiac arrest. Clinicians should have a high index of suspicion of acute coronary syndrome (ACS) occurrence in the setting of inflammatory conditions, such as SM and KS, and vice versa, where SM should be considered or ruled out in patients who present with anaphylaxis and cardiac arrest or myocardial infarction. We presented a patient with no risk factors for heart disease other than old age and history of SM and developed acute myocardial infarction. Management of ACS in SM is challenging leading to risk of allergy to medications and risk of anaphylaxis which will worsen plaque instability as described above. Premedication with anti-histamine and steroid was crucial in her management. Overall, multiple case reports and reviews have discussed the risk of cardiac events in systemic mastocytosis, yet large population studies are needed to prove the association.
Acknowledgements
• Department of Internal Medicine, University of Massachusetts Medical School-Baystate, Springfield, Massachusetts, USA.
• University of Texas Health Science Center at Houston, Texas, USA.
• Department of Internal Medicine, Overland Park Regional Medical Center-HCA Midwest Health, Overland Park, Kansas, USA.
• Department of Internal Medicine, University of Texas Health Science Center at San Antonio, USA.
• Department of Cardiology, State University of New York: Downstate Medical Center, Brooklyn, New York, USA.
• Department of Internal Medicine, State University of New York, Downstate Medical Center, Brooklyn, New York, USA.
LIST OF Abbreviations
- ACS
Acute Coronary Syndrome
- CAD
Coronary Artery Disease
- CVA
Cerebrovascular Accidents
- EKG
Electrocardiogram
- KS
Kounis Syndrome
- PCI
Percutaneous Coronary Intervention
- RCA
Right Coronary Artery
- SM
Systemic Mastocytosis
Care:
CARE guidelines were followed.
Consent for Publication
Verbal consent was obtained.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Shah P.K. Mechanisms of plaque vulnerability and rupture. J. Am. Coll. Cardiol. 2003;41(4) Suppl. S:15S–22S. doi: 10.1016/S0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 2.Stoll G., Bendszus M. Inflammation and atherosclerosis: Novel insights into plaque formation and destabilization. Stroke. 2006;37(7):1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 3.Lagraauw H.M., Wezel A., van der Velden D., Kuiper J., Bot I. Stress-induced mast cell activation contributes to atherosclerotic plaque destabilization. Sci. Rep. 2019;9(1):2134. doi: 10.1038/s41598-019-38679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Indhirajanti S., van Daele P.L.A., Bos S., Mulder M.T., Bot I., Roeters van Lennep J.E. Systemic mastocytosis associates with cardiovascular events despite lower plasma lipid levels. Atherosclerosis. 2018;268:152–156. doi: 10.1016/j.atherosclerosis.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Paratz E.D., Khav N., Burns A.T. Systemic mastocytosis, Kounis syndrome and coronary intervention: Case report and systematic review. Heart Lung Circ. 2017;26(8):772–778. doi: 10.1016/j.hlc.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Hermans M., Lennep J.R.V., van Daele P., Bot I. Mast cells in cardiovascular disease: From bench to bedside. Int. J. Mol. Sci. 2019;20(14):3395. doi: 10.3390/ijms20143395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broesby-Olsen S., Farkas D.K., Vestergaard H., et al. Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients with systemic mastocytosis: A nationwide population-based study. Am. J. Hematol. 2016;91(11):1069–1075. doi: 10.1002/ajh.24490. [DOI] [PubMed] [Google Scholar]
- 8.Bot I., Shi G.P., Kovanen P.T. Mast cells as effectors in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015;35(2):265–271. doi: 10.1161/ATVBAHA.114.303570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niccoli G., Montone R.A., Sabato V., Crea F. Role of allergic inflammatory cells in coronary artery disease. Circulation. 2018;138(16):1736–1748. doi: 10.1161/CIRCULATIONAHA.118.035400. [DOI] [PubMed] [Google Scholar]
- 10.Huang A L, Bosco J J, Peter K. 2017.
- 11.Erdogan O., Altun A., Gazi S., Ozbay G. Loratidine improves ischemic parameters of exercise stress test in patients with acute myocardial infarction. Am. Heart J. 2004;148(6):e24. doi: 10.1016/j.ahj.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 12.Ridolo E., Triggiani M., Montagni M., et al. Mastocytosis presenting as cardiac emergency. Intern. Emerg. Med. 2013;8(8):749–752. doi: 10.1007/s11739-013-1012-0. [DOI] [PubMed] [Google Scholar]
- 13.Rohr S.M., Rich M.W., Silver K.H. Shortness of breath, syncope, and cardiac arrest caused by systemic mastocytosis. Ann. Emerg. Med. 2005;45(6):592–594. doi: 10.1016/j.annemergmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Thomas D., Dragodanne C., Frank R., Prier A., Chomette G., Grosgogeat Y. Systemic mastocytosis with myo-pericardial localization and atrioventricular block. Arch. Mal. Coeur Vaiss. 1981;74(2):215–221. [PubMed] [Google Scholar]
- 15.Kounis N.G. Coronary hypersensitivity disorder: The Kounis syndrome. Clin. Ther. 2013;35(5):563–571. doi: 10.1016/j.clinthera.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Rigger J., Ehl N.F., Nägele R., Rickli H., Maeder M.T. Kounis syndrome revisited: Systemic mastocytosis and severe coronary artery disease. Int. J. Cardiol. 2016;214:510–511. doi: 10.1016/j.ijcard.2016.03.154. [DOI] [PubMed] [Google Scholar]
- 17.Kaartinen M., Penttilä A., Kovanen P.T. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90(4):1669–1678. doi: 10.1161/01.CIR.90.4.1669. [DOI] [PubMed] [Google Scholar]
- 18.Ridker P.M., Everett B.M., Thuren T., et al. CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 19.James S.K., Lindahl B., Siegbahn A., et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: A Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation. 2003;108(3):275–281. doi: 10.1161/01.CIR.0000079170.10579.DC. [DOI] [PubMed] [Google Scholar]
- 20.Chan A.W., Bhatt D.L., Chew D.P., et al. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation. 2003;107(13):1750–1756. doi: 10.1161/01.CIR.0000060541.18923.E9. [DOI] [PubMed] [Google Scholar]
- 21.Berwanger O., Santucci E.V., de Barros E., Silva P.G.M., et al. SECURE-PCI Investigators Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: the SECURE-PCI randomized clinical trial. JAMA. 2018;319(13):1331–1340. doi: 10.1001/jama.2018.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husebye T., Eritsland J., Arnesen H., et al. Association of interleukin 8 and myocardial recovery in patients with ST-elevation myocardial infarction complicated by acute heart failure. PLoS One. 2014;9(11):e112359. doi: 10.1371/journal.pone.0112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limalanathan S., Andersen G.Ø., Hoffmann P., Kløw N.E., Abdelnoor M., Eritsland J. Rationale and design of the POSTEMI (postconditioning in ST-elevation myocardial infarction) study. Cardiology. 2010;116(2):103–109. doi: 10.1159/000316965. [DOI] [PubMed] [Google Scholar]
- 24.Rymer J.A., Newby L.K. Failure to launch: Targeting inflammation in acute coronary syndromes. JACC Basic Transl. Sci. 2017;2(4):484–497. doi: 10.1016/j.jacbts.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tardif J.C., Kouz S., Waters D.D., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 26.Ridker P.M., Everett B.M., Pradhan A., et al. CIRT Investigators Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019;380(8):752–762. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiedler LR. Antibody based therapy in coronary artery disease and heart failure. 2017.