Abstract
In periodontitis, polymorphonuclear leucocytes (PMNs) are activated. They entrap and eliminate pathogens by releasing neutrophil extracellular traps (NETs). Abnormal NET degradation is part of a pro-inflammatory status, affecting co-morbidities such as cardiovascular disease. We aimed to investigate the ex vivo NET degradation capacity of plasma from periodontitis patients compared to controls (part 1) and to quantify NET degradation before and after periodontal therapy (part 2). Fresh NETs were obtained by stimulating blood-derived PMNs with phorbol 12-myristate 13-acetate. Plasma samples from untreated periodontitis patients and controls were incubated for 3 h onto freshly generated NETs (part 1). Similarly, for part 2, NET degradation was studied for 91 patients before and 3, 6 and 12 mo after non-surgical periodontal therapy with and without adjunctive systemic antibiotics. Finally, NET degradation was fluorospectrometrically quantified. NET degradation levels did not differ between periodontitis patients and controls, irrespective of subject-related background characteristics. NET degradation significantly increased from 65.6 ± 1.7% before periodontal treatment to 75.7 ± 1.2% at 3 mo post periodontal therapy, and this improvement was maintained at 6 and 12 mo, irrespective of systemic usage of antibiotics. Improved NET degradation after periodontitis treatment is another systemic biomarker reflecting a decreased pro-inflammatory status, which also contributes to an improved cardiovascular condition.
Keywords: Chronic periodontitis, innate immunology, PMN, polymorphonuclear leucocytes, neutrophils
Introduction
Periodontitis is a chronic inflammatory disease of the tooth-supporting tissues that potentially leads to tooth loss. Periodontitis has been found to be associated with atherosclerotic cardiovascular disease (ACVD).1–5 Even though the relation between periodontitis and ACVD has been broadly reported, the biological mechanisms and clinical relevance of this interaction are still under investigation.6,7 Nevertheless, previous research has shown beneficial effects of non-surgical periodontal therapy on several clinical and biochemical parameters of ACVD, including flow-mediated dilatation, intima media thickness, systolic blood pressure, and a decrease in activated platelets.8–14 Altogether, this may reduce the risk factors of an ACVD profile of periodontitis patients.
In periodontitis, circulatory polymorphonuclear leucocytes (PMNs) are present in higher numbers and in an activated state.15–17 This may be related to the chronic transmigration of oral bacteria from periodontal lesions into the blood circulation.1,6 Being the most abundant white blood cells in blood, PMNs have phagocytic and bacterial killing capacities to neutralise and remove micro-organisms from the circulation.18 Another antimicrobial strategy of PMNs is the release of neutrophil extracellular traps (NETs).19–21 NETs are web-like structures composed of a core DNA element, extranuclear histones and de-condensed nuclear chromatin combined with various antimicrobial compounds released from PMN granules that immobilise and kill pathogens.22,23 Various stimuli (e.g. phorbol 12-myristate 13-acetate (PMA), immune complexes or bacterial products) induce NET formation through the activation of the protein kinase C pathway.24 NET release by peripheral blood PMNs in periodontitis patients and healthy controls has been compared, and an independent association between elevated NET release and periodontitis was reported.25
It was initially proposed that increased NET production protects the host from infectious diseases by effective pathogen clearance. However, increased NET production or impaired NET degradation can also lead to pathological immune responses.26 In healthy individuals, NETs are degraded by multiple enzymes, in particular plasma DNases, which are endonucleases secreted by the pancreas. DNaseI degrades the phosphodiester linkages of the DNA backbone, thereby degrading both single- and double-stranded DNA.27 In the case of low levels or even in the absence of DNases, the degradation of NETs is heavily reduced.28
Accordingly, excessive accumulation of cytotoxic NET-associated compounds such as antimicrobial peptides, auto-immunogenic DNA (citrullinated histones and single-stranded DNA), enzymes (myeloperoxidase and elastase) and entrapped bacteria can amplify (chronic) immune reactions and potentially triggers the presentation of auto-Ags in the host, eventually leading to tissue damage.29,30 As such, NETs have been suggested as possible players in the development or exacerbation of autoimmune diseases such as rheumatoid arthritis (RA)29 and systemic lupus erythematosus (SLE).31–33 Furthermore, NETs can cause platelet adhesion, activation and aggregation, and thereby induce endothelial dysfunction by activation and damage of endothelial cells.34 These complications are risk factors for ACVD events.35,36 Furthermore, possibly via their negative effects on the endothelial lining of coronary arteries, for example, NETs have been found to play a causative role in the formation of atherosclerotic plaques and venous thrombi.37 Thus, any condition that can induce increased NETs and/or may result in insufficient degradation of NETs could be a risk factor for ACVD.
Another mechanism to explain the link between periodontitis and ACVD could be elevated NET levels. Since periodontal treatment has shown beneficial effects on clinical and biochemical parameters of ACVD,12 we hypothesised that periodontal treatment would lead to increased NET degradation levels. To date, only one study with matched patients and controls (n = 19 pairs) investigated NET degradation in periodontitis.25 The authors reported lower NET degradation in periodontitis patients compared to controls, which was explained by significantly lower DNaseI plasma concentration levels in the periodontitis patient group. Additionally, this investigation studied the effect of periodontal treatment with a 3-mo follow-up on NET degradation, and the NET degradation was found to be increased after periodontal treatment.25 Whether these ‘restored’ NET degradation levels remain stable for a longer follow-up period was not investigated. The current study was set up in a larger group of subjects to investigate NET degradation differences further in periodontitis patients and controls (n = 76, part 1) and to investigate NET degradation at baseline as well as at 3, 6 and 12 mo following non-surgical periodontal treatment (n = 91, part 2).
Materials and methods
Study populations
This study consisted of two parts. In the first part, the NET degradation capacity of plasma from periodontitis patients (n = 38) was compared to that of plasma from healthy control subjects (n = 38). In the second part, the NET degradation capacity of plasma from periodontitis patients (n = 91) before and after non-surgical periodontal treatment was analysed from samples which were available from the study of Bizzarro et al.38
Patients for the first part of the study were screened for eligibility between June 2014 and December 2016, and patients for the second part of the study were screened in the period 2008–2013. Eligible periodontitis patients for both parts of the study were asked to participate after their initial visit (intake consult) to the Department of Periodontology of the Academic Centre for Dentistry Amsterdam (ACTA), Amsterdam, The Netherlands, before the initiation of their periodontal treatment. The healthy control subjects from the first part of the study were dental patients from the educational clinics of ACTA or laboratory staff. Subjects willing to participate were invited for the research. The study protocol for part 1 was reviewed and approved by the Medical Ethical Committee of the VU University Medical Centre, Amsterdam, The Netherlands (approval number: 2014.246, revised on 12 March 2015), and part 2 was approved by the Medical Ethical Committee of the Academic Medical Centre of the University of Amsterdam, Amsterdam, The Netherlands (approval number: MEC 07/264).38 All participants were informed about the purpose of the study and signed an informed consent form. Researchers handling the plasma samples (C.G.J.M. and M.R.J.F.) could not retrieve the identity of the donors.
General characteristics
At the start of the study, demographic characteristics such as age, sex, ethnicity and education were recorded by means of a questionnaire. A participant was considered a smoker if he or she was currently smoking or had quit ≤6 mo before baseline examination. The body mass index (BMI) was calculated from height and weight.
Periodontitis patient and control subject characteristics
Periodontitis cases were defined as consented in a European Workshop,39 specifically the presence of proximal attachment loss of ≥ 5 mm in ≥ 30% of teeth present. Alveolar bone loss was confirmed on periapical radiographs. The healthy control subjects did not have more than one pocket of 4–5 mm, in the absence of proximal bone loss (third molars excluded). This was confirmed on bitewing radiographs not older than 1 yr, displaying a distance between the alveolar bone and the cemento-enamel junction of ≤ 3 mm. Periodontitis cases were excluded if they had received any form of periodontal therapy within the last 2 yr. Further exclusion criteria for both periodontitis and controls were pregnancy/lactation, the use of antimicrobials in the previous 6 mo, the presence of < 20 natural teeth, the use of omega-3/omega-6 fatty acids supplementation, systemic diseases (such as diabetes or RA) or the (regular) use of any medicine that could modulate the inflammatory response (such as statins or non-steroidal anti-inflammatory drugs) in the previous 2 wk.
Periodontal therapy
All periodontitis patients in part 2 of the study received non-surgical periodontal treatment in three appointments within 1 wk. Of all patients participating in part 2 of the study, 42 (randomly determined) received systemic antibiotics treatment in conjunction with periodontal therapy.38 Systemic antibiotics consisted of amoxicillin 375 mg and metronidazole 250 mg, both three times daily for 7 d. After completion of the active therapy with or without antibiotics, patients were seen for maintenance treatment every 3 mo until the end of the follow-up (1 yr).38
Sample collection
Plasma samples from patients and controls for both part 1 and part 2 of this study were available. Samples for the first part of the study were retrieved from previous yet unpublished studies (E.A.N., M.G.B. and E.L.). For part 2 of this study, 99 patients completed the study, including all follow-up appointments up to 12 mo.38 However, for part 2 of this study, plasma samples of 91 patients were available and used. In general, study participants were scheduled for blood collection between 8:00 am and 12:00 noon. Non-fasting blood was collected by venipuncture from the antecubital fossa into EDTA tubes (Vacuette®; Greiner Bio-One, Alphen a/d Rijn, the Netherlands) which were put on ice until further handling. The collected whole blood was processed (whole blood centrifugation, 2000 g, 10 min, at 4°C) within 2 h, and aliquots of all plasma samples were stored at −80°C until further analysis.
Circulatory PMN isolation for NET formation
To generate NETs for degradation experiments, PMNs were isolated from three healthy male donors (age 30–50 yr) which were not related to the aforementioned study population of either part 1 or part 2 of this study. From the three donors, each on a separate d, venous blood was obtained in lithium heparin tubes (Vacuette®; Greiner Bio-One, VWR, Amsterdam, The Netherlands). All three volunteers were informed about the purpose of the study and gave informed consent. In total, 90 ml blood was taken per donor to obtain a sufficient number of PMNs to study NET degradation of all plasma samples of part 1 and part 2 on 1 d.
Blood was diluted 1:1 in 1% PBS citrate (1.55 M sodium citrate, 0.10 M citric acid in sterile water, pH 7.4; both Merck Millipore, Darmstadt, Germany, pH 7.4), carefully layered on Lymphoprep (Axisshield Po CAS, Oslo, Norway), and centrifuged for 30 min at 800 g without brake at room temperature (RT; 21°C). The supernatant was removed. Remaining erythrocytes and PMNs were suspended in cold lysis buffer (1.5 M NH4Cl, 100 mM NaHCO3, 1 mM disodium EDTA; all Sigma–Aldrich, St Louis, MO, diluted 10× in sterile Milli-Q water). After erythrocytes were lysed (10 min), the samples were centrifuged (500 g, 10 min, 4°C), and the supernatant was discarded. Finally, PMNs were washed in cold PBS (4°C) and recovered in phenol-red free culture medium (Roswell Park Memorial Institute (RPMI) 1640; Gibco BRL, Paisley, UK).
NET formation
For NET formation, PMNs (5 × 105 in phenol red-free culture medium) were seeded onto a flat-bottom non-treated 96-well plate (Greiner Bio-One, Monroe, NC) pre-blocked overnight with 1% BSA in PBS. After 30 min baseline incubation at 37°C, selected wells were stimulated with 75 nM PMA (Sigma–Aldrich) for 3 h at 37°C to induce NET formation. Culture medium served as a negative control where no NETs formed.
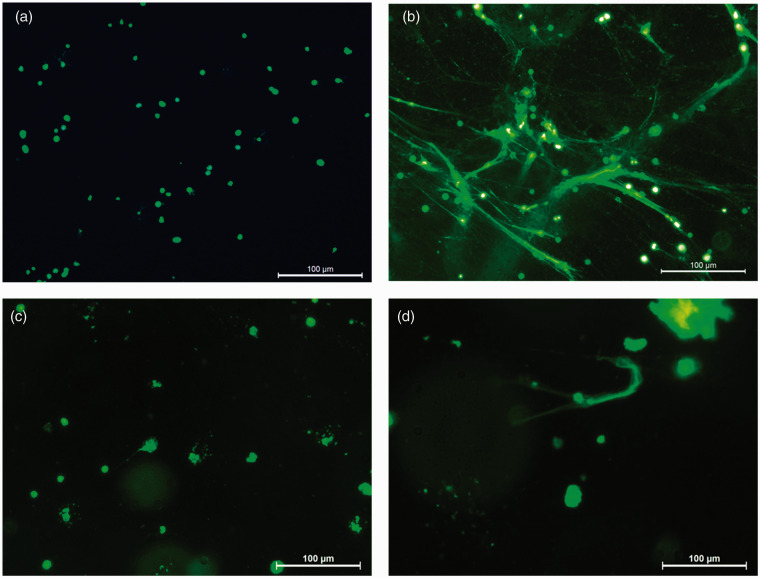
In addition to quantification, NET formation and degradation were microscopically validated with visualisation assays which were performed in the same manner as quantification assays. Briefly, PMNs (1 × 106) suspended in phenol red-free culture medium were seeded onto a transparent 48-well plate (Greiner Bio-One) pre-blocked with 1% BSA-PBS. After 30 min baseline incubation, selected wells were stimulated with 75 nM PMA. After 3 h of incubation at 37°C, 50 nM SYTOX™ green was added to each well and visualised with fluorescence microscopy (magnification 20×, Leica DFC320; Leica Microsystems, Wetzlar, Germany). The control condition contained unstimulated PMNs where no NETs were observed after 3 h (Figure 1a). Typical web-like structures of NETs were observed after 3 h of stimulation of PMNs with PMA (Figure 1b). For the visualisation of NET degradation, plasma samples from both a periodontitis patient and a healthy control were 10× diluted in PBS and added to NETs and incubated at 37°C for another 3 h. Degradation of NETs (procedures described below) by plasma from a healthy donor and periodontitis patient is shown in Figure 1c and d.
Figure 1.
NET. (a) PMNs without any NETs visible. (b) PMA-induced NET formation. (c) NET degradation post incubation with 10% plasma of a healthy subject (d) and a periodontitis patient. NETs were visualized using the extracellular DNA binding dye SYTOX™ Green. Magnification 20×, scale bars represent 100 µm.
NET degradation quantification
First, aliquots of plasma samples of part 1 and part 2 were simultaneously defrosted on ice. Thereafter, 20 µl of the plasma samples was individually transferred in duplicate to 96-well plates, and 180 µl cold PBS was added. This procedure was performed in triplicate to obtain a sufficient number of plates for each of the three experimental days. Thus, NET degradation by plasma samples of part 1 and part 2 was tested in duplicate on NETs which were generated from PMNs from three different blood donors. All plates containing all the plasma samples from both part 1 and part 2 of the study were stored at –80°C until the analysis.
To study in vitro NET degradation, the diluted plasma samples on 96-well plates were defrosted on ice and were carefully transferred onto the freshly generated NETs and incubated for 3 h at 37°C, as previously described.25 Next, 15 µl 14.3 IU/ml micrococcal nuclease (MNase; Invitrogen by Thermo Fisher Scientific, Eugene, OR) was added and incubated for 15 min at 37°C to digest any PMN bound NET DNA.25 Then, cells and debris were pelleted (10 min, 1800 g, RT; 21°C), and the supernatant was transferred to a black U-bottom 96-well plate (Greiner Bio-One) containing 50 nM SYTOX™ green (Invitrogen by Thermo Fisher Scientific). A fluorospectrophotometer (FLUOstar Galaxy, BMG; MTX Lab Systems, Bradenton, FL) was used to quantify NETs between 485 and 525 nm at 37°C and analysed with Fluostar Galaxy software v4.2. On the same plate, background absorption of plasma was quantified in a PMN-free condition, and these fluorescence values were subtracted from the final NET quantification readings. The percentage of NET degradation for each plasma sample was normalised to 100% and 0% degradation values. The 100% degradation was based on 3 h of NET digestion (instead of plasma) with 15 µl of 1 IU/ml MNase, and a similar condition without MNase represented the 0% degradation standard.25
Statistical analysis
Background characteristics of participants for part 1 and part 2 were analysed with IBM SPSS Statistics for Windows v25 (IBM Corp., Armonk, NY). Differences between patients and healthy controls (part 1) were tested with an unpaired parametric t-test for age and BMI, and chi-square tests (Fisher’s exact tests where appropriate) for sex, ethnicity, education and smoking. NET degradation results were analysed using GraphPad Prism v6.07 (GraphPad LLC, La Jolla, CA). The distribution of NET degradation percentages for part 1 and part 2 was assessed with D’Agostino–Pearson tests for normal distribution and found to be not normally distributed. Therefore, before proceeding with parametric statistics, log-transformed values were calculated. NET degradation results were presented in scatter plots with means ± SEM. For part 1, NET degradation differences between periodontitis patients and healthy subjects were first tested with an unpaired t-test. Thereafter, an analysis of covariance (ANCOVA), taking into account group variabilities such as smoking, ethnicity, age, BMI and education, was also performed using SPSS. These statistical tests yielded adjusted P Values (Padj). For part 2 of this study, the effect of non-surgical periodontal treatment over time on NET degradation was tested with ANOVA followed by Tukey multiple comparison tests for more than three comparisons. Differences with P < 0.05 were considered significant. Additionally, to explore the possible adjunctive effect of systemic antibiotics and other potential confounding factors for changes in NET degradation after non-surgical periodontal therapy, we first calculated the individual differences of NET degradation between baseline and 3 mo post therapy, and similarly for 6 and 12 mo. The obtained values were entered as a dependent variable in a multiple linear regression analysis. First, antibiotic usage during therapy for about half of the patients was explored as a possible predictor. Similarly, baseline NET degradation levels were explored as possible predictors. Potential patient characteristics were also tested as predictors. First, periodontitis severity (mean probing pocket depth, PPD) was explored and thereafter age, sex, ethnicity, education, smoking and BMI were entered as predictors in a fully adjusted model. The explorative modelling yielded adjusted P Values, and Padj <0.05 was considered significant.
Results
Characteristics of periodontitis patients and healthy subjects (part 1)
Baseline characteristics of the study population of part 1 are presented in Table 1. This study population consisted of 38 periodontitis patients and 38 controls with mean ages of 49 and 43 yr, respectively. There were more Caucasians among controls (89.5%) compared to patients (68.4%). The education level of high school or above in the control group was 100% and in the patient group it was 92%, but the difference was not statistically significant. More than half of the patient population smoked, while 81.6% did not smoke in the control group (P = 0.002). The mean BMI in the patient group was significantly higher than the control group (P = 0.009).
Table 1.
Baseline characteristics of periodontitis patients and healthy controls participating in part 1 of this study.
| Periodontitis patients (n=38) | Controls (n=38) | P Value | |
|---|---|---|---|
| Age (yr) | 49.1 ± 11.6 | 43.4 ± 12.2 | 0.043 |
| Sex | |||
| Female | 19 (50%) | 16 (42.1%) | 0.490 |
| Ethnicity | |||
| Caucasian | 26 (68.4%) | 34 (89.5%) | 0.047 |
| Education | |||
| ≥ High school | 35 (92.1%) | 38 (100%) | 0.240 |
| Smoking | |||
| Smokers | 20 (52.6%) | 7 (18.4%) | 0.002 |
| BMI (kg/m2) | 27.0 ± 4.5 | 24.6 ± 2.9 | 0.006 |
Values (age and BMI) represent means ± SD. Categorical data (sex, ethnicity, education and smoking) are presented as absolute numbers (percentages) of subjects. Statistical differences (P Values) between patients and controls are presented. P Values were calculated with unpaired parametric t-tests, and categorical data were compared with chi-square tests or Fisher’s exact tests where appropriate.
Similar NET degradation in healthy subjects and periodontitis patients (part 1)
NET degradation capacity of plasma from 38 periodontitis patients and 38 healthy controls was investigated. NET degradation did not differ significantly between periodontitis patients and healthy subjects (Figure 2; P = 0.1316); 62.8 ± 4.5% and 68.7 ± 4.3% NET degradation was shown in patients and controls (mean percentages ± SEM), respectively. In an ANCOVA model, we considered differences in subject characteristics between periodontitis patients and healthy controls such as age, sex, ethnicity, education, smoking and BMI. However, in this model, subject-related background characteristics did not influence the NET degradation differences between periodontitis patients and controls (Padj = 0.307). Accordingly, none of the potential confounders appeared a significant covariate.
Figure 2.
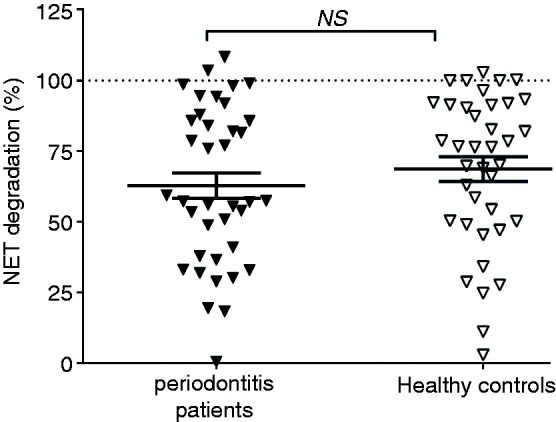
NET degradation by plasma from periodontitis patients and healthy controls. NET degradation levels of periodontitis patients (n=38) and healthy controls (n=38). Each individual data point represents the mean of three independent experiments from one plasma sample. Horizontal lines represent the overall mean percentages ± SEM. NS: not significant.
Characteristics of periodontitis patients receiving non-surgical periodontal therapy (part 2)
The study population of part 2 of this investigation consisted of 91 periodontitis patients who were seen at baseline (untreated) and at 3, 6 and 12 mo after non-surgical periodontal therapy.38 Patient characteristics were determined at baseline and are presented in Table 2. The study population comprised mainly Caucasians (87.9%) with a mean age of 48.5 ± 9.0 yr and a mean BMI of 25.3 ± 3.7 kg/m2. Of the 91 patients, 62 (68.1%) were smokers, and 42 (randomly assigned) patients received systemic antibiotics in conjunction with non-surgical periodontal therapy. The clinical results of non-surgical periodontal therapy have been recalculated for the current patient population and are presented in Supplementary Material Table S1.
Table 2.
Baseline characteristics of periodontitis patients participating in part 2 of this study.
| Periodontitis patients (n = 91) | |
|---|---|
| Age (yr) | 48.5 ± 9.0 |
| Sex | |
| Female | 37 (40.7%) |
| Ethnicity | |
| Caucasian | 80 (87.9%) |
| Education | |
| ≥ High school | 74 (81.3%) |
| Smoking | |
| Smokers | 62 (68.1%) |
| BMI (kg/m2) | 25.3 ± 3.7 |
| Systemic antibiotics | 42 (46.1%) |
| Number of teeth present | 27.0 ± 2.8 |
| Probing pocket depth (mm) | 3.9 ± 0.6 |
| Clinical attachment loss (mm) | 4.2 ± 1.0 |
| Bleeding on probing (%) | 66.3 ± 14.8 |
| Plaque (%) | 62.8 ± 24.2 |
| Sites with probing pocket depth ≥ 6 mm | 34.0 ± 17.7 |
Values represent means ± SD or absolute numbers (percentages).
NET degradation is significantly increased after periodontal treatment
In part 2 of this study, the plasma samples from the 91 periodontitis patients were individually added to fresh PMA-induced NETs to investigate NET degradation at baseline, and at 3, 6 and 12 mo following non-surgical periodontal treatment (Figure 3). Altogether, plasma-derived NET degradation was increased by approximately 10% after non-surgical periodontal therapy (overall P<0.0001). Accordingly, NET degradation was 65.6 ± 1.7% before periodontal treatment (baseline) and amounted to 75.7 ± 1.2%, 75.3 ± 1.2% and 74.9 ± 1.3% at 3, 6 and 12 mo post treatment, respectively (means ± SEM). The NET degradation for the three time points post treatment was significantly higher compared to baseline (P<0.0001 Tukey post hoc test; Figure 3). To explore whether the use of systemic antibiotics affected the positive effect of periodontal treatment on NET degradation, we performed a multiple linear regression analysis with antibiotic usage as a predictor. From this analysis, no effect of systemic antibiotics was found for changes in NET degradation from baseline to 3, 6 and 12 mo (P values = 0.868, 0.526 and 0.907, respectively). In contrast, the baseline NET degradation level of patients was a strong predictor for its increase after therapy (Padj<0.0001 for 3, 6 and 12 mo after treatment). With bivariate correlation analyses, we found high negative correlations (ρ = –0.699, –0.705 and –0.729 for 3, 6 and 12 mo, respectively), meaning that lower NET degradation levels at baseline predicted higher increases of NET degradation at 3, 6 and 12 mo post treatment. Furthermore, we investigated whether the severity of periodontitis at baseline would predict the improved NET degradation levels. As such, the mean PPD at baseline was tested as a predictor and found to be not significant (Padj =0.792). To investigate further whether other background characteristics of the patients were possibly associated with the improvement of NET degradation after therapy, we extended the model by also entering age, sex, ethnicity, education, smoking and BMI as predictors. From this fully adjusted model, it became apparent that a higher BMI at baseline was a significant predictor in the improvement of NET degradation at 3 mo (Padj = 0.026). At 6 and 12 mo, none of the potential confounders were significant, meaning that the NET degradation levels were maintained solely by periodontal therapy and subsequent maintenance care.
Figure 3.
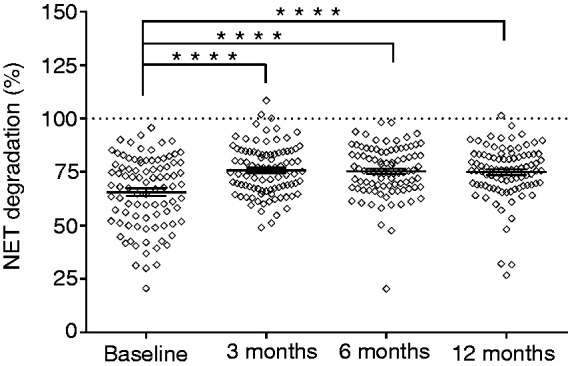
NET degradation by plasma from periodontitis patients pre and post non-surgical periodontal treatment. NETs were incubated with 10% plasma from periodontitis patients (n=91) obtained before and 3, 6 and 12 mo after non-surgical periodontal therapy. NET degradation pretreatment (baseline) and 3, 6 and 12 mo after periodontal treatment. Statistical significance was calculated using an ANOVA with repeated measures. Each individual data point represents the mean of three independent experiments for one plasma sample and accordingly representing one individual patient. Horizontal lines represent the overall mean percentages ± SEM. ****P<0.0001.
Discussion
The first objective of this study was to compare the NET degradation by plasma from periodontitis patients and healthy controls. Since we hypothesised that periodontal therapy might benefit NET degradation, the second objective was to quantify NET degradation by plasma from periodontitis patients at baseline and at 3, 6 and 12 mo after periodontal treatment. Altogether, our results showed that plasma-induced NET degradation did not differ significantly between periodontitis patients and healthy controls. Importantly, we found that non-surgical periodontal treatment increased the NET clearance capacity by 10% after 3 mo, being stably improved at 6 and 12 mo.
Although the authors of a previous investigation of NET production by circulatory PMNs in periodontitis found no difference between periodontitis patients and controls,25 normal NET production may be accompanied by impaired NET degradation. If so, this may potentially lead to NET accumulation, with negative consequences for tissue homeostasis in periodontitis and its systemic comorbidities.36,40 Therefore, in this study, we only investigated NET degradation.
White et al. compared NET degradation in periodontitis patients and matched controls, and showed that NET degradation and DNaseI levels were lower in periodontitis patients than in healthy controls.25 In the current unmatched study population about twice the size, we also found a slightly lower mean NET degradation in periodontitis patients compared to controls, but that did not differ significantly between patients and controls, even when we adjusted for variability in patient background characteristics. Periodontitis patients represent a heterogeneous population, and in most cases, the progression of periodontitis is not a linear process, but rather one consisting of periods of exacerbation and remission.41,42 Publications investigating plasma-induced NET degradation (in periodontitis and SLE) included age- ( ± 5 yr) and sex-matched controls.25,32 Unlike in our study, lifestyle factors of the subjects were matched in the aforementioned studies. However, in our study, testing for differences in NET degradation between periodontitis patients and controls with age- and sex-matched pairs resulted in non-significant differences.
In our study, plasma samples were obtained from periodontitis patients and healthy controls. Unlike White et al., we included smokers in our study group; more than half of our patient population (part 1: 52.6%; part 2: 68.1%) consisted of smokers. While the detrimental effects of smoking on the onset and progression of periodontitis and the association with a wide range of systemic diseases including ACVD is well known,43,44 there is limited knowledge on the effects of smoking on NET formation and degradation. However, a recent study showed the negative effects of smoking on PMN chemotaxis, reactive oxygen species formation and NET formation.45 Thus, smoking may also negatively influence NET formation and the associated degradation profiles of these subjects. The higher numbers of smokers in our periodontitis patient group may therefore have contributed to the absence of a difference in NET degradation. In general, differences in sociodemographic characteristics, smoking habits and BMI may have influenced the comparison of NET degradation in the first part of our study and may have been why no significant difference was found between two groups. Despite this, ANCOVAs resulted in non-significant differences, suggesting that this was not a result of a multifactorial cause–effect relationship.
The objective of part 2 of this study was to investigate whether a substantial reduction of the inflammatory state of periodontal tissues after non-surgical periodontal treatment would benefit NET degradation. We found that NET degradation increased significantly after non-surgical periodontal treatment. These results are in line with previous observations.25 However, our population was almost five times larger than that in the previous study, and we investigated two extra time points (6 and 12 mo after treatment). Although part of our study population (n = 42) received antibiotic treatment in addition to non-surgical periodontal treatment, this did not influence NET degradation levels. Furthermore, severity of periodontitis per se (mean PPD) was not a predictor for the increased NET degradation levels in our study. Thus, non-surgical periodontal treatment is beneficial not only for the clinical periodontal parameters38 of the periodontitis patient, but also for NET degradation levels, regardless of their PPD at baseline levels. Interestingly, patients with a higher BMI at baseline showed a greater improvement in NET degradation at 3 mo. This can possibly be explained by the fact that periodontal therapy also had a positive effect on the symptoms of metabolic syndrome.12 However, the confounding effect of BMI on NET degradation was absent at 6 and 12 mo, meaning that the NET degradation levels were solely maintained by periodontal therapy. From multiple regression and bivariate correlation analyses, it became apparent that NET degradation levels at baseline were significant predictors for the improvement of NET degradation at 3, 6 and 12 mo post periodontal treatment. In other words, lower degradation levels at baseline predict a larger increase in NET degradation after treatment, while high NET degradation levels at baseline were correlated with limited increases after therapy.
Systemic diseases evidently contribute to the onset and/or progression of periodontitis. Vice versa, periodontitis has been suggested to influence diabetes and ACVD negatively. While the biological mechanisms of these associations are still under investigation, a strong relationship between periodontitis, diabetes and ACVD has been reported.6,46–49 The ACVD and metabolic syndrome–related parameters of our study group were previously investigated, and we reported that periodontal treatment improved periodontal conditions and cardiovascular parameters such as systolic blood pressure and triglycerides, and the number of patients with metabolic syndrome also reduced.12 Interestingly, a related and significant area of NET research is the relationship between NETs and ACVD. Moreover, NETs play an important protective role by preventing bacterial dissemination into the vasculature and possibly lymphatics. On the other hand, the accumulation of NETs present at the injured endothelium could activate venous endothelium where PMNs and platelets are then recruited.34,50,51 This activated endothelium induces more NET formation, which creates a vicious cycle, resulting in increased vessel-wall damage. Eventually, accumulation of activated platelets possibly leads to the formation of microthrombi and potential obstruction of blood vessels. Increased NET production has been reported in ACVD patients37 but not in periodontitis patients.25 However, periodontal treatment has been proven to reduce endothelial dysfunction in patients with severe periodontitis.8–11 Therefore, we investigated whether periodontal treatment would benefit NET degradation and thus could be a possible reason for improved ACVD profiles. Using the same study population as Bizzarro et al., our finding that NET degradation was significantly increased 3 mo after periodontal treatment and remained at these levels after 6 and 12 mo is in line with previously reported ACVD clinical parameters.12 This suggests that periodontal treatment synergistically improves ACVD profiles, clinical periodontal parameters and NET degradation capacities.
Conclusion
We found that plasma-induced NET degradation did not quantitatively differ between periodontitis patients and healthy controls. We demonstrated that non-surgical periodontal treatment increased NET degradation capacity by 10% after 3 mo and that this remained stably elevated at 6 and 12 mo. Our findings are in line with previously reported improved ACVD clinical parameters such as systolic blood pressure and triglycerides.12,13 This suggests that non-surgical periodontal treatment synergistically affects ACVD profiles, periodontal inflammatory parameters and NET degradation. Unraveling NET formation and degradation mechanisms in periodontitis patients may improve the understanding of the pathophysiology of periodontitis and the aetiopathogenetic links between periodontitis and its systemic co-morbidities such as ACVD, while also enabling the development of new therapeutic approaches.
Supplemental Material
Supplemental material, INI889392 Supplemetal Material for Periodontal therapy increases neutrophil extracellular trap degradation by Carolyn GJ Moonen, Kirsten GD Buurma, Mouri RJ Faruque, Maria G Balta, Erol Liefferink, Sergio Bizzarro, Elena A Nicu and Bruno G Loos in Innate Immunity
Acknowledgements
The author(s) are grateful to Dr J Hirschfeld (University of Birmingham, UK) for her knowledge and advice on laboratory procedures.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD
Carolyn GJ Moonen https://orcid.org/0000-0003-1601-4768
Supplemental material
Supplemental material for this article is available online.
References
- 1.Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, et al. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ 2018; 27: 1327–1334. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation 2012; 125: 2520–2544. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich T, Sharma P, Walter C, et al. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Periodontol 2013; 84: S70–84. [DOI] [PubMed] [Google Scholar]
- 4.Beukers N, Van der Heijden G, Van Wijk AJ, et al. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J Epidemiol Commun Health 2017; 71: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphrey LL, Fu R, Buckley DI, et al. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med 2008; 23: 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol 2013; 84: S51–69. [DOI] [PubMed] [Google Scholar]
- 7.Aarabi G, Zeller T, Seedorf H, et al. Genetic susceptibility contributing to periodontal and cardiovascular disease. J Dent Res 2017; 96: 610–617. [DOI] [PubMed] [Google Scholar]
- 8.Teeuw WJ, Slot DE, Susanto H, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol 2014; 41: 70–79. [DOI] [PubMed] [Google Scholar]
- 9.Orlandi M, Suvan J, Petrie A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis 2014; 236: 39–46. [DOI] [PubMed] [Google Scholar]
- 10.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007; 356: 911–920. [DOI] [PubMed] [Google Scholar]
- 11.Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J 2005; 149: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 12.Bizzarro S, van der Velden U, Teeuw WJ, et al. Effect of periodontal therapy with systemic antimicrobials on parameters of metabolic syndrome: a randomized clinical trial. J Clin Periodontol 2017; 44: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arvanitidis E, Bizzarro S, Alvarez Rodriguez E, et al. Reduced platelet hyper-reactivity and platelet–leukocyte aggregation after periodontal therapy. Thromb J 2017; 15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal F, Cordovil I, Figueredo CMS, et al. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J Clin Periodontol 2013; 40: 681–687. [DOI] [PubMed] [Google Scholar]
- 15.Matthews JB, Wright HJ, Roberts A, et al. Neutrophil hyper-responsiveness in periodontitis. J Dent Res 2007; 86: 718–722. [DOI] [PubMed] [Google Scholar]
- 16.Chapple ILC, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 2007; 43: 160–232. [DOI] [PubMed] [Google Scholar]
- 17.Fine N, Hassanpour S, Borenstein A, et al. Distinct oral neutrophil subsets define health and periodontal disease states. J Dent Res 2016; 95: 931–938. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol 2010; 10:1325–1334. [DOI] [PubMed] [Google Scholar]
- 19.Kubes P. The enigmatic neutrophil: what we do not know. Cell Tissue Res 2018; 371: 399–406. [DOI] [PubMed] [Google Scholar]
- 20.Brinkmann V. Neutrophil extracellular traps in the second decade. J Innate Immun 2018; 10: 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moonen CGJ, Hirschfeld J, Cheng L, et al. Oral neutrophils characterized: chemotactic, phagocytic, and neutrophil extracellular trap (NET) formation properties. Front Immunol 2019; 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 23.Deniset JF, Kubes P. Neutrophil heterogeneity: bona fide subsets or polarization states? J Leukoc Biol 2018; 103: 829–838. [DOI] [PubMed] [Google Scholar]
- 24.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med 2017; 23: 279–287. [DOI] [PubMed] [Google Scholar]
- 25.White P, Sakellari D, Roberts H, et al. Peripheral blood neutrophil extracellular trap production and degradation in chronic periodontitis. J Clin Periodontol 2016; 43: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 26.Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, et al. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol 2017; 8: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munafo DB, Johnson JL, Brzezinska AA, et al. DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J Innate Immun 2009; 1: 527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiménez-Alcázar M, Rangaswamy C, Panda R, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017; 358: 1202–1206. [DOI] [PubMed] [Google Scholar]
- 29.Sohn DH. NETosis in autoimmune diseases. J Rheum Dis 2016; 23: 82–87. [Google Scholar]
- 30.Branzk N, Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol 2013; 35: 513–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leffler J, Martin M, Gullstrand B, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012; 188: 3522–3531. [DOI] [PubMed] [Google Scholar]
- 32.Hakkim A, Furnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 2010; 107: 9813–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahlenberg JM and Kaplan MJ. Mechanisms of Acute Inflammation and Vascular Injury in SLE. Duboi's Lupus Erythematosus and related Syndromes By Wallace DJ and Hahn BH. (2013) pp 166–174 doi: 10.1016/B978-1-4377-1893-5.00015-7 ISBN: 978-1-4377-1893-5: Elsevier Saunders; Philadelphia, PA, USA.
- 34.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010; 107: 15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight JS, Luo W, O’Dell AA, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res 2014; 114: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sørensen OE, Borregaard N. Neutrophil extracellular traps – the dark side of neutrophils. J Clin Invest 2016; 126: 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi H, Yang S, Zhang L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front Immunol 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bizzarro S, Van der Velden U, Loos BG. Local disinfection with sodium hypochlorite as adjunct to basic periodontal therapy: a randomized controlled trial. J Clin Periodontol 2016; 43: 778–788. [DOI] [PubMed] [Google Scholar]
- 39.Tonetti MS, Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research: Group C Consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol 2005; 32: 210–213. [DOI] [PubMed] [Google Scholar]
- 40.Yang H, Biermann MH, Brauner JM, et al. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol 2016; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015; 15: 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loos BG, Papantonopoulos G, Jepsen S, et al. What is the contribution of genetics to periodontal risk? Dent Clin North Am 2015; 59: 761–780. [DOI] [PubMed] [Google Scholar]
- 43.Leite FRM, Nascimento GG, Scheutz F, et al. Effect of smoking on periodontitis: a systematic review and meta-regression. Am J Prev Med 2018; 54: 831–841. [DOI] [PubMed] [Google Scholar]
- 44.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004; 43: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 45.White PC, Hirschfeld J, Milward MR, et al. Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression. J Periodontal Res 2018; 53: 525–535. [DOI] [PubMed] [Google Scholar]
- 46.Tonetti MS, Van Dyke TE. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol 2013; 40: 24–29. [DOI] [PubMed] [Google Scholar]
- 47.Chistiakov DA, Orekhov AN, Bobryshev YV. Links between atherosclerotic and periodontal disease. Exp Mol Pathol 2016; 100: 220–235. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Herrera M, Lopez-Domenech S, Silvestre FJ, et al. Chronic periodontitis impairs polymorphonuclear leukocyte–endothelium cell interactions and oxidative stress in humans. J Clin Periodontol 2018;1429–1439. [DOI] [PubMed] [Google Scholar]
- 49.Sanz M, Geriello A, Buysschaert M, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. Diabetes Res Clin Pract 2018; 137: 231–241. [DOI] [PubMed] [Google Scholar]
- 50.Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res 2017; 120: 736–743. [DOI] [PubMed] [Google Scholar]
- 51.Gaul DS, Stein S, Matter CM. Neutrophils in cardiovascular disease. Eur Heart J 2017; 38: 1702–1704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, INI889392 Supplemetal Material for Periodontal therapy increases neutrophil extracellular trap degradation by Carolyn GJ Moonen, Kirsten GD Buurma, Mouri RJ Faruque, Maria G Balta, Erol Liefferink, Sergio Bizzarro, Elena A Nicu and Bruno G Loos in Innate Immunity