Abstract
This study aimed to examine whether stromal cell-derived factor-1 (SDF-1) or C-X-C chemokine ligand 12 (CXCL12) participates in the development of lumbar disc degeneration, as implicated earlier by the level of CXCL12 correlating with this disease. It enrolled 145 patients with symptomatic lumbar intervertebral disc degeneration (IDD) and 130 asymptomatic healthy controls with no indication of IDD. Radiological assessment of the IDD patients was targeted at the lumbar vertebra region, based on Pfirrmann grade. Degeneration of the multifidus and psoas major muscles was evaluated using Goutallier classification. Visual Analogue Scale (VAS) and Oswestry Disability Index (ODI) scores were obtained for assessing the severity of manifestation. The levels of serum CXCL12, IL-6 and TNF-α were determined by ROC curve analysis, resulting in their prognostic value for Pfirrmann grading. Higher levels of serum CXCL12 were found in patients with IDD than in asymptomatic individuals, and were positively related to the Pfirrmann grade as well as multifidus muscle degeneration. Furthermore, serum CXCL12 concentration showed a significant correlation with the VAS and ODI scores. In addition, elevated serum CXCL12 levels were related to serum levels of TNF-α and IL-6. The ROC curve analysis implicated that CXCL12 could function as a biomarker of the early-mediate phase of IDD development. In summary, the serum CXCL12/SDF-1 level is positively related with lumbar IDD and its clinical severity.
Keywords: Stromal cell-derived factor-1, intervertebral disc degeneration, C-X-C chemokine ligand 12, disease progression
Introduction
Low back pain (LBP) is a prevailing disorder that affects about 12–30% of the population at any given time, while approximately 10% of these patients go on to develop chronic back pain.1 Intervertebral disc degeneration (IDD) was found to be a main factor that causes LBP and is a prevalent musculoskeletal disorder that contributes to a huge socioeconomic burden worldwide.2,3 The pathogenesis of IDD is complex, involving many contributing factors, such as genetic inheritance, age, inadequate metabolite transport and loading history.4 IDD may subsequently result in spinal instability, osteophyte formation and disc herniation, which can cause back and sciatic pain, as a result of compressing the spinal cord and nerve root.5
A present, IDD is mainly confirmed through magnetic resonance imaging (MRI) detection, almost always after symptoms of LBP or nerve root compressing have been reported.6 However, MRI-based images can only provide evidence during the intermediate or late stages of IDD, rather than detect early signs of degenerative alternations.7 In addition, certain biochemical alterations in the disc and diseases as well as the intensity of manifestations are not always detectable using conventional MRI sequences (e.g. T1- or T2-weighted).8 Therefore, rapid and reliable methods of diagnosing IDD are needed to impede and slow down degeneration at the early stage, in order to avoid the need for invasive surgery.
Biomarkers are indicators that can be measured and evaluated to determine either normal or pathogenic biological processes and responses to therapeutic interventions.9 Interest in using serum proteins as likely biomarkers to detect LBP or IDD pathologies has been growing.10 It has been demonstrated that disc cell stress caused by adverse mechanical forces may result in intracellular signal transduction, which leads to elevated gene expression and proteins being released into the blood.11
Chemokines are small cytokines with a molecular mass of 7–15 kDa that have the ability to guide chemotaxis of immune cells at designated sites, under certain physiological and pathological conditions.12 The existence of four cysteine residues at the NH2-terminal of chemokines allows them to be assigned to four subfamilies: C, CC, CXC and CX3C.13 Chemokines of the CXC subfamily that take part in the chemotaxis of neutrophils are the best known and most widely studied chemokines.14
C-X-C motif chemokine 12 (CXCL12) or stromal cell-derived factor 1 (SDF1), is a widely studied CXC chemokine that was first extracted from bone marrow stromal cells and was found to activate different types of cells by combining with the G-protein-coupled receptor, C-X-C motif chemokine receptor 4 (CXCR4).15 CXCL12/CXCR4 is essential for hematopoietic stem cell (HSC) maintenance and production of immune cells in the bone marrow.16 Induced deletion of CXCR4, in adult mice, resulted in severe reduction of HSC numbers and increased sensitivity to myelotoxic injury.16 CXCL12 is a strong chemoattractant that has an influence on angiogenesis, as well as leukocyte trafficking,17 and the CXCL12/CXCR4 axis is involved in various pathologies, including cancer18 and autoimmune and inflammatory disorders.19
Recent studies have unveiled the function of CXCL12/CXCR4 for the pathophysiology of IDD. At both mRNA and protein level, CXCL12 and CXCR4 levels are significantly higher in patients with IDD degeneration than in control individuals.20 CXCL12 has also been shown to accelerate the degradation of the extracellular matrix (ECM) and increase MMP expression in human disc endplate chondrocytes.21 In addition, using the NF-κB pathway, the CXCL12/CXCR4 axis can cause apoptosis of human degenerative nucleus pulposus cells.22
All these results imply that CXCL12 may be an important factor involved in IDD progression. However, as far as is known, no recent studies that investigate the circulatory CXCL12 concentration in patients with IDD and its influence on disease severity have been published. Hence, this study aimed to determine serum concentrations of CXCL12 in patients with IDD and its involvement in IDD progression, to identify if serum CXCL12 can be used as a potential biomarker for IDD.
Patients and methods
Study participants
We performed this cross-sectional study by consecutively recruiting 145 patients with complaint of LBP and on whom a lumbar spine MRI study was done between and inclusive of October 2017 and January 2019, at the clinic of our hospital. The lumbar spine MRI scans of the patients included were analyzed. To be included patients were required to meet the following criteria: a history of LBP that had persisted for over 6 wk, without or with leg pain, and presence or absence of lower extremity motor and/or sensorial deficits. Patients who met any of the exclusion criteria were not enrolled: a history of LBP other than that resulting from disc degeneration, including causes such as trauma, infection, spinal tumors, spondylolisthesis and spondylolysis. Patients were also excluded if they suffered from co-morbidities, including cancer, inflammatory bowel diseases, osteoarthritis, asthma, rheumatoid arthritis and amyotrophic lateral sclerosis, which may affect systemic CXCL12 levels. Meanwhile, 130 paired asymptomatic healthy controls, based on age and gender, who received a regular medical examination, were also enrolled. The control group included healthy subjects who did not have a history of LBP, regardless of cause, or systemic inflammatory diseases that may negate the results of this study. All these healthy controls did not show any signs of disc degeneration, as confirmed through a MRI. This study was approved by the Review Board at our Hospital, and guidelines for the ethical use of human subjects in medical research were adhered to. All participants provided written consent to participate, while the privacy of all patients and their collected data was ensured throughout the study.
Radiographic degeneration assessment using MRI
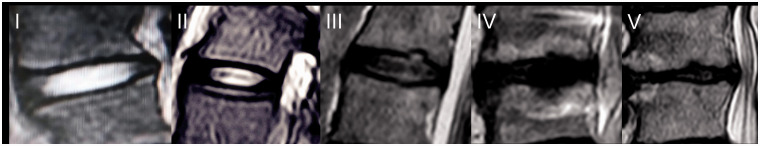
The Pfirrmann grading scale was used to identify lumbar IDD from L1–L2 to L5–S1 levels.23 T2-Weighted MRI, which in brief determines water content as denoted by signal intensity, forms the base of the system. Sagittal T2-weighted images were captured with the following settings: fast spin echo sequence, TR = 3500 ms, TE = 99.0 ms, section thickness = 4.0 mm, and field of view = 280 mm. Grade 1 was assigned to homologous bright white intervertebral discs with clear separation of the nucleus pulposus and annulus fibrosus. Grade 2 was assigned to heterologous intervertebral disc structures with the presence or absence of horizontal bands, as well as grade 1 imaging properties. Grade 3 was assigned to intervertebral disc structures that were heterologous and gray, with no clear separation of the nucleus pulposus and annulus fibrosus. Grade 4 was assigned to intervertebral disc structures that were gray to black, with a loss of separation of the nucleus pulposus and annulus fibrosus and a signal intensity of intermediate to hypointense. Grade 5 was assigned to heterologous and black intervertebral discs, with an obvious loss of separation of the nucleus pulposus and annulus fibrosus (Figure 1).23 The Pfirrmann grading scale demonstrates moderate to high levels of intra- and inter-observer stability. In our study, patients with Pfirrmann grade ≥ ⩾2 were enrolled. Pfirrmann grade 2 indicates mild change, while Pfirrmann grade 3 indicates moderate change, with Pfirrmann grade 4–5 implicating severe change. The most severely degenerated segment was selected for analysis. The results were read by one experienced radiologist and one advanced orthopedist. The Kappa value was also recorded.
Figure 1.
Pfirrmann grade for disc degeneration determined using MRI.
Evaluation of spinal muscle degeneration
Fatty degeneration of the multifidus and psoas major muscles was determined from L1–L2 to L5–S1, through T2-weighted axial MRI studies using the Goutallier classification system. The Goutallier classification system is as follows: grade 0, normal muscle; grade 1, fatty streaks within the muscle; grade 2, less fat present than muscle; grade 3, fat present equal to muscle; grade 4, more fat present than muscle.24 The most severely degenerated segment was selected for analysis.
Evaluation of pain and functional ability
We used the Visual Analogue Scale (VAS) and Oswestry Disability Index (ODI) to examine the clinical condition of patients with IDD. LBP was assessed through VAS, using a 10 cm-long straight line, with labels at each end to fasten the scale. Patients were requested to indicate the extent of their perception of pain on the scale, with 0 cm denoting no pain and 10 cm denoting unbearable pain.25 The patients were asked to attend pain test on the day of venous blood collection. ODI was used to determine the severity of functional impairment of patients with back pain.26 In ODI, patients were asked 10 questions that were scored between 0 and 5, based on their functional ability. A high score meant severe impairment. Scores from each question were summed up and calculated from a total of 50.
Laboratory examination
Vacutainer tubes were used to obtain fasting venous blood from all participants and the sample obtained was immediately centrifuged to prevent glycolysis. Serum samples were kept at −80°C until assayed. All samples were stored at −80°C for a period of 1 wk to 17 mo. Serum CXCL12 levels of patients were blindly assessed using a commercial Duoset ELISA Kit (R&D system, Minneapolis MN, US). In brief, standard recombinant human CXCL12 and serum samples were added into 96-well microtiter plates pre-coated using a polyclonal Ab against CXCL12 and incubated for 1 h at room temperature (20°C). Thereafter, the wells were washed seven times using a washing buffer and were incubated with a HRP-labeled mouse mAb against human CXCL12 for 30 min at 4°C. The substrate solution was added to each well after nine washes and the plate was incubated in the dark for 30 min at room temperature. Intra-assay and inter-assay coefficients of variation for CXCL12 were found to be 5.0% and 6.5%, respectively. The detection range was found to be 31.2–2,000 pg/ml. All procedures were done at room temperature, and the samples were measured in duplicate. Color reaction assay was performed at 450 nm. Other biomarkers, including TNF-α (R&D system, Minneapolis MN, US) and IL-6 (R&D system, Minneapolis MN, US), were also tested using the same procedure.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 6.0 software (San Diego, CA, USA). All results are expressed as mean ± SD or median. Kolmogorov–Smirnov test was used to determine the normal distribution of the data. The chi-square test, unpaired t-test or Mann–Whitney U test was utilized to evaluate the parameter variance of gender, age and body mass index (BMI). Student’s t-test or Mann–Whitney U tests was used to compare data between two groups, whereas one-way ANOVA or Kruskal–Wallis test was used to compare between three or more groups, followed by Tukey or Tamhan test for post-hoc analysis. Spearman or Pearson correlation analysis was conducted to identify the correlation between CXCL12 levels and other parameters. ROC analysis was conducted to analyze the diagnostic value of CXCL12, TNF-α and IL-6 in IDD. A P-value of < 0.05 was considered to indicate statistical significance.
Results
Demographic data
The mean patient age was 46.3 ± 7.2 (standard deviation) yr; 85 were men and 60 were women. The mean age was 45.9 ± 6.9 yr for the healthy controls, with 73 being male and 57 female. Significant differences were not found based on age, BMI and gender distribution between the IDD patients and controls (Table 1).
Table 1.
Demographic characteristics.
| IDD patients (n = 145) | Healthy controls (n = 130) | P-Value | |
|---|---|---|---|
| Age (yr) | 46.3 ± 7.2 | 45.9 ± 6.9 | 0.112 |
| Gender (F/M) | 60/85 | 57/73 | 0.857 |
| BMI (kg/m2) | 23.5 ± 3.0 | 23.2 ± 2.6 | 0.252 |
| VAS score | 4.8 ± 1.6 | / | |
| ODI index | 20.7 ± 6.7 | / | |
| Pfirrmann Grade (m/M/S) | 50/53/42 | / | |
| Multifidus degeneration (Y/N) | 83/62 | / | |
| Psoas major degeneration (Y/N) | 55/90 | / | |
| Leg pain (Y/N) | 59/86 | ||
| Most severely degenerated segment | 85/60 | ||
| Serum CXCL12 levels (pg/ml) | 308.2 ± 44.0 | 235.3 ± 31.0 | < 0.001 |
Results are expressed as mean ± standard deviation (mean ± SD). m: mild; M: moderate; S: severe; Y: yes; N: no.
Correlation between serum CXCL12 levels and Pfirrmann grade
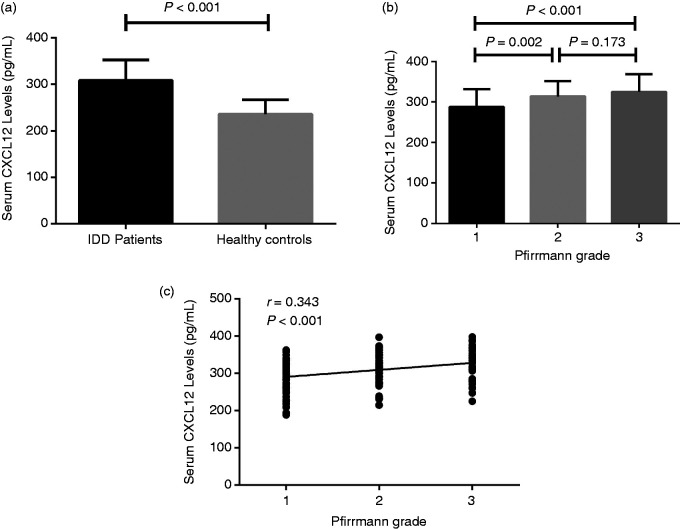
The Kappa value from evaluation of MRI results was 0.89. Serum CXCL12 concentrations were found to be significantly higher in symptomatic IDD patients (308.2 ± 44.0 pg/ml), compared with asymptomatic healthy individuals (235.3 ± 31.0 pg/ml) (P < 0.00; Figure 2a). Based on the Pfirrmann grading system, patients with IDD were divided into three subgroups: 50 patients were assigned to the mild degeneration group (marked as 1), 53 to the moderate degeneration group (marked as 2) and 42 to the severe degeneration group (marked as 3). Serum CXCL12 concentrations were found to be markedly higher in patients with moderate degeneration (313.8 ± 37.9 pg/ml), compared with that of patients with mild degeneration (288.0 ± 43.4 pg/ml) (P = 0.002). In addition, although no significant difference in serum CXCL12 levels was found between the severe (325.3 ± 43.3 pg/ml) and moderate (313.8 ± 37.9 pg/ml) degeneration groups (P = 0.173), patients with severe degeneration IDD still had significantly increased serum CXCL12 levels (325.3 ± 43.3 pg/ml) compared with mild degeneration group patients (288.0 ± 43.4 pg/ml) (P < 0.001; Figure 2b). We also found that serum CXCL12 levels were significantly higher in the mild degeneration group (288.0 ± 43.4 pg/ml) compared with that of the healthy control group (235.3 ± 31.0 pg/ml) (P < 0.001; Figure 2b). Overall, it was found that serum CXCL12 levels are positively correlated with Pfirrmann grade (r = 0.343, P < 0.001; Figure 2c; Table 1).
Figure 2.
(a) Comparison of serum CXCL12 levels between IDD patients and healthy controls. (b) Comparison of serum CXCL12 levels among different Pfirrmann grades and healthy controls. (c) Correlation between serum CXCL12 levels and Pfirrmann grade.
Correlation between serum CXCL12 levels and muscle degeneration
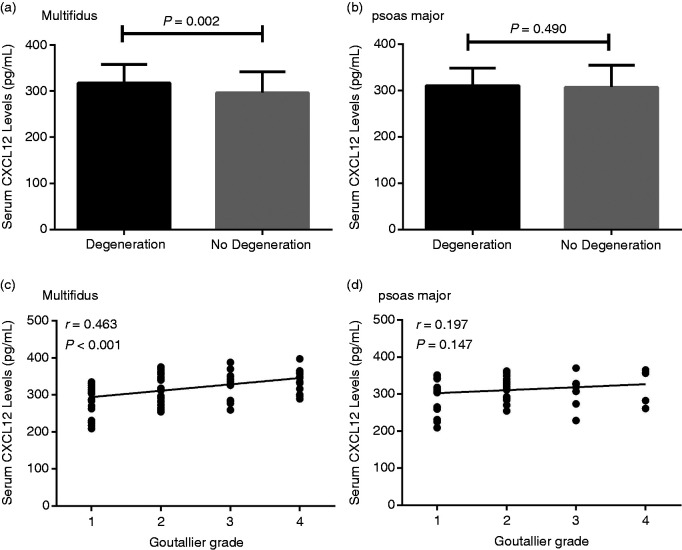
Next, the correlation between CXCL12 levels and the multifidus and psoas major muscle degeneration was examined using MRI and was based on the Goutallier classification system. Eighty-three of the patients were found to have multifidus degeneration, while 55 patients had psoas major muscles degeneration. CXCL12 levels were found to be significantly elevated in multifidus, but not psoas major muscles degeneration IDD patients, compared with non-degeneration IDD patients (multifidus: 317.2 ±40.7 pg/ml and 296.1 ± 45.7 pg/ml, respectively (P = 0.002); psoas major: 310.4 ± 37.9 pg/ml and 306.8 ± 47.5 pg/ml, respectively (P = 0.490); Figure 3a and b). Serum CXCL12 levels were also found to be significantly and positively associated with Goutallier grade in the multifidus degeneration group (r = 0.463, P < 0.001; Figure 3c). However, a significant association between serum CXCL12 levels and the psoas major degeneration group, based on Goutallier grade, was not found (r = 0.197, P = 0.147; Figure 3d).
Figure 3.
(a) Comparison of serum CXCL12 levels between multifidus degeneration and non-degeneration IDD patients. (b) Comparison of serum CXCL12 levels between psoas major degeneration and non-degeneration IDD patients. (c) Correlation between serum CXCL12 levels and multifidus degeneration, based on Goutallier grade. (d) Correlation between serum CXCL12 levels and psoas major degeneration, based on Goutallier grade.
Correlation between serum CXCL12 levels and clinical severity
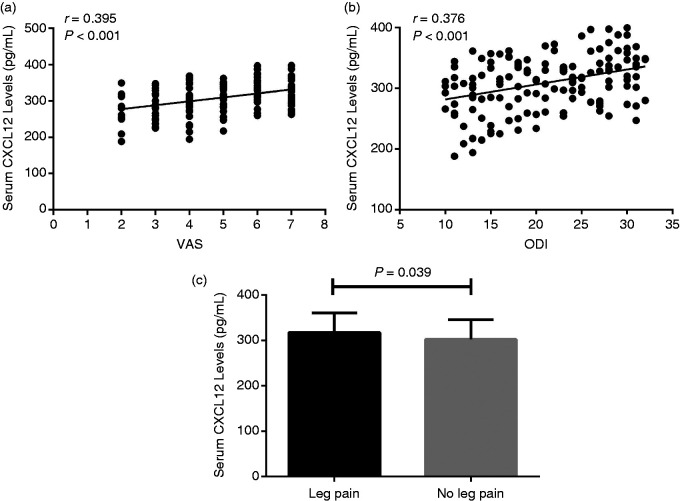
In order to further investigate whether increased serum CXCL12 levels are related to clinical severity in patients with IDD, we explored the relationships between serum CXCL12 concentrations and VAS, as well as ODI. We found that serum CXCL12 levels are positively associated with clinical intensity assessed using VAS (r = 0.395, P < 0.001; Figure 4a) and ODI (r = 0.376, P < 0.001; Figure 4b). In addition, serum CXCL12 levels were significantly increased in IDD patients with leg pain (317.3 ± 43.2 pg/ml) compared with IDD patients without leg pain (302.0 ± 43.7 pg/ml) (P = 0.039; Figure 4c).
Figure 4.
(a) Relationship between serum CXCL12 levels and VAS among IDD patients. (b) Relationship between serum CXCL12 levels and ODI among IDD patients. (c) Comparison of serum CXCL12 levels between IDD patients with leg pain and IDD patients without leg pain.
Correlation of serum CXCL12 levels with biochemical indices and ROC analysis
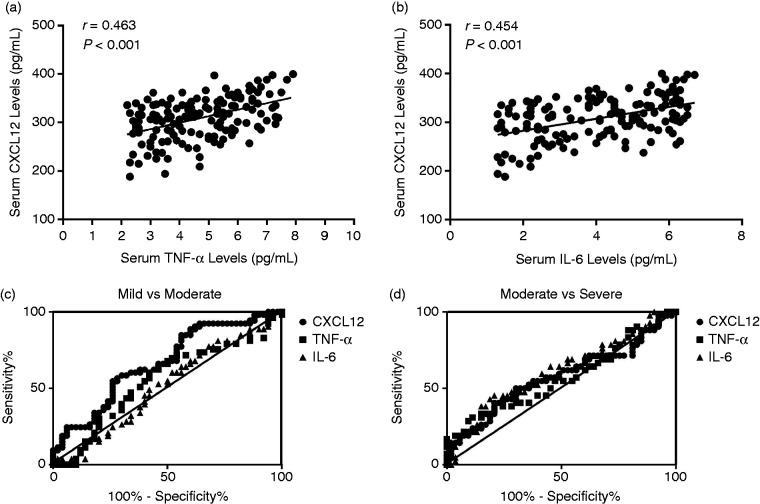
We next investigated the association of serum CXCL12 levels with serum TNF-α and IL-6 levels. We observed serum CXCL12 levels were positively correlated with serum TNF-α levels (r = 0.463, P < 0.001; Figure 5a) and serum IL-6 levels (r = 0.454, P < 0.001; Figure 5b). Finally, a ROC curve analysis was conducted to identify the diagnostic functionality of CXCL12, IL-6 and TNF-α, with regard to Pfirrmann grade. As demonstrated in Figure 4, between Pfirrmann grade-based mild degeneration and moderate degeneration, ROC analysis shows that CXCL12 exhibits a larger area under curve (AUC) (AUC = 0.668, P = 0.003), than that for TNF-α (AUC = 0.551, P = 0.373) and IL-6 (AUC = 0.514, P = 0.805) (Figure 5c, Table 2). However, between Pfirrmann moderate degeneration and severe degeneration, CXCL12 possesses a smaller AUC (AUC = 0.574, P = 0.022) than that for IL-6 (AUC = 0.625, P = 0.049) (Figure 5d, Table 3). These results indicate that serum CXCL12 levels may act as an early-mediate marker that can reflect disc degeneration, as determined by Pfirrmann grade at the early-mediate stage.
Figure 5.
(a) Relationship between serum CXCL12 levels and serum TNF-α levels in IDD patients. (b) Relationship between serum CXCL12 levels and serum IL-6 levels in IDD patients. (c) ROC curve analysis of CXCL12, TNF-α and IL-6 levels between Pfirrmann mild grade and moderate grade. (d) ROC curve analysis of CXCL12, TNF-α and IL-6 levels between Pfirrmann moderate grade and severe grade.
Table 2.
Calculation of the AUC for CXCL12, TNF-α and IL-6 levels between Pfirrmann mild and moderate grades.
| AUC | SE | 95% CI | P-Value | |
|---|---|---|---|---|
| CXCL12 | 0.668 | 0.053 | 0.563–0.772 | 0.003 |
| TNF-α | 0.551 | 0.058 | 0.438–0.664 | 0.373 |
| IL-6 | 0.514 | 0.057 | 0.401–0.627 | 0.805 |
SE: Standard Error; 95%CI: 95% confidence interval.
Table 3.
Calculation of area under curve (AUC) for CXCL12, TNF-α and IL-6 levels between Pfirrmann moderate and severe grades.
| AUC | SE | 95% CI | P-Value | |
|---|---|---|---|---|
| CXCL12 | 0.574 | 0.061 | 0.455–0.693 | 0.218 |
| TNF-α | 0.569 | 0.060 | 0.450–0.688 | 0.247 |
| IL-6 | 0.625 | 0.059 | 0.512–0.733 | 0.049 |
SE: Standard Error; 95%CI: 95% confidence interval.
Discussion
The findings of this study have, for the first time, identified that significantly increased levels of serum chemokine CXCL12 are found in individuals with symptomatic lumbar disc degeneration, compared with those of the asymptomatic control group that showed no signs of disc degeneration when observed through MRI. We also observed that serum CXCL12 levels are independently and positively related with IDD progression. More importantly, study participants of corresponding age, gender and BMI value were paired, in order that the effect of these parameters on the results could be eliminated or minimized. Our findings verified the results of other previous studies, based on animal models and affected human disc tissue in vitro, which indicate that CXCL12 is involved in the process of disc degeneration. Therefore, systemic CXCL12 levels may be molecular candidates that can be used to diagnose and monitor patients with clinically relevant IDD.
Increasing evidence suggests that IDD may involve the immune response and inflammatory reaction of the disc, and that degenerated nucleus pulposus tissue is considered to be an Ag that can immediately induce inflammation.27 Studies done during recent years have confirmed the central role of inflammation in spinal degenerative mechanisms.28,29 As degeneration progresses, elevated levels of inflammatory cytokines enhance aggrecan and collagen degradation, leading to structural changes and spinal instability.30 In recent years, serum inflammatory cytokines have been used as potential biomarkers for IDD.31 The verification of detectable serum inflammatory markers could revolutionize the diagnostic process of IDD, especially for those with undefined origins of LBP symptoms.31
Chemokines have been regarded as important cytokines that participate in IDD progression. For example, CCL5 is a substance that attracts motile cells of a certain type and is produced by degenerative intervertebral discs,32 and systemic CCL5 and CXCL6 levels were found to have increased and be correlated with medium to high intensities of lumbar disc degeneration.33 In addition, CCL5 CXCL12 is the most studied chemokine regarding IDD progression.
We first found that increased CXCL12 levels are positively associated with disc and multifidus degeneration, as defined by the Pfirrmann and Goutallier classification systems. In this study, we selected the most severely degenerated disc for assessment, in order that it may directly reflect disease severity. The CXCL12/CXCR4 axis can accelerate apoptosis in degenerative nucleus pulposus cells in vitro,22 indicating that elevated CXCL12 levels are related to the extent of disc degeneration. Degeneration of lumbar paravertebral muscles occurs frequently in patients with IDD, with a tendency to be most pronounced in multifidus degeneration patients.34 We found that serum CXCL12 levels were also related to Goutallier classification, suggesting that circulating CXCL12 may also be involved in multifidus degeneration.
Next, we found that serum CXCL12 levels were significantly related to pain and functional severity, as defined by VAS and ODI. At present, a growing number of studies using animal experiments have demonstrated that under pathological pain states, CXCL12 is expressed at important points along the pain transmission pathway.35 The CXCL12/CXCR4 axis has been found to be a key pro-inflammatory mediator for the pathogenesis and persistence of neuropathic pain36 and critical for pain sensitization.37 In various pain-related models, CXCL12 and CXCR4 expressions have been found to be drastically elevated in the dorsal root ganglion, and it was shown that inhibition of CXCR4 can reverse pain-related behaviors.38,39 The results of these studies are consistent with our findings that serum CXCL12 levels are significantly elevated in patients with leg pain, implicating that a nerve root compressed by a herniated disc may induce both local and systemic CXCL12 production.
The roles of cytokines IL-6 and TNF-α have been confirmed as key causes of inflammation in the lumbar disc degeneration process. We observed that serum CXCL12 levels are positively related with serum expressions of IL-6 and TNF-α. Licciardone et al. found that IL-6 is a main influencer of LBP pathology and is correlated with pain severity and indicates somatic dysfunction.40 In another study, patients with disc degeneration disease were found to have higher serum IL-6 levels compared with control subjects.41 IL-6 levels were shown to be correlated with the symptom duration of patients with IDD.41 TNF-α levels were found to be higher in degenerate nucleus pulposus (NP) tissues, than in normal human and rat tissues, and a previous study also identified an obvious increase in TNF-α levels in patients with IDD and Lumbar Disc Herniation (LDH).42 Patients with chronic pain as a result of disc herniation have elevated blood and cerebrospinal fluid TNF-α and IL-6 levels compared with controls.43,44 Based on the ROC curve analysis, we can use CXCL12 to detect patients with early stage IDD and TNF-α levels to detect patients with medium to late stage IDD.
Our study has several limitations that should be pointed out. First, the current study was performed on a relatively small sample size and was a single-center study on Han Chinese individuals at our hospital. Therefore, future studies using larger sample sizes and multi-center studies are needed. Second, only serum CXCL12 levels were examined, while levels of its receptor, CXCR4, and other chemokines were not analyzed. Third, we did not examine the bone mineral density (BMD) of all participants, since CXCL12 has been reported to be associated with BMD in previous studies.45 However, BMD would have provided more valuable information for the association between CXCL12 and bone metabolism. Finally, our study is a cross-sectional study. In order to identify if serum CXCL12 levels can predict clinical prognosis and outcome in IDD patients, a prospective study is required in the future.
Collectively, serum CXCL12/SDF-1 level was found to be positively related with lumbar IDD and clinical severity, thus improving our understanding of IDD progression.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Xue-Jun Chen https://orcid.org/0000-0002-1568-4383
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine 2006; 18: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong J, Reed C, Novick D, et al. Costs associated with treatment of chronic low back pain: An analysis of the UK General Practice Research Database. Spine (Phila Pa 1976) 2013; 38: 75–82. [DOI] [PubMed] [Google Scholar]
- 4.Dowdell J, Erwin M, Choma T, et al. Intervertebral disc degeneration and repair. Neurosurgery 2017; 80: S46–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehra M, Hill K, Nicholl D, et al. . The burden of chronic low back pain with and without a neuropathic component: A healthcare resource use and cost analysis. J Med Econ 2012; 15: 245–252. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Hou J, Lv D, et al. Multimodal quantitative magnetic resonance imaging for lumbar intervertebral disc degeneration. Exp Ther Med 2017; 14: 2078–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: A systematic review and meta-analysis. AJNR Am J Neuroradiol 2015; 36: 2394–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenty M, Crescenzi R, Fry B, et al. Novel imaging of the intervertebral disc and pain. Global Spine J 2013; 3: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 10.Poole AR. Biologic markers and disc degeneration. J Bone Joint Surg Am 2006; 88: 72–75. [DOI] [PubMed] [Google Scholar]
- 11.Peng B, Wu W, Hou S, et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br 2005; 87: 62–67. [PubMed] [Google Scholar]
- 12.Miller MC, Mayo KH. Chemokines from a structural perspective. Int J Mol Sci 2017; 18: pii: E2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol 2003; 15: 15–21. [DOI] [PubMed] [Google Scholar]
- 14.Sanz MJ, Kubes P. Neutrophil-active chemokines in in vivo imaging of neutrophil trafficking. Eur J Immunol 2012; 42: 278–283. [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa T. CXCL12/SDF-1 and CXCR4. Front Immunol 2015; 6: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–988. [DOI] [PubMed] [Google Scholar]
- 17.Janssens R, Struyf S, Proost P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev 2018; 44: 51–68. [DOI] [PubMed] [Google Scholar]
- 18.Meng W, Xue S, Chen Y. The role of CXCL12 in tumor microenvironment. Gene 2018; 641: 105–110. [DOI] [PubMed] [Google Scholar]
- 19.Villalvilla A, Gomez R, Roman-Blas JA, et al. SDF-1 signaling: A promising target in rheumatic diseases. Expert Opin Ther Targets 2014; 18: 1077–1087. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Zhang L, Chen L, et al. Stromal cell-derived factor-1 and its receptor CXCR4 are upregulated expression in degenerated intervertebral discs. Int J Med Sci 2014; 11: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Zhu T, Zhang L, et al. Stromal cell-derived factor 1 induces matrix metalloproteinase expression in human endplate chondrocytes, cartilage endplate degradation in explant culture, and the amelioration of nucleus pulposus degeneration in vivo. Int J Mol Med 2018; 41: 969–976. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Ma C, Shen J, et al. SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus pulposus cells via the NF-κB pathway. Mol Med Rep 2016; 14: 783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001; 26: 1873–1878. [DOI] [PubMed] [Google Scholar]
- 24.Goutallier D, Postel JM, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994; 304: 78–83. [PubMed] [Google Scholar]
- 25.Bird SB, Dickson EW. Clinically significant changes in pain along the visual analog scale. Ann Emerg Med 2001; 38: 639–643. [DOI] [PubMed] [Google Scholar]
- 26.Zigler JE, Delamarter RB. Oswestry Disability Index. J Neurosurg Spine 2014; 20: 241–242. [PubMed] [Google Scholar]
- 27.Lv F, Leung VY, Huang S, et al. In search of nucleus pulposus-specific molecular markers. Rheumatology (Oxford) 2014; 53: 600–610. [DOI] [PubMed] [Google Scholar]
- 28.Wuertz K, Haglund L. Inflammatory mediators in intervertebral disc degeneration and discogenic pain. Global Spine J 2013; 3: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol 2014; 10: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 2006; 88: 10–14. [DOI] [PubMed] [Google Scholar]
- 31.Khan AN, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann N Y Acad Sci 2017; 1410: 68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pattappa G, Peroglio M, Sakai D, et al. CCL5/RANTES is a key chemoattractant released by degenerative intervertebral discs in organ culture. Eur Cell Mater 2014; 27: 124–136. [DOI] [PubMed] [Google Scholar]
- 33.Grad S, Bow C, Karppinen J, et al. Systemic blood plasma CCL5 and CXCL6: Potential biomarkers for human lumbar disc degeneration. Eur Cell Mater 2016; 31: 1–10. [DOI] [PubMed] [Google Scholar]
- 34.Kalichman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int 2017: 2562957. [DOI] [PMC free article] [PubMed]
- 35.Luo X, Wang X, Xia Z, et al. CXCL12/CXCR4 axis: An emerging neuromodulator in pathological pain. Rev Neurosci 2016; 27: 83–92. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Huang X, Di Y, et al. Effect of CXCL12/CXCR4 signaling on neuropathic pain after chronic compression of dorsal root ganglion. Sci Rep 2017; 7: 5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson NM, Jung H, Ripsch MS, et al. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun 2011; 25: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhangoo SK, Ren D, Miller RJ, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun 2007; 21: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubovy P, Klusakova I, Svizenska I, et al. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol 2010; 133: 323–337. [DOI] [PubMed] [Google Scholar]
- 40.Licciardone JC, Kearns CM, Hodge LM, et al. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: Results from the OSTEOPATHIC Trial. J Am Osteopath Assoc 2012; 112: 596–605. [DOI] [PubMed] [Google Scholar]
- 41.Weber KT, Alipui DO, Sison CP, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther 2016; 18: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiler C, Nerlich AG, Bachmeier BE, et al. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: A study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 2005; 30: 44–53. [DOI] [PubMed] [Google Scholar]
- 43.Wang K, Bao JP, Yang S, et al. A cohort study comparing the serum levels of pro- or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J 2016; 25: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 44.Kraychete DC, Sakata RK, Issy AM, et al. Serum cytokine levels in patients with chronic low back pain due to herniated disc: Analytical cross-sectional study. Sao Paulo Med J 2010; 128: 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carbone LD, Bůžková P, Fink HA, et al. Association of plasma SDF-1 with bone mineral density, body composition, and hip fractures in older adults: The Cardiovascular Health Study. Calcif Tissue Int 2017; 100: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]