Abstract
Introduction
Mechanical thrombectomy is standard treatment for large vessel occlusion (LVO) in adults. There are no randomized controlled trials for the pediatric population. We report our single-center experience with thrombectomy of LVO in a series of pediatric patients, and perform a review of the literature.
Methods
Retrospective review of consecutive pediatric thrombectomy cases between 2011 and 2018. Demographic variables, imaging data, technical aspects and clinical outcome were recorded.
Results
In a period of 7 years, 7 children were treated for LVO at our center. Median age was 13 (2–17), and median Ped-NIHSS was 15 (3–24), and the median ASPECTS was 8 (2–10). Five patients had cardiac disease, and 2 of them were under external cardiac assistance. Median time from onset of symptoms to beginning of treatment was 7h06m (2h58m–21h38m). Five patients had middle cerebral artery occlusions. Thrombectomy was performed using a stentriever in 3 patients, aspiration in 3 patients, and combined technique in 1 patient. Six patients had good recanalization (TICI 2 b/3). There were no immediate periprocedural complications. At 3 months, 4 patients (57%) were independent (mRS score <3). Two patients died, one after haemorrhagic transformation of an extensive MCA infarct, and one due to extensive brainstem ischemia in the setting of varicella vasculitis.
Discussion
Selected pediatric patients with LVO may be treated with mechanical thrombectomy safely. In patients under external cardiac assistance and under anticoagulation, thrombectomy is the only alternative for treatment of LVO. A multidisciplinary approach in specialized pediatric stroke centers with trained neurointerventionalists are essential for good results.
Keywords: Stroke, paediatric, thrombectomy
Introduction
Acute ischemic stroke is a common disease in the adult population, but has a lower incidence in children, especially after one month of age, where incidence is estimated to be between 3 and 13/100,000/year.1,2 Etiology, presentation and outcome in pediatric ischemic stroke have specificities when compared to stroke in adults.3 The prevalence of large vessel occlusion in children is unknown, but is estimated to be around 7% of all ischemic strokes.4
The recent guideline inclusion of mechanical thrombectomy for large vessel occlusion strokes5 has led to increasing numbers of patients with acute ischemic stroke undergoing treatment. Even more patients might now be included after the publication of studies like DAWN,6 increasing the time window for large vessel occlusions up to 24 h. Patients under 18 years old were initially excluded from acute ischemic stroke guidelines. New orientations on pediatric stroke were recently published,7 suggesting that children should be treated in dedicated pediatric acute care centers, in a multidisciplinary approach, as has been developed for adult ischemic stroke. The approach for pediatric acute ischemic stroke is in all similar to adults: the time window for IV tPA is 4,5 h after symptoms, the total dose of IV tPA is calculated likewise, as 0.9 mg/kg.7
Regarding large vessel occlusions treatment, much less is known in the pediatric population. Several case reports and case series have been published that demonstrate the efficacy and safety of thrombectomy.8 Recently, some reviews have gathered the published cases of endovascular treatment reported worldwide, and conclude that thrombectomy leads to high recanalization rates in children, similarly to adults, with excellent clinical outcome.4,9,10 However, even in reviews, numbers of treated cases are still low, reporting as few as 29 cases in one review of the literature between 2008 and 2015,9 38 cases in a national database study in the US, representing 1% of the total pediatric ischemic stroke cases,2 and 44 cases in one review between 1990 and 2016.11 Despite the published literature, prospective evidence is still lacking.12,13 Reports of consecutive series of cases remain relevant in this context.
We hereby report our single center experience using mechanical thrombectomy for large vessel occlusion in pediatric acute ischemic stroke. We also reviewed the literature on mechanical thrombectomy in the pediatric age.
Methods
Retrospective analysis of all consecutive pediatric patients (aged 28 days to 18 years) with acute ischemic stroke and large vessel occlusion, submitted to endovascular treatment at our center, between December 2011 and March 2018. The study was approved by the local Ethics Committee. All study protocols were conducted in accordance with the Declaration of Helsinki.
A systematic search of PubMed was performed of all papers published until December 2019 using the search terms: “pediatric” AND “ischemic stroke”, “endovascular thrombectomy” AND “pediatric” or “children” or “child”. We included all case reports, case series and systematic reviews that referred to mechanical thrombectomy of large vessel occlusion in patients under 18 years old.
Patients
Demographic and clinical data, and radiological characteristics of stroke were collected. Pediatric NIHSS was used to quantify neurologic deficits at admission. All patients underwent noncontrast CT and CT angiography (CTA) on admission. ASPECTS score (Alberta Stroke Programme Early CT Score)14 was used to quantify ischemia on admission CT whenever applicable.
Endovascular treatment
Treatment included mechanical thrombectomy, alone or in combination with IV r-tPA. IV tPA was administered according to guidelines at 0.9 mg/kg, as a 10% bolus, and perfusion of remaining dose in 1 hour.5
Time from symptom onset to imaging and time from symptom onset to beginning of treatment and to recanalization were recorded.
Efficient recanalization was defined as thrombolysis in cerebral infarction (TICI) grade 2 b–3.15 Partial recanalization was defined as TICI = 1–2a. Absence of recanalization was defined as TICI = 0.
Outcome
Imaging (computed tomography or magnetic resonance) was performed 24 hours after treatment. Symptomatic intracranial haemorrhage was considered for any haemorrhage causing clinical deterioration (increase in NIHSS by >4). Clinical outcome evaluation was performed by experienced pediatric neurologists, using the modified Rankin Scale (mRS) score at 3-month follow-up.
Data analysis
Patient demographics, procedural characteristics, and outcome are reported as median (interquartile range/total range) or as frequency.
Results
Study population
Between December 2011 and March 2018, 7 patients (5 girls and 2 boys) were admitted to our center for acute ischemic stroke due to large vessel occlusion. Median age was 13 (range 2–17). The median Ped-NIHSS was 15 (range 3–24).
In 5 patients, a pre-existing cardiopathy was known, and 2 of those patients were under external cardiac assistance, while waiting for cardiac transplant. Of the 5 cardiopathies, 2 had a right-left shunt, one was a dilated cardiomyopathy, and 2 had mitral regurgitation. Of the remaining 2 patients, one had a post-infeccious vasculopathy (varicella zoster vasculopathy confirmed by PCR of virus in the cerebrospinal fluid), and in the other, etiology of stroke was unknown. Median time from symptom onset to imaging was 4h45m (range 1h15m–21h). In only 2 patients MR was performed before treatment. Median ASPECTS was 8 (range 2–10). The patient treated with an ASPECTS of 2 was under external cardiac assistance, and rescue thrombectomy was considered as a condition to remain in the cardiac transplant list. All patients had documented large vessel occlusions, involving the middle cerebral artery in 6 patients, and the basilar artery in one patient (Table 1).
Table 1.
Patient characteristics.
| Age,y | Gender | Etiology | PedNIHSS | ASPECTS | Clot location | rtPA | Endovascular technique | mTICI | Time to recanalisation (min) | ICH | mRS at 90 days | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | F | ComplexCardiopathy | 14 | 10 | Right M1 | No | Stent retriever | 3 | 220 | No | 1 |
| 2 | 14 | F | ComplexCardiopathy | 3 | 10 | Right M2 | No | Stent retriever | 2c | 492 | No | 2 |
| 3 | 10 | F | Unknown | 21 | 8 | Left M1 | No | Stent retriever | 2b | 504 | No | 3 |
| 4 | 2 | M | Post-infeccious arteriopathy | 15 | NA | Basilar artery | No | Combined | 0 | 1460 | No | 6 |
| 5 | 13 | M | ComplexCardiopathy | 16 | 10 | Left M2 | No | Aspiration | 3 | 478 | No | 2 |
| 6 | 17 | F | ComplexCardiopathy | 5 | 8 | Left M2 | Yes | Aspiration | 2b | 325 | No | 2 |
| 7 | 8 | F | ComplexCardiopathy | NA | 2 | Right M1 | No | Aspiration | 2c | NA | Yes | 6 |
ASPECTS: Alberta Stroke Program Early CT Score; ICH: intracranial hemorrhage; mRS: modified Rankin Scale; mTICI: modified treatment in cerebral infarction; NA: not applicable; pedNIHSS: Pediatric National Institutes of Health Stroke Scale; rtPA: Recombinant tissue plasminogen activator.
Endovascular treatment and angiographic outcome
Only one patient had IV tPA; in the remaining 6 patients, IV TPA was contraindicated (one patient was beyond 4.5 h after symptoms, 5 patients were under heparin/oral anticoagulation). Median time from onset of symptoms to beginning of endovascular treatment was 7h06m (range 2h58m–21h38m). Middle cerebral artery occlusions were proximal (M1) in 3 patients and post bifurcation (M2) in 3 patients. In 50% of the patients, occlusions were on the right side.
Endovascular treatment was performed in a dedicated angio suite, by experienced neurointerventionalists. A transfemoral approach was used in all patients, using a 5 F sheath, and a 5 F guiding catheter. Per protocol, no heparin was used during the procedure, however, catheters were flushed via a pressurized bag of heparinized saline (5000 U heparin/liter). Thrombectomy was performed using a single technique in 6 patients – in 3 patients a stentriever alone was used (Solitaire® or Trevo®), and in 3 patients aspiration alone was performed using the Penumbra® system (Ace 64 and/or 3Max). A maximum of 3 passes were performed. All six patients had good recanalization (TICI 2 b/3).
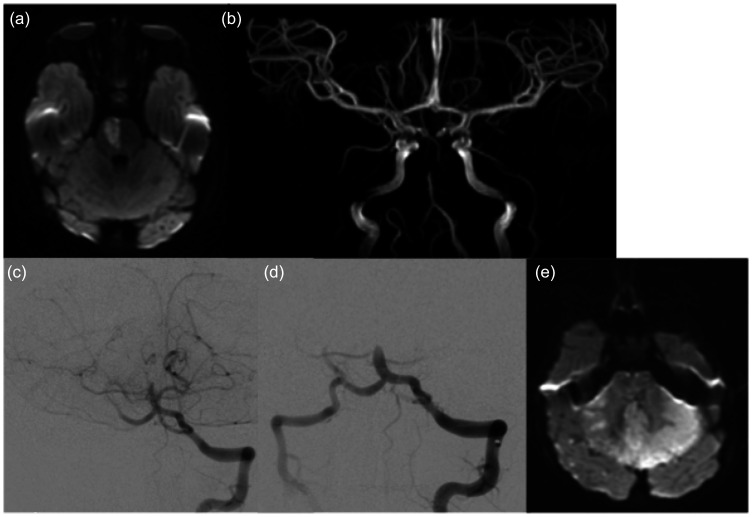
In one patient, a combined technique with stentriever and aspiration was used, with no recanalization achieved (TICI 0) after 3 passages. This patient was later proved to have a varicella-zoster vasculopathy (Figure 1).
Figure 1.
A 2 year-old child presented with acute hemiparesis and somnolence. MRI was performed at 20 hours after onset, showing acute paramedian pons ischemia on the DWI (a), and occlusion of the mid-basilar artery on the MR angiogram (b). Findings were thought basilar artery was confirmed (c), and several attempts to recanalize it failed (d). Diffusion weighted imaging at 24 h post treatment showed extensive ischemia of the brainstem and cerebellum (e), and the patient did not survive.
There were no immediate haemorrhagic complications or other periprocedural complications in all patients.
Clinical outcome
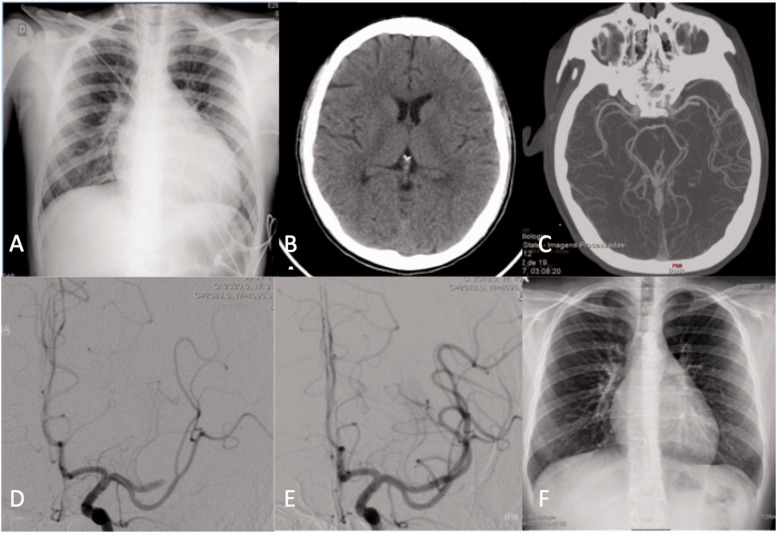
Five patients had a favourable clinical evolution during hospital stay, with recovery of neurological symptoms. At 3 months, 4 patients (57%) were independent (mRS score = 0–2), and one patient had a mRS of 3 (residual mild aphasia). One of the patients under external cardiac assistance had a cardiac transplant weeks after thrombectomy, having had a full neurological recovery (Figure 2).
Figure 2.
A 13 year-old patient presented with acute aphasia and right hemiparesis. The patient was under cardiac external assistance, on a cardiac transplant list after myocarditis and cardiac failure. Chest radiography showed an increased cardiothoracic index (a). CT scan performed 4 hours after onset of symptoms showed no lesion (ASPECTS 10) (b), and a left M2 occlusion on CT angiography (c). The patient was transported to the angio suite, and thrombectomy was started at 7h30m after symptom onset. Occlusion of the anterior division of the left MCA was confirmed (d), and the clot was aspirated, with complete recanalization (e). The patient recovered completely, and had a cardiac transplant some weeks after. Follow up chest radiography showed normal cardiac dimensions (f).
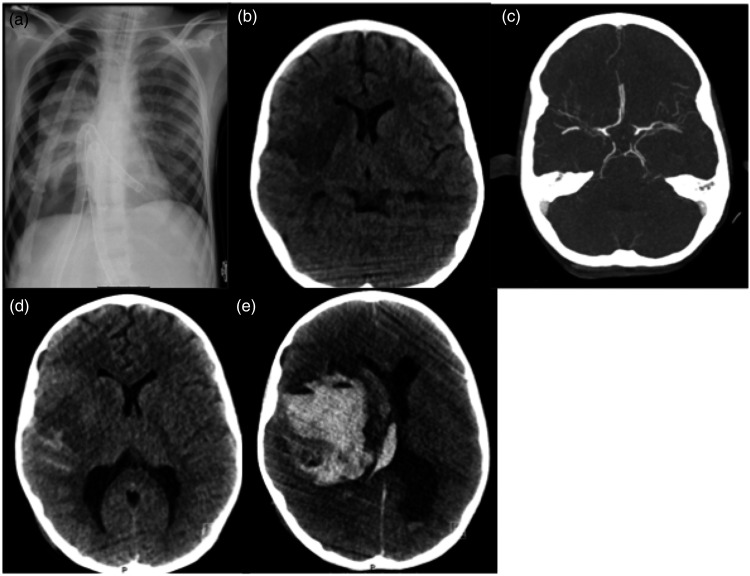
One patient had a fatal haemorrhage 10 days after treatment, that was related to haemorrhagic transformation of an extensive MCA infarct in a patient with a pre-treatment ASPECTS of 2. This patient was under external cardiac assistance, and was under heparin perfusion (Figure 3). The patient with a basilar occlusion due to varicella zoster vasculopathy, and with no recanalization after thrombectomy, evolved to a fatal ischemia of the brainstem and cerebellum (Figure 1).
Figure 3.
An 8 year-old child under external left ventricular assist device, as shown on a plain chest x-ray (a), under sedation and ventilated, had an unknown onset of left hemiparesis. Head CT scan showed an already established acute ischemic lesion affecting the perforator and superior division of the right MCA (b). CT angiogram showed a proximal M1 occlusion, with good collaterals (c). Considering that thrombectomy was the only alternative of treatment, and that only part of the MCA territory was infarcted, a decision to treat was made, and complete recanalization was obtained. CT at 24 h showed the same ischemic lesion, and slight subarachnoid haemorrhage (d). The patient was kept under sedation. At 10 days post thrombectomy, the patient developed sudden signs of intracranial hypertension, and assimetric pupils. Emergent CT showed massive hemorrhagic transformation of the ischemic lesion (e), and the patient did not survive.
Discussion
Our small cohort of consecutive pediatric patients with acute ischemic stroke and large vessel occlusion demonstrates the feasibility and safety of mechanical thrombectomy.
There are several case reports and series of cases on mechanical thrombectomy in children, documenting the efficacy of this technique, as well as its safety, in a similar fashion as in adult patients.4,8,10,13,16–21 Presently, several literature reviews and systematic reviews have already been published on thrombectomy in large vessel occlusion pediatric stroke.2,8,9,11,12,22–25 Table 2 summarizes the largest recent series of cases in the literature. Main conclusions are that: mechanical thrombectomy is effective in recanalizing large vessel occlusion – TICI 2 b/3 recanalization rates reaching over 65% and up to 90%; there is a relatively high percentage of complications, reaching 29% of cases9 in some reports, however, only a small proportion of these are symptomatic, between 1 and 5.9%. Functional independence is achieved in over two thirds of patients (65.7%–92%), paralleling, and even overcoming adults, and mortality reaches 10%.2,9,24
Table 2.
Summary of the larger case series of mechanical thrombectomy in pediatric patients.
| Reference | n | Median age | Etiology | Initial PedNIHSS | Clot location | Technique | Time to groin puncture | mTICI2b/3 | Intracerebral hemorrhage | Favorable clinical outcome (mRS 0-2 90 days) |
|---|---|---|---|---|---|---|---|---|---|---|
| Tabone et al., 20178 | 13 | 3.7–16.6 | Arteriopathy 61.5% | 10 (1–21) | Anterior circulation 85% | – | 4h (3–4,5) | – | 0 | 92% |
| Wilson et al., 20172 | 38 | 10.2 | Cardiac 42% | – | Anterior circulation 96% | – | – | – | 7% | 55% |
| Bigi et al., 201817 | 16 | 11.0 | Undetermined 42.7%,cardiac 18%, arteriopathy 17.3% | 13.5 (IQR 11.5–21.3) | Anterior circulation 68.8% | – | – | 63% | 6,2% | – |
| Shoirah et al., 201919 | 19 | 10.9 | Cardiac 47.4% | 13.9 | Anterior circulation 79% | Stentretriever 52.6% | 323m | 89.5% | 0 | 89.5% |
| Sun et al., 201920 | 11 | 2.1 (9mo-4y) | Cardiac 60% | 8–21 | Anterior circulation 73% | Stentretriever 64% | 12h (4–50) | 58% | 9% | 64% |
| Sporns et al., 201925 | 73 | 11.3 (IQR 7–15) | Cardiac 44%, undetermined 37% | 14 (IQR 9.2–30) | Anterior circulation 86% | Stentretriever 82% | Onset to recanalization4h (IQR 3.0–6.9) | 87% | 1% | 85% |
mRS: modified Rankin Scale; mTICI: modified treatment in cerebral infarction; pedNIHSS: Pediatric National Institutes of Health Stroke Scale.
We are still lacking controlled studies to demonstrate the safety and indication of this technique in children. Randomized trials, such as the TIPS trial,26 have been prematurely stopped due to poor enrolment, which will certainly be a limitation for every study on ischemic stroke in children. For now, class IIb recommendations have been made for mechanical thrombectomy in patients with severe stroke and large vessel occlusion, in centers with documented experience in pediatric angiography.22
Experience seems to be an important determinant of success in recanalization, but above all, of safety. This is particularly relevant in the pediatric population, with smaller vessels, more prone to spasm. Therefore, what is unanimous from the literature is that pediatric endovascular stroke should be treated in dedicated stroke centers with experience in performing endovascular procedures in children.
Thrombectomy has been performed in all pediatric ages, the youngest reported at 28 days.8 There are also series specifically addressing the adolescent patient,18 showing equivalent results to the adult population. Optimal imaging for children with suspected stroke has been reviewed, and MRI is suggested as the best imaging method.27 However, in most series, like in ours, treatment decisions are still based in CT and CTA as primary imaging techniques, due to easy access and speed. Children with dilated cardiomyopathy and under external cardiac assistance cannot perform MRI, therefore diagnosis of stroke and vessel occlusion must be done with CT and CTA.
In what concerns the technical aspects of thrombectomy, the use of either stentrievers or aspiration has been described, with similar efficacy as for the adult population, reaching more than 85% of successful recanalization rates (TICI 2 b/3).9 This was in line with our results. In most studies, stent retrievers are used in up to 82% of patients as a first option,9,25,28–35 reflecting the guidelines for stroke treatment in adults. In our center, aspiration as first intention has now replaced stentrievers, and was used in 4 (57%) of our patients. In the literature, aspiration is increasingly reported as effective in pediatric stroke.36,37 Some series also report the use of balloon angioplasty.38,39
Compatibility with devices used for adults seems to be an issue only until 5 years of age, from which point on intracranial vessels will have reached the adult proportions.20,40 The use of smaller catheters/stent retrievers should be considered in smaller children. In the youngest patient in our series (2 year-old), a 3 mm stentriever was used, as well as a 3 F aspiration catheter.
Etiology of stroke is unique in children, when compared to adults. Arteriopathy can be a cause of stroke in up to 49% of patients, cardiac disorders are the cause in almost 30% of patients, as are prothrombotic disorders.41 Anedoctal cases on rare etiologies of stroke and thrombectomy have also been reported, such as embolic atrial myxoma42 or hematologic malignancies.43Two challenging stroke etiologies stand out from our series: the patients under external cardiac assistance and the patient with intracranial vasculitis.
Patients under external cardiac support are in cardiac transplant lists, and have a high risk of ischemic stroke, that can reach 16%.44 Stroke and neurological deficit are contraindications for transplant, and therefore, the decision to rapidly treat these patients is critical. The use of IV tPA in contraindicated, since these patients are under heparin. Mechanical thrombectomy will remain the only choice in this scenario, however, it is not without risks, that add up to the serious systemic condition of most of them: intubated, ventilated and under aminergic support. Cardioembolic strokes, in particular in patients under external cardiac assistance devices, have been shown to benefit from mechanical thrombectomy, as the sole alternative of treatment.45 Our series adds two patients with external cardiac assistance treated with thrombectomy by aspiration alone, to a total of only 4 cases previously reported,45–48 three using stentriever, and one using intra-arterial thrombolysis. To our knowledge, we report the first 2 cases using aspiration alone. One of the patients in our series suffered a fatal late hemorrhagic transformation of a large ischemic lesion, despite thrombectomy, whereas the other patient had a total recovery and had a cardiac transplant shortly after. These two cases also illustrate the importance of a multidisciplinary approach in the treatment of acute stroke in children,7 with decisions involving the pediatric cardiology team, pediatric neurology and neurointerventional teams.
Intracranial arteriopathy is an important cause of pediatric stroke, reaching 49–64% of cases in some series41; of those, around 5.3% will be secondary vasculitis.49 In these cases, endovascular treatment will not solve the occlusion. In fact, the manipulation of an inflamed vessel wall might aggravate the spasm and occlusion, or increase the risk of rupture.25 The only patient in our series with vasculitis had a late diagnosis, and was initially misinterpreted as a possible cardioembolic stroke, and submitted to thrombectomy, with no success. The diagnosis of vasculitis has to be kept in mind in the pediatric patient, and additional laboratory testing and accurate clinical history are crucial in the setting of acute ischemic stroke.
We treated a patient with a very low ASPECTS score. In younger patients, intracranial pial collaterals are thought to be present in a higher extent,50 and also neuronal plasticity is higher in children than in adults, possibly justifying treatment of patients beyond the time window, and also with larger lesions on imaging. In fact, delayed treatment seems to be the rule in children stroke,22,51 also in our series, with a median 7h06m from symptom onset to treatment, however, results are still favourable in terms of recanalization and outcome.
Clinical outcome after stroke in children is poor:41,52 epilepsy will develop in 15–20%, 75% will have motor impairment. The mRS scale was not designed for use in children, and a proper follow-up should include behaviour and psychosocial outcome.53,54 This was a limitation of our study. However, most studies in the literature also use the mRS scale as a measure of outcome, and report very favourable outcomes, with the largest study, the Save ChildS study reporting median mRS scores of 1 at 6 and 24 months.
Our study has other limitations, namely its retrospective design, and very small sample size. However, the small number of patients reflects the low prevalence and low recognition of ischemic stroke in children. We also probably have a bias towards cardioembolic strokes, and towards patients under cardiac external support, due to our association to a tertiary reference pediatric cardiology center.
Conclusion
This small cohort of pediatric patients with large vessel occlusion shows that mechanical thrombectomy is feasible and safe if performed in selected patients. A review of the available literature underlines our results. Careful selection of patients under external cardiac assistance and early suspicion and diagnosis of vasculitis are crucial for procedural safety. Multidisciplinary work in specialized pediatric stroke centers is essential for good results.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Isabel Fragata https://orcid.org/0000-0002-7037-7458
References
- 1.Giroud M, Lemesle M, Madinier G, et al. Stroke in children under 16 years of age. Clinical and etiological difference with adults. Acta Neurol Scand 1997; 96: 401–406. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JL, Eriksson CO, Williams CN. Endovascular therapy in pediatric stroke: Utilization, patient characteristics, and outcomes. Pediatr Neurol 2017; 69: 87–92.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felling RJ, Sun LR, Maxwell EC, et al. Pediatric arterial ischemic stroke: epidemiology, risk factors, and management. Blood Cells Mol Dis 2017; 67: 23–33. [DOI] [PubMed] [Google Scholar]
- 4.Sporns PB, Kemmling A, Hanning U, et al. Thrombectomy in childhood stroke. J Am Heart Assoc; 8. Epub ahead of print 2019. DOI: 10.1161/JAHA.118.011335. [DOI] [PMC free article] [PubMed]
- 5.Pw J, Ra A, Teri A, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018. ; 49: e46–e99. 2018; [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 7.Rivkin MJ, Bernard TJ, Dowling MM, et al. Guidelines for urgent management of stroke in children. Pediatr Neurol 2016; 56: 8–17. [DOI] [PubMed] [Google Scholar]
- 8.Desguerre I, Lesage F, Husson B, et al. Regional pediatric acute stroke protocol: initial experience during 3 years and 13 recanalization treatments in children. Stroke 2017; 48: 2278–2281. [DOI] [PubMed] [Google Scholar]
- 9.Satti S, Chen J, Sivapatham T, et al. Mechanical thrombectomy for pediatric acute ischemic stroke: review of the literature. J NeuroIntervent Surg 2017; 9: 732–737. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia K, Nbi Kortman H, Blair C, et al. Mechanical thrombectomy in pediatric stroke: systematic review, individual patient data meta-analysis, and case series. Journal of Neurosurgery: Pediatrics 2019; 24: 558–571. [DOI] [PubMed] [Google Scholar]
- 11.Cobb MIPH, Laarakker AS, Gonzalez LF, et al. Endovascular therapies for acute ischemic stroke in children. Stroke 2017; 48: 2026–2030. [DOI] [PubMed] [Google Scholar]
- 12.Pacheco JT, Siepmann T, Barlinn J, et al. Safety and efficacy of recanalization therapy in pediatric stroke: a systematic review and meta-analysis. Eur J Paediatr Neurol 2018; 22: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 13.Barry M, Hallam DK, Bernard TJ, et al. What is the role of mechanical thrombectomy in childhood stroke? Pediatr Neurol 2019; 95: 19–25. [DOI] [PubMed] [Google Scholar]
- 14.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta Stroke Programme Early CT Score. Lancet (London, England ) 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 15.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109-37. [DOI] [PubMed] [Google Scholar]
- 16.Stidd DA, Lopes DK. Successful mechanical thrombectomy in a 2-year-old male through a 4-French guide catheter. Neurointervention 2014; 9: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigi S, Dulcey A, Gralla J, et al. Feasibility, safety, and outcome of recanalization treatment in childhood stroke. Ann Neurol 2018; 83: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 18.Bhogal P, Hellstern V, Almatter M, et al. Mechanical thrombectomy in children and adolescents: report of five cases and literature review. Stroke Vasc Neurol 2018; 3: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoirah H, Shallwani H, Siddiqui AH, et al. Endovascular thrombectomy in pediatric patients with large vessel occlusion. J Neurointerv Surg 2019; 11: 729–732. [DOI] [PubMed] [Google Scholar]
- 20.Sun LR, Felling RJ, Pearl MS. Endovascular mechanical thrombectomy for acute stroke in young children. J Neurointerv Surg 2019; 11: 554–558. [DOI] [PubMed] [Google Scholar]
- 21.Bodey C, Goddard T, Patankar T, et al. Experience of mechanical thrombectomy for paediatric arterial ischaemic stroke. Eur J Paediatr Neurol EJPN off J Eur Paediatr Neurol Soc 2014; 18: 730–735. [DOI] [PubMed] [Google Scholar]
- 22.Ellis MJ, Amlie-Lefond C, Orbach DB. Endovascular therapy in children with acute ischemic stroke. Neurology 2012; 79: S158–164. [DOI] [PubMed] [Google Scholar]
- 23.Madaelil TP, Kansagra AP, Cross DT, et al. Mechanical thrombectomy in pediatric acute ischemic stroke: clinical outcomes and literature review. Interv Neuroradiol 2016; 22: 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cappellari M, Moretto G, Grazioli A, et al. Primary versus secondary mechanical thrombectomy for anterior circulation stroke in children: an update. J Neuroradiol 2018; 45: 102–107. [DOI] [PubMed] [Google Scholar]
- 25.Sporns PB, Strater R, Minnerup J, et al. Feasibility, safety, and outcome of endovascular recanalization in childhood stroke: the Save ChildS Study. JAMA Neurol. Epub Ahead of Print October 2019. DOI: 10.1001/jamaneurol.2019.3403. [DOI] [PMC free article] [PubMed]
- 26.Rivkin MJ, Ichord RN, Chan A, et al. Thrombolysis in pediatric stroke (TIPS) study. Stroke 2015; 46: 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirsky DM, Beslow LA, Amlie-Lefond C, et al. Pathways for neuroimaging of childhood stroke. Pediatr Neurol 2017; 69: 11–23. [DOI] [PubMed] [Google Scholar]
- 28.Buompadre MC, Andres K, Slater L-A, et al. Thrombectomy for acute stroke in childhood: a case report, literature review, and recommendations. Pediatr Neurol 2017; 66: 21–27. [DOI] [PubMed] [Google Scholar]
- 29.Serra Martínez M, Avellaneda-Gómez C, Cayuela Caudevilla N, et al. Endovascular treatment of arterial ischaemic stroke in paediatric patients: a case-report. Neurologia 2020; 35: 52–54. [DOI] [PubMed] [Google Scholar]
- 30.Sainz de la Maza S, De Felipe A, Matute MC, et al. Acute ischemic stroke in a 12-year-old successfully treated with mechanical thrombectomy. J Child Neurol 2014; 29: 269–273. [DOI] [PubMed] [Google Scholar]
- 31.Savastano L, Gemmete JJ, Pandey AS, et al. Acute ischemic stroke in a child due to basilar artery occlusion treated successfully with a stent retriever. J Neurointerv Surg 2016; 8: e33 LP-e33. [DOI] [PubMed] [Google Scholar]
- 32.Nicosia G, Cicala D, Mirone G, et al. Childhood acute basilar artery thrombosis successfully treated with mechanical thrombectomy using stent retrievers: case report and review of the literature. Childs Nerv Syst 2017; 33: 349–355. [DOI] [PubMed] [Google Scholar]
- 33.Pandey AS, Savastano L, Gemmete JJ, et al. Late recanalization of basilar artery occlusion in a previously healthy 17-month-old child. J Neurointerv Surg 2018; 10: e17–e17. [DOI] [PubMed] [Google Scholar]
- 34.Futch HS, Corliss BM, Polifka AJ, et al. Solitaire stent retriever mechanical thrombectomy in a 6-month-old patient with acute occlusion of the internal carotid artery terminus: Case report. World Neurosurg 2019; 126: 631–637. [DOI] [PubMed] [Google Scholar]
- 35.Huded V, Kamath V, Chauhan B, et al. Mechanical thrombectomy using solitaire in a 6-year-old child. J Vasc Interv Neurol 2015; 8: 13–16. [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner GM, Feroze RA, Agarwal N, et al. Successful manual aspiration thrombectomy in a pediatric patient. Pediatr Neurol 2016; 61: 107–113. [DOI] [PubMed] [Google Scholar]
- 37.Lena J, Eskandari R, Infinger L, et al. Basilar artery occlusion in a child treated successfully with mechanical thrombectomy using ADAPT. J Neurointerv Surg 2017; 9: e2. [DOI] [PubMed] [Google Scholar]
- 38.Kirton A, Wong JH, Mah J, et al. Successful endovascular therapy for acute basilar thrombosis in an adolescent. Pediatrics 2003; 112: e248-51–e251. [DOI] [PubMed] [Google Scholar]
- 39.Cognard C, Weill A, Lindgren S, et al. Basilar artery occlusion in a child: ‘clot angioplasty’ followed by thrombolysis. Childs Nerv Syst 2000; 16: 496–500. [DOI] [PubMed] [Google Scholar]
- 40.He L, Ladner TR, Pruthi S, et al. Rule of 5: angiographic diameters of cervicocerebral arteries in children and compatibility with adult neurointerventional devices. J Neurointerv Surg 2016; 8: 1067–1071. [DOI] [PubMed] [Google Scholar]
- 41.deVeber GA, Kirton A, Booth FA, et al. Epidemiology and outcomes of arterial ischemic stroke in children: the Canadian Pediatric Ischemic Stroke Registry. Pediatr Neurol 2017; 69: 58–70. [DOI] [PubMed] [Google Scholar]
- 42.Vega RA, Chan JL, Anene-Maidoh TI, et al. Mechanical thrombectomy for pediatric stroke arising from an atrial myxoma: case report. J Neurosurg Pediatr 2015; 15: 301–305. [DOI] [PubMed] [Google Scholar]
- 43.Mahadevan J, Bacchi S, Kiley M, et al. Paediatric acute lymphoblastic leukaemia causing acute leukaemic occlusion of the proximal middle cerebral artery: treatment with endovascular thrombectomy. J Clin Neurosci off J Urosci 2019; 68: 336–338. [DOI] [PubMed] [Google Scholar]
- 44.Huang JY, Ignjatovic V, Sheridan BJ, et al. Bleeding and thrombotic events occur early in children on durable ventricular assist devices. Thromb Res 2019; 173: 65–70. [DOI] [PubMed] [Google Scholar]
- 45.Stowe RC, Kan P, Breen DB, et al. Mechanical thrombectomy for pediatric acute stroke and ventricular assist device. Brain Dev 2018; 40: 81–84. [DOI] [PubMed] [Google Scholar]
- 46.Rhee E, Hurst R, Pukenas B, et al. Mechanical embolectomy for ischemic stroke in a pediatric ventricular assist device patient. Pediatr Transplant 2014; 18: E88–92. [DOI] [PubMed] [Google Scholar]
- 47.Alnaami I, Buchholz H, Ashforth R, et al. Successful use of solitaire FR for stroke in a pediatric ventricular assist device patient. Ann Thorac Surg 2013; 96: e65-7–e67. [DOI] [PubMed] [Google Scholar]
- 48.Byrnes JW, Williams B, Prodhan P, et al. Successful intra-arterial thrombolytic therapy for a right middle cerebral artery stroke in a 2-year-old supported by a ventricular assist device. Transpl Int 2012; 25: e31–e33. 3. [DOI] [PubMed] [Google Scholar]
- 49.Wintermark M, Hills NK, De Veber GA, the VIPS Investigators et al. Clinical and imaging characteristics of arteriopathy subtypes in children with arterial ischemic stroke: results of the vips study. AJNR Am J Neuroradiol 2017; 38: 2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal S, Scoffings DJ, Jones PS, et al. Interaction of age with the ischaemic penumbra, leptomeningeal collateral circulation and haemodynamic variables in acute stroke: a pilot study. J Neurol Neurosurg Psychiatry 2013; 84: 271–276. [DOI] [PubMed] [Google Scholar]
- 51.Kulhari A, Dorn E, Pace J, et al. Acute ischemic pediatric stroke management: an extended window for mechanical thrombectomy? Front Neurol 2017; 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.deVeber GA, MacGregor D, Curtis R, et al. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol 2000; 15: 316–324. [DOI] [PubMed] [Google Scholar]
- 53.B, Gs AC, MA, et al. Long-term outcome after arterial ischemic stroke in children and young adults. Neurology 2015; 84: 1941–1947. [DOI] [PubMed] [Google Scholar]
- 54.Gordon AL, Anderson V, Ditchfield M, et al. Factors associated with six-month outcome of pediatric stroke. Int J Stroke 2015; 10: 1068–1073. [DOI] [PubMed] [Google Scholar]