Abstract
Background
Optimal antiplatelet inhibition is vital during cerebrovascular stenting procedures, yet no standardized recommendation exists for antithrombotic therapy in these scenarios. Cangrelor is an intravenous P2Y12 inhibitor with a favorable pharmacokinetic profile for use during neuroendovascular stenting.
Methods
A retrospective review of all neuroendovascular patients who underwent stenting between 1 January 2019 and 22 March 2020 and were treated with cangrelor was conducted. Thirty-seven patients met inclusion criteria.
Results
All patients were administered a bolus of 5 mcg/kg of cangrelor followed by a maintenance infusion. Antiplatelet effects of cangrelor were monitored using platelet reactivity units (PRU). Based on the initial PRU, seven patients’ doses were adjusted with subsequent PRUs in or near the goal range of 50–150. One patient experienced an acute intraprocedural occlusion likely related to a subtherapeutic PRU which subsequently resolved with cangrelor dose adjustment and intra-arterial tirofiban administration, and one patient experienced a post-procedure stent occlusion which required a thrombectomy and intra-arterial tirofiban administration. No hemorrhagic complications occurred.
Discussion
Cangrelor utilization during neuroendovascular stenting with maintenance doses of <2 mcg/kg/min with dose adjustments based on platelet function testing has not been previously described. Cangrelor presents many advantages compared to standard therapy in patients undergoing stent placement related to its pharmacokinetic profile, rapid onset of action, ease of transition to oral P2Y12 antiplatelet agents, and measurability.
Conclusion
Cangrelor is a promising alternative to currently available therapies, especially in patients with a high hemorrhagic risk.
Keywords: Antiplatelet therapy, cangrelor, cerebrovascular, stenting, stroke, subarachnoid hemorrhage
Introduction
Indications for acute cerebrovascular intervention have continued to increase, and major trials showing the benefit of emergent intervention to provide revascularization in patients presenting with an acute large vessel occlusion or symptomatic critical stenosis have vastly increased the usage of intracranial and carotid stents.1 More recently, the use of stents—including flow-diverting devices—has gained increasing popularity even in the setting of an acutely ruptured aneurysms causing subarachnoid hemorrhage.2
Antiplatelet therapy remains an integral part of treatment in order to maintain stent patency, at the expense of exposing patients to the known risks of antiplatelet therapy. Although limited data exist for the use of antiplatelet agents in this patient population during unplanned stent-deployment procedures, no current rapid-acting intravenous antiplatelet agents are approved by the US Food and Drug Administration for use during neuroendovascular procedures. Currently, the use of glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors is limited. Large variation exists in the use of antiplatelet regimens among providers as no standard recommendation exists.3
A need exists for an antiplatelet medication for use during neuroendovascular stenting that has easily measurable antiplatelet effects to allow dose optimization, a rapid onset and offset of action, organ-independent metabolism, and the ability to easily transition to an oral antiplatelet. Cangrelor (Kengreal; Chiesi, Parma, Italy) is a newer-generation intravenous P2Y12 inhibitor which has been studied in percutaneous coronary interventions (PCI) that possesses these advantageous qualities. Currently, the data available for cangrelor use in neuroendovascular procedures are limited.4–7 In addition, maintenance infusion rates of <2 mcg/kg/min of cangrelor with utilization of dose adjustment with platelet function testing have not been previously reported. Here, we report our initial experience with the utilization of dose-adjusted cangrelor with platelet function testing during neuroendovascular stent deployment.
Materials and methods
Objective and design
The objective of this retrospective review was to evaluate the safety and efficacy of cangrelor administration to maintain stent patency during neuroendovascular interventions.
Study approval
We received Institutional Review Board approval for this retrospective review of patients administered cangrelor for neuroendovascular stenting. Patient consent was not obtained because use of cangrelor in neuroendovascular stenting was approved by the necessary institutional committees.
Patient population
A retrospective chart review of all endovascular patients who underwent intervention between 1 January 2019 and 22 March 2020 at a regional Comprehensive Stroke Center was undertaken. Patients 18 years of age or older undergoing carotid artery or intracranial stent placement for any underlying pathology were considered. Those who received cangrelor during the intervention were included in this review. Demographic information, indication for intervention and type of stent placed, cangrelor dosing utilized, platelet reactivity unit (PRU) results, pertinent information related to patient outcome, and information related to any acute (intraprocedural) or subacute (within 48 h) complications were recorded.
Cangrelor protocol
In all patients, cangrelor was administered as a 5 mcg/kg bolus over 30 s followed by a maintenance infusion of either 0.75 or 1 mcg/kg/min. The rationale for this dosing regimen is given in the “Discussion” section. In general, a PRU goal utilizing the VerifyNow assay (Accriva Diagnostics; San Diego, USA) of 50–150 is preferred with an acceptable lower limit of 10 and an acceptable upper limit of 180 depending on the patient scenario. Cangrelor dose adjustments were made with the intent to maintain the patients PRU within the above range if possible. If feasible, a VerifyNow assay was obtained 5–10 min after bolus administration and again post-procedure to assess the need for dose adjustment, and additional PRUs were sent if deemed appropriate for dose adjustment. Blood samples for PRU testing were sent to the hospital central laboratory and were not available as point of care results. Thus, there was at times a delay in PRU results being available to adjust the dose of cangrelor. If the cangrelor infusion rate was changed, it was changed in increments of 0.25 mcg/kg/min. The cangrelor infusion was then continued until the patient was able to receive an oral antiplatelet agent. When it was appropriate, the patient was given a loading dose of crushed ticagrelor, and the cangrelor infusion was discontinued 1 h after the loading dose of ticagrelor was administered. In addition, if aspirin was not previously administered, all patients were started on aspirin upon discontinuation of cangrelor. The patients were maintained on oral ticagrelor and aspirin as per our usual protocol.
Study outcomes
The primary study objective was to assess the effectiveness of dose-adjusted cangrelor utilizing platelet function testing and secondarily to evaluate the safety and efficacy of cangrelor in this patient population.
Results
After a retrospective chart review, 37 patients met criteria for inclusion. Twenty-one of these patients presented with an acute ischemic stroke or symptomatic critical stenosis (Table 1), eight suffered from ruptured intracranial aneurysms resulting in subarachnoid hemorrhage, four had unruptured aneurysms, one had a ruptured carotid pseudoaneurysm (Table 2), two had carotid blowouts/lacerations resulting in active extravasation, and one had a direct cavernous carotid fistula repaired with a flow diverter stent (Table 3).
Table 1.
Acute ischemic stroke/critical stenosis subjects’ baseline characteristics.
| Subject | Age | Sex | Baseline NIHSS | Lesion | Core | Penumbra | Device |
|---|---|---|---|---|---|---|---|
| 1 | 43 | F | 0 | R VA dissection | N/A | N/A | Enterprise |
| 2 | 67 | M | 15 | L ACA occlusion, L MCA stenosis | N/A | N/A | Enterprise x2 |
| 3 | 53 | M | 5 | L MCA occlusion | 0 | 33 | Enterprise |
| 4 | 63 | M | 18 | R ICA and MCA tandem occlusion | 6 | 191 | Wallstent |
| 5 | 67 | M | 0 | R ICA critical stenosis | N/A | N/A | XACT |
| 6 | 55 | F | Unknown | R ICA occlusion | N/A | N/A | XACT, Wallstent, Enterprise x3 |
| 7 | 45 | M | 8 | Basilar occlusion | 0 | 116 | Enterprise |
| 8 | 81 | F | 17 | L MCA occlusion | 0 | 85 | Enterprise |
| 9 | 67 | M | 7 | L MCA occlusion | 0 | 130 | Enterprise x2 |
| 10 | 64 | M | 16 | L MCA occlusion | 4 | 183 | Enterprise |
| 11 | 57 | F | 5 | L ICA occlusion | 0 | 113 | XACT |
| 12 | 55 | M | 13 | R ICA occlusion | 17 | 136 | Wallstent |
| 13 | 88 | M | 6 | R ICA occlusion | 0 | 71 | XACT |
| 14 | 64 | M | 5 | R ICA occlusion | 4 | 167 | Wallstent x2 |
| 15 | 87 | M | 9 | R MCA occlusion | 14 | 140 | Enterprise |
| 16 | 57 | M | 6 | L ICA occlusion | 16 | 99 | Wallstent x2 |
| 17 | 76 | M | 1 | L ICA occlusion | N/A | N/A | XACT |
| 18 | 73 | M | 15 | L ICA occlusion | 0 | 133 | Wallstent |
| 19 | 51 | M | 14 | R ICA occlusion | 39 | 389 | Enterprise |
| 20 | 76 | M | 30 | L ICA occlusion | 149 | 243 | Wallstent |
| 21 | 74 | M | 5 | R ICA occlusion | 0 | 338 | XACT x2 |
ACA: anterior cerebral artery; ICA: internal carotid artery; L: Left; MCA: middle cerebral artery; N/A: not applicable; R: right; VA: vertebral artery.
Table 2.
Aneurysm subjects’ baseline characteristics.
| Subject | Age | Sex | Grade | Baseline GCS | Aneurysm type | Maximum dome size (mm) | Neck size (mm) | Aneurysm location | Device | EVD |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 72 | M | HH5, F4, WFNS 5 | 3T | Saccular | 11.2 | 5 | ACOM | Pipeline Flex | No |
| 23 | 67 | F | HH3, F3, WFNS 3 | 12 | Saccular | 1.5 | 1.5 | R ICA | Pipeline Flex | Yes |
| 24 | 55 | F | HH1, F3, WFNS 1 | 15 | Saccular | 2 | 2 | R MCA | LVIS Jr | No |
| 25 | 42 | F | Un-ruptured | N/A | Saccular | 6.1 | 3.1 | ACOM | LVIS Jr + Coils | No |
| 26 | 52 | F | HH1, F2, WFNS 1 | 15 | Saccular | 5 | 3.2 | L PCA | Pipeline | No |
| 27 | 77 | M | Un-ruptured | 15 | Pseudoaneurysm | 4 | 2 | L ICA | Pipeline Flex | Yes |
| 28 | 57 | F | Un-ruptured | N/A | Saccular | 6 | 4 | R ICA | Enterprise + Coils | No |
| 29 | 53 | F | HH3, F4, WFNS 3 | 10T | Saccular | 4.6 | 4 | R ICA | Pipeline Flex x2 | Yes |
| 30 | 69 | F | HH3, F3, WFNS 2 | 13 | Saccular | 2.8 | 2 | ACOM | Enterprise + Coils | Yes |
| 31 | 66 | F | Un-ruptured | N/A | Saccular | 7.2 | 4.7 | R PCA | Pipeline Flex | No |
| 32 | 51 | F | Un-ruptured | N/A | Saccular | 6.5 | 4.7 | PCA | Enterprise + Coils | No |
| 33 | 77 | F | HH4, F4, WFNS5 | 3T | Saccular | 11.6 | 4.2 | ACOM | Neuroform Atlas + Coils | Bilateral |
| 34 | 56 | F | HH2, F4, WFNS1 | 15 | Fusiform | 13 | N/A | PCA | Neuroform Atlas x2 + Coils | Yes |
ACA: anterior cerebral artery; ACOM: anterior communicating artery; EVD: external ventricular drain; F: Fisher; GCS: Glasgow Coma Scale; HH: hunt hess; ICA: internal carotid artery; L: left; MCA: middle cerebral artery; PCA: posterior communicating artery; R: right; VA: vertebral artery; WFNS: World Federation of Neurosurgery.
Table 3.
Miscellaneous (non-ischemic stroke, non-aneurysmal) patient characteristics.
| Subject | Age | Sex | Indication | Device |
|---|---|---|---|---|
| 35 | 69 | F | Direct cavernous carotid fistula | Pipeline Flex |
| 36 | 67 | M | R ICA hemorrhagic injury due to tumor (previously radiated) | LVIS Jr + Viabahn |
| 37 | 57 | M | L ICA hemorrhagic injury following surgical manipulation | Viabahn |
ICA: internal carotid artery; L: left; R: right.
Cangrelor dosing and PRU results
Initially, patients were treated with a 5 mcg/kg bolus over 30 seconds followed by a 0.75 mcg/kg/min maintenance infusion. Following an evaluation of preliminary results after the first 14 consecutive patients, the decision was made to increase the empiric starting maintenance dose from 0.75 to 1 mcg/kg/min due to the need to increase in the cangrelor infusion rate more often than was felt acceptable. Thus, dose adjustments are evaluated for each group separately below.
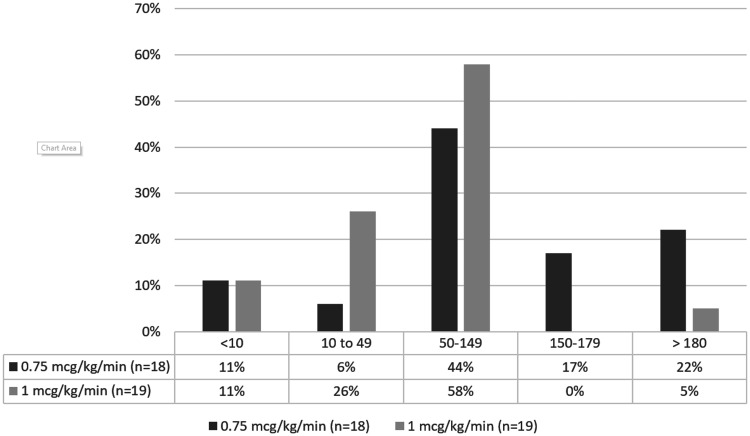
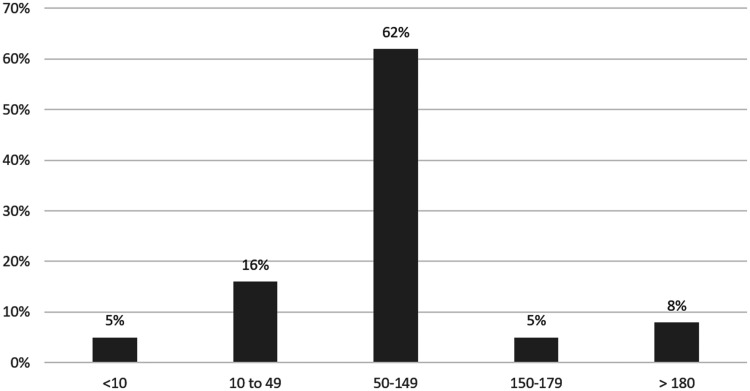
For those receiving the 0.75 mcg/kg/min initial maintenance dose, 8 out of 18 (44%) patients were in the ideal PRU range (50–150) and 12 out of 18 (67%) patients were in the acceptable range (10–180) based on the first recorded PRU (Figure 1). For those receiving the 1 mcg/kg/min initial maintenance dose, 11 out of 19 (58%) were in the ideal range and 16 out of 19 (84%) of patients were in the acceptable range (10–180) based on similarly-timed PRUs (Figure 1). Based on the last recorded PRU while on cangrelor after all dose adjustments were accounted for, 23 out of 37 (62%) were in the ideal range (50–150) and 31 out of 37 (84%) were in the acceptable range (10–180) (Figure 2). A total of seven patients required a dose adjustment (Tables 4 and 5).
Figure 1.
Immediate post-bolus PRU results following initial cangrelor administration (either 0.75 or 1 mcg/kg/min boluses as marked) in our cohort (n = 37).
Figure 2.
PRU results following all cangrelor dose adjustments (n = 37).
Table 4.
Cangrelor dosing and platelet reactivity dosing results.
| Subject | Initial dose (mcg/kg/min) | Post-bolus PRU | Dose adjustment | Post-procedure PRU | Post-ticagrelor PRU | Complication |
|---|---|---|---|---|---|---|
| 1 | 0.75 | 109 | None | 96 | 85 | |
| 2 | 0.75 | 121 | None | 123 | 126 | |
| 3 | 0.75 | 207 | None | 165 | 164 | |
| 4 | 0.75 | 103 | None | 61 | 41 | |
| 5 | 0.75 | 220 | Raised 0.25 mcg/kg/min | 103 | Not obtained | |
| 6 | 0.75 | 8 | Lowered 0.25 mcg/kg/min | 77 | 185 | |
| 7 | 0.75 | 49 | None | Not obtained | 37 | |
| 8 | 0.75 | 66 | None | Not obtained | 7 | |
| 9 | 0.75 | 142 | None | 120 | 84 | |
| 10 | 0.75 | 80 | None | 59 | 44 | |
| 11 | 1 | 70 | None | Not obtained | 224 | |
| 12 | 1 | 117 | None | Not obtained | 82 | |
| 13 | 1 | Not obtained | None | 126 | 63 | |
| 14 | 0.75 | Not obtained | None | 51 | 7 | |
| 15 | 1 | Not obtained | None | 57 | 69 | |
| 16 | 1 | Not obtained | None | 110 | 27 | |
| 17 | 1 | Not obtained | Lowered 0.25 mcg/kg/min | 10 | 230 | |
| 18 | 1 | Not obtained | None | 7 | 53 | |
| 19 | 1 | Not obtained | None | 44 | 2 | |
| 20 | 1 | Not obtained | None | 64 | 43 | |
| 21 | 1 | 22 | None | 32 | 19 | |
| 22 | 0.75 | 108 | None | 89 | 43 | |
| 23 | 0.75 | 178 | Raised 0.25 mcg/kg/min | 134 | 68 | |
| 24 | 0.75 | 214 | Raised 0.25 mcg/kg/min | 3 | 99 | Thrombosis |
| 25 | 0.75 | 168 | None | Not obtained | 179 | |
| 26 | 1 | 88 | None | 33 | 113 | |
| 27 | 0.75 | 3 | Lowered 0.5 mcg/kg/min | 51 | 4 | |
| 28 | 1 | 9 | None | 75 | 9 | |
| 29 | 1 | 89 | None | 190 | 92 | Thrombosis |
| 30 | 1 | 89 | None | Not obtained | 136 | |
| 31 | 1 | 205 | None | Not obtained | 137 | |
| 32 | 1 | 83 | None | 79 | 34 | |
| 33 | 0.75 | 182 | Raised 0.25 mcg/kg/min | 103 | 129 | |
| 34 | 1 | 132 | None | Not obtained | 158 | |
| 35 | 1 | 33 | None | Not obtained | 38 | |
| 36 | 1 | 18 | None | Not obtained | 43 | |
| 37 | 0.75 | 168 | None | 81 | 103 |
Note:
The seven subjects who required dose adjustments are bolded.
PRU: platelet reactivity units.
Table 5.
Dose and PRU details of patients whose dose was adjusted.
| Subject # | Initial dose (mcg/kg/min) | Initial PRU | Subsequent dose (mcg/kg/min) | PRU post adjustment |
|---|---|---|---|---|
| Dosage titrated up (n = 4) | ||||
| 5 | 0.75 | 220 | 1 | 103 |
| 23 | 0.75 | 178 | 1 | 134 |
| 24 | 0.75 | 214 | 1 | 3 |
| 33 | 0.75 | 182 | 1 | 103 |
| Dosage titrated down (n = 3) | ||||
| 6 | 0.75 | 8 | 0.5 | 77 |
| 17 | 1 | 10 | 0.75 | 185 |
| 27 | 0.75 | 3 | 0.25 | 51 |
PRU: platelet reactivity units.
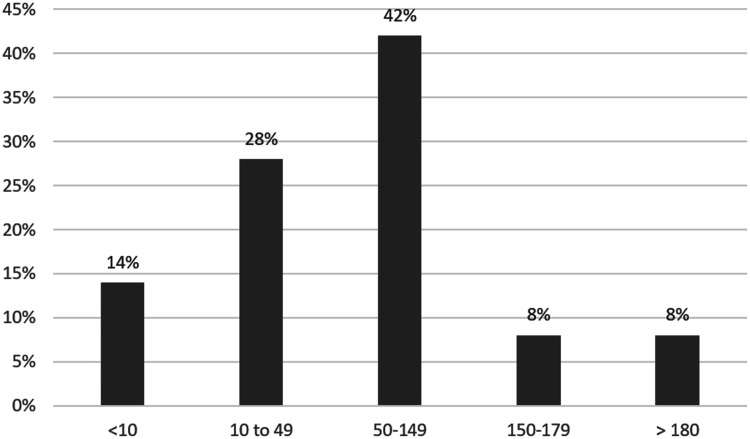
Transition to ticagrelor resulted in 16 out of 37 (43%) of patients in the ideal range (50–150) and 29 out of 37 (78%) in the acceptable range (10–180) (Figure 3).
Figure 3.
PRU results following ticagrelor loading doses (n = 37).
Patient outcomes
No patients in our cohort experienced hemorrhagic complications, including those related to groin puncture. One patient (subject 24) had an acute intraprocedural occlusion of the stent placed in the right middle cerebral artery (MCA), which resolved after microcatheter injection of tirofiban and raising the cangrelor dosage, and no new neurological deficit following the procedure. A second patient (subject 29), who had two flow-diverting stents placed across a ruptured right intracranial internal carotid artery (ICA) aneurysm (with an external ventricular drain (EVD) in place) had an occlusion of the proximal right ICA while on cangrelor which required a thrombectomy and subsequent intra-arterial tirofiban administration, also with resolution of the clot radiographically and no new post-procedural neurological deficit.
Example cases
Multiple patients within our case series necessitate further mention to underscore the scenarios where the advantages of cangrelor were especially apparent:
Subject 22 presented after a fall with an 11 mm subdural hemorrhage (SDH), multiple smaller SDHs, and subarachnoid hemorrhage from a ruptured anterior communicating artery (ACOM) aneurysm. He was maintained on warfarin for prior embolic stroke of unclear etiology approximately 10 years prior. His therapeutic INR was reversed with vitamin K and prothrombin complex concentrate. Cangrelor was utilized during placement of a flow diverter stent with no change in his SDH size.
Subject 35 initially presented with an eye infection which did not improve with topical antibiotics. Subsequent imaging revealed a superior ophthalmic vein thrombosis extending into the cavernous sinus for which she was started on warfarin. Symptoms continued to worsen and the patient presented with significantly worsening eye swelling and proptosis. Due to persistent symptoms, an angiogram was performed to exclude a carotid-cavernous fistula which revealed a direct cavernous carotid fistula. Due to the worsening eye swelling, endovascular repair was performed in an urgent fashion. Due to the need for urgent repair, cangrelor was utilized. A flow diverting stent was utilized for repair with a therapeutic INR (2.7 on morning of procedure) due to recent diagnosis of superior ophthalmic vein thrombosis; no hemorrhagic complications were noted.
Subject 37 had a history of total laryngectomy for squamous cell carcinoma and recent development of a pharyngocutaneous fistula. He presented with profuse bleeding from the fistula site from a carotid artery blowout and active pulsatile bleeding. An initial covered (Viabahn) stent was placed without initiation of antithrombotic therapy likely due to active, significant bleeding by a different surgical service, which quickly occluded. Intraoperative endovascular neurosurgery consult was requested and cangrelor was then started and a second covered stent was placed successfully over the first in telescoping fashion, which remained patent with cessation of active bleeding.
Eight subjects (six with EVDs in place at the time of the procedure) presented with aneurysmal subarachnoid hemorrhage and were repaired with flow diversion or stent-assisted coiling successfully without hemorrhagic complications.
Discussion
To the authors’ knowledge, this is the first report of utilizing low dose (<2 mcg/kg/min) cangrelor during neuroendovascular stenting. Furthermore, the utilization of platelet function testing to adjust doses of cangrelor in neuroendovascular stenting has not been previously reported.
Platelet activation is a complex process resulting from binding of a number of different activator substances to receptors on the platelet surface, including thromboxane (TXA2), thrombin, and collagen. Upon binding, platelet activation ensues and is amplified by the release of substances from platelet granules, specifically nucleosides, one of which is adenosine diphosphate (ADP). ADP binds to two different G-protein coupled receptors, P2Y1 and P2Y12; this binding results in platelet shape change and activation of the GP IIb/IIIa receptor resulting in platelet aggregation. Activation of the P2Y1 receptor results in weak and non-complete activation while activation of the P2Y12 receptor is critical to full platelet activation. Two classes of antiplatelet P2Y12 receptor antagonists are available for clinical use: thienopyridines (clopidogrel and prasugrel) and nucleoside analogues (ticagrelor and cangrelor). Thienopyridines are indirect acting agents and require metabolic activation and covalently bind to the P2Y12 receptor, irreversibly inhibiting the platelet for its entire lifespan. Cangrelor and ticagrelor are ADP analogues that directly antagonize the P2Y12 receptor in a reversible fashion. Cangrelor is cleared by plasma ectonucleotidases and thus displays organ-independent metabolism.8 The unique properties of cangrelor when compared to the thienopyridines with respect to its pharmacology and pharmacokinetics make it an ideal agent for use during endovascular stenting. These properties translate into clinical and practical advantages because cangrelor has a rapid onset and offset, is easily transitioned to an oral antiplatelet, and the antiplatelet effect of cangrelor can be measured.
Cangrelor achieves a rapid onset of effect with extensive platelet inhibition within 2 min of bolus administration and initiation of a continuous infusion.9 Similarly, it exhibits rapid offset of antiplatelet effect within 9 min in 90% of patients (with average half-life between 3 and 5 min).9 A majority (80%–90%) of patients returned to baseline platelet function in 60–90 min after stopping the infusion in the absence of introducing another agent.9 Furthermore, cangrelor presents a number of advantages over currently available GP IIb/IIIa inhibitors (eptifibatide/tirofiban). This includes significantly shorter duration of antiplatelet effect, ability to measure patient antiplatelet response, and organ-independent metabolism (e.g. no need for dose adjustment in patients with renal impairment) (Table 6).9,10
Table 6.
Comparison of cangrelor and glycoprotein IIb/IIIa inhibitors.
| Cangrelor | Tirofiban | Eptifibatide | |
|---|---|---|---|
| Half-life | 3–5 min | 2.5 h | 2 h |
| Time to return of normal platelet function | 60–90 min | 4–8 h | 4–8 h |
| Primary site of metabolism | Plasma (serum enzymes) | Renal | Renal |
Evidence for transitioning patients from continuous infusions of antiplatelet agents to oral medications is variable and many different strategies and protocols exist, but proper conversion is paramount in the immediate period after intracranial or carotid stent placement to minimize thrombotic complications.11 Some GP IIb/IIIa inhibitors interfere with the VerifyNow assay that many centers utilize for monitoring patients’ response to P2Y12 inhibitors, which can complicate this transition.12 Conversely, when transitioning cangrelor to an oral antiplatelet agent, the VerifyNow assay can be utilized which aids in optimizing a proper transition. Beyond the usual considerations, the choice of which oral antiplatelet agent to utilize when transitioning from cangrelor is important. Many centers utilize clopidogrel which, if utilized in transition from cangrelor, should not be administered until the cangrelor infusion has been completely discontinued due to the possibility of cangrelor blocking the initial binding of clopidogrel.13 Our center has been utilizing ticagrelor prior to beginning utilization of cangrelor, and we developed a transition strategy of administering a loading dose of crushed ticagrelor 1 h prior to discontinuation of the cangrelor infusion. Based on our limited data, this transition strategy appeared to be effective because the majority of our patients maintained a PRU in the therapeutic range during transition and we observed no complications during transition.
In related interventional cardiology procedures, cangrelor has shown efficacy in reducing stent thrombosis and thrombosis-related complications compared to loading with oral clopidogrel. Three large, randomized, placebo-controlled trials (RCT) (i.e. the CHAMPION trials) assessed the efficacy of cangrelor in patients undergoing PCI. A pooled analysis of these studies showed that cangrelor significantly decreased myocardial infarction, ischemia-driven revascularization, and stent thrombosis.14 The clear and robust decrease in stent thrombosis by 50% in these trials is likely the most relevant outcome to using cangrelor in neuroendovascular procedures. In the largest of the three RCTs, stent thrombosis at 48 h was decreased from 1.4% in the clopidogrel group to 0.8% in the cangrelor group.15 Additionally, intraprocedural stent thrombosis was decreased from 1% in the clopidogrel group to 0.6% in the cangrelor group.14
Our initial institutional experience with cangrelor shows significant promise for its use in stent placement in neuroendovascular procedures. Cangrelor was shown to be effective in several different patient scenarios. Cangrelor proved to be especially valuable in patients with a high risk of bleeding who required stent placement, with no observed hemorrhagic complications.
The pharmacokinetic profile of cangrelor is ideal for stent placement in patients who have a high risk of bleeding because the dose can be titrated to effect utilizing the VerifyNow assay, and in the event of significant bleeding, the drug’s effects will rapidly dissipate once discontinued. Patients undergoing neuroendovascular stenting for ruptured aneurysms are unique in that they have experienced very recent intracranial hemorrhage and could be at risk of extra-aneurysmal bleeding due to EVD placement but also necessitate antiplatelet therapy. Some evidence supports a platelet aggregate dissolution effect of GP IIb/IIIa inhibitors which theoretically would increase the risk of re-bleeding in patients with recently ruptured aneurysmal hemorrhage.16 A recent study underscored this potential risk by emphasizing that the aneurysm be packed with coils prior to tirofiban administration and stent placement to guard against the risk of re-bleeding due to a potential platelet dissolving effect.17 Furthermore, because of the lack of measurability, optimal dosing of GP IIb/IIIa inhibitors in patients with a high risk of intracranial bleeding is uncertain. There are some limited data that reversible P2Y12 inhibitors may not be effective at dispersing established arterial thrombi and thus cangrelor may have a lower risk of re-bleeding in stenting performed in patients with ruptured aneurysms.18
Utilizing flow diversion devices in ruptured aneurysms is an off-label indication and an area of evolving practice but for some aneurysms it is the only practical treatment option.19 Two patients experienced thrombotic complications during cangrelor utilization. Subject 24 presented with subarachnoid hemorrhage and a right MCA blister aneurysm. A right MCA flow diverting stent was placed, which occluded during the procedure. Subject 29 was treated with two flow diversion devices across a ruptured right ICA aneurysm and experienced a carotid artery occlusion shortly after procedure completion. Other centers have reported results from using flow diversion devices in ruptured aneurysms, and thrombotic complications were documented.20 Varying antithrombotic regimens were utilized, and the numbers of patients in our series and other centers series are small making comparisons of complication rates difficult. Other centers have reported good outcomes with the use single antiplatelet therapy in conjunction with modified flow diversion devices with reduced thrombogenicity.21 In addition, multiple flow diversion devices with reduced thrombogenicity are under investigation which may decrease the rate of thrombotic and hemorrhagic complications.22 To balance the risks of thrombosis versus hemorrhage in patients with ruptured aneurysms, cangrelor may be a useful antithrombotic agent in this scenario given its short half-life, ease of dose titration, and measurability. Consideration could be given to utilizing a higher empiric dose (1.25–2 mcg/kg/min) in patients with flow diversion utilization and ruptured aneurysms.
A primary goal of our initial utilization of cangrelor was to evaluate what dosing strategy is most appropriate for neuroendovascular procedures based on patient outcomes and PRU testing utilizing the VerifyNow assay. The dose used in PCI with cangrelor is a 30 mcg/kg bolus and a 4 mcg/kg/min continuous infusion. In reviewing the pre-clinical development of cangrelor, it was felt that this dose would likely be excessive for neuroendovascular interventions. The aim of the dose-determining studies during cangrelor development was to decide on a dose that would ensure near 100% platelet inhibition in as close to 100% of patients as possible. It was thought that this level of platelet inhibition would lead to an unacceptably high risk of bleeding in the neuroendovascular population.
Indeed, pre-clinical data showed that at a maintenance dose of 1 mcg/kg/min, the mean percentage inhibition of platelet ADP-induced inhibition approached 100%. Furthermore, at a maintenance dose of 1 mcg/kg/min, more than 90% of patients achieved greater than 80% platelet inhibition.23 Other studies have shown that a dose of 2 mcg/kg/min following a 15 mcg/kg loading dose showed nearly identical amounts of platelet inhibition compared to a bolus of 30 mcg/kg and an infusion of 4 mcg/kg/min.9 In addition, in the BRIDGE study, a dose of 0.75 mcg/kg/min lead to PRUs at or below 100 in most patients.24 Furthermore, other case reports and case series highlight that a dose of 0.5 mcg/kg/min or 0.75 mcg/kg/min is likely to yield a PRU result between 50 and 150 in the majority of patients.25,26 Initially (based on the above data), a dose of 0.75 mcg/kg/min was decided as the most likely to achieve our PRU goal of 50–150 for the majority of patients. The bolus dose of 5 mcg/kg was chosen based on the pre-clinical data that a bolus dose 7.5-fold higher than the maintenance infusion rate is likely appropriate to achieve a reasonable amount of immediate platelet inhibition.23 After experience with utilizing cangrelor and in light of the observation that a number of patients necessitated a dosage increase, a dose of 1 mcg/kg/min was chosen as an empiric infusion starting rate. On the other hand, we also observed some patients necessitating a dose decrease, and this observation is also validated by pre-clinical data during cangrelor development that low doses (<1 mcg/kg/min) exhibit significant variations in platelet inhibition.23
The utility of PRU testing in the neuroendovascular population is controversial and there is considerable heterogeneity among institutions in both its usage and protocols.27 Other institutions have reported utilizing dose adjustment protocols based on platelet function testing for patients on oral antiplatelet agents but no study has previously reported utilizing dose adjustments with platelet function testing with cangrelor.28 Our institution relies on platelet function testing and incorporated this into our cangrelor protocol. A major reason for this is when cangrelor is utilized at low doses, limited data show that there can be significant variability in patient response. Data from the BRIDGE study and case reports/case series have shown that occasionally some patients will respond to very low doses of cangrelor (0.25 mcg/kg/min) while some need considerably higher doses (2 mcg/kg/min) to maintain PRUs at the therapeutic goal.24–26 Given the possibility of significant variability in response to cangrelor, we incorporated VerifyNow platelet function testing into our cangrelor protocol by assessing patients’ response to the drug shortly after initiation during the procedure and again immediately post-procedure.
Utilizing platelet function testing necessitates defining a therapeutic and/or threshold cut-offs for alteration of a patient’s antiplatelet regimen. Significant effort has been invested to define such a therapeutic window within the interventional cardiology and neuroendovascular literature, with no current consensus.29,30 Our institution initially derived our PRU range from the interventional cardiology literature and defined a PRU of 100 as being optimal. Despite the heterogeneity of the available literature in regards to cut-offs for low and high platelet reactivity, an argument can be made that targeting a PRU of 100 is reasonable to balance the risk of bleeding and thrombosis.29,31 A number of studies have shown an increased risk of bleeding with PRUs of <50–80, and this lead to our cut-off of 50 as being optimal to define high platelet reactivity.32–35 More variability exists in defining a cut-off for low platelet reactivity with some studies showing no correlation between high PRUs and thrombosis and some studies showing a correlation with PRUs in the range of 150–250.36–38 Keeping values below 150 is an effort to be conservative and to ensure a low risk of thrombosis.
Furthermore, there is significant variability in PRU responses in many patients, and these results can be altered by a number of different variables not related to platelet reactivity.39,40 Because of this, the physiologic meaning (and clinical relevance) of a PRU result that is 150 versus 200 or 10 versus 50 is unclear, which leads to our utilization of optimal versus acceptable cut-offs depending on the clinical scenario. In general, our institution’s practice is if a patient’s PRU falls within the ideal range there is no indication to alter the antiplatelet regimen, but patient-specific factors and provider preference may justify a change in therapy.
Data supporting the use of cangrelor during neuroendovascular stenting are limited but growing (Table 7).4–7 Our study has several differences compared to other published studies, specifically regarding dose adjustments based on PRU values and our utilization of the lower dose protocol for cangrelor as previously discussed. There is mounting evidence that cangrelor is a viable option for use in many different scenarios during neuroendovascular stenting and appears to be safe and effective. Additionally, these reports highlight the challenge of proper dosing of cangrelor in neuroendovascular stenting and whether or not platelet function should be utilized, both of which require further study.
Table 7.
Studies utilizing cangrelor during neuroendovascular stenting.
| Article | Number of patients | Population | Cangrelor dose | Platelet function testing | Complications |
|---|---|---|---|---|---|
| Aguilar-Salinas et al.4 | 8 | Stroke (7); Unruptured aneurysm (1) |
Bolus: 15 mcg/kg Infusion: 2 mg/kg/min |
Recorded (all patients below PRU 200); no dose adjustments | None |
| Abdennour et al.5 | 7 | Aneurysm (5 ruptured; 2 unruptured) |
Bolus: 30 mcg/kg Infusion: 4 mcg/kg/min |
Not utilized | One ICH after switching to ticagrelor |
| Elhorany et al.6 | 12 | Stroke | Bolus: 30 mcg/kg/min Infusion: 4 mcg/kg/min |
Not utilized | Two asymptomatic ICH; one symptomatic ICH; one retroperitoneal hematoma |
| Linfante et al.7 | 10 | Stroke (4); ruptured aneurysm (4); vertebral artery dissection (1) | Bolus: 30 mcg/kg/min Infusion: 4 mcg/kg/min |
Not utilized | One thrombosis (evolution of infarct, no in-stent thrombosis); two minor ICH extension |
ICH: intracranial hemorrhage; PRU: platelet reactivity unit.
Conclusion
Cangrelor utilization during neuroendovascular stenting shows promise as an alternative to currently available antiplatelet therapies. Our experience suggests cangrelor is generally safe and effective during stenting of patients with acute ischemic stroke, ruptured and un-ruptured intracranial aneurysms. Additionally, because the antiplatelet effects of cangrelor can be monitored, it may be particularly advantageous in patients with ruptured intracranial aneurysms or who are at high risk of hemorrhagic complications. Due to variability in antiplatelet response at low doses of cangrelor, further studies are needed to define the optimal dose and monitoring strategy to optimize outcomes.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Pouya Entezami https://orcid.org/0000-0003-0208-049X
References
- 1.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 2.Murchison AG, Young V, Djurdjevic T, et al. Stent placement in patients with acute subarachnoid haemorrhage: when is it justified? Neuroradiology 2018; 60: 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faught RW, Satti SR, Hurst RW, et al. Heterogeneous practice patterns regarding antiplatelet medications for neuroendovascular stenting in the USA: a multicenter survey. J Neurointerv Surg 2014; 6: 774–779. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Salinas P, Agnoletto GJ, Brasiliense LBC, et al. Safety and efficacy of cangrelor in acute stenting for the treatment of cerebrovascular pathology: preliminary experience in a single-center pilot study. J Neurointerv Surg 2019; 11: 347–351. [DOI] [PubMed] [Google Scholar]
- 5.Abdennour L, Sourour N, Drir M, et al. Preliminary experience with cangrelor for endovascular treatment of challenging intracranial aneurysms. Clin Neuroradiol. Epub ahead of print 15 July 2019. DOI: 10.1007/s00062-019-00811-2. [DOI] [PubMed]
- 6.Elhorany M, Lenck S, Degos V, et al. Cangrelor and stenting in acute ischemic stroke: monocentric case series. Clin Neuroradiol. Epub ahead of print 7 May 2020. DOI: 10.1007/s00062-020-00907-0. [DOI] [PubMed]
- 7.Linfante I, Ravipati K, Starosciak AK, et al. Intravenous cangrelor and oral ticagrelor as an alternative to clopidogrel in acute intervention. J Neurointerv Surg. Epub ahead of print 15 May 2020. DOI: 10.1136/neurintsurg-2020-015841. [DOI] [PubMed]
- 8.Wallentin L. P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J 2009; 30: 1964–1977. [DOI] [PubMed] [Google Scholar]
- 9.Akers WS, Oh JJ, Oestreich JH, et al. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol 2010; 50: 27–35. [DOI] [PubMed] [Google Scholar]
- 10.De Luca G, Savonitto S, van’t Hof AW, et al. Platelet GP IIb-IIIa receptor antagonists in primary angioplasty: back to the future. Drugs 2015; 75: 1229–1253. [DOI] [PubMed] [Google Scholar]
- 11.Angiolillo DJ, Rollini F, Storey RF, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation 2017; 136: 1955–1975. [DOI] [PubMed] [Google Scholar]
- 12.VerifyNow P2Y12 platelet reactivity test. Accriva diagnostics, http://www.accriva.com/uploads/literature/vn5004_05_verifynow_user_manual.pdf (accessed 27 February 2020).
- 13.Steinhubl SR, Oh JJ, Oestreich JH, et al. Transitioning patients from cangrelor to clopidogrel: pharmacodynamic evidence of a competitive effect. Thrombosis Res 2008; 121: 527–534. [DOI] [PubMed] [Google Scholar]
- 14.Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 2013; 382: 1981–1992. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 2013; 368: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 16.Moser M, Bertram U, Peter K, et al. Abciximab, eptifibatide, and tirofiban exhibit dose-dependent potencies to dissolve platelet aggregates. J Cardiovasc Pharmacol 2003; 41: 586–592. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Park IS, Lee JM, et al. Stent-assisted coil embolization using only a glycoprotein IIb/IIIa inhibitor (Tirofiban) for ruptured wide-necked aneurysm repair. J Cerebrovasc Endovasc Neurosurg 2018; 20: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speich HE, Bhal V, Houser KH, et al. Signaling via P2Y12 may be critical for early stabilization of platelet aggregates. J Cardiovasc Pharmacol 2014; 63: 520–527. [DOI] [PubMed] [Google Scholar]
- 19.Patel PD, Chalouhi N, Atallah E, et al. Off-label uses of the pipeline embolization device: a review of the literature. Neurosurg Focus 2017; 42: E4. [DOI] [PubMed] [Google Scholar]
- 20.Cagnazzo F, di Carlo DT, Cappucci M, et al. Acutely ruptured intracranial aneurysms treated with flow-diverter stents: a systematic review and meta-analysis. Am J Neuroradiol 2018; 39: 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning NW, Cheung A, Phillips TJ, et al. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J Neurointerv Surg 2019; 11: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkes H, Bhogal P, Aguilar Perez M, et al. Anti-thrombogenic coatings for devices in neurointerventional surgery: case report and review of the literature. Interv Neuroradiol 2019; 25: 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. FDA briefing document for the Cardiovascular and Renal Drugs Advisory Committee. Presented at Silver Spring, MD. See section 3.2.4.1.2, https://wayback.archive-it.org/7993/20170405211438/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM385234.pdf (2014, accessed 27 February 2020).
- 24.Angiolillo DJ, Firstenberg MS, Price MJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA 2012; 307: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowman S, Gass J, Weeks P. Antiplatelet therapy bridging with cangrelor in patients with coronary stents: a case series. Ann Pharmacother 2019; 53: 171–177. [DOI] [PubMed] [Google Scholar]
- 26.Laehn SJ, Feih JT, Saltzberg MT, et al. Pharmacodynamic-guided cangrelor bridge therapy for orthotopic heart transplant. J Cardiothorac Vasc Anesth 2019; 33: 1054–1058. [DOI] [PubMed] [Google Scholar]
- 27.Gupta R, Moore JM, Griessenauer CJ, et al. Assessment of dual-antiplatelet regimen for Pipeline Embolization Device placement: a survey of major academic neurovascular centers in the United States. World Neurosurg 2016; 96: 285–292. [DOI] [PubMed] [Google Scholar]
- 28.Wirtz MM, Schirmer CM, Goren O, et al. Utility of platelet function testing in stent-assisted coiling of cerebral aneurysms. Interv Neuroradiol. Epub ahead of print 21 December 2019. DOI: 10.1177/1591019919894140. [DOI] [PMC free article] [PubMed]
- 29.Kim KS, Fraser JF, Grupke S, et al. Management of antiplatelet therapy in patients undergoing neuroendovascular procedures. J Neurosurg 2018; 129: 890–905. [DOI] [PubMed] [Google Scholar]
- 30.Taylor LI, Dickerson JC, Dambrino RJ, et al. Platelet testing in flow diversion: a review of the evidence. Neurosurg Focus 2017; 42: E5. [DOI] [PubMed] [Google Scholar]
- 31.Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013; 62: 2261–2273. [DOI] [PubMed] [Google Scholar]
- 32.Delgado Almandoz JE, Crandall BM, Scholz JM, et al. Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the Pipeline Embolization Device. J Neurointerv Surg 2013; 5: iii3–iii10. [DOI] [PubMed] [Google Scholar]
- 33.Delgado Almandoz JE, Crandall BM, Scholz JM, et al. Last-recorded P2Y12 reaction units value is strongly associated with thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients with cerebral aneurysms treated with the pipeline embolization device. Am J Neuroradiol 2014; 35: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daou B, Starke RM, Chalouhi N, et al. P2Y12 reaction units: effect on hemorrhagic and thromboembolic complications in patients with cerebral aneurysms treated with the Pipeline Embolization Device. Neurosurgery 2016; 78: 27–33. [DOI] [PubMed] [Google Scholar]
- 35.Ajadi E, Kabir S, Cook A, et al. Predictive value of platelet reactivity unit (PRU) value for thrombotic and hemorrhagic events during flow diversion procedures: a meta-analysis. J Neurointerv Surg 2019; 11: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 36.Skukalek SL, Winkler AM, Kang J, et al. Effect of antiplatelet therapy and platelet function testing on hemorrhagic and thrombotic complications in patients with cerebral aneurysms treated with the pipeline embolization device: a review and meta-analysis. J Neurointerv Surg 2016; 8: 58–65. [DOI] [PubMed] [Google Scholar]
- 37.Adeeb N, Griessenauer CJ, Foreman PM, et al. Use of platelet function testing before pipeline embolization device placement: a multicenter cohort study. Stroke 2017; 48: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 38.Tan LA, Keigher KM, Munich SA, et al. Thromboembolic complications with Pipeline Embolization Device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg 2015; 7: 217–221. [DOI] [PubMed] [Google Scholar]
- 39.Rollini F, Franchi F, Singh K, et al. Impact of timing from blood sampling to pharmacodynamic assessment on measures of platelet reactivity in patients treated with P2Y12 receptor inhibitors. Thromb Haemost 2016; 116: 1060–1069. [DOI] [PubMed] [Google Scholar]
- 40.Choi SY, Kim MH. Comparison of factors affecting platelet reactivity in various platelet function tests. Platelets 2019; 30: 631–636. [DOI] [PubMed] [Google Scholar]