Abstract
Objective
PulseRider is a novel self-expanding nickel-titanium (nitinol) stent for treatment of wide-necked aneurysms, which is commonly located at the arterial branches in the brain. This systematic review and meta-analysis aims to assess the efficacy and safety of PulseRider for treatment of wide-necked intracranial aneurysm.
Method
We performed a systematic literature search on articles that evaluate the efficacy and safety of PulseRider-assisted coiling of the wide-necked aneurysm from several electronic databases. The primary endpoint was adequate occlusion, defined as Raymond-Roy Class I + Raymond-Roy Class II upon immediate angiography and at six-month follow-up.
Results
There were a total of 157 subjects from six studies. The rate of adequate occlusion on immediate angiography was 90% (95% CI, 85%–94%) and 91% (95% CI, 85%–96%) at six-month follow-up. Of these, Raymond-Roy Class I can be observed in 48% (95% CI, 41%–56%) of aneurysms immediately after coiling, and 64% (95% CI, 55%–72%) of aneurysms on six-month follow-up. Raymond-Roy Class II was found in 30% (95% CI, 23%–37%) of aneurysms immediately after coiling, and 25% (17–33) after six-month follow-up. Complications occur in 5% (95% CI, 1%–8%) of the patients. There were three intraoperative aneurysm rupture, three thrombus formation, three procedure-related posterior cerebral artery strokes, one vessel dissection, and one delayed device thrombosis. There was no procedure/device-related death.
Conclusions
PulseRider-assisted coiling for treatment of patients with wide-necked aneurysm reached 90% adequate occlusion rate that rises up to 91% at sixth month with 5% complication rate.
Keywords: Coiling, endovascular, intracranial aneurysm, PulseRider, wide-necked aneurysm
Introduction
Despite recent advances in endovascular technology, treatment of wide-necked aneurysm remains a considerable challenge today.1 Several methods are available to assist wide-necked aneurysm coiling, these include, but not limited to balloon-assisted coiling, double microcatheter, stents deployment using Waffle Cone techniques, T-stenting, and Y-stenting; novel devices such as PulseRider™ (Cerenovus, New Brunswick, NJ, USA), pCANvas™ (Phenox, Bochum, Germany), pCONUS™ (Phenox), Woven EndoBridge™ (Sequent Medical, Aliso Viejo, CA, USA), eClips™ (Evasc Medical, Vancouver, Canada), Comaneci (Rapid Medical, Israel), and honeycomb microporous stent (NCVC-CS1). Y-stents are often used to facilitate coiling procedures; however, it involves multiple steps and requires technical prowess to perform. Each step of the procedure is a possible source of complication.2 The abundance of metal elements and continuous manipulation of blood vessel promotes thromboembolism. Indeed, thromboembolism is the most common complication in a Y-stent-assisted coiling.3 Hence, there is a need to reduce the steps needed, the risk for complications, and at the same time, increases the success rate, especially in complex lesions.
PulseRider™ is a self-expanding stent implant for treatment of wide-necked aneurysms, which are commonly located at the arterial branches in the brain. This device is made of nickel-titanium (nitinol) and has a Y or T shape, which will provide scaffolding for the placement of endovascular coils inside the aneurysmal sac. The scaffolding like-function of this device prevents the protrusion of endovascular coils even in the wide-necked anatomy and thus facilitating thrombus formation.4 This device is also easier to deploy and requires fewer steps compared to Y-stent. Pulserider is a versatile device and can be positioned within the wings of daughter branches, this device also has a lower metal-to-artery ratio, which prevents jailing of daughter branches.5,6 This systematic review and meta-analysis aim to assess the efficacy and safety of PulseRider for treatment of wide-necked intracranial aneurysm.
Materials and methods
Search strategy
We performed a systematic literature search on articles that evaluate the efficacy and safety of PulseRider-assisted coiling of the wide-necked aneurysm with broad search strategy using keyword [“pulserider”] to ensure the broadest result possible. Several electronic databases including PubMed, EuropePMC, ScienceDirect, ProQuest, Clinicaltrials.gov, and hand-sampling from potential articles cited by other studies were used to identify published studies from the beginning of time up until December 2019. The records were then systematically evaluated by applying inclusion and exclusion criteria. Two researchers performed initial search independently, discrepancies that arose during the process were resolved by discussion. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the literature search strategy of studies is presented in Figure 1.
Figure 1.
Study flow diagram.
Inclusion and exclusion criteria
The inclusion criteria for this systematic review and meta-analysis are all original articles/case series that evaluate the efficacy and safety of PulseRider-assisted coiling in wide-necked aneurysm. The exclusion criteria were case reports, case series <5 patients, review articles, and non-English language articles.
Data extraction
Two independent authors performed data extraction and quality assessment using standardized extraction form which comprises of rows and columns for first author, year of publication, study design, sample size, dome/neck ratio, complications, Raymond-Roy Class I (RR1), Raymond-Roy Class II (RR2), Raymond-Roy Class III (RR3), and RR1 + RR2 immediately after coiling and at six-month follow-up. RR1 is defined as a complete obliteration of aneurysm and RR2 as a residual neck. Wide-necked aneurysm in this study was defined as aneurysm with a neck size of ≥4 mm and/or a dome-to-neck ratio of ≤2.
The primary endpoint of this study was adequate occlusion, defined as RR1 + RR2 upon immediate angiography and at six-month follow-up. RR3 is considered as an inadequate occlusion. The secondary endpoint of this study was the rate of complication, RR1, and RR2 upon immediate angiography and at six-month follow-up.
Statistical analysis
STATA 16.0 (StataCorp 2019 LLC) was used to perform meta-analysis. Meta-analysis of proportion was used to pool primary and secondary outcomes of the PulseRider-assisted coiling.
Results
Study selection and characteristics
We found a total of 133 results, in which 62 records remained after duplicates removal. After screening the title and abstracts, 51 records were excluded leaving 11 eligible studies. After the full-texts for eligibility; we excluded five because (1) overlapping cohorts (n = 2), (2) case reports or small case series (n = 3). We included six studies in the qualitative and quantitative syntheses (Figure 1). There were a total of 157 subjects from six studies4,5,7–10 (Table 1). Out of these studies, one was a retrospective cohort study, two were single-arm registries, one was a single-arm clinical trial, and two were case series. All studies used dual antiplatelet therapy for 3–6 months after coiling, followed by single antiplatelet therapy (usually aspirin). The studies have varying dome/neck ratio of 1.2 to 1.6. 3 studies assessed up to six-month follow-up. One study followed the patients for up to 12 months, and the other was up to 24 months.
Table 1.
Studies included in the systematic review.
| Author | Design | Center | Antiplatelets | Dome/Neck ratio | Initial sample size | Complications | Adequate occlusion (immediate) | Adequate occlusion (6 months) | RR 1 (immediate) | RR 1 (6 months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Limbucci et al.8 | Retrospective cohort study | Single-center | Aspirin 300 mg + CPG 75 mg / Ticlopidine 250 mg started 10 days prior until 6 months, then switched into Aspirin 100/150 mg | 1.36 | 32 | Intraoperative thrombus formation (1) | 31/32 (96.9%) | 27/32 (84.3%) | 22/32 (68.8%) | 20/32 (62.5%) |
| Srinivasan et al.4 | Single-arm Prospective Registry | Multicenter | DAPT for 3–6 months, followed by SAPT (usually aspirin 81 mg) | 1.2 | 52 | Procedure-related posterior cerebral artery strokes (3)Device-related intraoperative aneurysm rupture (1) Delayed device thrombosis (1) | 36/52 (69.1%) | 26/28 (92.7%) | 21/52 (40.3%) | 18/28 (64.2%) |
| Sakai et al.10 | Single-arm Registry Study | Single-center | Aspirin 100 mg + CPG 75 mg started 7 days prior until 3 months, followed by SAPT | 1.6 | 8 | None reported | 6/8 (75%) | 7/8 (87.5%) | 1/8 (12.5%) | 6/8 (75%) |
| Spiotta et al.5(ANSWER Trial) | Single-arm clinical trial | Multicenter | DAPT for 6 months, followed by aspirin 325 mg | Unclear | 34 | Intraoperative aneurysm rupture (1) Vessel dissection (1)Thrombus formation (1) | 27/34 (79.4%) | 29/33 (87.8%) | 18/34 (52.9%) | 20/33 (60.6%) |
| Gory et al.7 | Retrospective case series | Multicenter | Aspirin 325 mg and 600 mg a day before procedure or Aspirin 75 mg + CPG 75 mg started 5 days prior or until 3 months, followed by aspirin 75–325 mg | 1.51 | 19 | Intraoperative aneurysm rupture (1) | 17/10 (89.5%) | 18/19 (94.7%) | 11/19 (57.9%) | 12/19 (63.2%) |
| Mukherjee et al.9 | Prospective Case Series | Multicenter | Aspirin 600 mg and 600 mg evening before procedure; Aspirin 75 mg + CPG 75 mg post-procedure until 3 months, followed by aspirin 75 | Not reported | 10 | Asymptomatic thrombus formation (1) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) |
CPG: clopidogrel; DAPT: dual antiplatelet therapy; RR: Raymond-Roy Class; SAPT: single antiplatelet therapy.
The rate of occlusion
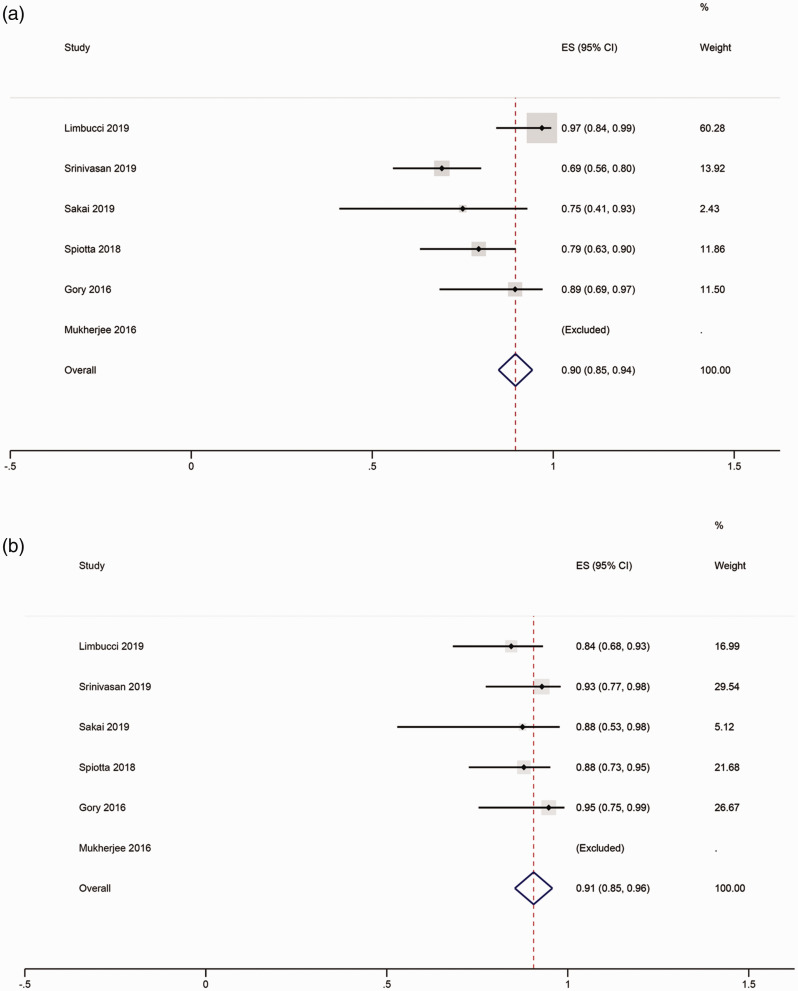
Meta-analysis showed that the rate of adequate occlusion on immediate angiography was 90% (95% CI, 85%–94%; Figure 2(a)) and was 91% (95% CI, 85%–96%; Figure 2(b)) at six-month follow-up. Of these, RR1 can be observed in 48% (95% CI, 41%–56%) of the aneurysms immediately after coiling (Figure 3(a)), and 64% (95% CI, 55%–72%) of the aneurysms on six-month follow-up (Figure 3(b)). RR2 was found in 30% (95% CI, 23%–37%; Figure 3(c)) of aneurysms immediately after coiling, and 25% (95% CI, 17%–33%) of the aneurysms after six-month follow-up (Figure 3(d)). The occlusion rate in Mukherjee et al.’s study cannot be pooled in the meta-analysis due to a 100% RR1 rate.
Figure 2.
PulseRider and adequate occlusion. (a) shows RR1 + RR2 immediately post-coiling and (b) shows a six-month follow-up status. RR: Raymond-Roy Classification.
Figure 3.
PulseRider and RR class 1. (a) shows RR class 1 immediately post-coiling, and (b) shows a six-month follow-up status. (c) shows RR class 2 immediately post-coiling, and (d) shows a six-month follow-up status. RR: Raymond-Roy Classification.
The rate of adequate occlusion and RR1 was greater at six-month follow-up compared to immediately after coiling. Spiotta et al. provided a 12-month follow-up in 34 patients showing a 90% adequate occlusion, slightly higher compared to 89% at six-month follow-up but with less sample size (30 vs. 33). Sakai et al. provided a 24-month follow-up, assessed using magnetic resonance imaging and magnetic resonance angiography, showing no recanalization. Limbucci et al.’s study compared PulseRider-assisted coiling against Y-stenting without PulseRider, both achieved a similar rate of adequate occlusion (94.5% vs. 96.9%), but Y-stenting achieved a higher but not statistically significant occlusion rate at six months (93.1% vs. 84.3%).
Complications
Meta-analysis showed that complications occurred in 5% (95% CI, 1%–8%) of the patients (Figure 4). There were three intraoperative aneurysm rupture, three thrombus formation, three procedure-related posterior cerebral artery strokes, one vessel dissection, and one delayed device thrombosis. There were no deaths caused by the procedure; one death was reported due to metastatic cancer in Spiotta et al.’s study.
Figure 4.
PulseRider and complications. The figure shows the rate of complications. RR: Raymond-Roy Classification.
Characteristics of aneurysm and rate of adequate occlusion
Limbucci et al. study showed that the rate of six months adequate occlusion was lower (n = 2, 40% vs. n = 26, 96.3%) in patients with large aneurysm defined as size ≥10 mm. However, on a study-level meta-analysis of Limbucci et al., Gory et al., and Sakai et al.’s studies, large aneurysm was not associated with adequate occlusion at six months (odds ratio 0.27 (0.06, 1.16), p = 0.08, I2: 56%, p = 0.10, n = 59). A patient-level analysis of 37 patients derived from Sakai et al., Gory et al., and Mukherjee et al.’s studies showed that there was no significant difference between the rate of adequate occlusion on immediate/six months angiography and dome size (p = 0.997, p = 0.701), dome/neck ratio (p = 0.824, p = 0.565), and large aneurysm (p = 1 for both). However, a larger sample size might be needed due to the low rate of inadequate occlusion upon immediate angiography (n = 4, 10.8%) and six-month follow-up (n = 2, 5.4%).
Discussion
This systematic review and meta-analysis of six studies demonstrated that PulseRider-assisted coiling for patients with wide-necked aneurysm has initial adequate occlusion rate of 90% and 91%, on immediate and six months delayed angiography, respectively. Complications occurred in 5% and there was no device-associated mortality.
PulseRider can be positioned proximal to the aneurysm neck, intra-aneurysm, or with only one arch in the aneurysm. After PulseRider deployment, an endovascular coiling catheter can be inserted into the aneurysms to initiate coiling. PulseRider will then be detached after the completion of coiling.10 Compared to reconstruction using X and Y-stent, PulseRider has a lower metal-to-artery ratio along with the highest metal density to cover aneurysm neck and support coil mass, avoiding jailing of daughter branches.9 Furthermore, it is fully retrievable and can be easily repositioned or recaptured.9
Stent-assisted coiling has been shown to increase the success rate compared to conventional coiling.11,12 A study in patients with wide-necked aneurysm showed that total occlusion (RR1) was achieved in 46.7% of balloon group, 75% of the double microcatheter group, and 78.6% of the stent group.13 Stent-assisted coiling in wide-necked aneurysm has also been shown to be superior compared to balloon-assisted coiling in a study of 101 patients (75.4% vs. 50%).14 These results indicate the superiority of the stent-assisted coiling compared to balloon-assisted coiling. T-stent-assisted coiling of wide-necked aneurysm showed 83.3% complete occlusion which increases to 90% in 30-month follow-up along with 13.7% periprocedural complication rate in a multicenter study of 102 patients.15 In a multicenter case series of 45 patients evaluating Y-stenting, it was shown that the initial adequate occlusion rate was 84% and rose to 92% in 9.8-month follow-up.16 The efficacy is similar to PulseRider-assisted coiling; however, T-stenting and Y-stenting were associated with periprocedural complications in 13.7% and 11% of the patients, respectively, compared to 5% in patients undergoing PulseRider-assisted coiling. The technical requirement for PulseRider is less demanding compared to Y-stenting, the device allow fewer steps and potentially reduces maneuver-related complications.10 A previous meta-analysis on Neuroform Atlas (Stryker Neurovascular, Fremont, CA, USA) assisted coiling in wide-neck aneurysms showed 88% RR1 + RR2 rate on six-month follow-up and 6% periprocedural complication rate.17 A multicenter study evaluated the use of LVIS Jr stent (MicroVention-Terumo, Inc., Tustin, CA, USA) for Y-stent-assisted coiling embolization and found that the adequate occlusion rate was 89.6%. However, the rate of periprocedural complications was alarming (5/29, 17.2%), in which one patient suffered a permanent neurological disability. The Barrel vascular reconstruction device (Medtronic/Covidien, Irvine, CA, USA) achieved 78.9% RR1 + RR2 out of 19 patients; periprocedural stroke occurred in a patient.18 Another device that can be used to facilitate treatment of wide-necked intracranial aneurysm is the Woven EndoBridge device, a meta-analysis showed an initial adequate occlusion rate of 77% and 80% in the short term.19 The aforementioned device has a concerning rate of mortality, reaching that of 7%, albeit 28.6% of the patients had ruptured aneurysms. There was no procedure/device-related mortality in PulseRider-assisted coiling, although it was based on a limited number of patients and short follow-up. PulseRider was also shown to be potentially useful for treating a ruptured wide-necked basilar aneurysm, as shown in a small case series.20 One of the drawbacks of the PulseRider device is the lack of flow-diverting properties observed in braided, X, or Y-stents.9pCANvas devices have 31% RR1 + RR2 at a median of 6.1 months with 0% periprocedural complications rate in a series of 17 patients.21 pCONUS devices have 60% complete occlusion rate at 9.9 ± 3.8 months follow-up with 7% periprocedural complication rate in a meta-analysis.22 Our meta-analysis only provided data at six-month follow-up which showed 64% complete occlusion rate along with 5% perioperative complications, a longer term follow-up is necessary to compare the efficacy. The hydrophilic polymer coating (pHPC, Phenox) is a glycan-based biopolymer that inhibits fibrinogen and von Willebrand factor to the stent surface, thus inhibiting platelet adhesion.23 In vitro studies have shown that pHPC coating reduces thrombogenicity of endovascular device24,25 and this surface modification may prevent the need for dual antiplatelet therapy.26 A study on pCONUS HPC device in an acutely ruptured aneurysm showed 66.6% initial RR1 + RR2 and 45.5% at a median of five-month follow-up.27 Patients in the study received aspirin before and after the procedure which was then followed by a lifetime 100 mg aspirin.27 There was intraprocedural thrombus formation requiring further treatment in 20% of the patients;27 however, there was no periprocedural or postprocedural aneurysm re-rupture. A study on eClips devices demonstrated 81% modified RR1 + RR2 at an average of eight-month follow-up along with 12% periprocedural complications (2 transient ischemic attacks and 2 asymptomatic thrombotic events).28 A case series on Comaneci devices showed that complete occlusion was achieved in 28 out of 29 patients in the third-month follow-up. A trial assessing the use of honeycomb microporous covered stent (NCT02907229) is currently underway.29
Limbucci et al.’s study demonstrated that the large aneurysms were associated with a higher risk of inadequate occlusion;8 however, the conclusion was based on small sample size. We found no significant difference in a study-level meta-analysis, a larger prospective study is required for a definite conclusion. On a study-level meta-analysis, the dome-to-neck ratio did not statistically differ in those with adequate occlusion compared to inadequate occlusion, similar to the finding in Limbucci et al.’s study.
The limitation of this systematic review and meta-analysis is the small sample size, consequently, the analysis on anatomical features lacked statistical power. At the time this article was written, randomized controlled trials that provide direct comparison between PulseRider and other devices for wide-neck base aneurysm is unavailable. The studies were also short-term, most of them reported up until six-month period. A longer follow-up is necessary to evaluate long-term safety and efficacy.
Conclusion
PulseRider-assisted coiling for treatment of wide-necked intracranial aneurysm has initial adequate occlusion rate of 90%, increasing to 91% upon six-month follow-up. Complications occurred in 5% of the patients and there was no procedure/device-associated mortality. However, further prospective studies are needed to assess the long-term efficacy and safety compared to other devices.
Footnotes
Ethical statement: This study did not require ethical clearance because it did not involve human or animal subjects directly.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Raymond Pranata https://orcid.org/0000-0003-3998-6551
References
- 1.Fiorella D, Arthur AS, Chiacchierini R, et al. How safe and effective are existing treatments for wide-necked bifurcation aneurysms? Literature-based objective performance criteria for safety and effectiveness. J Neurointerv Surg 2017; 9: 1197–1201. [DOI] [PubMed] [Google Scholar]
- 2.Spiotta AM, Lena J, Chaudry MI, et al. Y-stenting for bifurcation aneurysm coil embolization: what is the risk? Stroke Res Treat 2014; 2014: 762389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cagnazzo F, Limbucci N, Nappini S, et al. Y-stent-assisted coiling of wide-neck bifurcation intracranial aneurysms: a meta-analysis. Am J Neuroradiol 2019; 40: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan VM, Srivatsan A, Spiotta AM, et al. Early postmarket results with PulseRider for treatment of wide-necked intracranial aneurysms: a multicenter experience. J Neurosurg. Epub ahead of print 8 November 2019. DOI: 10.3171/2019.5.JNS1931. [DOI] [PubMed] [Google Scholar]
- 5.Spiotta AM, Chaudry MI, Turner RD, et al. An update on the adjunctive neurovascular support of Wide-Neck Aneurysm Embolization and Reconstruction Trial: 1-year safety and angiographic results. Am J Neuroradiol 2018; 39: 848–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPheeters MJ, Vakharia K, Munich SA, et al. Wide-necked cerebral artery aneurysms: where do we stand? Endovasc Today 2019; 70–79. [Google Scholar]
- 7.Gory B, Spiotta AM, Di Paola F, et al. PulseRider for treatment of wide-neck bifurcation intracranial aneurysms: 6-month results. World Neurosurg 2017; 99: 605–609. [DOI] [PubMed] [Google Scholar]
- 8.Limbucci N, Cirelli C, Valente I, et al. Y-stenting versus PulseRider-assisted coiling in the treatment of wide-neck bifurcation aneurysms: role of anatomical features on midterm results. Neurosurgery. Epub ahaed of print 3 December 2019. DOI: 10.1093/neuros/nyz490. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee S, Chandran A, Gopinathan A, et al. PulseRider-assisted treatment of wide-necked intracranial bifurcation aneurysms: safety and feasibility study. J Neurosurg 2017; 127: 61–68. [DOI] [PubMed] [Google Scholar]
- 10.Sakai N, Imamura H, Arimura K, et al. PulseRider-assisted coil embolization for treatment of intracranial bifurcation aneurysms: a single-center case series with 24-month follow-up. World Neurosurg 2019; 128: e461–e467. [DOI] [PubMed] [Google Scholar]
- 11.Chalouhi N, Tjoumakaris S, Gonzalez LF, et al. Coiling of large and giant aneurysms: complications and long-term results of 334 cases. Am J Neuroradiol 2014; 35: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascitelli JR, Oermann EK, Mocco J, et al. Predictors of success following endovascular retreatment of intracranial aneurysms. Interv Neuroradiol 2015; 21: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J, Xiao F, Szeder V, et al. Stent, balloon-assisted coiling and double microcatheter for treating wide-neck aneurysms in anterior cerebral circulation. Neurol Res 2013; 35: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 14.Chalouhi N, Starke RM, Koltz MT, et al. Stent-assisted coiling versus balloon remodeling of wide-neck aneurysms: comparison of angiographic outcomes. Am J Neuroradiol 2013; 34: 1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aydin K, Stracke CP, Barburoglu M, et al. Long-term outcomes of wide-necked intracranial bifurcation aneurysms treated with T-stent–assisted coiling. J Neurosurg. Epub ahead of print 6 December 2019. DOI: 10.3171/2019.9.JNS191733. [DOI] [PubMed] [Google Scholar]
- 16.Fargen KM, Mocco J, Neal D, et al. A multicenter study of stent-assisted coiling of cerebral aneurysms with a Y configuration. Neurosurgery 2013; 73: 466–472. [DOI] [PubMed] [Google Scholar]
- 17.Pranata R, Yonas E, Deka H, et al. Stent-assisted coiling of intracranial aneurysms using a nitinol-based stent (Neuroform Atlas): a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2020; 43(7): 1049–1061. [DOI] [PubMed]
- 18.Gory B, Blanc R, Turjman F, et al. The Barrel vascular reconstruction device for endovascular coiling of wide-necked intracranial aneurysms: a multicenter, prospective, post-marketing study. J Neurointerv Surg 2018; 10: 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S-M, Liu L-X, Ren P-W, et al. Effectiveness, safety and risk factors of woven endobridge device in the treatment of wide-neck intracranial aneurysms: systematic review and meta-analysis. World Neurosurg 2020; 136: e1–e23. [DOI] [PubMed]
- 20.Folzenlogen Z, Seinfeld J, Kubes S, et al. Use of the PulseRider device in the treatment of ruptured intracranial aneurysms: a case series. World Neurosurg 2019; 127: e149–e154. [DOI] [PubMed] [Google Scholar]
- 21.Lylyk P, Chudyk J, Bleise C, et al. Treatment of wide-necked bifurcation aneurysms. Clin Neuroradiol 2019; 29: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorenson T, Iacobucci M, Murad M, et al. The pCONUS bifurcation aneurysm implants for endovascular treatment of adults with intracranial aneurysms: a systematic review and meta-analysis. Surg Neurol Int 2019; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henkes H, Bhogal P, Aguilar Pérez M, et al. Anti-thrombogenic coatings for devices in neurointerventional surgery: case report and review of the literature. Interv Neuroradiol 2019; 25: 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenz-Habijan T, Bhogal P, Peters M, et al. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: initial results in vitro. Cardiovasc Intervent Radiol 2018; 41: 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenz-Habijan T, Brodde M, Kehrel BE, et al. Comparison of the thrombogenicity of a bare and antithrombogenic coated flow diverter in an in vitro flow model. Cardiovasc Intervent Radiol 2020; 43: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhogal P, Lenz-Habijan T, Bannewitz C, et al. The pCONUS HPC: 30-day and 180-day in vivo biocompatibility results. Cardiovasc Intervent Radiol 2019; 42(7): 1008–1015. [DOI] [PMC free article] [PubMed]
- 27.Aguilar Perez M, AlMatter M, Hellstern V, et al. Use of the pCONus HPC as an adjunct to coil occlusion of acutely ruptured aneurysms: early clinical experience using single antiplatelet therapy. J Neurointerv Surg. Epub ahead of print 2020. DOI: 10.1136/neurintsurg-2019-0157 [DOI] [PMC free article] [PubMed]
- 28.Chiu AH, De Vries J, O’Kelly CJ, et al. The second-generation eCLIPs Endovascular Clip System: initial experience. J Neurosurg 2018; 128: 482–489. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto H, Hamasaki T, Onda K, et al. Evaluating the safety and technical effectiveness of a newly developed intravascular ’flow isolator’ stent for the treatment of intracranial aneurysms: study protocol for a first-in-human single-arm multiple-site clinical trial in Japan. BMJ Open 2019; 9: e020966. [DOI] [PMC free article] [PubMed] [Google Scholar]