Abstract
Background
The NeVa™ thrombectomy device (Vesalio LLC, Nashville, USA) has been reported to succeed in large vessel occlusion thrombectomy in animal, in-vitro, and clinical studies. Designed with Drop Zone technology, a closed distal tip, and strong expansive radial force, the device demonstrated particular efficiency in resistant “white” thrombi in preclinical research. Our goal is to determine the safety and performance of this novel stent retriever on first-pass rates and overall recanalization.
Methods
The Interventional Neurology Database is a prospectively maintained database of anterior and posterior circulation stroke thrombectomy cases. We retrospectively analyzed cases where the NeVa™ thrombectomy device was used as the first-line treatment strategy. Data collection occurred between January 2019 and January 2020. First-pass recanalization, final recanalization, 90-day functional outcome, complication, and bleeding rates are reported.
Results
One hundred eighteen patients were treated with the NeVa™ thrombectomy device. The mean patient age was 69 ± 14 years, the median baseline National Institutes of Health Stroke Scale was 14, and the median initial Alberta Stroke Program Early Computed Tomography score was 8. The median time from groin puncture to successful recanalization was 29 min (interquartile range (IQR): 20–40). First-pass recanalization rates were 56.8% (modified treatment in cerebral infarction (mTICI) 2b/3) and 44.9% (mTICI 2c/3). Final successful recanalization rate was 95.8% (thrombolysis in cerebral infarction 2b/3). Favorable functional outcome (modified Rankin Scale 0–2) was 53% in the “first-pass” subgroup and 42.4% in the total patient population. The median number of passes to achieve the final recanalization score was 1 (IQR 1–2). The rate of embolization into new territory was 1.7%. Four patients (3.3%) had symptomatic hemorrhage.
Conclusions
In our experience, the NeVa™ device demonstrated high first-pass and overall recanalization rates along with a good safety profile.
Keywords: Acute stroke, excellent outcome, first-pass recanalization, NeVa™ device, thrombectomy device
Introduction
The efficacy of stent-retriever thrombectomy in acute ischemic stroke patients with large vessel occlusions (LVOs) has been proven in both prospective and retrospective trials and is currently accepted as standard of care for selected patients.1–4 Technological advancements in device design and improved thrombectomy techniques have allowed recanalization rates up to 92.5%.5,6 Several recent analyses have shown fast and complete recanalization of the occluded vessel in the first pass to be extremely important for good functional outcome.7–9 Although different stent retrievers have a relatively similar mechanism of action, design differences affect the achievement of recanalization success.5 Despite the high rates of recanalization documented, there are still incidents where stent retrievers fail. Vessel tortuosity and white clots can be counted among the circumstances that render successful recanalization impossible.10,11 The NeVa™ thrombectomy device, designed with Drop Zone technology, a closed distal tip, and strong radial force, has been shown to succeed in these resistant cases in animal and in-vitro models.12,13 Our goal in this study is to share the clinical success and safety features of the NeVa™ device observed during our initial experience.
Methods
Prospectively collected data from the Interventional Neurology Database allowed us to identify two stroke centers where the NeVa™ device was used regularly between January 2019 and January 2020. We retrospectively analyzed 118 consecutive, LVO stroke patients where NeVa was employed as the first-line thrombectomy device in these centers. Patient demographic data, cardiovascular risk factors, procedural details (first pass and final Thrombolysis in Cerebral Infarction (TICI) reperfusion scores, number of passes to achieve recanalization, and complications) were recorded.
The study protocol was approved by the institutional review board or ethics committee at each participating site. Written patient consent was provided by every patient or a legal representative before enrollment.
Imaging and patient selection
Acute LVO stroke patients between 18 and 90 years of age were included in the study. LVO was confirmed with noncontrast computed tomography (CT) and contrast-enhanced neck–brain CT angiography. Patients with intracranial hemorrhage on baseline imaging were excluded from the analysis. The decision for endovascular treatment was based on CTP with RAPID (iSchema View, Menlo Park, CA, USA) software.
We defined inclusion criteria as baseline National Institutes of Health Stroke Scale (NIHSS) score of 6–25, pre-stroke modified Rankin Scale (mRS) of 0–1, Alberta Stroke Program Early Computed Tomography (ASPECT) score above 5, and angiographically proven occlusion in the internal carotid artery, middle cerebral artery (M1 or M2 segments), posterior cerebral artery, or basilar artery.
One hundred sixteen of 118 identified eligible patients were treated with mechanical thrombectomy within 8 h of symptom onset. Patients who arrived within 4.5 h were administered IV tissue-type plasminogen activator (tPA) (0.6 mg/kg of body weight) before being transferred to the angio suite. Patients with contraindication to IV rtPA underwent endovascular treatment only.
Clinical assessment
Successful recanalization was defined as modified thrombolysis in cerebral infarction (mTICI) 2b-3 and excellent recanalization as mTICI 2c-3. The primary outcome measure we examined was first-pass success without recourse to rescue therapy. Successful and excellent recanalization within two or three NeVa passes was also included in the analysis. Good functional outcome (measured by 90-day mRS score) and complication rates (intracerebral hemorrhage, distal embolization to previously unaffected territory, vessel perforation, dissection, and vasospasm) were recorded. Post-procedure CT was utilized to assess hemorrhagic transformations (HI1, HI2, PH1, PH2), which were categorized as symptomatic if they were associated with a worsening of the NIHSS score by more than 4 points, as per ECASS-3 criteria.14
Device
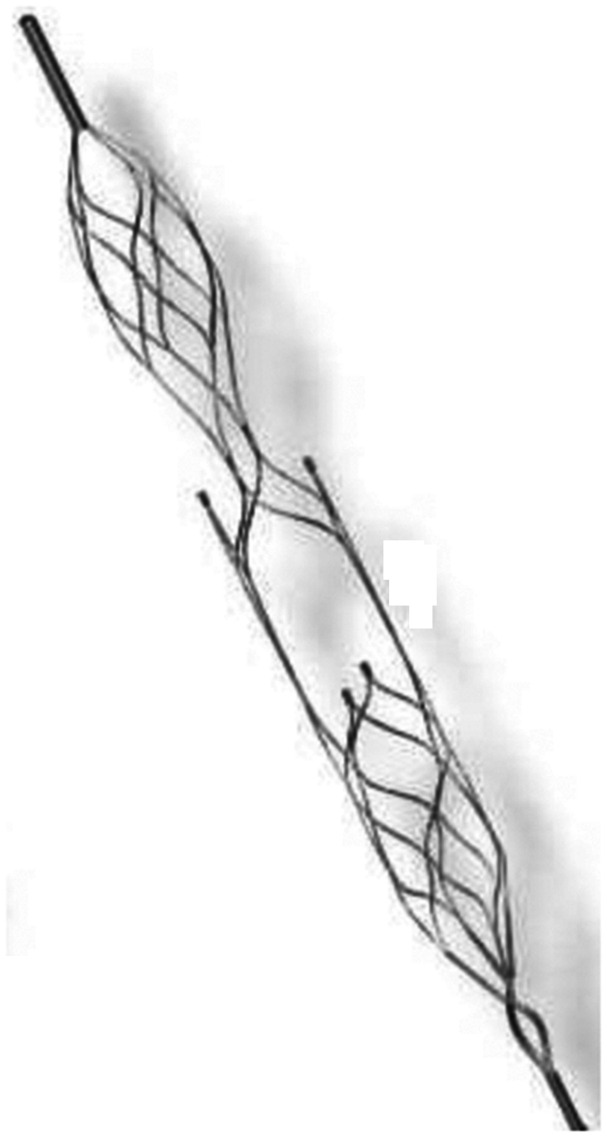
The NeVa™ thrombectomy device is available in multiple sizes to adapt to different anatomical and procedural challenges. Our experience to this point has been accumulated using the three initially commercialized sizes: M1-S (4 × 22 mm), M1 (4 × 30 mm) (Figure 1), and T (4.5 × 37 mm). The final segment is a closed-ended basket, which retains thrombus that has entered the enlarged “Drop Zone” openings. The NeVa™ M1-S differs from the M1 and T sizes in that there is no proximal “flow restoration zone.” These sizes are compatible with microcatheters with a minimum internal diameter of 0.021 in. In our series, the Headway 27 catheter (MicroVention, Valencia, USA) was routinely used with all sizes.
Figure 1.
The Neva™ M1 (4 × 30 mm) which has an identical design with T (4.5 x 37).
The NeVa™ stent scaffolding includes three distinct parts going from the proximal to the distal section of the basket. The initial segment is a closed-cell stent structure which compresses thrombus and provides immediate flow restoration (flow restoration zone). The second segment consists of two large, open areas with minimal metal coverage, named “Drop Zones.” These are offset at 90° to each other and provide entry points for hard, white, fibrin-rich thrombi that are resistant to penetration by typical stent scaffolding (Figure 2(a) and (b)).13
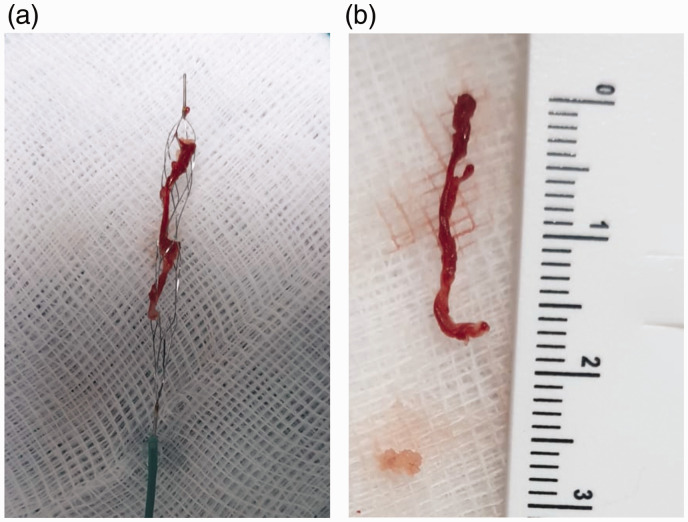
Figure 2.
(a) The NeVa™ M1 (4 × 30 mm) with the recovered thrombus. (b) A long clot retrieved with the NeVa™ M1.
The NeVa™ device is deployed with the proximal marker (device-pusher wire junction) at the leading edge of the occlusion. In general, the M1 or M1-S is utilized for M1 and M2 Middle cerebral artery (MCA) occlusions while the T is utilized for Internal carotid artery (ICA) and proximal M1 occlusions. After deployment, we typically left the device in place for up to few seconds and then slowly retrieved, either into a distal access catheter (DAC) or into a proximal balloon guide catheter (BGC). In our study group, the choice of proximal flow control strategy (DAC vs BGC vs both) was left up to the discretion of the interventionalist.
Thrombectomy technique
The procedure was carried out under general or local anesthesia depending on the patient’s medical condition.
In the anterior circulation occlusions, either combined aspiration or proximal flow arrest strategies were used for access and flow control. In some cases, NeVa™ thrombectomy device was used alone under proximal flow arrest with the 9 French Merci BGC (Stryker Neurovascular, California, USA). Whenever the Neurosheath (Neuron 088 Max (Penumbra, California, USA) or Terumo Destination (Terumo, Japan)) could be advanced into the ICA or the Common carotid artery (CCA), combined aspiration was performed with the 6 Fr large-bore catheter and the NeVa thrombectomy device (Solumbra technique). For posterior circulation occlusions, a combined aspiration and stent retriever technique was performed navigating a 6 French Neurosheath through the dominant vertebral artery.
During the procedure, up to three passes were allowed with the NeVa™ thrombectomy device. If successful or excellent recanalization could not be achieved after the third attempt, rescue therapy was permitted. Rescue therapy was defined as the use of another technique (ADAPT,15 ADVANCE16), of a different device (Trevo, EmboTrap, Solitaire, etc.), or permanent stenting.
Results
One hundred eighteen consecutive patients—58 men (49.2%) and 60 women (50.8%)—were treated with NeVa as the first-line thrombectomy device from January 2019 to January 2020. The mean age was 69 (±14), and the median baseline NIHSS was 14 (interquartile range (IQR): 12–18). The median initial ASPECT score was 8 (IQR: 7–9). Fifty-six of the 118 patients (47.4%) received IV tPA before endovascular treatment. We identified tandem ICA–MCA occlusion in 10 (8.5%) patients, carotid T occlusion in 22 (18.6%) patients, M1 occlusion in 44 (37.3%) patients, M2 occlusion in 32 (26.3%) patients, basilar occlusion in 7 (5.9%) patients, anterior cerebral artery A1 occlusion in 2 patients (1.7%), and posterior cerebral artery P1 occlusion in 1 patient (0.8%). Patient baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of patients.
| Characteristics (n = 118) | n (%) |
|---|---|
| Mean age | 69 (+14) |
| Gender (Female) | 60 (50.8) |
| NIHSS score on admission, median (IQR) | 14 (12–18) |
| Vascular risk factors, n (%) | |
| Hypertension | 102 (86) |
| Diabetes mellitus | 23 (19.5) |
| Atrial fibrillation | 38 (32) |
| Coronary artery disease | 13 (11) |
| Current smoking | 28 (24) |
| Stroke etiology, n (%) | |
| Cardioembolism | 68 (57.6) |
| Large artery atherosclerotic disease | 8 (6.8) |
| Carotid dissection | 2 (1.7) |
| Unknown etiology | 40 (33.9) |
| Intravenous rtPA, n (%) | 56 (47.4) |
| CT ASPECT score, median (IQR) | 8 (7–9) |
| Occlusion site, n (%) | |
| Carotid T | 22 (18.6) |
| MCA/ICA tandem | 10 (8.5) |
| M1 middle cerebral artery | 44 (37.3) |
| M2 middle cerebral artery | 32 (27.1) |
| Anterior cerebral artery A1 | 2 (1.7) |
| Basilar artery | 7 (5.9) |
| Posterior cerebral artery P1 | 1 (0.8) |
Values are mean (SD), median (IQR), or n (%) as appropriate.
ASPECT: Alberta Stroke Program Early CT Score for MCA territory stroke; CT: computed tomography; IQR: interquartile range; NIHSS: National Institutes of Health Stroke Scale.
The median time from symptom onset or last known well to groin puncture was 222.5 min (IQR: 160–289). The median time from groin puncture to successful recanalization was 29 min (IQR: 20–40).
Successful first-pass recanalization (mTICI 2b/3) was achieved in 56.8% (67/118) and excellent first-pass recanalization (mTICI 2c/3) was achieved in 44.9% (53/118). The rate of excellent recanalization (mTICI 2c/3) within two NeVa passes was 60.2% (71/118) and within three NeVa passes was 71.2% (84/116). Successful final recanalization (mTICI 2b/3) was achieved in 95.8% (113/118) of patients. Of these, 38.1% (45/118) had an mTICI score of 3 (complete reperfusion), 34.7% (41/118) had an mTICI score of 2c, and 22.9% (27/118) had an mTICI score of 2b. The median number of thrombectomy passes necessary for final reperfusion was 1 (IQR 1–2). Reperfusion scores are summarized in Table 2.
Table 2.
Details of endovascular treatment.
| Procedural Characteristics | |
|---|---|
| Onset to groin puncture (min) | 205 (180–251) |
| Onset to recanalization (min) | 270 (240–340) |
| Balloon guide catheter, n (%) | 16 (13.6) |
| Solumbra technique, n (%) | 109 (92.4) |
| Reperfusion outcome, n (%) | |
| Successful final recanalization (mTICI 2b–3) | 113 (95.8%) |
| Excellent final recanalization (mTICI 2c–3) | 86 (72.9%) |
| First-pass mTICI 2b–3 recanalization | 67 (56.8%) |
| First-pass mTICI 2c–3 recanalization | 53 (44.9%) |
| mTICI 2c–3 recanalization within two passes | 71 (60.2%) |
| mTICI 2c–3 recanalization within three passes | 84 (71.2%) |
| Rescue treatment | 9 (7.6%) |
| Number of passes, median (IQR) | 1 (1–2) |
Values are mean (SD), median (IQR), or n (%) as appropriate.
IQR: interquartile range; mTICI: modified treatment in cerebral infarction; SD: standard deviation.
The combined aspiration–stent retriever technique was utilized in 92.4% (109/118) of patients. A BGC was used in 16 of 118 cases (13.6%). Rescue therapy was required in only nine patients (7.6%), none of whom required intracranial or extracranial stenting (Table 2).
Complications
The rate of embolization into new territory was 1.7% (2/118). Four patients (3.3%) had symptomatic hemorrhage (NIHSS >4) with type 2 parenchymal hematoma and seven patients (5.9%) had asymptomatic type 1 parenchymal hematoma. Four patients (3.4%) experienced asymptomatic petechial type 1 hemorrhage, and five patients (4.2%) had asymptomatic type 2 petechial type 2 hemorrhage (4.2%). Follow-up CT revealed an asymptomatic sulcal subarachnoid hemorrhage that did not require further treatment in two cases (1.7%). There were two instances of extracranial internal carotid artery dissection due to a procedure that did not require stenting (Table 3).
Table 3.
Endovascular complications and clinical outcome.
| Radiological and clinical outcomes | |
|---|---|
| Embolization to new territory, n (%) | 2 (1.7%) |
| Intracranial hemorrhage, n (%) | |
| Hemorrhagic infarction 1 | 4 (3.4) |
| Hemorrhagic infarction 2 | 5 (4.2) |
| Parenchymal hematoma 1 | 7 (5.9) |
| Parenchymal hematoma 2 | 4 (3.3) |
| Subarachnoid hemorrhage | 2 (1.7) |
| Procedure-related dissection, n (%) | 2 (1.7) |
| Outcome, n (%) | |
| Modified Rankin Scale 0–2 | 50 (42.4) |
| Modified Rankin Scale 3–6 | 68 (57.6) |
| All-cause mortality at 90 days | 17 (14.4) |
Values are mean (SD), median (IQR), or n (%) as appropriate.
IQR: interquartile range; SD: standard deviation.
Clinical outcome
Good functional outcome (mRS 0–2) was 52.2% (35/67) in the “first-pass” (mTICI 2b/3) sub-group and 42.4% in the total patient (50/118) population. All-cause mortality was 14.4% (17/118). (Detailed clinical results can be found in Table 3.)
Discussion
Our retrospective analysis of 118 patients treated with NeVa™ as the first-line device in our stroke centers constitutes the largest patient series reported to date with this device. We achieved successful recanalization (mTICI ≥ 2b) in 95.8% and first-pass success (mTICI ≥ 2b) in 56.8%. Fifty of 118 patients (42.4%) had functional independence (mRS ≤ 2) at 3 months. Our analysis demonstrates that the NeVa™ thrombectomy device performs rapidly and safely without major complications.
Conventional stent retrievers have identical working mechanisms.17–21 They work by opening within the thrombus and by tearing it away from the artery wall. Clot incorporation within the stent is brought about by the administration of expansive radial force to the artery wall. For first-pass removal, the diameter and length of the stent retriever need to be significant enough to cover the full length of the clot.
Radial force and the cell size gain further importance when dealing with organized, white clots. When radial force is not sufficiently high and cell size not sufficiently large, integration of clot within the stent structure becomes impossible and clot remains stuck between the stent basket and the artery wall.11,22,23
The NeVa™ device has relatively elevated expansive radial force. Its Drop Zones could be fostering the extraction of thrombi that adhere firmly to the artery wall. These features could be favoring the efficiency of this particular design in removing all clot types.
Experimental models have endorsed this hypothesis and established NeVa™ to be more efficient than other retrievers.13,24 Furthermore, both pre-clinical and recent clinical evaluations reported no significant adverse effects caused by the device. Pre-clinical animal studies reported artery wall abrasion due to NeVa™ use to be comparable to other stent retrievers.12,13,25
The first published clinical study reporting on 30 consecutive patients treated with the NeVa™ device recorded first-pass recanalization rates of 63% (mTICI ≥ 2b) and 47% (mTICI ≥ 2c), a successful final recanalization rate of 93%, and a favorable outcome of 53% (mRS ≥ 2). The same publication documented an asymptomatic intracranial hemorrhage rate of 23%, no symptomatic ICH, no device-related adverse events, and an all-cause mortality rate of 17%.25
Advances in stent retriever design and thrombectomy technique contributed to the improvement of first-pass and final recanalization rates (Table 4). Nevertheless, these remain below 50% and 95%, respectively.5,6,20,26 A recent study reported a first-pass recanalization rate of 61%.9 Therefore, rescue techniques and multiple passes remain a necessity. Repeated attempts delay time-to-recanalization and cause damage to the arterial wall, resulting in poor clinical outcomes,9,27 while full or near-full recanalization in the first pass has been demonstrated to correlate with favorable clinical outcomes.1,9 Some studies have shown repeated thrombectomy attempts to be correlated with bad clinical outcomes but have been unable to prove the effect to be statistically significant.28,29 Additionally, repeated thrombectomy attempts have been reported to be associated with intracranial hemorrhage.30
Table 4.
Characteristics of reperfusion therapy studies.
| First-pass mTICI 2b | First-pass mTICI 2c–3 or 3 | First-pass mTICI 2b–3 | First-pass mRS 0–2 | OverallmTICI 2b, 2c, 3 | OverallmRS 0–2 | Overall sICH | Device-related adverse events | Procedure-related adverse events | Overall mortality | EDT | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| COMPASS (stent-retriever group n = 136)36 | NA | NA | 51% | 52% | 89% | 50% | 6% | 0 | 14% | 16% | 2% |
| García-Tornel et al. (n = 457)29 | 14.3% | 24.9% | % | 58.6% | 84.5% | 42.4% | 4.9% | NA | 1.8% | 13.2% | NA |
| Ribo et al. (n = 30)25 | 16.6% | 47% | 63% | NA | 93% | 53% | 0 | 0 | 10% | 17% | NA |
| Sakai et al. (n = 5)37 | 0 | 45.5% | 0 | 80% | 100% | 72.7% | 0 | 0 | 0 | 9% | NA |
| Jindal et al. (n = 205)32 | 7.3% | 17% | % | 54% | 78% | 46% | NA | NA | NA | 19% | NA |
| Bourcier et al. (n = 80)30 | 6.3% | 42.5% | 48.4% | 72% | 90% | 62.8% | 6.3% | 0 | 3.8% | ||
| Ernst et al. (n = 101)38 | NA | NA | 63.4% | NA | 73.3% | 32.7% | 1% | 0 | 2% | 16.8% | NA |
| Zaidat et al. (n = 354)39 | NA | 63.1% | % | 61.3% | 72.3% | 42% | 9.9% | 0 | 30% | 5.5% | |
| Garcia-Tornel et al. (stent-retriever group n = 378)40 | NA | 66% | % | 57.5% | 83.6% | 57.4% | 4.7% | NA | 3.9% | 14% | NA |
| Zaidat et al. (n = 227)9 | 11.4% | 40% | 51.5% | NA | 92.5% | 67.3% | 5.3% | 0 | 4.8% | 9% | NA |
| Current study (n = 118) | 11.8% | 45% | 56% | 53% | 95.8% | 42.4% | 3.3% | 0 | 3.4% | 14.4% | 1.7% |
EDT: emboli of different territory; mRS: modified Rankin Scale; mTICI: modified treatment in cerebral infarction; sICH: symptomatic intracranial hemorrhage.
A study evaluating first-pass rates as a function of thrombectomy technique revealed rates of 14% for stent retriever, 17% for aspiration, and 26% for combined techniques.31 Another study by Garcia-Tornel et al. noted a 90-day functional independence (mRS ≥ 2) rate of 57.7% for a first-pass recanalization rate of 39%.29 Two recent studies have shown 90-day functional independence (mRS ≥ 2) rates of 61% and 64% for first-pass recanalization rates of 22% and 25%.9,32 Zaidat et al. have shown 90-day functional independence (mRS ≥ 2), sICH, mortality, distal embolization, and embolization into new territory rates respectively as 61.3%, 5.6%, 16.3%, 5.7%, and 2.3% in the patient group that had first-pass recanalization versus 42%, 9.9%, 30%, 16.4%, and 5.5% in the total patient population.9
Previous studies reported about procedure-related complications of 10% with stent retrievers26 and up to 14% in difficult anatomy.33,34 The ASTER study reported 3-month mortality to be 19.2% in the stent retriever subgroup and in roughly half of them intracranial hemorrhage was observed (HI1: 13%, HI2: 13.6%, PH1: 10.3%, PH2: 7.6%). Symptomatic intracranial hemorrhage rate was 6.5%, while 15.9% of patients had procedure-related adverse events such as vasospasm (6.4%), perforation (1.6%), and dissection (1.1%). Embolization in a new vascular territory was reported as 2.7% and in a different vascular territory as 8.5%.35 The COMPASS study reports results similar to the ASTER study.36 In our study, 4 patients (3.3%) had symptomatic hemorrhage (NIHSS score increase >4 points) with type 2 parenchymal hematoma and 16 patients (13.6%) had asymptomatic hemorrhage. Our hemorrhage rates seem slightly better than previous reports.
Another important point is that we did not need to wait after deploying the NeVa™ device in the clot. This resulted in reduced time to recanalization. In our experience, when compared with former stent designs, this novel design with Drop Zone technology and high radial force serves for better clot integration and thrombus capture.
The main limitation of our study is its non-randomized design and thus lack of a control group. Furthermore, we did not perform histological analysis of retrieved clots hence cannot provide a comparative evaluation of retrieval capabilities per clot type.
Conclusion
The high first-pass and final recanalization rates noted in our series allow us to conclude that the NeVa™ thrombectomy device can be used as the first-line choice in LVO stroke thrombectomy. It could also serve as a rescue option in resistant thrombectomy procedures. The high reperfusion rates could be due to the particular design of NeVa™. With the Drop Zones, good radial force, and the closed distal tip, NeVa™ may indeed be more efficient than conventional stent-retriever designs in retrieving all clot types. Our findings need to be confirmed by larger, multi-center studies.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Cetin K Akpinar https://orcid.org/0000-0001-9512-1048
References
- 1.Tung EL, McTaggart RA, Baird GL, et al. Rethinking thrombolysis in cerebral infarction 2b: which thrombolysis in cerebral infarction scales best define near complete recanalization in the modern thrombectomy era? Stroke 2017; 48: 2488–2493. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mis-match between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 4.Touma L, Filion KB, Sterling LH, et al. Stent retrievers for the treatment of acute ischemic stroke: a systematic review and meta-analysis of randomized clinical trials. JAMA Neurol 2016; 73: 275–281. [DOI] [PubMed] [Google Scholar]
- 5.Saber H, Rajah GB, Kherallah RY, et al. Comparison of the efficacy and safety of thrombectomy devices in acute stroke: a network meta-analysis of randomized trials. J Neurointerv Surg 2018; 10: 729–734. [DOI] [PubMed] [Google Scholar]
- 6.Kleine JF, Wunderlich S, Zimmer C, et al. Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg 2017; 9: 117–121. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Fargen KM, Turk AS, et al. 2b-2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg 2014; 6: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, Van der Lugt A, et al. HERMES collaborators. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 9.Zaidat OO, Bozorgchami H, Ribó M, et al. Primary results of the multicenter ARISE II study (Analysis of revascularization in ischemic stroke with EmboTrap). Stroke 2018; 49: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 10.Machi P, Jourdan F, Ambard D, et al. Experimental evaluation of stent retrievers mechanical properties and effectiveness. J Neurointerv Surg 2017; 9: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuki I, Kan I, Vinters HV, et al. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. Am J Neuroradiol 2012; 33: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulm A, Khachatryan T, Grigorian A, et al. Preclinical evaluation of the NeVa™ stent retriever: safety and efficacy in the swine thrombectomy model. Intervent Neurol 2018; 7: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machi P, Ulm A, Bernava G, et al. Experimental evaluation of the NeVa™ thrombectomy device a novel stent retriever conceived to improve efficacy of organized clot removal. J Neuroradiol 2019; 46: 163–167. [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1795–1806. [DOI] [PubMed] [Google Scholar]
- 15.Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2018; 10: 4–7. [DOI] [PubMed] [Google Scholar]
- 16.Gurkas E, Akpinar CK, Aytac E. ADVANCE: an effective and feasible technique in acute stroke treatment. Interv Neuroradiol 2017; 23: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira VM, Gralla J, Davalos A, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke 2013; 44: 2802–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.San Roman L, Obach V, Blasco J, et al. Single center experience of cerebral artery thrombectomy using the TREVO device in 60 patients with acute ischemic stroke. Stroke 2012; 43: 1657–1659. [DOI] [PubMed] [Google Scholar]
- 19.Kurre W, Aguilar-Perez M, Schmid E, et al. Clinical experience with pREset stent retriever for the treatment of acute ischemic stroke: a review of 271 consecutive cases. Neuroradiology 2014; 56: 397–403. [DOI] [PubMed] [Google Scholar]
- 20.Kahles T, Garcia-Esperon C, Zeller S, et al. Mechanical thrombectomy using the new ERIC retrieval device is feasible, efficient and safe in acute ischemic stroke: a Swiss stroke center experience. Am J Neuroradiol 2016; 37: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabbasch C, Mpotsaris A, Liebig T, et al. First-in-man procedural experience with the novel EmboTrap® revascularization device for the treatment of ischemic stroke: a European multicenter series. Clin Neuroradiol 2016; 26: 221–228. [DOI] [PubMed] [Google Scholar]
- 22.Mordasini P, Frabetti N, Gralla J, et al. In vivo evaluation of the first dedicated combined flow-restoration and mechanical thrombectomy device in a swine model of acute vessel occlusion. Am J Neuroradiol 2011; 32: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenger KJ, Nagle F, Wagner M, et al. Improvement of stent retriever design and efficacy of mechanical thrombectomy in a flow model. Cardiovasc Intervent Radiol 2013; 36: 192–197. [DOI] [PubMed] [Google Scholar]
- 24.Haussen DC, Rebello LC, Nogueira RG. Optimizating clot retrieval in acute stroke: the push and fluff technique for closed cell stentrievers. Stroke 2015; 46: 2838–2842. [DOI] [PubMed] [Google Scholar]
- 25.Ribo M, Requena M, Macho J, et al. Mechanical thrombectomy with a novel stent retriever with multifunctional zones: initial clinical experience with the NeVa™ thrombectomy device. J Neuroradiol 2020; 47: 301–305. [DOI] [PubMed] [Google Scholar]
- 26.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 27.Arai D, Ishii A, Chihara H, et al. Histological examination of vascular damage caused by stent retriever thrombectomy devices. J Neurointerv Surg 2016; 8: 992–995. [DOI] [PubMed] [Google Scholar]
- 28.Flottmann F, Leischner H, Broocks G, et al. Recanalization rate per retrieval attempt in mechanical thrombectomy for acute ischemic stroke. Stroke 2018; 49: 2523–2525. [DOI] [PubMed] [Google Scholar]
- 29.García-Tornel A, Requena M, Rubiera M, et al. When to stop detrimental effect of device passes in acute ischemic stroke secondary to large vessel occlusion. Stroke 2019; 50: 1781–1788.31177974 [Google Scholar]
- 30.Bourcier R, Saleme S, Labreuche J, et al. ; ASTER Trial Investigators. More than three passes of stent retriever is an independent predictor of parenchymal hematoma in acute ischemic stroke. J Neurointerv Surg 2019; 11: 625–629. [DOI] [PubMed] [Google Scholar]
- 31.Hesse AC, Behme D, Kemmling A, et al. Comparing different thrombectomy techniques in five large-volume centers: a ‘real world’ observational study. J Neurointerv Surg 2018; 10: 525–529. [DOI] [PubMed] [Google Scholar]
- 32.Jindal G, Carvalho HDP, Wessell A, et al. Beyond the first pass: revascularization remains critical in stroke thrombectomy. J NeuroInterv Surg 2019; 11: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 33.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 34.Lavine SD, Cockroft K, Hoh B, et al. Training guidelines for endovascular ischemic stroke intervention: an international multi-society consensus document. J Neurointerv Surg 2016; 8: 989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion. The ASTER Randomized Clinical Trial. JAMA 2017; 318: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turk AS, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 2019; 393: 998–1008. [DOI] [PubMed] [Google Scholar]
- 37.Sakai N, Imamura H, Adachi H, et al. First-in-man experience of the Versi Retriever in acute ischemic stroke. J NeuroIntervent Surg 2018; 9: 1--4. [DOI] [PubMed] [Google Scholar]
- 38.Ernst E, Papanagiotoua P, Politia M, et al. Safety and effectiveness of CATCH+ as a first-line device for revascularization in the treatment of acute ischemic stroke. J Neuroradiol 2019; 17: 30442--30447. [DOI] [PubMed] [Google Scholar]
- 39.Zaidat OO, Castonguay AC, Linfante I, et al. First Pass Effect A New Measure for Stroke Thrombectomy Devices. Stroke 2018; 9: 60--666. [DOI] [PubMed] [Google Scholar]
- 40.García-Tornel A, Rubiera M, Requena M, et al. Sudden Recanalization, A Game-Changing Factor in Endovascular Treatment of Large Vessel Occlusion Strokes. Stroke 2020; 1: 313--1316. [DOI] [PubMed] [Google Scholar]