Abstract
Background
Obesity is the fifth leading risk factor for mortality in the world and it has increased among patients with ulcerative colitis in recent years. We examined the impact of obesity on the hospitalized patients admitted primarily with a diagnosis of ulcerative colitis.
Methods
We used the National Inpatient Sample data for the year 2016 to identify patients with ulcerative colitis and compared obese and non-obese patients in terms of length of hospital stay, total charges, and mortality. We used multiple imputations to estimate missing values and survey analysis to estimate the outcomes, and we adjusted for confounders by implementing the inverse probability of treatment weighting using propensity score.
Results
A total of 61,075 admissions with ulcerative colitis were identified. Among these, 6020 were diagnosed with obesity. Baseline hospital and patient characteristics between the 2 groups were notable for differences in age and sex. Patients with obesity were found to have a mean hospital stay longer by 0.57 days (95% confidence interval [CI] 0.22-0.93; P=0.002) and charges $6341.71 higher (95%CI 2499.72-10,183.71; P=0.001) compared to non-obese patients. There was no difference in hospital mortality, with an odds ratio of 0.28 (95%CI 0.04-2.05; P=0.212).
Conclusion
In a comprehensive review of inpatient admissions in 2016, primarily for ulcerative colitis, obesity was associated with a longer hospital stay and higher total charges per admission after balancing of confounders.
Keywords: Ulcerative colitis, obesity, length of hospital stay, hospital cost
Introduction
Obesity is a significant problem in the American healthcare system. It has a prevalence of 39.8% and affected approximately 93.3 million US adults in 2015-2016 [1]. It has been identified as the fifth leading global risk factor for mortality in the world [2]. Despite the historically lower body mass index (BMI) in patients with ulcerative colitis (UC) compared to the general population; there is an increasing trend in obesity rates among patients with UC in recent years [3,4].
Obesity has been associated with an increase in inflammatory markers such as C-reactive protein, erythrocyte sedimentation rate, and other cytokines [5-7]. There are multiple potential pathways by which obesity may worsen outcomes in UC. A reduction in bacterial diversity, with accompanying dysbiosis of the gut, and higher intestinal permeability may contribute to worsening chronic inflammatory diseases by inducing a persistent low-grade inflammatory process [6]. Some studies also suggest direct involvement of adipose tissue and mesenteric fat in creating a proinflammatory environment for developing inflammatory bowel disease (IBD) in obese patients [7-9].
Previous work has found that BMI is linked to greater mortality and length of hospital stay, with a similar trend in patients admitted for asthma, chronic obstructive pulmonary disease, cardiovascular disease, and cancer [10]. Similarly, there is an association between obesity and worse clinical outcomes in patients with autoimmune diseases, such as rheumatoid arthritis, psoriasis, and multiple sclerosis [11-14]. However, there are only scarce and conflicting data regarding the effect of obesity on patients with UC.
While some studies showed that obesity increases healthcare burden and utilization for patients with UC compared to non-obese patients [15-17], others showed no association between obesity and UC-related emergency department visits, hospitalizations or surgical procedures [18]. Furthermore, one study found that high BMI has a favorable effect on the prognosis of patients with UC [19]. In light of the above, we examined the impact of obesity on the length of hospital stay, healthcare expenses, mortality and other clinical outcomes among hospitalized patients with UC, using the National Inpatient Sample (NIS).
Materials and methods
Data source
We used the NIS database for the year 2016. The NIS is an all-payer, annually collected abstraction of approximately 7 million hospital discharges from 46 states in addition to the District of Columbia, representing more than 97% of the US population. It is available through the Healthcare Cost and Utilization Project (HCUP) for research purposes. Since 2012, there have been changes in the sampling strategy of the NIS data to improve its precision and decrease sample error. These strategies include sampling 20% from all participating hospitals instead of sampling hospitals, using statewide data from the HCUP to feed the NIS database, and removing all identifiers of hospitals or states, as well as data not uniformly available in all states [20].
Patient selection
Patients who had a primary diagnosis of UC were identified using the tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) code (K51) in the first 2 discharge diagnoses. This code has been used in multiple previous studies [21,22].
We excluded all subjects less than 18 years of age, and anyone with a diagnosis of underweight (ICD-10 R636) or a BMI less than 20 kg/m2 (ICD-10 Z681). We then defined patients with obesity based on ICD-10 codes of obesity or a BMI >30 kg/m2 (Supplementary Table 1 (1.7MB, pdf) ) and compared them to other patients in the study population.
The weighted Charlson Comorbidity Score [23-25], which unlike other indexes does not include obesity as one of its components, was calculated for all individuals in the study population, based on the ICD-10 code’s definition of the included comorbidities, and was used to adjust between obese and non-obese patients. Demography (age, race, sex), median household income for the patient’s ZIP Code based on the current year, the expected primary payer for the patient’s hospital stay, patient location (based on a 6-category urban–rural classification scheme for the United States counties developed by the National Center for Health Statistics), whether the patient was admitted to the hospital electively, and the type of facility that the patients were transferred from (if any) were considered as potential confounders.
Missing data
The randomness of the missing values in our data was evaluated using the missing data pattern plot to recognize if there was any systematic pattern. We used multiple imputations by chained equations [26], a method recommended by the HCUP for computing missing values in the NIS database [27]. Five imputed datasets were created; we ran the analyses repeatedly on each one and then combined the results.
Outcomes
Our primary outcomes of interest were: 1) length of hospital stay; 2) total charges of hospital admission; and 3) in-hospital mortality.
We also examined other secondary outcomes including: 1) rate of IBD-related surgeries; 2) venous thromboembolism; 3) infections, including pneumonia, urinary tract infection, and any bacterial or viral infection; and 4) cardiac events during the patients’ hospitalization.
Statistical analysis
R version 3.5.1 (R foundation for statistical computing, Vienna, Austria) was used for the analysis. Descriptive statistics were used to describe the baseline hospital and patient characteristics. We used the chi-square test to compare categorical variables and 2-group analysis of variance to compare continuous variables, while the Kruskal and Fisher tests were used for variables not fitting a normal distribution. We then estimated propensity scores by applying logistic regression models to all 5 imputed datasets to estimate the log odds of the probability of being obese based on the possible confounders listed above. The common support of propensity score distribution between the obese and non-obese groups was evaluated visually using Kernel density and box-and-whiskers plots. We then calculated the inverse probability of treatment weighting (IPTW) using propensity scores. We evaluated the covariate balance between the obese and non-obese groups to confirm adequate balance with acceptable absolute standardized effect sizes below 0.1 standard deviations [28]. All analyses were performed using survey-adjusted methods, accounting for NIS-specific hospital weighting and the IPTW. As a confirmatory measure, we re-estimated propensity scores using generalized boosting modeling (GBM) and compared the final results to the previous ones.
Results
Descriptive analysis
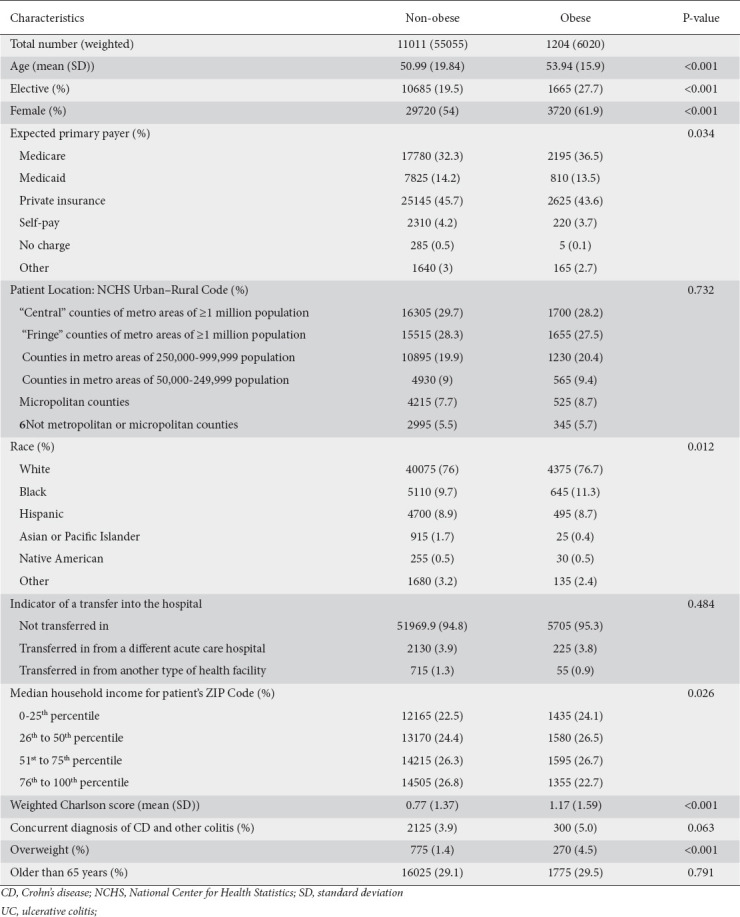
We identified a total of 12,215 patients (61,075 weighted sample size) with a primary discharge diagnosis of UC. Among those, 1204 patients (6020 weighted sample size) fulfilled the criteria for obesity based on their BMI or ICD-10 coding of obesity. These patients tended to be older (54 vs. 51 years), were more likely to be female (62% vs. 54%), to be admitted electively to the hospital (28% vs. 20%), and to have Medicare (37% vs. 32%). Overall, patients with UC had a lower Weighted Charlson Comorbidity Score; however, obese patients tended to have a higher score (1.17 for patients with obesity vs. 0.77 for non-obese), and more specifically, a higher rate of congestive heart failure, chronic obstructive pulmonary disease, diabetes mellitus, mild liver disease and renal disease (Supplementary Table 2 (1.7MB, pdf) ). Other characteristics in both groups are summarized in Table 1.
Table 1.
Baseline characteristics of patients with UC comparing obese and non-obese patients
Missing data
The total amount of missing data was 6.8%. Most of the missing values referred to race (4.3%), followed by median household income for the patient’s ZIP Code (1.7%), indicator of a transfer into the hospital (0.5%), elective admissions (0.3%), patient location (0.3%), expected primary payer (0.1%), and sex (0.1%). The missing data pattern plot showed that data appeared to be missing at random (Supplementary Fig. 1 (1.7MB, pdf) ).
Propensity score and IPTW
After propensity scores had been created using a regression model on all 5 imputed datasets, the common support was reviewed using Kernel density plots and box-and-whiskers plots and was judged by the authors as sufficient to use (Supplementary Figs. 2-5 (1.7MB, pdf) ). IPTW was calculated for each individual based on their propensity score. The covariate balance was achieved by using the IPTW, based on absolute standardized effect sizes below 0.1 standard deviations in all the covariates (Supplementary Tables 3,4, Supplementary Fig. 6 (1.7MB, pdf) ).
Outcomes
The length of hospital stay was greater in obese patients, with a mean difference (MD) of 0.57 days (95% confidence interval [CI] 0.22-0.93; P=0.002) compared to non-obese patients. Similarly, the MD of total charges was $6,341.71 higher (95%CI 2499.7-10183.71; P=0.001) obese patients compared to non-obese. There was no significant difference in hospital mortality, with an odds ratio (OR) of 0.28 and a wide CI that included the null value (95%CI 0.04-2.05; P=0.212; Fig. 1).
Figure 1.
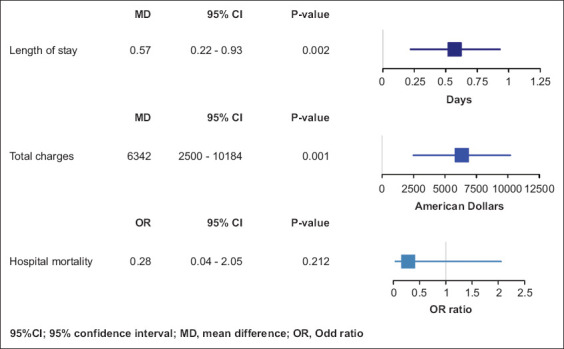
Outcomes plot of hospitalized patients with ulcerative colitis comparing obese and non-obese patients in relation to length of stay, total charges and hospital mortality
CI, confidence interval; MD, mean difference; OR, odds ratio
Our results from estimating propensity scores using GBM were similar, with length of stay MD 0.56 days (95%CI 0.14-0.97; P=0.008), total charges MD $5657.59 (95%CI 1439.66-9875.52; P=0.009), and no significant difference in hospital mortality with OR 0.56 (95%CI 0.08-4.07; P=0.566). Additionally, these results were consistent in subgroup analyses that stratified patients based on the presence of a simultaneous diagnosis of Crohn’s disease or other types of colitis, being overweight, or being younger than 65 years (Supplementary Figs. 7,8 (1.7MB, pdf) ).
For the secondary outcomes, there was no statistical differences between the 2 groups in the rate of IBD-related surgery, venous thromboembolic events or the rate of infections, with ORs of 1.12 (95%CI 0.88-1.43; P=0.354), 1.21 (95%CI 0.94-1.55; P=0.13), and 1.24 (95%CI 0.96-1.6; P=0.1), respectively. On the other hand, obese patients appeared to have a higher risk of having myocardial infarction, with an OR of 1.26 (95%CI 1.03-1.55; P=0.025).
Discussion
In this article, we studied the impact of obesity on the outcomes of hospitalized patients with UC using the NIS database. Our results show that obesity is associated with a greater length of hospital stay and total hospital charge per admission, even after adjustment for other comorbidities. Nevertheless, we did not find a significant difference in hospital mortality between obese and non-obese patients.
This effect is likely to be multifactorial given the complex nature of obesity, which could affect both the medical and surgical management of patients with UC. The proinflammatory process induced by obesity may complicate the clinical picture of patients with UC, interfere with biological treatment dosing, or worsen some of the side effects of systematic steroid use, such as hyperglycemia. In addition, obesity has been found to increase IBD-related surgery in patients with UC [4], while multiple studies have shown that obesity is an independent risk factor for postoperative complications, including wound infections, postoperative myocardial infarction, stoma problems, and other postsurgical complications [29-31], that might contribute to an increase in the length of hospital stay and hospital charges.
These results are in alignment with other studies that focused on outpatient settings and showed an increase in annual charges, the number of hospitalized days per year, and surgical complications [4,15,32]. A cohort study that used the Nationwide Readmissions Database to estimate the burden of obesity in patients with IBD showed that the obese group spent a median of 8 days with a median charge of $17,277, compared to 5 days and $11,847 in the non-obese group [15]. Nonetheless, Christian et al found that obesity is one of the predictors of early hospital readmission within 30 days for patients with IBD and increased readmissions with an OR of 1.72 (95%CI 1.20-2.47) [16].
Our study was based on data obtained from a discharge sample of hospitalized patients, and interpreting the results out of this context should be avoided. Furthermore, we used the diagnostic ICD-10 codes to identify the patient population, and our results are dependent on the accuracy of these codes; however, we used verified codes that have been used in many other studies. Identifying patients with obesity was a challenging part of our project, as obesity is known for under-coding [33]. Obesity defined by BMI has been accepted in population-based studies and was found to be a better predictor for cardiovascular mortality compared to other obesity indicators [34,35]. We also used ICD-10 code E66 to identify obesity, which has been verified in a study and found to have a specificity of 99% and a sensitivity of 7.8% [33].
Patients with UC already have an increased risk of coronary artery disease [36-38], diabetes [39] and stroke [40], regardless of their body weight. In our study, we showed that obesity increases the healthcare costs of hospitalized patients with UC, probably by worsening their medical and surgical condition. Further studies are needed to study the etiology of this relation and the best way to manage it using a multidisciplinary approach, including nutrition, lifestyle changes, and possibly altering some of the UC-related medications.
In conclusion, in a comprehensive review of inpatient admissions in 2016, primarily for UC, obesity was associated with a longer hospital stay and higher total charges per admission after balancing of confounders, including demographics and comorbidities. Future studies are needed to better understand obesity’s impact on disease management and to identify methods for improving care in patients with UC.
Summary Box.
What is already known:
• The proinflammatory effect of obesity can affect the clinical course of chronic and inflammatory diseases
• The prevalence of obesity has increased in patients with ulcerative colitis (UC) in recent years
• There are conflicting data regarding the effect of obesity on the clinical outcomes of patients with UC
What the new findings are:
• Hospitalized patients with UC and obesity have a higher rate of comorbidities compared to non-obese patients
• Obesity is associated with a longer hospital stay and greater cost for patients admitted primarily for UC, after adjustment for comorbidities and other independent factors
• The data are insufficient to support a significant different in hospital mortality between obese and non-obese patients with UC
Acknowledgment
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health
Biography
Cambridge Health Alliance and Harvard Medical School, Cambridge, MA; Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; Brigham and Women’s Hospital and Harvard Medical School, Boston, MA; Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Footnotes
Conflict of Interest: None
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth:United States, 2015-2016. NCHS Data Brief. 2017;288:1–8. [PubMed] [Google Scholar]
- 2.World Health Organization. Global Health Risks:Mortality and burden of disease attributable to selected major risks. World Health Organization. 2009 [Google Scholar]
- 3.Back IR, Marcon SS, Gaino NM, Vulcano DSB, Dorna MS, Sassaki LY. Body composition in patients with Crohn's disease and ulcerative colitis. Arq Gastroenterol. 2017;54:109–114. doi: 10.1590/S0004-2803.201700000-02. [DOI] [PubMed] [Google Scholar]
- 4.Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts. 2009;2:370–372. doi: 10.1159/000262276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations:a systematic review and meta-analysis. Obes Rev. 2013;14:232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 6.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 7.Karagiannides I, Pothoulakis C. Substance P, obesity, and gut inflammation. Curr Opin Endocrinol Diabetes Obes. 2009;16:47–52. doi: 10.1097/MED.0b013e328321306c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertin B, Desreumaux P, Dubuquoy L. Obesity, visceral fat and Crohn's disease. Curr Opin Clin Nutr Metab Care. 2010;13:574–580. doi: 10.1097/MCO.0b013e32833cf0f4. [DOI] [PubMed] [Google Scholar]
- 9.Bilski J, Mazur-Bialy A, Wojcik D, et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9:780. doi: 10.3390/biom9120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinyemiju T, Meng Q, Vin-Raviv N. Association between body mass index and in-hospital outcomes:Analysis of the nationwide inpatient database. Medicine (Baltimore) 2016;95:e4189. doi: 10.1097/MD.0000000000004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases:not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Iannone F, Lopalco G, Rigante D, Orlando I, Cantarini L, Lapadula G. Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun Rev. 2016;15:447–450. doi: 10.1016/j.autrev.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD:epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110–121. doi: 10.1038/nrgastro.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero-García JJ, Carrera-Quintanar L, López-Roa RI, Márquez-Aguirre AL, Rojas-Mayorquín AE, Ortuño-Sahagún D. Multiple sclerosis and obesity:possible roles of adipokines. Mediators Inflamm. 2016;2016:4036232. doi: 10.1155/2016/4036232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen NH, Ohno-Machado L, Sandborn WJ, Singh S. Obesity is independently associated with higher annual burden and costs of hospitalization in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:709–718. doi: 10.1016/j.cgh.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Christian KE, Jambaulikar GD, Hagan MN, et al. Predictors of early readmission in hospitalized patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1891–1897. doi: 10.1097/MIB.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 17.Pavelock N, Masood U, Minchenberg S, Heisig D. Effects of obesity on the course of inflammatory bowel disease. Proc (Bayl Univ Med Cent) 2019;32:14–17. doi: 10.1080/08998280.2018.1542887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2857–2863. doi: 10.1097/MIB.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 19.Stabroth-Akil D, Leifeld L, Pfützer R, Morgenstern J, Kruis W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis. 2015;30:237–242. doi: 10.1007/s00384-014-2051-3. [DOI] [PubMed] [Google Scholar]
- 20.HCUP-US NIS Overview. [Accessed 5 January 2021]. Available from:https://www.hcup-us.ahrq.gov/nisoverview.jsp .
- 21.Jones GR, Lyons M, Plevris N, et al. IBD prevalence in Lothian, Scotland, derived by capture-recapture methodology. Gut. 2019;68:1953–1960. doi: 10.1136/gutjnl-2019-318936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen MB, Njor S, Ingeholm P, Andersen B. Effectiveness of colorectal cancer screening in detecting earlier-stage disease-a nationwide cohort study in Denmark. Gastroenterology. 2018;155:99–106. doi: 10.1053/j.gastro.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies:development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 25.Radovanovic D, Seifert B, Urban P, et al. AMIS Plus Investigators. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002-2012. Heart. 2014;100:288–294. doi: 10.1136/heartjnl-2013-304588. [DOI] [PubMed] [Google Scholar]
- 26.van Buuren S, Groothuis-Oudshoorn K. MICE:Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 27.Houchens R. Missing data methods for the NIS and the SID. 2015. HCUP Methods Series Report #2015-01 ONLINE. U.S. Agency for Healthcare Research and Quality; 2015. Jan 22, [Accessed 5 January 2021]. Available from:http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp . [Google Scholar]
- 28.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendall KA, Raniga S, Kennedy R, Frizelle FA. The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum. 2007;50:2223–2237. doi: 10.1007/s10350-007-9051-0. [DOI] [PubMed] [Google Scholar]
- 30.Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31:556–560. doi: 10.1007/s00268-006-0305-0. [DOI] [PubMed] [Google Scholar]
- 31.Glance LG, Wissler R, Mukamel DB, et al. Perioperative outcomes among patients with the modified metabolic syndrome who are undergoing noncardiac surgery. Anesthesiology. 2010;113:859–872. doi: 10.1097/ALN.0b013e3181eff32e. [DOI] [PubMed] [Google Scholar]
- 32.Boutros M, Maron D. Inflammatory bowel disease in the obese patient. Clin Colon Rectal Surg. 2011;24:244–252. doi: 10.1055/s-0031-1295687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin BJ, Chen G, Graham M, Quan H. Coding of obesity in administrative hospital discharge abstract data:accuracy and impact for future research studies. BMC Health Serv Res. 2014;14:70. doi: 10.1186/1472-6963-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease:an update. Prog Cardiovasc Dis. 2018;61:103–113. doi: 10.1016/j.pcad.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Ortega FB, Sui X, Lavie CJ, Blair SN. Body mass index, the most widely used but also widely criticized index:would a criterion standard measure of total body fat be a better predictor of cardiovascular disease mortality? Mayo Clin Proc. 2016;91:443–455. doi: 10.1016/j.mayocp.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernstein CN, Wajda A, Blanchard JF. The incidence of arterial thromboembolic diseases in inflammatory bowel disease:a population-based study. Clin Gastroenterol Hepatol. 2008;6:41–45. doi: 10.1016/j.cgh.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. doi: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 38.Gandhi S, Narula N, Marshall JK, Farkouh M. Are patients with inflammatory bowel disease at increased risk of coronary artery disease? Am J Med. 2012;125:956–962. doi: 10.1016/j.amjmed.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Kang EA, Han K, Chun J, et al. Increased risk of diabetes in inflammatory bowel disease patients:a nationwide population-based study in Korea. J Clin Med. 2019;8:343. doi: 10.3390/jcm8030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S, Singh H, Loftus EV, Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease:a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382–393.e1. doi: 10.1016/j.cgh.2013.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


