Abstract
Background
COVID-19 is now a critical threat to global public health. Although the majority of patients present with respiratory illness, several studies have described multiorgan involvement. This study evaluated the prevailing patterns of liver enzymes in COVID-19 patients on admission and their association with clinical outcomes.
Methods
This was a single-center retrospective analysis of all inpatients with COVID-19. Demographic and clinical factors, and liver enzyme tests, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), were noted on admission. The association of liver enzyme elevation with outcomes such as inpatient death, need for intubation, and vasopressor use was determined using the chi-square test and multivariate regression analysis.
Results
Among 200 patients, AST and ALT elevation was seen in 55% and 20%, respectively. Alkaline phosphatase elevation was seen in 28%. AST elevation was associated with inpatient death (odds ratio [OR] 1.03, 95% confidence interval [CI] 1.01-1.05; P=0.035), need for vasopressors (OR 1.034, 95%CI 1.015-1.055; P=0.001), and intubation (OR 1.03, 95%CI 1.01-1.05; P=0.002). An AST/ALT ratio of 2 or more was seen in 34% of patients and was associated with need for intubation (OR 2.678, 95%CI 1.202-5.963; P=0.016), and need for vasopressors (OR 3.352, 95%CI 1.495-7.514; P=0.003).
Conclusion
Serum aminotransferase levels are useful markers of hepatocellular injury. Patients with elevated AST or AST/ALT ratio are at higher risk of severe disease, as evidenced by intubation, vasopressor use, and inpatient death. These patients should be monitored closely given their propensity for severe disease.
Keywords: Liver enzymes, COVID-19, outcomes, aspartate/alanine aminotransferase ratio
Introduction
COVID-19, caused by SARS-CoV-2, has recently been declared a pandemic by the World Health Organization [1]. SARS-CoV-2 pneumonia was first reported in December 2019 in Wuhan, China. COVID-19 infection is now a critical threat to global public health. Nearly 79 million confirmed cases in 212 countries and territories, and over 1.7 million deaths have been reported as of December 2020 [1]. As the number of confirmed cases escalates, we continue to learn more about the epidemiology and clinical characteristics of patients infected with COVID-19. Most patients present with mild respiratory illness; however, in more extreme cases, respiratory failure, septic shock and/or multiple organ dysfunction have been reported. Many cases are acute and resolve quickly; however, COVID-19 infection can also be fatal, with a reported case mortality rate of 3% [2].
Several large-scale case studies have now detailed the clinical features of patients with COVID-19 [2-5]. Evidence of extrapulmonary organ involvement has been associated with greater mortality [2]. This evolving evidence has shed some light on liver function abnormalities in COVID-19 patients. One study revealed that SARS-CoV-2 may have the potential to bind to angiotensin-converting enzyme 2 (ACE2) positive cholangiocytes, possibly leading to cholangiocyte dysfunction [6]. Another study of liver biopsy specimens from a patient who died from COVID-19 showed some microvesicular steatosis, mild lobular as well as portal involvement, indicating that SARS-CoV-2 is associated with liver injury [7]. At present, there is a shortage of studies that have systematically described liver function abnormalities in COVID-19 patients [8]. The aim of this study was to report the liver enzyme test results, including aspartate (AST), alanine (ALT) aminotransferase, and the AST/ALT ratio in COVID-19 patients on admission, and their association with the patients’ clinical course and outcomes.
Patients and methods
Study design
This study was a single-center retrospective analysis of all patients >18 years of age with a confirmed diagnosis of COVID-19 via polymerase chain reaction (PCR). We excluded patients who were still in hospital, based on chart-review, and those who had incomplete data on outcomes and liver enzyme test results. Demographic and clinical factors were recorded, including age, sex, race and comorbidities.
The primary outcome was to assess the prevailing patterns of liver enzyme tests, including the utility of the AST/ALT ratio, among patients admitted with COVID-19. The other objectives were to assess the associations between liver enzyme tests and clinical outcomes such as inpatient mortality, need for intubation/mechanical ventilation, and the use of vasopressors.
Statistical analysis
Demographic variables were presented using descriptive statistics and frequencies. Categorical variables were analyzed with chi-square testing. Demographic and clinical variables were tabulated according to an AST/ALT ratio ≥2, compared to <2. The chi-square test was used to analyze the relationship between individual liver test parameters (AST, ALT, alkaline phosphatase [ALP], bilirubin level) and an AST/ALT ratio ≥2, and rates of inpatient death, need for intubation and vasopressor use. Multivariate logistic regression was used to examine the associations of liver enzyme tests, as well as to adjust for other factors associated with the above clinical outcomes. Odds ratios (OR) 95% confidence intervals (CI) were calculated and are presented when appropriate.
Results
A total of 389 patients were evaluated in our hospital and tested positive via PCR for COVID-19. Of these, 122 were excluded as they were still in hospital at the time of the study and so their outcomes could not be evaluated. Another 67 patients who lacked clinical data such as liver enzyme tests were excluded, leaving a final sample of 200 patients analyzed (Fig. 1).
Figure 1.
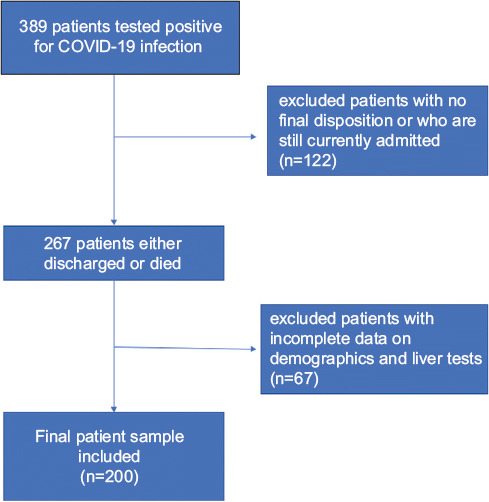
Study sample population
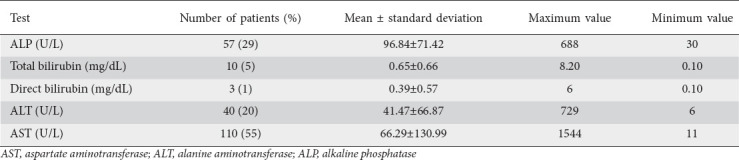
In this final sample, the mean age was 66.52±14.38 years, 45% were female and 62% were African American. The general liver enzyme test pattern in the population consisted of mild ALT and AST elevation with mean values of 41 and 66, respectively. There was mild to moderate total bilirubin elevation (range 0.1-8.20 mg/dL) with predominance of direct bilirubinemia. The ALP pattern had a mean of 96.84 with range 30-688 IU/L) (Table 1). Of the sample population, 55% had AST elevation and 20% had ALT elevation. The current upper limit of serum ALT and AST, though varying among different laboratories, is generally around 40 IU/L and this was used as the upper normal limit in our study. AST elevation was associated with inpatient death (OR 1.03, 95%CI 1.01-1.05; P=0.035), need for vasopressors (OR 1.034, 95%CI 1.015-1.055; P=0.001), and intubation (OR 1.03, 95%CI 1.01-1.05; P=0.002).
Table 1.
Liver test parameters in overall sample population
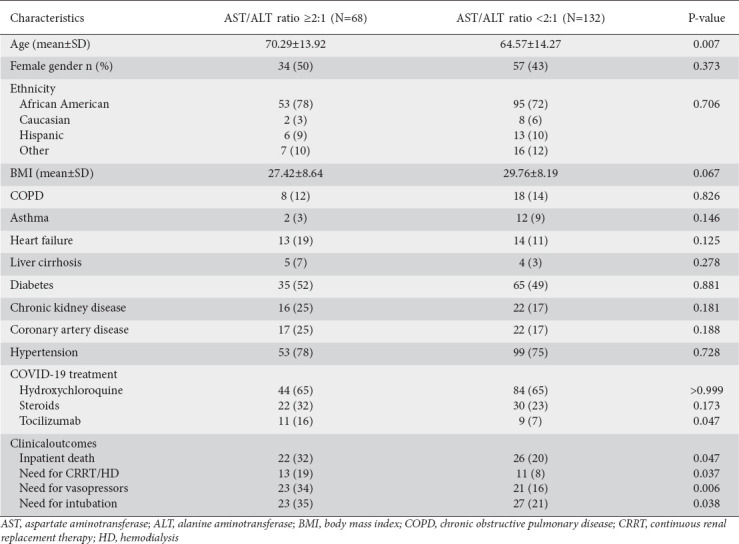
Among the 200 patients, 34% had an AST/ALT ratio ≥2. Patients with an AST/ALT ratio of ≥2 were significantly older (70.29 vs. 64.57 years, P=0.007). Aside from this, there were no significant differences in the distribution of other medical comorbidities. An AST/ALT ratio ≥2 was associated with a significantly higher rate of inpatient death (32% vs. 20%, P=0.047). It was also associated with higher rates of vasopressor use, need for intubation, and need for continuous renal replacement therapy. However, after adjustment for age, sex, race and comorbidities via multivariate logistic regression, the only significant associations that remained were with the need for intubation (OR 2.678, 95%CI 1.202-5.963; P=0.016) and the need for vasopressors (OR 3.352, 95%CI 1.495-7.514; P=0.003) (Table 2).
Table 2.
AST/ALT ratio and associated demographics and clinical outcomes
Discussion
Anecdotally, liver injury was noted in patients with 2 other highly pathogenic coronaviruses: SARS-CoV and Middle East Respiratory Syndrome (MERS) coronavirus. Liver injury in those cases was associated with severe disease [7]. Similarly, in COVID-19 patients several studies have reported liver test abnormalities and their association with disease severity. As almost all patients had liver tests done on admission, we attempted to look at the pattern of abnormalities, if any, and their association with the patients’ clinical course. Our study evaluated the medical records of 200 hospitalized COVID-19 patients, of whom about half had abnormal liver enzymes. More specifically, 55% of the patients had abnormal AST and 20% had abnormal ALT levels. Notably, Zhang et al reported a liver injury prevalence of 16-53% among their patients [9], while Cai et al found that about half of their patients had abnormal liver tests at admission [10].
Overall, our patients appeared to have mild AST and ALT elevation on admission. We also noticed that AST elevation was more frequent and significant than ALT elevation in patients with severe disease. AST levels also had the highest correlation with severe disease compared to other indicators reflecting liver injury. In our population, AST elevation was associated with inpatient death (OR 1.03, 95%CI 1.01-1.05; P=0.035), need for vasopressors (OR 1.034, 95%CI 1.015-1.055; P=0.001), and intubation (OR 1.03, 95%CI 1.01-1.05; P=0.002). Guan et al also described elevated AST levels in 18.2% of patients with non-severe disease and 39.4% of patients with severe disease, thus establishing a link between AST elevation and disease severity. Disease severity in this study was defined based on the clinical practice guidelines published by the American Thoracic Society and the Infectious Disease Society of America for the management of community acquired pneumonia [11]. Similarly, Huang et al showed that elevation of AST was observed in 8 of 13 (62%) patients admitted to the Intensive Care Unit (ICU), compared to 7 of 28 (25%) patients who did not require ICU care [3]. Wang et al also observed higher ALT and AST levels among those admitted to the ICU [12]. In 2 other studies, patients in a subclinical phase of COVID-19 infection had a lower incidence of AST abnormality compared to patients with severe disease [2,13].
Serum aminotransferase levels are 2 of the most useful markers of hepatic cellular injury. AST is also found in heart, skeletal muscle, kidney and brain tissue, in addition to the liver. Serum ALT and the serum AST/ALT ratio are often measured clinically as biomarkers of liver health. Elevation in the AST/ALT ratio can be seen in patients with alcoholic liver disease. In addition, patients with Wilson’s disease or cirrhosis due to viral hepatitis may have AST greater than ALT, though the ratio may not be greater than 2. In our study, we noticed that AST elevation prevailed overall on admission compared to ALT, so we attempted to establish the utility of the AST/ALT ratio in COVID-19 patients. Among our patients, 34% had an AST/ALT ratio ≥2. An AST/ALT ratio of 2 or more was associated with the need for intubation (OR 2.678, 95%CI 1.202-5.963; P=0.016) and need for vasopressors (OR 3.352, 95%CI 1.495-7.514; P=0.003). In fact, in our study an AST/ALT ratio ≥2 was more strongly associated with disease severity compared to AST or ALT alone. Given that the source of AST is not limited to the liver alone, elevation of AST out of proportion to ALT may suggest multiorgan involvement in COVID-19 patients. This could explain the association between an AST/ALT ratio ≥2 and poor outcomes, as mentioned above. This needs further validation, as it has not been studied previously, but this was a notable finding in our study. The impact of COVID-19 in those with preexisting chronic liver diseases, such as viral hepatitis, nonalcoholic fatty liver disease, and alcohol related liver disease, is yet to be evaluated further. One study from China reported that patients with underlying chronic hepatitis B infection did not have any higher risk of liver injury or severe disease compared to the overall population [2], whereas other studies have shown that SARS-CoV-2 may aggravate liver injury in patients with viral hepatitis [14,15]. Similarly, Moon et al also reported a higher case fatality rate among non-cirrhotic chronic liver disease patients compared to the general population [16]. These data need to be validated further. Mean ALP level largely remained normal and there was mild to moderate bilirubin elevation, with a predominance of direct bilirubinemia. However, a statistically significant association could not be established with severe outcomes, such as ICU admission, vasopressor use or death.
Liver injury in COVID-19 patients might occur from viral infection of hepatocytes, or as a response to cytokine storm resulting in inflammation [9,17]. This needs to be further corroborated by direct clinical evidence. SARS virus has been isolated in liver tissue based on some case series, although viral inclusions were not observed and the overall viral titer was low [18]. On the other hand, in an alternative case report of a pathological analysis of a patient who died from COVID-19, viral inclusions in hepatic tissue were not noted [7].
In conclusion, liver injury indicators may help foresee disease severity. Patients with elevated AST and AST/ALT ratio are at higher risk of severe disease, as evidenced by intubation, vasopressor use, and inpatient death. These patients should be monitored closely, given their propensity for severe disease.
Summary Box.
What is already known:
Liver function abnormalities have been observed in patients with COVID-19 infection
Evidence of extrapulmonary organ involvement, including hepatic injury, has been associated with higher mortality in patients with COVID-19 infection, based on previously published reports
Several mechanisms of liver injury have been described thus far, including potential binding of the virus to angiotensin-converting enzyme 2 positive cholangiocytes, and causing cholangiocyte dysfunction, along with mild lobular and portal involvement and progression to microvesicular steatosis
What the new findings are:
An elevated aspartate (AST)/alanine (ALT) aminotransferase ratio of 2 or more can serve as a marker for multiorgan involvement, given that AST can be produced by several other organs apart from the liver; in our study, an elevated AST/ALT ratio was associated with severe clinical outcomes, as evidenced by increased need for intubation, intensive care unit admission, and vasopressor use
Patients with severe COVID-19 infection were more frequently noted to have AST elevation compared to ALT elevation
AST levels had the highest correlation with severe disease compared to other individual markers reflecting liver injury
Biography
Cooper University Hospital, NJ; Albert Einstein Medical Center, Philadelphia, USA
Footnotes
Conflict of Interest: None
References
- 1.World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. [Accessed 18 December 2020]. Available from:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports .
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 Feb 04; [Online ahead of print]. doi:10.1101/2020.02.03.931766. [Google Scholar]
- 7.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom P, Meyerowitz EA, Reinus Z, et al. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. 2020 May 16; doi: 10.1002/hep.31326. [Online ahead of print]. doi:10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Shi L, Wang FS. Liver injury in COVID-19:management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q, Huang D, Yu H, et al. COVID-19:Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China:a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic:AASLD expert panel consensus statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis:preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido I, Liberal R, Macedo G. Review article:COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chau TN, Lee KC, Yao H, et al. SARS-associated viral hepatitis caused by a novel coronavirus:report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]


