Abstract
Objective
To evaluate the outcomes of immunosuppressive therapy (IST) discontinuation in patients with neuromyelitis optica spectrum disorder (NMOSD) after a sustained remission period.
Methods
We retrospectively reviewed the medical records of 17 patients with antiaquaporin-4 antibody-positive NMOSD who discontinued IST after a relapse-free period of ≥3 years.
Results
IST was discontinued at a median age of 40 years (interquartile range [IQR], 32–51) after a median relapse-free period of 62 months (IQR, 52–73). Among the 17 enrolled patients, 14 (82%) relapsed at a median interval of 6 months (IQR, 4–34) after IST discontinuation, 3 (18%) of whom experienced severe attacks; notably, all 3 of these patients had a history of severe attack before IST. These 3 patients received steroids, followed by plasma exchange for acute treatment, but 2 exhibited poor recovery and significant disability worsening at 6 months after relapse.
Conclusions
IST discontinuation may increase the risk of relapse in seropositive patients with NMOSD even after 5 years of remission. Given the potentially devastating consequence of a single attack of NMOSD, caution is advised with IST discontinuation, particularly in patients with severe attack before IST.
Neuromyelitis optica spectrum disorder (NMOSD) is a rare and severe inflammatory disorder of the CNS associated with aquaporin-4 (AQP4) antibodies.1 Because of the high morbidity associated with NMOSD relapse, early initiation of immunosuppressive therapy is recommended.2 Rituximab, mycophenolate mofetil (MMF), and azathioprine (AZA) are the most commonly prescribed therapeutic agents for preventing relapse in NMOSD. Although these agents are generally well-tolerated, the long-term use of these drugs may lead to an increased risk of opportunistic infections, particularly in elderly patients, resulting in increased economic burden. However, there is no current consensus regarding the optimum duration of immunosuppressive therapy (IST), and a common clinical dilemma is the feasibility of treatment withdrawal in patients who have achieved a sustained period of remission. Here, we aimed to evaluate the outcomes of IST discontinuation in patients with NMOSD with a sustained remission period before discontinuation.
Methods
Participants and Data Collection
We retrospectively evaluated medical records of 252 patients with NMOSD, according to the 2015 NMOSD criteria,1 who were positive for AQP4 antibodies and underwent continuous IST with MMF, AZA, or rituximab for ≥3 years at the National Cancer Center, Korea, between May 2005 and March 2020. Of these patients, we identified 18 patients who discontinued IST after ≥3 years of relapse-free period on IST. One patient was lost to follow-up and was thus excluded. Finally, we included 17 patients in this study. The study was approved by the Institutional Review Board of the National Cancer Center, Korea. Collected data included patient demographics, treatment history, relapse history, and disabilities (Expanded Disability Status Scale [EDSS]). A severe relapse was defined as an EDSS score of ≥6.0, or as new worsening of visual acuity of ≤0.1 points at the nadir of the attack.
Treatment Protocols
The maintenance AZA dose was 2–3 mg/kg/d, and the MMF dose was 1,500–2,000 mg/d. Rituximab was administered based on the frequency of memory B cells in peripheral blood according to our previously published protocol.3
Laboratory Testing
Blood samples were obtained during the follow-up once per year at the clinic. AQP4 antibodies were measured using an in-house live cell-based assay.4
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
The characteristics of the 17 patients are presented in table. IST was discontinued after a median relapse-free period of 62 months (interquartile range [IQR], 48–73 months). Reasons for IST discontinuation included the patient's decision (n = 8, 47%), provider advice (n = 8, 47%), and preparing for pregnancy (n = 1, 6%).
Table.
Clinical and Demographic Characteristics of Patients With Neuromyelitis Optica Spectrum Disorder
Fourteen patients (82%) experienced a relapse at a median interval of 6 months (IQR, 4–34 months) after discontinuation; the median interval was 4 months (IQR, 3–18 months) for patients (n = 12) who discontinued AZA or MMF and 85 months and 54 months for 2 patients who discontinued rituximab, respectively. Two patients who discontinued AZA and MMF maintained a stable disease course for 30 and 65 months, respectively. One patient restarted IST with MMF after 6 months of discontinuation without relapse because of concern about relapse. In our cohort, 2 patients started IST after a single attack, and both relapsed after discontinuation of IST. Eight patients (47%) experienced severe attacks before IST, whereas 3 (21%) of 14 patients experienced severe attacks after discontinuation of IST; notably, all 3 patients had a history of severe attack before IST. These 3 patients received steroids, followed by plasma exchange for acute treatment, but 2 exhibited poor recovery and EDSS worsening at 6 months after the attack.
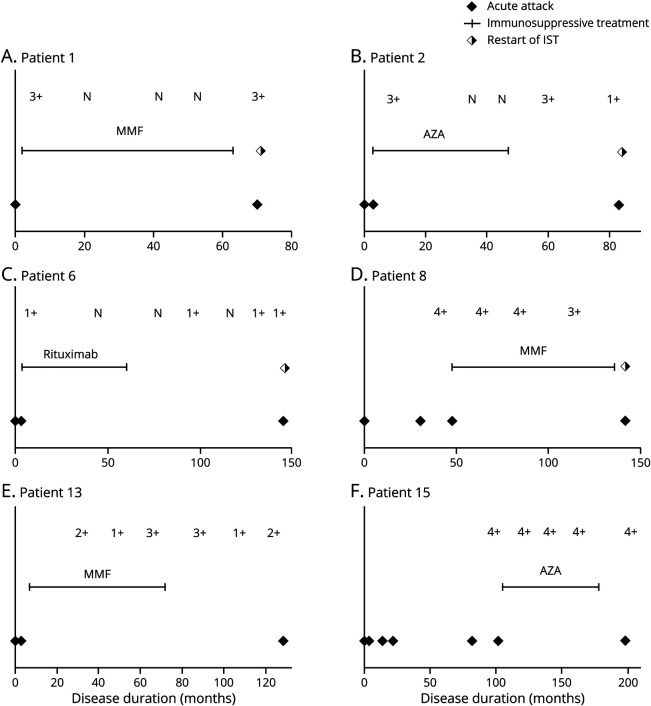
Five (29%) were seronegative for AQP4 antibodies at the time of discontinuation, but 4 (80%) of them exhibited seroreversion (from seronegative to seropositive) after IST discontinuation (figure 1, A–C, figure e-1, links.lww.com/NXI/A380). The remaining 11 patients were continuously seropositive for AQP4 antibodies regardless of the treatment (figure 1D, figure e-1), and 2 patients were in a remission state without IST for 65 and 30 months (figure 1, E and F).
Figure. Temporal Association of Clinical Relapse With Discontinuation of Immunosuppressive Therapy and AQP4 Antibody Status in 6 Patients With Neuromyelitis Optica Spectrum Disorder.
N, AQP4 antibody negative, 1–4+ semiquantitative AQP4 antibody positive score. AQP4 = aquaporin-4; AZA = azathioprine; MMF = mycophenolate mofetil.
The 14 patients who relapsed after IST discontinuation restarted IST immediately. All but 2 had a stable disease course for a median duration of 29 months (IQR, 13–62 months) since IST was restarted. Notably, the 2 patients who attempted IST discontinuation again after 14 months and 49 months of AZA and MMF treatment relapsed again after 3 and 10 months of discontinuation, respectively. There were no significant safety issues among the 17 patients during the IST period.
Discussion
In our cohort of patients with NMOSD with a median relapse-free period of 62 months before IST discontinuation, 82% of patients relapsed after a median interval of 6 months. Among them, 3 (21%) had severe attacks, 2 of whom did not recover despite high dose steroids and plasma exchange. These findings indicate that IST discontinuation leads to an increased risk of relapse in seropositive patients with NMOSD despite a lengthy relapse-free period. Particular caution is required in patients with a history of severe attack before IST because of the risk of irreversible disability even from a single attack.
Data regarding the outcomes of IST discontinuation in patients with NMOSD are lacking. Weinfurtner et al.5 suggested that stopping rituximab treatment after disease stabilization is a viable option for some patients with NMOSD. However, among their 4 cases, 2 eventually relapsed 3 and 5 years after the last infusion of rituximab. Likewise, in our cohort, the 2 patients previously treated with rituximab relapsed long after rituximab discontinuation. Based on a longer remission after rituximab discontinuation compared with that of AZA or MMF, we suggested that rituximab treatment before pregnancy is more advantageous for a successful pregnancy in patients with NMOSD.6 Nevertheless, long-term remission under IST does not guarantee lifelong remission after IST discontinuation.
Our study was unable to determine the factors that would predict remission maintenance after IST discontinuation because only 2 patients maintained remission after IST discontinuation. Some expert suggested that in patients with NMOSD who do not disseminate in time either clinically or radiologically, long-term therapy (>5 years) may be unnecessary.7 However, 2 patients who experienced only a single attack before IST relapsed after IST discontinuation. In patients with MS, the benefits of disease-modifying therapies (DMT) may decrease with age because of diminishing inflammatory activity or having already reached significant disability, hence it may be feasible to discontinue DMT in some older patients.8 Nevertheless, unlike MS, late-onset NMOSD (age, ≥50) are reported to be associated with worse outcomes because of high frequency of severe myelitis.9,10 Thus, the prevention of relapse by IST is still an important treatment goal in older patients with NMOSD, and IST discontinuation in older patients may not particularly more feasible. In our cohort, monitoring of AQP4 antibody serostatus was not helpful in predicting relapse after IST discontinuation. More than 60% of patients were continuously seropositive. Moreover, among 5 patients with negative seroconversion at the time of discontinuation, all except one exhibited positive seroreversion after IST discontinuation, and the time interval from seroreversion to relapse varied between patients (figure 1, A–C).
This study was limited by its retrospective and single-center design. In particular, the referral bias toward more severe cases should be taken into account. The number of enrolled patients was not large because most patients with NMOSD were unwilling to stop treatment even after a lengthy relapse-free period. Nevertheless, this is the largest case series exploring the feasibility of IST discontinuation in patients with NMOSD. Owing to the uncontrolled nature of a case series, the outcome of IST discontinuation should be interpreted with caution. However, all patients who relapsed after IST discontinuation became stable after restarting and maintaining IST. Moreover, 2 patients who restarted and stopped again relapsed several months after discontinuation. Thus, long-term remission is indeed associated with IST, not a spontaneous regression of disease activity.
In conclusion, although evaluation of the balance between risk and benefit of maintaining long-term IST should be individualized, this study advocates continuing relapse prevention therapy in seropositive patients with NMOSD, until future studies enable us to determine when and in which patients IST can be safely discontinued and/or until validated biomarkers to predict relapse well in advance are available.
Glossary
- AQP4
aquaporin-4
- AZA
azathioprine
- DMT
disease-modifying therapies
- EDSS
Expanded Disability Status Scale
- IQR
interquartile range
- IST
immunosuppressive therapy
- MMF
mycophenolate mofetil
- NMOSD
neuromyelitis optica spectrum disorder
Appendix. Authors
Contributor Information
Su-Hyun Kim, Email: herena20@ncc.re.kr.
Min Young Lee, Email: 74687@ncc.re.kr.
Jae-Won Hyun, Email: jacksy12@naver.com.
Study Funding
This work was supported by the National Research Foundation of Korea (Grant No. NRF-2018R1A5A2023127 & NRF-2016R1D1A1A09916480).
Disclosure
S.-H. Kim received a grant from the National Research Foundation of Korea. H. Jang, N.Y. Park, Y. Kim, S.-Y. Kim, M.Y. Lee, and J.-W. Hywn report no financial disclosures. H.J. Kim received a grant from the National Research Foundation of Korea; received consultancy/speaker fees from Alexion, Aprilbio, Celltrion, Eisai, Hanall Biopharma, Merck Serono, Novartis, Sanofi Genzyme, Teva-Handok, and Viela Bio; serves on a steering committee for MedImmune/Viela Bio; is a coeditor for the Multiple Sclerosis Journal and an associated editor for the Journal of Clinical Neurology. Go to Neurology.org/NN for full disclosures.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol 2014;10:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SH, Jeong IH, Hyun JW, et al. . Treatment outcomes with rituximab in 100 patients with neuromyelitis optica: influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol 2015;72:989–995. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, Kim G, Kong BS, et al. . Large-scale in-house cell-based assay for evaluating the serostatus in patients with neuromyelitis optica spectrum disorder based on new diagnostic criteria. J Clin Neurol 2017;13:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinfurtner K, Graves J, Ness J, Krupp L, Milazzo M, Waubant E. Prolonged remission in neuromyelitis optica following cessation of rituximab treatment. J Child Neurol 2015;30:1366–1370. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Huh SY, Jang H, et al. . Outcome of pregnancies after onset of the neuromyelitis optica spectrum disorder. Eur J Neurol 2020;27:1546–1555. [DOI] [PubMed] [Google Scholar]
- 7.Shosha E Disease-modifying therapies should be stopped in NMOSD patients in remission–yes. Mult Scler 2019;25:1217–1218. [DOI] [PubMed] [Google Scholar]
- 8.Hua LH, Fan TH, Conway D, Thompson N, Kinzy TG. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler 2019;25:699–708. [DOI] [PubMed] [Google Scholar]
- 9.Collongues N, Marignier R, Jacob A, et al. . Characterization of neuromyelitis optica and neuromyelitis optica spectrum disorder patients with a late onset. Mult Scler 2014;20:1086–1094. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Mealy MA, Levy M, et al. . Racial differences in neuromyelitis optica spectrum disorder. Neurology 2018;91:e2089–e2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.