1. Introduction
Adolescence is a developmental period characterized by marked change and maturation across a number of physical, social, and affective domains. It is also a time of heightened sensitivity to reward, an increase in risk-taking behavior, and a dramatic rise in psychopathology. Evolutionarily, this increase in risk-taking behaviors is a normative part of healthy development, emboldening adolescents to explore their environments, affiliate with peers and romantic partners, and gain new knowledge and skills in order to increase their social status and achieve self-relevant goals. However, adolescence has also been associated with a host of negative outcomes such as suicide, accidents, unprotected sex, depression, substance abuse, and other forms of psychopathology, which may be linked to high reward sensitivity (Force, 1996; Ozer et al., 2002).
The period of adolescence cannot be fully understood without considering the significant hormonal changes that define this stage of development. The uptick in reward sensitivity and sensation-seeking observed among adolescents has been associated with level of pubertal maturation (Forbes and Dahl, 2010; Spear, 2000; Steinberg, 2008). The current paper examines associations between pubertal hormones—specifically, testosterone and estradiol—and adolescents’ reward system functioning, as indexed by brain responses to monetary reward as well as functional connectivity during reward processing. It is important to examine links between adolescents’ hormone levels and 1) their reward-related task-based functional activation and 2) the strength of functional connectivity in reward circuitry in order to further clarify the extent to which pubertal maturation influences normative reward system development and functioning in adolescence. Surprisingly, little is known about the degree to which circulating levels of testosterone and estradiol are associated with functional connectivity in reward circuitry among human adolescents. In addition to furthering knowledge of the mechanisms of pubertal development, understanding the association between estradiol, testosterone, and neural reward circuitry could inform brain-based interventions that harness teens’ neural plasticity to address the reward-related health problems that emerge at adolescence.
1.1. Reward system development during adolescence
A number of neuroimaging studies have employed reward and risk-taking paradigms to examine the neural correlates of reward processing among adolescents and adults. Both the adolescent (e.g., Forbes et al., 2010) and adult (e.g., Breiter et al., 2001; Delgado, 2007; McClure et al., 2004) literature has found that the dorsal and ventral striatum (encompassing regions such as the NAcc, caudate, and putamen), in addition to the anterior cingulate cortex (ACC) and ventromedial prefrontal cortex (vmPFC), activate to the receipt of a monetary reward. The NAcc core is centrally involved in the processing of motivation and reward (Haber and Knutson, 2010). Closely linked to the striatum, both the dorsal and rostral areas of the ACC are involved in reward-related decision making and assessment (Bush et al., 2002). In addition to striatal regions and the ACC, the vmPFC is involved in encoding a reward’s subjective value (Clithero and Rangel, 2013) and reliably activates upon the receipt of a reward (Knutson et al., 2003). Regions in the ventral-rostral mPFC—including the dmPFC—have also been implicated in reward anticipation and may be particularly sensitive to reward in social contexts (Bogdan and Pizzagalli, 2006). Given the influence of hormone levels on the structure and function of this region, the present study also examined the dlPFC. While not typically considered a central reward-related region, some research has found that the dlPFC is involved in reward anticipation, value-based decision-making, and the modulation of striatal activation during reward processing (e.g., Ballard et al., 2011; Morris et al., 2014; Staudinger et al., 2011).
Adolescence is a time of many changes in brain structure, function, and connectivity. The adolescent brain undergoes extensive growth, with increases in synaptic pruning and myelination, leading to more efficient brain processing and connectivity between distal regions. Adolescence is associated with increases in structural and functional connectivity between many reward-related cortical and sub-cortical regions (Blakemore, 2012; Casey et al., 2005; Dosenbach et al., 2010), and significant development of frontal regions involved in executive functioning and cognitive control (Braams et al., 2015; Chein et al., 2011; Galvan et al., 2007; Van Leijenhorst et al., 2010). For example, neuroimaging studies have shown increased recruitment of the ACC, dmPFC, and dlPFC from childhood to adulthood during executive functioning tasks (Crone and Ridderinkhof, 2011). In addition, many subcortical brain regions—such as those involved in the processing of reward (e.g., NAcc)—show changes in functional activation during adolescence. Pubertal maturation could contribute to reward-drive behavior through the influence of puberty-linked hormones on the structural and functional changes observed in reward-related regions across adolescence, but the extent of this influence is poorly understood.
Several studies find increased striatal activation to reward in adolescents compared to children and adults (e.g., Galvan et al., 2006), but a few find blunted activation (Bjork et al., 2004; Bjork et al., 2010). On the one hand, adolescents may engage reward-related regions to a greater extent than children and adults, which may underlie the increase in risk-taking behaviors observed during this developmental period. From this perspective, rewarding stimuli may be more salient and therefore lead to the increased activation of reward-related regions among adolescents. On the other hand, lower brain reactivity may reflect a blunted response to typical rewards, causing adolescents to seek out more intense rewards in order to maximize reward activation and achieve a similar behavioral effect compared to children and adults. However, very few studies of reward processing in adolescence account for the potential contributions of testosterone or estradiol levels despite evidence that neural reward sensitivity may serve as a mechanism linking adolescents’ pubertal maturation and risk behavior (e.g., Op de Macks et al., 2016). It is possible that the variability in neural reward system functioning in adolescent studies may be due to individual differences in hormone levels.
1.1.1. Theoretical model
Puberty-linked hormones such as testosterone and estradiol may affect reward related brain reactivity and connectivity through both “organizational” and “activational” mechanisms (Phoenix et al., 1959; see Vigil et al., 2011 for a review). First, puberty-estradiol and testosterone may be “organizational” (impacting the development of the brain) in the sense that these hormones may shape structure and function of the brain during adolescence by stimulating neuronal outgrowth, synapse quantity, dendritic branching, and myelination (Cooke and Woolley, 2005; Perrin et al., 2008). For example, a large longitudinal structural MRI study supported this theoretical model and found that testosterone and estradiol levels were linked to increased white matter, gray matter, right amygdala, and bilateral caudate volumes during early pubertal maturation (as indexed by lower hormone levels), followed by less robust—and potentially even reversals in growth—by late puberty (Herting et al., 2014).
Secondly, estradiol and testosterone may be “activational” in that they activate neural systems to favor specific behavioral responses—including reward-seeking behaviors—in adolescence (Schulz et al., 2009). The present study focuses on the “activational” effects of testosterone and estradiol on adolescent reward processing during puberty. Researchers have theorized that high levels of estradiol and testosterone may increase response in neural affective and reward processing regions that activate differently in adolescence compared to other developmental stages (e.g. NAcc, vmPFC, dmPFC, ACC, and dlPFC; Casey et al., 2011) and, in turn, lead to greater reward-seeking behaviors (see SIPN model: Nelson et al., 2005).
Moreover, it is hypothesized that functional connectivity among these rapidly developing reward-processing regions may also be linked to reward-seeking behaviors in adolescence (Casey et al., 2011). Increases in testosterone or estradiol that co-occur with these brain changes represent an important variable potentially impacting the level of functional connectivity between reward-related regions among adolescents. Testosterone and estradiol may potentiate heightened or blunted connectivity within the reward processing system. No research to date has explored the relationship between either testosterone or estradiol and functional connectivity. One possibility is that adolescents with higher levels of these hormones would demonstrate lower connectivity between subcortical and cortical reward processing regions, as low connectivity between these regions can be regarded as facilitating intense, potentially maladptive reward-seeking behavior, while higher levels of testosterone and estradiol have been linked with higher levels of risky reward-seeking behaviors in adolescents (e.g., Op de Macks et al., 2016). The precise role of testosterone and estradiol on neural reward processing among adolescents (Doremus-Fitzwater et al., 2010) is a notable gap in the literature, given that testosterone and/or estradiol may serve a mechanistic role linking pubertal maturation to the increase in risk-taking behavior observed across this sensitive developmental period.
1.2. Puberty and reward system development
Research supports theoretical claims of both organizational and activational effects of estradiol and testosterone on the adolescent brain. Our review will focus on activational effects of testosterone and estradiol most relevant to the current study, such as those that occur with the onset of reproductive changes rather than the early-life influences of hormones on brain development. To begin, pubertal development influences adolescents' brain structure and function, including brain systems implicated in reward processing. At the onset of puberty, the hypothalamic-hypophyseal-gonadal axis and the hypothalamic-pituitary-adrenal (HPA) axis are activated, stimulating the release of gonadotropin-releasing hormone by the hypothalamus (see Buck Louis et al., 2008 for a review). This, in turn, promotes the pituitary gland, which releases both the follicle stimulating hormone (FSH) and luteinizing hormones (LH), causing the ovaries to secrete estrogen and progesterone in females and the testes to secrete testosterone in males. Most studies of hormone production in early adolescents (i.e., ages 10 to 13) tend to focus on testosterone and estrogen or estradiol levels (Forbes et al., 2010). Increased levels of these hormones are a biomarker of pubertal development and serve as a more precise measurement of an adolescent’s level of pubertal maturation compared with age or subjective measures, such as self-reported pubertal stage or, for females, age at first menarche (see Dorn et al., 2006, for a review of pubertal measurement). Because female adolescents also produce testosterone and male adolescents also produce detectable estradiol—both of which play a role in sexual and other reward-related behaviors—the current study measures estradiol and testosterone in both males and females.
Functionally, a few studies have examined the relationship between pubertal hormone levels, particularly testosterone, and brain function in reward processing regions among adolescents. Forbes et al. (2010) found that, for boys aged 12–13 years, higher testosterone levels were correlated with increased striatal activity (specifically caudate) during the anticipation of a monetary reward. Conversely, both male and female adolescents’ testosterone levels were associated with lower caudate activation during the receipt of a reward (Forbes et al., 2010). Similarly, another study found that testosterone levels were positively correlated with striatal activation when receiving a monetary reward among girls and boys ages 10 to 16 (Op de Macks et al., 2011). In another study of 11 to 13-year-old girls, the same authors found that higher testosterone levels were associated with increased risk-taking during an economic decision-making task, which was mediated by increased medial orbitofrontal cortex activation, a region inferior to the vmPFC (Op de Macks et al., 2016). In addition, higher estradiol levels among female adolescents have been associated with increased activation in the dorsal striatum and medial PFC (including the dmPFC) in response to the receipt of a monetary reward (Op de Macks et al., 2011), and increased NAcc activation to riskier “play” trials compared to the safer “pass” trials (Op de Macks et al., 2016). Finally, in a longitudinal study of 299 participants (M age = 14.15), Braams et al. (2015) found that adolescent testosterone levels correlated with both higher self-reported reward sensitivity and higher NAcc activation to a monetary reward. Altogether, the extant literature suggests that testosterone and estradiol may both lead adolescents to exhibit increased activation of striatal and possibly reward-related prefrontal regions during reward processing. The current study builds upon this literature by examining the role of testosterone and estradiol on adolescents’ functional activation in reward-related regions upon the receipt of a monetary reward.
1.3. Pubertal hormones: links to neural functional connectivity
Testosterone and estradiol may also be related to changes in functional connectivity between brain regions during adolescence. Functional connectivity is defined as the temporal correlation of distant neurophysiological events, such as activation in separate brain regions (Friston et al., 1993); functional connectivity is thought to reflect communication between anatomically-separated brain regions during rest or during a task. Unfortunately, no studies to date have examined how testosterone and estradiol are associated with functional connectivity during the processing of reward. In two studies of adult women, Weis et al. (2008, 2011) found that higher levels of estradiol and progesterone during the luteal phase of the menstrual cycle were correlated with enhanced functional connectivity between hemispheres during both verbal and spatial cognitive tasks.
With regard to testosterone, a handful of EEG and fMRI studies suggest that testosterone may reduce functional communication between subcortical and cortical brain areas (Peper et al., 2011; Schutter and Honk, 2004). For example, higher testosterone levels predicted lower amygdala-PFC connectivity among healthy male adults during a socio-emotional processing task (Volman et al., 2011). Thus, some initial research with adults suggests that estradiol is associated with increased neural connectivity and that testosterone may be associated with decreased subcortical-cortical connectivity. However, to our knowledge, no prior research has investigated how testosterone and estradiol among adolescents are associated with functional connectivity between key subcortical and cortical regions during the processing of a reward.
1.4. The current study
The overarching goal of the current fMRI study was to investigate the relationship between pubertal hormone levels (testosterone, estradiol) and neural reward processing in a sample of adolescents ages 12–14. Current theoretical models posit that adolescents’ heightened reward sensitivity may be driven by increased activation in striatal regions and limited recruitment of control-related prefrontal regions in response to rewarding stimuli; however, few studies have investigated how pubertal maturation may influence neural reward processing. Therefore, the first aim was to examine links between adolescents’ pubertal hormone (estradiol, testosterone) levels and activation in key regions of interest (NAcc, ACC, vmPFC, dmPFC, and dlPFC) in response to obtaining a monetary reward. Based on prior studies examining the role of testosterone, estradiol, and reward-related functional activation (e.g., Braams et al., 2015; Forbes et al., 2010; Op de Macks et al., 2011, 2016), we hypothesized that higher levels of testosterone and estradiol would be correlated with higher activation in the reward processing ROIs (NAcc, ACC, vmPFC, dmPFC) and negatively correlated with activation in the dlPFC upon the receipt of a monetary reward. Our second aim was to investigate how pubertal hormone levels were related to functional connectivity between a key subcortical reward region—the NAcc—and the other reward-related ROIs during the receipt of monetary reward. Due to the dearth of literature on testosterone, estradiol, and reward-related functional connectivity, we did not have specific hypotheses regarding the direction of the association between hormone levels and functional connectivity. Notably, ours is the first study to explicitly investigate how adolescents’ levels of testosterone and estradiol may impact functional connectivity in the context of a monetary reward.
2. Method
2.1. Participants
Participants were 67 early adolescents ages 12–14 years (36 boys, M age = 12.51, SD = 0.61). The majority of adolescents were European American (76.1%), with 7.5% Mixed race, 6.0% Hispanic/Latino, 7.5% Asian, and 3.0% African American. Median annual household income was > $100,000 (77.6%), 7.5% between $75,000 and $100,000, 9.0% between $60,000 and 74,999, 1.5% between $50,000 and 59,999, 3.0% between 25,000 and 34,999, and 1.5% not reported. Both the racial and income composition of our sample were representative of the local community from which participants were recruited. Participants were recruited from a larger behavioral study on gender, emotion, parent-adolescent interactions, and adolescent substance use and risk behaviors. For the larger study, participants and their parents were recruited from mailings and flyers sent out to households and schools in a suburban area in the mid-Atlantic United States. Inclusion criteria for the larger study were adolescents: 1) with an IQ > 80, 2) possessing adequate English proficiency to complete questionnaires, and 3) without pervasive developmental disabilities or psychotic disorders.
Adolescents in the larger study were invited to participate in an additional MRI scanning session if they met additional MRI inclusion criteria (MRI safe, no history of congenital brain defect or traumatic brain injury, and no current psychiatric medication use). Of those invited to participate, 80 adolescents were interested in and eligible to participate in the MRI session. Of those 80 adolescents, eight were removed from the scanner before completing the reward task and five were excluded due to excessive head motion, resulting in a final sample of 67. Excluded adolescents did not significantly differ on measures of demographic (race, gender, family income, age), psychopathology, or emotion regulation from those included.
We examined self-reported pubertal status of the adolescents using adolescents’ report on the Pubertal Development Scale (PDS; Petersen et al., 1988), a widely-used measure that correlates with physician-assessed Tanner stages. We scored it based on Carskadon and Acebo (1993). In the present sample, the majority of girls were in middle or late puberty (6.7% early puberty, 16.7% middle puberty, 73.3% late puberty, 3.3% post puberty) and the majority of boys were in early or middle puberty (2.9% pre-puberty, 34.3% early puberty, 40% middle puberty, and 22.9% late puberty). This was consistent with sex differences in the onset of pubertal maturation (Carskadon and Acebo, 1993).
2.2. Procedure
Participants and their primary caregivers completed three study sessions. The first session was a questionnaire/interview session in which participants completed questionnaires regarding emotion regulation, psychopathology, and other constructs of interest in the larger study. At the first session, parent consent and adolescent assent were obtained. The second session (the laboratory session) included a laboratory task and also the collection of saliva samples for testosterone and estradiol. The third session was the fMRI session, which was scheduled for approximately 2–6 weeks after the second session. Three adolescents were scanned 6 months after saliva sampling because they had metal braces during the 2–6 week period. However, results were run with and without these adolescents and the pattern of results remained unchanged and thus we included them in our analyses. The University’s Institutional Review Board (IRB) approved all study protocols.
2.3. Laboratory session
The laboratory session was scheduled for approximately 4:00 pm. This allowed us to control for possible diurnal fluctuations in pubertal hormone levels. During the session, the adolescent participated in several tasks as part of the larger study and also provided saliva samples. Samples were collected from the adolescent participants using the “passive drool” method, per Shirtcliff et al.’ (2001) recommendations. After the collection of saliva samples, samples were placed immediately on ice, placed in a − 80° degree freezer, and later assayed.
Samples were assayed in duplicate to assess levels of testosterone and estradiol—the two most common hormones associated with puberty—using a highly sensitive enzyme immunoassay (Salimetrics, PA). Salimetrics reports an inter-assay coefficient of variation ranging from 5.0% to 7.35%. In accordance with previous studies and Salimetrics recommendations, we used the average value for testosterone and estradiol from the two assays (Ferree et al., 2011). Salimetrics reports that the lower limit of detectable values for estradiol is between 0 pg/ml and 0.1 pg/ml and the lower limits of detectable values for testosterone are about 1.5 pg/ml. Both testosterone and estradiol fell within the range of detectable values for boys and girls in our sample and are similar to values reported by Op de Macks et al. (2016) (see Table 1 for descriptive statistics). Testosterone and estradiol were highly correlated with one another (r = 0.45, p ≤ .001).
Table 1.
Descriptive statistics for pubertal hormones.
| Mean (SD) | Range | |
|---|---|---|
| Testosterone (pg/ml) | ||
| Males | 41.30 (17.24) | 23.12–89.19 |
| Females | 50.46 (23.24) | 16.18–91.92 |
| Estradiol (pg/ml) | ||
| Males | 1.91 (0.61) | 1.01–3.91 |
| Females | 1.85 (0.73) | 0.77–4.48 |
SD = standard deviation.
2.4. fMRI session
Upon arriving at the fMRI session, adolescents were rescreened for MRI safety and MRI procedures were explained to them. Additionally, adolescents completed practice items for the MRI tasks on a laptop and were given the option to lie in a mock scanner to increase comfort with being in the scanner. Participants then completed a 60-min MRI scan session that included the reward task and three other tasks, T1-weighted structural scans, and a Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE). Participants were scanned using a Siemens 3T scanner with a single-channel birdcage head coil. To minimize movement and enhance comfort in the scanner, adolescents were provided with head padding and ear plugs. While in the scanner, adolescents viewed stimuli through a high-resolution rear-projected display at the head of the bore. A mirror on the head cage allowed participants to view the display. Additionally, adolescents used 2 buttons on a 5-button box to record their responses to stimuli during the reward task.
2.5. Reward task
The reward task was a card-guessing task that has been used in previous studies with adolescents (Forbes et al., 2009). Stimuli were presented using E-prime software. The task was a single-run slow event-related design comprising a total of 24 trials: 12 potential win trials and 12 potential loss trials. Trials were presented in a pseudo-randomized order and each trial lasted 20 s. The 20 s consisted of 4 s viewing a question mark and guessing whether the card would be greater than or < 5 (card values ranged from 1 to 9) using two buttons on the button box. Next, there were 6 s of viewing shuffling hands and either an up or down arrow to indicate whether it was a potential win or potential loss trial. Next, there were 500 ms of viewing the actual card and 500 ms of viewing feedback, which was an up or down arrow indicating a win or loss, or a yellow circle, indicating neutral feedback (i.e., they neither won nor lost money). The last phase of each trial included 9 s of viewing a crosshair, which served as an inter-stimulus interval (see Fig. 1). For the 12 potential win trials the feedback was either win or neutral; for the 12 potential loss trials, the feedback was either loss or neutral.
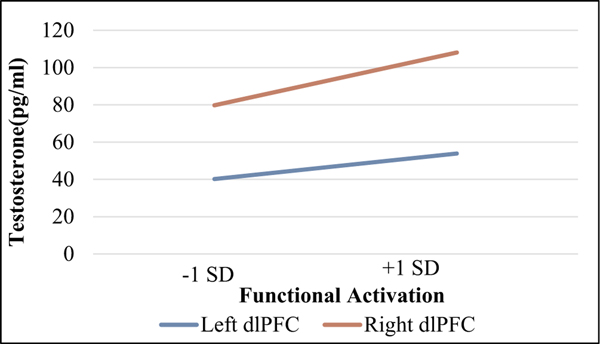
Fig. 1.
Testosterone Functional Activation Regression Lines
SD = standard deviation
dlPFC = dorsolateral prefrontal cortex
*Note: lines are significant bootstrapped regression lines with reported pubertal stage as a covariate.
Adolescents were told they could win $1 on potential win trials and lose 50 cents on potential loss trials. A trained research assistant told them that they would calculate their earnings at the end and provide them with that amount. In actuality, the paradigm was programmed in such a way that each adolescent won 6 of the potential win trials and lost 6 of the potential loss trials; however, they were led to believe the outcomes were contingent on their guessing performance. Given that outcomes were fixed, each adolescent “won” $6 in addition to their compensation for participating in the fMRI session.
Contrasts were computed between the potential win-win outcome trials minus the potential win-neutral outcome trials. The win-win and win-neutral trials were identical except for the 500 ms of viewing the card and the 500 ms of viewing feedback, which indicated a win for the win-win trials and a neutral outcome for the win-neutral trials. In other words, analyses focused on contrasting brain activity when adolescents “won” compared to brain activity when adolescents did not win. Thus, analyses isolated adolescents’ brain activation in response to the receipt of a monetary reward.
2.6. Image acquisition
Functional images of hemodynamic response were captured using the T2-weighted gradient echoplanar images (GE-EPI) (TR/TE: 2250/30 ms; flip = 70o; FOV: 192 mm; matrix size: 64 × 64; 40 axial 3 mm thick/1 mm gap slices). Structural images were captured using T1-weighted magnetization with magnetization prepared rapid-acquisition gradient echo (MPRAGE) pulse sequence (TR/TE = 2300/3 ms; FOV = 260 mm; matrix size = 256 × 256; 160 1 mm thick slices).
2.7. Data analytic plan
Raw data were preprocessed and motion corrected, slice time corrected, and B0 unwarped. We used FSL (http://www.fmrib.ox.ac.uk/fsl/) and MATLAB to analyze ROI and connectivity data during the reward task. Subjects with head motion of > 3 mm in any direction for one TR were excluded which is consistent with previous research on adolescents (Bjork et al., 2004). Data were co-registered onto each participant’s MPRAGE using the Montreal Neurological Institute (MNI) brain template. Regressors for onset and duration of reward task events were convolved using double gamma functions and temporal derivatives to create explanatory variables. Motion parameters (and temporal masks if applicable) were added in the first level of analyses. Linear regressions were conducted at each voxel, using generalized least squares with a voxel-wise temporally and spatially regularized auto-correlation model and drift fit with a Gaussian-weighted running line smoother (96 s FWHM). Contrasts of parameter estimates (COPEs) for the win-win trials and the win- neutral trials were calculated for each participant where the duration was the entire trial. Following this, a contrast between activation in win-win minus win-neutral was created.
2.8. Covariates
We did not include demographic variables as covariates given that our sample was fairly homogenous in terms of age, race, and income level. We explored if gender moderated the relationship between estradiol and testosterone and our brain variables. Because it did not significantly moderate the relationship for any of our variables, we did not include gender in subsequent analyses. Additionally, we examined if handedness (4 adolescents were left-handed) was significantly related to estradiol levels, testosterone levels, or brain function and/or connectivity in/between our regions of interest (ROIs). Handedness was not significantly related to our variables and thus we did not include it as a covariate. We also considered as a covariate menstrual cycle phase on the salivary pubertal hormone collection day (e.g. follicular, ovulatory, luteal). We determined menstrual cycle phase based on adolescent report of when the first day of their most recent menstruation occurred. Then, we coded whether they were in menstruating (day 1 to 3) or in the follicular (day 4 to 13), ovulatory (day 14), or luteal phase (15–28) in accordance with Weis et al. (2008) and Wismann and Willoughby (2006). Of the 31 girls, 8 were not menstruating or not regularly menstruating. Of the remaining 23 girls, 13% were in the menstrual phase, 52% were in follicular phase, and 34% were in the luteal phase. We examined whether menstrual cycle phase was correlated with estradiol, testosterone levels or brain function or connectivity. It was not significantly correlated with our variables of interest, and thus we did not include it further in our analyses. Of note, none of the girls were taking hormonal oral contraceptives. Lastly, we examined if reported pubertal stage on the PDS was related to testosterone and estradiol and it was significantly related to testosterone (r = 0.41, p ≤ .001), but not estradiol (r = 0.15, p ≥ .05). Thus, we added the PDS as a covariate in all of our analyses to examine the relationship with hormone levels and the brain above and beyond the relationship between reported pubertal stage and the brain.
2.9. Exploratory whole brain analyses
Whole-brain voxel-wise analyses investigating reward win > neutral outcomes were estimated using FSL’s FLAME mixed effects model. Results were corrected for multiple comparisons using a voxel-based threshold of z > 2.3 and cluster-based correction of p < .05. Estradiol and testosterone were then included as predictors in separate whole-brain group-level analyses.
2.10. Functional activation analyses
Our functional reward analyses focused on a priori ROIs that have been shown in the literature to be associated with reward processing and also correlated with pubertal hormone levels (bilateral NAcc, vmPFC, dlPFC, dmPFC, and ACC). Subcortical ROIs were created using FSL’s Harvard Oxford Atlas (http://www.fmrib.ox.ac.uk/fsl/) and cortical ROIs were created using the Automated Anatomical Labels atlas (AAL; Tzourio-Mazoyer et al., 2002). Notably, since the dmPFC is not included in either the Harvard-Oxford Atlas or the AAL atlas, we used a spherical ROI of 10 mm radius centered around the following MNI co-ordinates from a previous study: dmPFC (x,y,z = 8, 32, 52) (Tavares et al., 2008). Our dmPFC region was bilateral due to its small size and medial location in the brain. Linear regression analyses were conducted to determine whether levels of estradiol and testosterone significantly predicted brain activity in a priori ROIs in the win-win minus win-neutral conditions. To control for multiple comparisons, a Benjamini-Hochberg False Discovery Rate (FDR; Benjamini and Hochberg, 1995) correction was applied at p < .05. FDR-corrected and uncorrected values with estimates of effect size are reported (Table 2). Effect sizes for ROIs and connectivity analyses were determined using the f2 statistic recommended for use in multiple linear regression analyses which is calculated by taking the value of R2 from the analysis (the amount of variance explained by the predictors) divided by 1-R2 to yield an f2 value. According to Cohen (1992), a small effect is an f2 value of 0.02, a medium effect is an f2 value of 0.15, and a large effect is an f2 value of 0.40 (Cohen, 1992).
Table 2.
Results from reward-related connectivity and activation analyses.
| Estradiol |
Testosterone |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Win- Neutral M (SD) | B value | Uncorrected p value | FDR-corrected p value | f2 | B value | Uncorrected p value | FDR-corrected p value | f2 | |
| L dlPFC | 3.23 (19.18) | −0.01 | 0.102 | 0.318 | 0.14 | −0.36 | 0.010* | 0.045* | 0.34 |
| R dlPFC | 1.11 (20.40) | −0.01 | 0.054 | 0.318 | 0.18 | −0.37 | 0.003** | 0.027* | 0.38 |
| L NAcc & L dlPFC | −0.003 (0.077) | −3.67 | 0.014* | 0.049* | 0.18 | −69.96 | 0.017* | 0.175 | 0.24 |
| L NAcc & R dlPFC | 0.012 (0.082) | −3.03 | 0.013* | 0.049* | 0.12 | −53.40 | 0.098 | 0.336 | 0.24 |
| L NAcc & L ACC | −0.010 (0.131) | −2.14 | 0.004** | 0.035* | 0.22 | −28.40 | 0.120 | 0.390 | 0.23 |
| L NAcc & R ACC | −0.004 (0.108) | −2.66 | 0.005** | 0.035* | 0.18 | −41.82 | 0.089 | 0.336 | 0.24 |
Note. L dlPFC = left dorsolateral prefrontal cortex; R dlPFC = right dorsolateral prefrontal cortex; &= connectivity between; L NAcc= left nucleus accumbens; L ACC = left anterior cingulate cortex; R ACC= right anterior cingulate cortex.
p < .05.
p < .01.
2.11. Functional connectivity analyses
We additionally examined task-based functional connectivity between one subcortical seed region involved in reward processing (L&R NAcc) and our ROIs implicated in reward processing and pubertal hormone levels. In order to examine functional connectivity between NAcc and our other regions of interest during the win-neutral trials, we performed psycho-physiological interaction (PPI) analyses using activity in the left or right NAcc and the win-neutral condition as a covariate (Friston et al., 1993). These analyses assess if the activity in one region of the brain (in this case left and right NAcc) can be explained by the interaction of activation in another region of the brain and an experimental condition (win > win-neutral). Our PPI analyses included: (1) a psychological variable (activation in win-neutral), (2) a time series physiological variable (the time series of each seed region), and (3) the interaction between those two variables.
Our seed regions of the left and right NAcc were extracted using FSL’s Harvard Oxford Atlas (http://www.fmrib.ox.ac.uk/fsl/). Then the BOLD time series for each subject’s functional response in the left and right NAcc during win-neutral were extracted. Lastly, at the combined group level, voxel-wise PPI analyses were conducted to identify if our regions (ACC, vmPFC, dmPFC, and dlPFC) exhibited significant increases in functional correlation with the NAcc during win-neutral at a threshold of p < .05 (minimum voxel extent 4) with false discovery rate correction. The interaction variables for the correlations between function in the NAcc and the ACC, vmPFC, dmPFC and dlPFC, respectively, were used in analyses as outcome variables, with estradiol and testosterone levels as predictor variables. The NAcc was used as our seed region due to its importance in neural reward function (e.g. Ernst et al., 2005; Galvan, 2010).
3. Results
3.1. Preliminary data analyses
Primary study variables were examined for outliers (values > 3 standard deviations above or below the mean). L and R NAcc and R dlPFC activation to the win-neutral condition each had one outlier. The connectivity between R NAcc and R dlPFC had one outlier and the connectivity between L NAcc and L vmPFC, L dlPFC, and L ACC each had one outlier. Lastly, the connectivity between L NAcc and R vmPFC and R ACC both had two outliers. Outliers were set to 3 SDs above or below the mean; similar procedures have been used in prior fMRI studies (e.g. Price et al., 2014).
Additionally, variables were examined for normality. Our estradiol variable had high kurtosis (> 2) and thus violated the assumption of normality. Consequently, all analyses were performed with bias-corrected accelerated bootstrapped regressions, with 1000 bootstraps performed for all analyses given that bootstrapping does not assume normality and can yield accurate estimates of standard errors with non-normal data (Barber and Thompson, 2000). No other variables were skewed or kurtotic (skewness and kurtosis < 1).
3.2. Adolescent hormone levels and ROI activation
Adolescents’ testosterone levels were significantly inversely related to R and L dlPFC activation for the win-neutral condition after FDR correction (p < .05) with large effect sizes (f2 = 0.34 and 0.38 respectively; Cohen, 1992). Adolescents’ testosterone and estradiol levels were not significantly associated with ACC, NAcc, vmPFC, or dmPFC activation. Adolescents with higher testosterone levels had lower dlPFC activation in response to the receipt of a reward (win-neutral contrast). Significant bootstrapped R & L dlPFC regression lines covarying reported pubertal stage are depicted in Fig. 1.
3.3. Adolescent hormone levels and functional connectivity
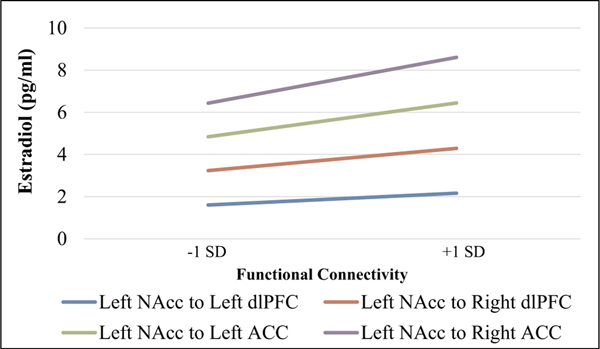
Results indicated a significant negative association between adolescent estradiol levels and functional connectivity between the L NAcc with L & R dlPFC during the win-neutral contrast after FDR correction, with a medium (for L dlPFC: f2 = 0.18) and small to medium (for R dlPFC: f2 = 0.12) effect size (Cohen, 1992). Thus, higher estradiol levels were associated with lower functional connectivity between L NAcc and dlPFC, see Fig. 2 and Fig. 3.
Fig. 2.
Estradiol Functional Connectivity Regression Lines
SD = standard deviation
NAcc =Nucleus Accumbens
dlPFC = dorsolateral prefrontal cortex
ACC = anterior cingulate cortex
*Note: lines are significant bootstrapped regression lines with reported pubertal stage as a covariate.
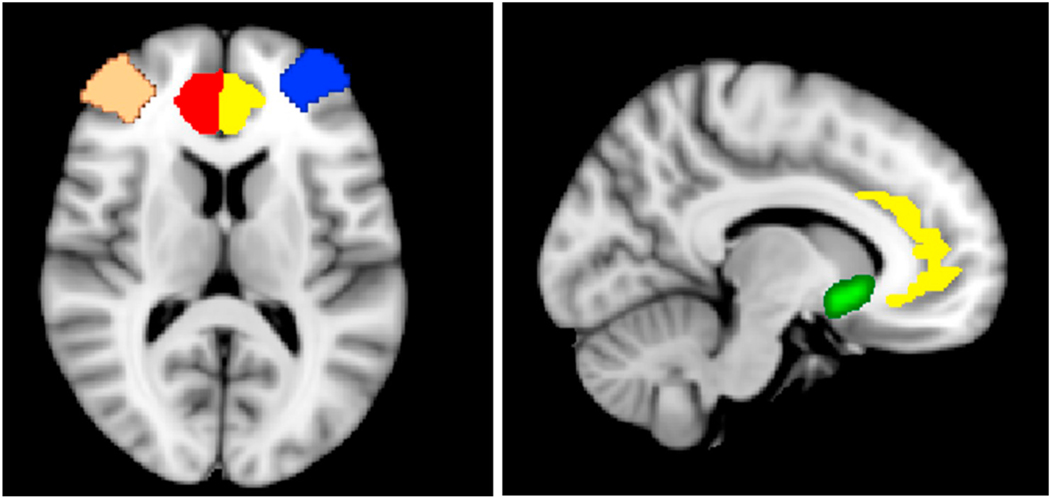
Fig. 3.
Regions with significant activation or connectivity
 = Left Nucleus Accumbens (NAcc)
= Left Nucleus Accumbens (NAcc)
 = Left dorsolateral prefrontal cortex (dlPFC)
= Left dorsolateral prefrontal cortex (dlPFC)
 = Right dorsolateral prefrontal cortex (dlPFC)
= Right dorsolateral prefrontal cortex (dlPFC)
 = Left Anterior Cingulate Cortex (ACC)
= Left Anterior Cingulate Cortex (ACC)
 = Right Anterior Cingulate Cortex (ACC).
= Right Anterior Cingulate Cortex (ACC).
Results also indicated a significant negative association between estradiol levels and connectivity between L NAcc and L & R ACC, after FDR correction, with a medium to large effect size with L ACC (f2 = 0.22) and a medium effect size with R ACC (f2 = 0.18; Cohen, 1992). Testosterone levels were not significantly associated with NAcc connectivity with our regions of interest after FDR correction. In sum, higher estradiol levels (but not testosterone levels) were associated with lower functional connectivity between L NAcc and ACC, see Fig. 2 and Fig. 3.
3.4. Nonlinearity
Given the dearth of literature in this area, we wanted to test whether there is a point at which the direction of the relationship between testosterone, estradiol, and brain variables may change. To test for this possibility of nonlinear effects, we ran the above analyses with an inverse squared function of testosterone and estradiol holding constant the simple effect of testosterone and estradiol. However, these analyses did not yield significant findings and thus we did not include them in our final results figures.
4. Discussion
The findings from this study provide further support for the link between testosterone, estradiol, and neural reward processing and suggest that the onset of puberty is not only linked to brain function but also to connectivity between regions involved in reward processing. Our ROI analyses revealed that adolescents’ testosterone levels were associated with lower bilateral dlPFC activation in response to the receipt of a reward, whereas adolescents’ estradiol levels were not associated with activation in any of the reward ROIs. Functional connectivity analyses showed that estradiol was broadly related to decreased connectivity between the L NAcc and some frontal regions, including the ACC and bilateral dlPFC. This is the first paper to date that examines how testosterone and estradiol are associated with not only striatal and frontal responses to reward, but also functional connectivity between these regions during reward processing.
4.1. Pubertal hormones and adolescent neural response to reward
Functional ROI findings revealed that higher testosterone levels were associated with lower activation in the bilateral dlPFC upon the receipt of a monetary reward. However, inconsistent with our hypotheses, adolescents’ estradiol levels were not related to functional activation in any of the ROIs. Broadly speaking, this suggests that adolescents who have higher levels of testosterone may be less likely to engage frontal regions implicated in decision-making and cognitive control—such as the dlPFC—in the context of reward. Van Leijenhorst et al. (2010) found that dlPFC activation was positively related to lowrisk decisions during a monetary gambling task, suggesting that this region is implicated in the healthy modulation of reward processing. Notably, we did not find any associations between adolescents’ estradiol levels and dlPFC activation. Although results for estradiol were trending towards significance with respect to the dlPFC, they were not as robustly associated with dlPFC as testosterone; therefore, we may have been underpowered to detect possible smaller effects of estradiol on dlPFC activation. Our findings converge with prior research (e.g., Op de Macks et al., 2011, 2016) suggesting that testosterone may perpetuate risky decision-making among youth. However, while prior research has generally found that higher testosterone levels are linked to increased activation of prefrontal regions with links to limbic regions (including those in the striatum), we found that testosterone was linked to decreased engagement of the dlPFC during reward processing, at least during early adolescence when levels of testosterone and estradiol are initially rising.
While not typically considered a putative reward region, the dlPFC has been implicated in a number of executive functioning processes, such as working memory, risky and moral decision-making, cognitive flexibility, planning, inhibition, attention, and performance feedback during reward processing (Choi et al., 2013). The results of the current study suggest that adolescents with higher levels of testosterone may be less able to recruit prefrontal regions to guide higher-level cognitive processes, such as the allocation of attentional resources or effective reward-related regulation. Alternatively, perhaps moderately high levels of testosterone are adaptive for regulating a lower reward region response, while very high levels may be associated with higher engagement of these regions. This may be why other studies have found links between higher testosterone and stronger reward system responses. Notably, however, the current study does not support this hypothesis given that our test of curvilinear effects did not yield significant results. Studies with larger sample sizes should test for the possibility of non-linear relationships between testosterone/estradiol and reward-related ROI activation. Overall, the current study provides preliminary support for the link between testosterone levels and blunted dlPFC activation during reward; future neuroimaging studies should consider examining activation in this region and the role of reward processing and risky decision-making among youth. The blunted activation of these regions may underlie the increase in risk behavior observed during adolescence.
Notably, we did not find associations between either testosterone or estradiol levels and NAcc, ACC, vmPFC, or dmPFC responses to reward. Past studies have suggested that these regions are activated in response to reward among adolescents (e.g., Ernst et al., 2005; Forbes et al., 2010), and some of these regions have also been associated with testosterone levels (e.g., Forbes et al., 2010; Op de Macks et al., 2011, 2016); thus, it is surprising that neither testosterone nor estradiol were associated with altered activation in these regions. For example, prior studies of early adolescents have found associations between higher testosterone levels and higher NAcc (Op de Macks et al., 2011) and vmPFC (Forbes et al., 2010) activation to reward. Perhaps participants in the current study may have experienced more psychological symptoms and consequently different patterns of brain response compared to those in the prior studies, both of which only included healthy adolescents who had no current or lifetime psychiatric conditions. Of note, we did have significantly lower variability in NAcc activation compared to that of our other ROIs (including the dlPFC). This restricted range may reflect the nature of our younger, community-based sample of youth and may at least partially account for our lack of functional activation findings in the NAcc in the present study. Future studies would benefit from including samples with wider age ranges and/or longitudinal designs in order to adequately capture the full range of variability in this important striatal region.
We also did not find any associations between adolescents’ estradiol levels and striatal activation. This is not surprising given that the vast majority of prior literature has either excluded or yielded null effects for estradiol, with the exception of one study which found that girls’ estradiol levels were linked to heightened NAcc activation (Op de Macks et al., 2016). Perhaps estradiol is more closely related to prefrontal circuitry than striatal region activation; alternatively, estradiol could also be more closely linked to “organizational” changes and/or changes in functional connectivity. Future research is needed in order to further clarify how estradiol may impact the level of responsivity in striatal and associated frontal regions (including the dlPFC) during reward processing among early adolescents. Instead of solely examining the level of striatal/NAcc activation, it may be more informative to investigate the extent to which the NAcc is also communicating with other reward-related structures during reward processing.
4.2. Pubertal hormones and reward-related functional connectivity
Our functional connectivity analyses revealed that early adolescents’ higher estradiol levels were related to decreased functional connectivity between the L NAcc and the bilateral dlPFC and bilateral ACC. This pattern of findings suggests that estradiol levels are broadly related to reduced connectivity between a key striatal region, the NAcc, and reward-related cortical regions when early adolescents are engaged in reward processing. Surprisingly, the only two studies to date that have examined estradiol in relation to functional connectivity found that higher levels of during the luteal phase of the menstrual cycle were correlated with enhanced functional communication between hemispheres during both verbal and spatial cognitive tasks (Weis et al., 2008, 2011). However, neither of these studies included adolescents or examined connectivity in the context of a reward task. Given the high number of pubertal hormone receptors in striatal regions and prior research linking estradiol levels to increases in NAcc activation in reward processing and during risky decision-making among adolescents (e.g., Op de Macks et al., 2016), it is plausible that estradiol contributes to decreased communication between striatal and frontal regions in rewarding contexts during early adolescence.
We did not find any significant associations between adolescents’ testosterone levels and their striatofrontal connectivity during reward processing. However, paralleling our findings with respect to estradiol, although they did not survive FDR correction, higher testosterone levels were associated with lower connectivity between the L NAcc and R dlPFC. Although non-significant, this dovetails with our functional ROI findings showing reduced dlPFC engagement among adolescents with higher pubertal hormone levels (Stanton et al., 2009). Testosterone has also been linked to lower connectivity between subcortical and cortical regions, including those involved in reward processing (Peper et al., 2011; Schutter and Honk, 2004). Developmentally, functional connectivity between distal brain regions strengthens over the course of adolescent development (Casey et al., 2005; Dosenbach et al., 2010; Supekar et al., 2009). However, no study to date has examined how testosterone or estradiol may influence functional communication between key reward-related regions.
It is possible that both estradiol and testosterone do not directly contribute to the increased connectivity observed across adolescence; instead, organizational or activational effects on neural reward responsivity or cognitive processing may mediate the effects of testosterone or estradiol. Finally, there may be other maturational factors that account for this increased communication between distal brain regions. At least among early adolescents and adults, surges (or high levels) of testosterone or estradiol in certain individuals may temporarily (or permanently) allow for under-controlled reactivity to reward in the brain due to lower dlPFC engagement and lower NAcc-prefrontal connectivity. As adolescents mature, this temporary reduction in communication between distal brain regions may increase across later adolescence and into young adulthood. Alternatively, testosterone and especially estradiol may influence neural reward processing differentially based on an individuals’ level of pubertal maturation. For example, the eating disorder literature suggests that hormone levels that are too low or too high may lead to disruptions in normative development and predispose adolescents to engage in dysregulated behaviors such as binge eating (Culbert et al., 2014). Future studies with larger sample sizes should test the possibility of curvilinear relationships between adolescents’ hormone levels and patterns of neural reward processing. Our exploratory functional connectivity results provide preliminary evidence consistent with the extant adult literature suggesting that estradiol (and potentially testosterone) may decrease subcortical-cortical connectivity, particularly during the processing of reward. Specifically, adolescents with higher testosterone show decreased frontal engagement (dlPFC) and that adolescents with higher levels of estradiol exhibit reduced striatofrontal connectivity during reward processing which, in turn, may be a possible mechanism underlying increases in sensation-seeking and risk behavior in adolescence.
4.3. Limitations and future directions
The present study is the first to date to investigate how early adolescents’ testosterone and estradiol levels are associated with both functional activation and striatal-cortical connectivity during the processing of a monetary reward. Despite the intriguing pattern of findings, there are a number of limitations that may inform future research. First, the current study only included the assessment of testosterone and estradiol; future studies employing female participants should also consider measuring progesterone, another pubertal hormone that has been found to influence mesolimbic dopaminergic system functioning. Second, given that the concentration of testosterone and estradiol tends to be lower in saliva, this may have led to lower variability and decreased power to find associations in our study. Blood measures may be useful for future studies. Third, consistent with prior research (e.g., Forbes et al., 2010), the current study focused on adolescent neural processing upon the receipt of a monetary reward. Future investigators should consider examining other types of reward, including social rewards. Finally, consistent with virtually all prior research on this topic, the current study was cross-sectional; future studies should examine how pubertal hormone levels longitudinally contribute to their neural reward processing.
Overall, results of the current study indicate that testosterone and estradiol may result in lower cortical activation in dlPFC and reduced functional connectivity between cortical brain regions and NAcc during reward processing, respectively. In order to fully elucidate the links between puberty, reward-related processing, and risky decision-making, future studies should investigate how neural reward sensitivity may serve as a mechanism linking testosterone and estradiol with engagement in risk behavior across time. These findings provide further support for the link between estradiol, testosterone, and neural reward processing, and may meaningfully inform preventative efforts targeted at reducing adolescents’ vulnerability to risky behaviors. For example, our findings suggest that in early adolescence, early rises in testosterone and estradiol may lead to (potentially temporary) lowered levels of cognitive control over reward system activation, suggesting that interventions for early adolescents should target youth with low cognitive control during that sensitive developmental period.
Acknowledgement
This study was was supported through a NIH/NIDA Award (R01-DA033431, R01-DA033431-S1; PI: Chaplin).
References
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA, 2011. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J. Neurosci 31 (28), 10340–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JA, Thompson SG, 2000. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat. Med 19 (23), 3219–3236. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol 289–300. [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW, 2004. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci 24, 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW, 2010. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One 5, e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, 2012. Imaging brain development: the adolescent brain. Neuroimage 61, 397–406. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA, 2006. Acute stress reduces reward responsiveness: implications for depression. Biol. Psychiatry 60, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA, 2015. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P, 2001. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30, 619–639. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Gray LE, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, … Bourguignon JP, 2008. Environmental factors and puberty timing: expert panel research needs. Pediatrics 121 (Supplement 3), S192–S207. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR, 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci 99, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, 1993. A self-administered rating scale for pubertal development. J. Adolesc. Health 14 (3), 190–195. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA, 2005. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol 15, 239–244. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones Rebecca M., Somerville Leah H., 2011. Braking and accelerating of the adolescent brain. J. Res. Adolesc 21 (1), 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L, 2011. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WH, Son JW, Kim YR, Oh JH, Lee SI, Shin CJ, … Ha TH, 2013. An FMRI study investigating adolescent brain activation by rewards and feedback. Psychiatry Investig. 10, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A, 2013. Informatic parcellation of the network involved in the computation of subjective value. Soc. Cogn. Affect. Neurosci 9, 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1992. A power primer. Psychol. Bull 112, 155. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS, 2005. Gonadal hormone modulation of dendrites in the mammalian CNS. Dev. Neurobiol 64, 34–46. [DOI] [PubMed] [Google Scholar]
- Crone EA, Ridderinkhof KR, 2011. The developing brain: from theory to neuroimaging and back. Dev. Cogn. Neurosci 1, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, Sisk CL, Nigg JT, Klump KL, 2014. The effects of circulating testosterone and pubertal maturation on risk for disordered eating symptoms in adolescent males. Psychol. Med 44 (11), 2271–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, 2007. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP, 2010. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 72 (1), 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F, 2006. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl. Dev. Sci 10, 30–56. [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, … Barnes KA, 2010. Prediction of individual brain maturity using fMRI. Science 329, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, … Pine DS, 2005. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage 25, 1279–1291. [DOI] [PubMed] [Google Scholar]
- Ferree NK, Kamat R, Cahill L, 2011. fluences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Conscious. Cogn 20, 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, 2010. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 72, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE, 2009. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatr 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, … Dahl RE, 2010. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry 49, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force E (1996). USPST. Guide to clinical preventive services. Alexandria, VA: International Medical Publishing. [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ, 1993. Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 13, 5–14. [DOI] [PubMed] [Google Scholar]
- Galvan A, 2010. Adolescent development of the reward system. Front. Hum. Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ, 2006. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci 26, 6885–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ, 2007. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 10, 8–14. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER, 2014. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum. Brain Mapp 35, 5633–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D, 2003. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18, 263–272. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD, 2004. Separate neural systems value immediate and delayed monetary rewards. Science 306, 503–507. [DOI] [PubMed] [Google Scholar]
- Morris RW, Dezfouli A, Griffiths KR, Balleine BW, 2014. Action-value comparisons in the dorsolateral prefrontal cortex control choice between goal-directed actions. Nat. Commun 5, 4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS, 2005. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med 35 (2), 163–174. [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Moor BG, Overgaauw S, Güroğlu B, Dahl RE, Crone EA, 2011. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev. Cogn. Neurosci 1, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks ZAO, Bunge SA, Bell ON, Wilbrecht L, Kriegsfeld LJ, Kayser AS, Dahl RE, 2016. Risky decision-making in adolescent girls: the role of pubertal hormones and reward circuitry. Psychoneuroendocrinology 74, 77–91. [DOI] [PubMed] [Google Scholar]
- Ozer EM, Macdonald T, Irwin CEJ, 2002. Adolescent health care in the United States: Implications and projections for the new millennium In: Mortimer JT, Larson RW (Eds.), Changing Adolescent Experience: Societal Trends and the Transition to Adulthood. Cambridge University Press, Cambridge. [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Pol HEH, van Honk J, 2011. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology 36, 1101–1113. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Hervé PY, Leonard G, Perron M, Pike GB, Pitiot A, … Paus, T., 2008. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J. Neurosci 28, 9519–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc 17 (2), 117–133. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC, 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. [DOI] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, Ryan ND, 2014. Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depress. Anxiety 31, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL, 2009. Back to the future: the organizational–activational hypothesis adapted to puberty and adolescence. Horm. Behav 55, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ, Honk JV, 2004. Decoupling of midfrontal delta–beta oscillations after testosterone administration. Int. J. Psychophysiol 53, 71–73. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ, 2001. Use of salivary biomarkers in bio-behavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology 26, 165–173. [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Wirth MM, Waugh CE, Schultheiss OC, 2009. Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biol. Psychol 81, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H, 2011. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb. Cortex 21, 2578–2588. [DOI] [PubMed] [Google Scholar]
- Steinberg L, 2008. A social neuroscience perspective on adolescent risk-taking. Dev. Rev 28, 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares JVT, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC, 2008. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage 42, 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SA, Westenberg PM, Crone EA, 2010. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage 51, 345–355. [DOI] [PubMed] [Google Scholar]
- Vigil P, Orellana RF, Cortés ME, Molina CT, Switzer BE, Klaus H, 2011. Endocrine modulation of the adolescent brain: a review. J. Pediatr. Adolesc. Gynecol 24, 330–337. [DOI] [PubMed] [Google Scholar]
- Volman I, Toni I, Verhagen L, Roelofs K, 2011. Endogenous testosterone modulates prefrontal–amygdala connectivity during social emotional behavior. Cereb. Cortex 21, 2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Hausmann M, Stoffers B, Vohn R, Kellermann T, Sturm W, 2008. Estradiol modulates functional brain organization during the menstrual cycle: an analysis of interhemispheric inhibition. J. Neurosci 28, 13401–13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Hausmann M, Stoffers B, Sturm W, 2011. Dynamic changes in functional cerebral connectivity of spatial cognition during the menstrual cycle. Hum. Brain Mapp 32, 1544–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismann J, Willoughby D, 2006. Gender differences in carbohydrate metabolism and carbohydrate loading. J. Int. Soc. Sports Nutr 3 (1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]