Abstract
COVID-19 is generally a benign or asymptomatic infection in children, but can occasionally be severe or fatal. Delayed presentation of COVID-19 with hyperinflammation and multi-organ involvement was recently recognized, designated the Multisystem Inflammatory Syndrome in Children (MIS-C). Six children with MIS-C with molecular and serologic evidence of SARS-CoV-2 infection were admitted to our hospital between May 5, 2020 and June 25, 2020. All had fever and weakness; 4/6 presented with gastrointestinal symptoms. Two children had features of complete Kawasaki disease, 3 had incomplete Kawasaki disease, while 1 had terminal ileitis with delayed onset of circulatory shock. Treatment consisted of intravenous immunoglobulin and aspirin for Kawasaki-like disease. Remdesivir, corticosteroids, and infliximab were used when indicated. Median hospitalization was 7 days. Immediate treatment resulted in rapid clinical improvement. In children presenting with hyperinflammatory syndromes without cardiac manifestations, testing for SARS-CoV-2 RNA and antibodies, with close cardiac monitoring should be pursued due to the manifold presentations of SARS-CoV-2 infection in children.
Keywords: COVID-19, ileitis, Kawasaki disease, MIS-C, myocarditis, SARS-CoV-2
Introduction
SARS-CoV-2 was identified in December 2019 as causing a cluster of pneumonia in Wuhan City, China, although the first affected individual (“patient zero”) may have been an adult in Hubei province on November 17, 2019.1 The disease, termed COVID-19, was declared a global pandemic on March 11, 2020.1 SARS-CoV-2 infections in adults can range from the asymptomatic to the fatal, with the most feared illnesses being severe interstitial pneumonia and hyperactivation of the inflammatory cascade.2,3 By way of contrast, an early systematic literature review of pediatric SARS-CoV-2 infection indicated that children typically had asymptomatic infection or mild respiratory symptoms such as dry cough, fever, and fatigue.4 More recently, there emerged reports of children in Europe presenting with a severe hyperinflammatory syndrome and multi-organ involvement with some deaths temporally related to SARS-CoV-2 infection, leading to widespread concerns among physicians and parents alike.5,6 This has since been identified in the United States, prompting the Centers for Disease Control and Prevention (CDC) to issue a warning and provide a case definition and guidance on diagnosis and management on what is now termed Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) in the United States and Pediatric Inflammatory Multisystem Syndrome (PIMS) in Europe.5-7 Within a span of 7 weeks, we encountered 6 children with MIS-C with myriad clinical presentations, all clearly linked to SARS-CoV-2 infections. The first 5 were hospitalized within the first 2.5 weeks of this time interval and the sixth a month later, mirroring the level of SARS-CoV-2 disease activity in Southeast Michigan. This report describes their clinical presentations, management, and short-term outcomes.
Materials and Methods
Children younger than 18 years of age admitted to Beaumont Children’s Hospital, Royal Oak, Michigan between May 5, 2020 and June 25, 2020 with molecular and/or serologic evidence of SARS-CoV-2 infection were eligible for the study. These included 6 children diagnosed with MIS-C.
SARS-CoV-2 infection was confirmed with RNA detection in nasopharyngeal swabs using the Centers for Disease Control and Prevention 2019-nCoV Real-Time Reverse Transcriptase-PCR Diagnostic Panel (CDC qPCR) and/or by detection of SARS CoV-2 IgG and IgA responses by enzyme immunoassay (EUROIMMUN AG, Germany). MIS-C was defined according to the United States CDC criteria as enumerated in Table 1. Diagnostic criteria for Kawasaki Disease are also described in Table 1.
Table 1.
Diagnostic Criteria for Multisystem Inflammatory Syndrome in Children Associated with COVID-19 (MIS-C) and Kawasaki Disease.
| Multisystem Inflammatory Syndrome in Children Associated with COVID-19 (MIS-C)a: |
| - Age <21 years |
| - Fever ≥38°C or report of subjective fever for ≥24 hours |
| - Severe illness requiring hospitalization |
| - ≥2 organ systems involved (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological) |
| - Laboratory evidence of inflammation (one or more of the following: elevated C-reactive protein, erythrocyte sedimentation rate, fibrinogen, procalcitonin, lactic acid dehydrogenase, d-dimer, fibrinogen, or interleukin-6, elevated neutrophil count, reduced lymphocyte count, and low serum albumin |
| - No alternative plausible diagnosis |
| AND |
| - Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or exposure to a suspected or confirmed COVID-19 case within the 4 weeks prior to onset of symptoms |
| Kawasaki Disease b |
| - Fever lasting ≥5 days |
| AND |
| - At least 4 of the following criteria (complete Kawasaki disease) or 2–3 criteria (incomplete Kawasaki disease): |
| Rash |
| Cervical lymphadenopathy (≥1.5 cm in diameter) |
| Bilateral conjunctival injection |
| Oral mucosa changes |
| Peripheral extremity changes |
Echocardiograms were performed for children meeting the criteria for complete or incomplete Kawasaki disease or if they required inotropic support. Treatment consisted of a combination of intravenous gammaglobulin (IGIV), high-dose aspirin, and/or methylprednisolone, as determined by the treating physicians. IGIV was given as a 2 g/kg infusion over 10 to 12 hours, methylprednisolone at a dose of 2 mg/kg per day, and aspirin at 80 to 100 mg/kg per day in 4 divided doses followed by low-dose aspirin at 3 to 5 mg/kg once daily for 6 to 8 weeks. Infliximab (5 mg/kg once daily for 2 days) was used for 1 child with IGIV-resistant Kawasaki disease.
Ethical Approval and Informed Consent
The study was approved by the Institutional Review Board (IRB# 2020-106) for Beaumont Health. A waiver for informed consent was granted by the IRB as this was part of a large registry for a study entitled “In House COVID-19 Testing Experience at a Tertiary Children’s Hospital in the Metropolitan Detroit Area; A Case Series.” The electronic health records were reviewed for demographic information, clinical presentation, laboratory and imaging data, treatment modalities, and outcomes. Outpatient follow up data were included where available.
Results
Between May 5, 2020 and June 25, 2020, 6 children were treated at Beaumont Children’s Hospital, 5 in the Pediatric Intensive Care Unit (PICU), for SARS-CoV-2-related MIS-C. Demographic data and presentation features are summarized in Table 2. The median age was 8 years (range, 9 months-14 years). Four were girls. None had underlying cardiac disease. Pre-existing comorbidities were limited to 1 with asthma and 1 who had a controlled seizure disorder.
Table 2.
Demographic and Clinical Characteristics (N = 6).
| Characteristic | Value, N (%) |
|---|---|
| Age (years) | |
| Median | 8 years |
| Age <1 year | 2 (33.3) |
| Age 1–5 years | 0 (0) |
| Age 6–10 years | 2 (33.3) |
| Age 11–16 years | 2 (33.3) |
| Sex | |
| Female | 4 (66.7) |
| Male | 2 (33.3) |
| Race | |
| African American | 2 (33.3) |
| Caucasian | 2 (33.3) |
| Hispanic | 1 (16.7) |
| Biracial | 1 (16.7) |
| Comorbidity | |
| None | 4 (66.7) |
| Asthma | 1 (16.7) |
| Seizure disorder | 1 (16.7) |
| Clinical characteristics | |
| Fever | 6 (100) |
| Weakness | 6 (100) |
| Gastrointestinal symptoms | 4 (66.7) |
| Respiratory difficulties | 3 (50) |
| Rash | 4 (66.7) |
| Conjunctival injection | 3 (50) |
| Cervical adenopathy | 1 (16.7) |
SARS-CoV-2 infection was confirmed in all 6 patients. Nasopharyngeal swab PCR detected SARS-CoV-2 RNA in 4 out of 6 children (66.7%). Five were positive for SARS-CoV-2 IgA and IgG, but 1 child was not tested (case 2). Case 3 had 3 negative nasopharyngeal swabs by PCR but had IgG and IgA antibodies for SARS-CoV-2.
All 6 children presented with daily high fever for 1 to 9 days (median, 3 days), with temperatures ranging from 38.2°C to 40°C. The children also presented with “weakness,” and 4 had gastrointestinal symptoms (vomiting, abdominal pain, and/or diarrhea). Three children required inotropic support for 3 to 6 days upon admission. Case 3 developed hypotension and pulmonary disease requiring fluid boluses after hospitalization, but no inotropic agents. Two were diagnosed with features consistent with complete Kawasaki disease and 3 others had features of incomplete Kawasaki disease (or Kawasaki-like MIS-C). Case 3 presented with peritonitis and was diagnosed with MIS-C when SARS-CoV-2 antibodies were found to be positive.
Laboratory data, imaging and echocardiographic findings, and treatments received are summarized in Table 3. Peak d-dimer values ranged from 1124 to 5203 ng/mL (normal 0-499 ng/mL), peak BNP level from 368 to 1317 pg/mL (normal 0-100 pg/mL), and troponin I between <0.01 and 2.44 ng/mL (normal 0.00-0.03 ng/mL) among those tested in our series. Four of 6 children were noted to have lymphopenia on initial laboratory tests. Gallbladder hydrops was noted in 1 infant (case 1).
Table 3.
Laboratory, Imaging, and Treatment Summary Data.
| Laboratory test | Case 1 (M, 9 months) | Case 2 (F, 10 months) | Case 3 (M, 7 years) | Case 4 (F, 9 years) | Case 5 (F, 12 years) | Case 6 (F, 14 years) | Median | Normal range |
|---|---|---|---|---|---|---|---|---|
| D-dimer (ng/mL), peak | 2372 | 1124 | ND | 2174 | 4499 | 5203 | 2372 | 0–499 |
| BNP (pg/mL), peak | 409 | ND | ND | 1317 | 807 | 368 | 608 | 0–100 |
| Troponin I (ng/mL), peak | <0.01 | 0.01 | ND | 0.04 | 0.21 | 2.44 | 0.04 | ≤0.03 |
| CRP (mg/L), peak | 44.8 | 50.1 | 326.17 | 173.7 | 260.6 | 61.2 | 117.5 | 0.0–7.9 |
| Ferritin (ng/mL), peak | 184 | 148 | ND | 1362 | 965 | 486 | 486 | 12–207 |
| Fibrinogen (mg/dL) | ND | 505 | ND | 624 | 606 | 418 | 555.5 | 200–400 |
| Lactate dehydrogenase (U/L), peak | ND | 402 | ND | 381 | 364 | ND | 381 | 140–280 |
| PT (seconds) | ND | 13.5 | ND | 12 | 12.5 | 17.6 | 13 | 9.2–13.5 |
| Admission WBC (bil/L) | 17.1 | 14.1 | 14.2 | 16 | 10.7 | 5.3 | 14.15 | 5–14 |
| Admission lymphocyte count (bil/L) | 4.6 | 5.1 | 0.8 | 1.1 | 0.7 | 0.3 | 0.95 | 1.5–6.5 |
| Chest radiograph | Normal | Small airway disease | Right-sided airspace opacities; right pleural effusion | Normal | Normal | Bibasilar interstitial opacities | NA | NA |
| Echocardiogram, day 1 of admission | Normal, EF 69.7% (day 5 of fever) | Normal, EF 67% (day 4 of fever) | Normal, EF 57% (day 7 of fever) | Mild dilation of LAD (3.3 mm), EF 55% (day 8 of fever) | Trivial to small pericardial effusion, EF 82% (day 3 of illness) | Normal, EF 60% (day 5 of fever) | NA | NA |
| Echocardiogram, day 5 of admission | ND | ND | ND | Stable dilation of LAD (3 mm), EF 65% (day 12) | Mildly dilated LMCA (4.7 mm), moderately dilated proximal LAD (4.4 mm), small aneurysm at left coronary artery bifurcation (5.3 mm), EF 68% (day 8 of illness) | Trivial pericardial effusion, EF 54–56% (day 9 of illness) | NA | NA |
| Echocardiogram at 2-week follow up | Normal, EF 63% | ND | Normal, EF 70% | Normal, EF 63% | Improved coronary artery dilation and pericardial effusion, LMCA (4.2 mm), LAD (3.3 mm), EF 63% | Normal, EF 67% | NA | NA |
| Echocardiogram 1 month after presentation | ND | ND | ND | ND | Coronary artery dilation and pericardial effusion resolved, EF 57% | ND | Normal, EF 64% | NA |
| Echocardiogram 6–8 weeks after presentation | Normal, EF 69% | Normal, EF 63% | ND | Normal, EF 66% | Normal, EF 57% | Normal, EF 64%a | NA | NA |
| Treatment | ||||||||
| IGIV | + | + | – | + | + | + | NA | NA |
| Corticosteroids | – | – | + | – | + | + | NA | NA |
| High-dose aspirin | + | + | – | + | + | – | NA | NA |
| Remdesivir | – | – | – | – | – | + | NA | NA |
| Infliximab | – | – | – | – | + | – | NA | NA |
| Inotropic support | – | – | – | + | + | + | NA | NA |
| Supplemental oxygen | – | – | + | – | – | + | NA | NA |
Abbreviations: BNP, B type natriuretic peptide; CRP, C-reactive protein; EF, ejection fraction; F, female; IGIV, intravenous immunoglobulin; LAD, left anterior descending artery; LMCA, left main coronary artery; M, male; NA, not applicable; ND, not done; PT, prothrombin time; WBC, white blood cell count.
Echocardiogram done 15 weeks after presentation, not 6 to 8 weeks.
Three children had echocardiographic abnormalities, 2 of whom had coronary artery dilation/aneurysm. These 3 children, along with cases 1 and 2 with an initial diagnosis of incomplete Kawasaki disease, were treated with IGIV and high-dose aspirin for 2-4 days. Case 6 was additionally treated with methylprednisolone for 5 days and remdesivir due to requirement of supplemental oxygen by high flow nasal cannula and evidence of multifocal pneumonia on chest radiographs.
Case 3 was initially thought to have peritonitis. He had a history of recurrent, almost identical, febrile illnesses with abdominal pain occurring at irregular intervals during the preceding year, each lasting for 2 to 4 days. A periodic fever syndrome such as familial Mediterranean fever was suspected. CT scan of the abdomen and pelvis (with oral and intravenous contrast) revealed terminal ileitis and mesenteric lymphadenopathy, without appendicitis (Figure 1). He became hypotensive and had pulmonary infiltrates on the second hospital day, was treated with 5 days of intravenous methylprednisolone only, and quickly improved. The child was diagnosed with MIS-C after the detection of SARS-CoV-2 IgG and IgA antibodies. His mother also harbored IgG and IgA antibodies to SARS-CoV-2 but was never ill. Subsequent genetic testing for periodic fever-associated gene mutations was negative. The child never developed coronary artery abnormalities.
Figure 1.
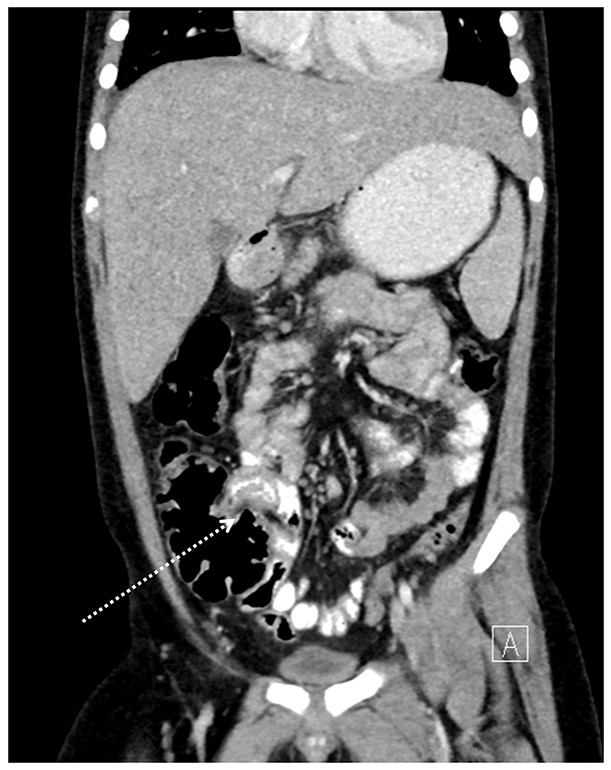
Coronal reconstruction of contrast-enhanced CT demonstrates wall thickening of the terminal ileum (arrow).
The 12-year-old girl (case 5) was African American and her first elevated body temperature of 38.2°C was noted on admission. She had limited clinical improvement after 2 IGIV doses, high-dose aspirin, and 5 days of methylprednisolone. Within less than 24 hours of stopping methylprednisolone, she had relapse of her Kawasaki-like disease with high fever, intense eye redness, and palmar erythema. She improved quickly after 2 infliximab doses, and was sent home on a 2-week tapering regimen of prednisone.
Clinical and hemodynamic improvement was noted within 24 hours of initiation of treatment in the 5 other children. Median duration of hospitalization was 7 days (range, 4-10 days). All had follow up evaluations at various intervals after hospital discharge. Follow up echocardiograms were obtained on 5 children 2 weeks after their initial presentation, 4 of which were normal. For case 5, coronary artery dilation resolved a month after hospital discharge. Case 2 was initially lost to follow-up, but an echocardiogram 6 to 8 weeks after hospital discharge was normal (Table 3).
Five of the 6 children in this case series were hospitalized within a 2.5-week time period between May 5, 2020 and May 23, 2020, about 4 to 6 weeks after Michigan reported its peak number of COVID-19 cases. The number of COVID-19 cases declined considerably after the mandatory lockdown imposed by the State of Michigan, and only 1 infant with MIS-C was admitted to our hospital a month after the initial cluster of 5 children.
Discussion
We report a case series of 6 children with multisystem inflammatory syndrome associated with COVID-19, presenting largely with findings compatible with complete or incomplete Kawasaki disease. All had evidence of SARS-COV-2 infection, of whom 5 had mounted IgA and IgG responses to the virus (one infant was not tested) suggesting that the clinical illnesses are predominantly post-infectious hyperinflammatory manifestations. The varied clinical presentations make the diagnosis of MIS-C challenging and needs to be considered in a broad range of circumstances. The frequency of gastrointestinal manifestations, older median age at onset, hypotension, echocardiographic evidence of cardiac dysfunction more than coronary artery involvement, and strong association with SARS-CoV-2 infection seem to separate this illness into a distinct clinical entity. Gastrointestinal complaints in febrile children may be the sole presenting feature for SARS-COV-2-related MIS-C, as described in case 3.8
Kawasaki disease is an acute febrile vasculitis of the medium-sized vessels with multisystem involvement, which typically affects children less than 5 years of age.9 In the acute phase of the disease, children with Kawasaki disease might have hemodynamic instability, a condition known as Kawasaki disease shock syndrome (KDSS).10 Multisystem inflammatory syndrome in children has been recognized to have features similar to Kawasaki disease, bacterial sepsis, toxic shock syndrome, and macrophage activation syndrome. It can present with peculiar abdominal symptoms and marked elevation of inflammatory markers.11
A study from France and Switzerland reported acute cardiac decompensation due to an inflammatory state secondary to SARS-CoV-2 infection.5 This emerging condition manifested by features overlapping with toxic shock syndrome and atypical Kawasaki disease together with evidence of cardiac inflammation is termed multisystem inflammatory syndrome in children (MIS-C).5 The overlapping characteristics of MIS-C and Kawasaki disease are thought to be due to similarity in pathophysiology.5 Antibodies against SARS-CoV-2 can be detected in the middle and later stage of the illness.12 Their presence generally indicates exposure to the virus about 3 weeks or longer prior to admission.5
Another case study of 8 patients in the United Kingdom demonstrated a phenomenon of hyperinflammatory syndrome and multiorgan involvement.6 They reported presentations with unrelenting fever, variable rash, conjunctivitis, peripheral edema, and significant gastrointestinal symptoms. The children had no significant respiratory involvement but progressed to vasoplegic shock requiring noradrenaline and milrinone for hemodynamic support. Unlike our study, children tested negative for SARS-CoV-2 RNA on bronchoalveolar lavage or nasopharyngeal aspirate samples, and a common echocardiographic finding was echobright coronary vessels that progressed to a giant coronary aneurysm in 1 child within a week.
In a case series of 58 children in England with PIMS, 43% required respiratory support with mechanical ventilation, while in our study none required this level of respiratory care.13 In another case series of 17 children with MIS-C from New York City, children presented with fever, gastrointestinal symptoms and required inotropic support with zero requiring mechanical ventilation, similar to our study. They reported left ventricular dysfunction without coronary dilatation, unlike 2 children in our study that had coronary abnormalities as mentioned in Table 3.14 In all of the above studies, children were treated with IGIV and aspirin with/without corticosteroids as well as other immunomodulators.
In a recent study of 576 children <18 years of age hospitalized in the United States with laboratory confirmed COVID-19 reported to COVID-NET (COVID-19-Associated Hospitalization Surveillance Network), 208 had their charts carefully reviewed. A third required admission to PICUs, 4.8% needed vasopressor support, 9.1% received corticosteroids, and 6.7% were administered IGIV. Only 9 had a discharge diagnosis of MIS-C, 5 of whom were 2 to 4 years of age and 3 were 5 to 17 years of age.15 In a systematic review by the Centers for Disease Control and Prevention of all published MIS-C cases reported between April 25, 2020 and June 29, 2020, 8 studies comprising 440 diagnosed children from various countries were included. The median ages across studies were 7.3 to 10 years, of whom only 13% to 69% had positive SARS-CoV-2 RNA and 75% to 100% had positive serologic test results against the virus. Gastrointestinal symptoms were the most common (87%), followed by dermatologic (73%) and cardiac (71%) manifestations; 0% to 2% of children across the studies died from MIS-C.16
We noted clinical improvement within 24 hours of initiation of treatment with IGIV, aspirin, and/ or corticosteroids in 5 children. Case 5 in our series had features of IGIV-resistant Kawasaki disease. She had progression of coronary dilatation with aneurysm formation after 2 doses of IGIV. Rapid improvement in coronary dilatation was noted upon treatment with infliximab. Case 3 was treated with only intravenous methylprednisolone due to a history of recurrent fever and concern for a periodic fever syndrome; there was low suspicion for COVID-19 infection with lack of features of Kawasaki disease. This child was found to have terminal ileitis and mesenteric lymphadenopathy, and then developed hypotension requiring saline fluid boluses. He had IgA and IgG antibodies against SARS-CoV-2. Testing for genetic mutations associated with periodic fever syndromes was later shown to be negative. All children were discharged after a median of 7 days.
MIS-C appears to be a post-infectious complication of SARS-CoV-2 infection, possibly related to IgG development, although an 11-year-old girl who quickly died of this condition had histologic pancarditis with the identification viral particles consistent with coronaviruses within several cardiac muscle cells, capillary endothelial cells, and endocardium. SARS-CoV-2 RNA was detected in cardiac and lung tissues.17 A recent study comparing echocardiographic findings among 28 children with MIS-C, 20 controls, and 20 with classic Kawasaki disease found that only 4% of those with MIS-C had coronary artery dilation. Left ventricular systolic and diastolic function was worse among children with MIS-C as compared to those with Kawasaki disease.18 In a case series of 4 children with myocarditis and MIS-C with no detectable SARS-CoV-2 RNA in nasopharyngeal, respiratory, and stool samples, but positive serologic test results for viral antibodies, cardiac MRI revealed diffuse myocardial edema without gadolinium enhancement, which was suggestive of replacement fibrosis or focal necrosis. This was felt to support a post-infectious immune-mediated myocarditis.19
Antibodies to the virus can appear as early as the second week of infection, and it is not possible to retrospectively determine the timing of initial SARS-CoV-2 infection.20 Regardless of exact etiopathogenesis, children with MIS-C have hyperinflammation with high levels of interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-17, and interferon-γ, high CD64 expression on neutrophils and monocytes, and B and T cell lymphopenia, all of which return to normal after recovery.21
In conclusion, multisystem inflammatory syndrome in children (MIS-C) presents frequently with Kawasaki-like disease and immediate initiation of appropriate treatment with IGIV, aspirin, and/or corticosteroids typically resulted in rapid clinical improvement in our case series. In children presenting with hyperinflammatory syndromes without cardiac manifestations, testing for SARS-CoV-2 RNA and antibodies, with close cardiac monitoring, should be part of the medical management.
Footnotes
Author Contributions: Drs. Shikhare, Iqbal, Tariq, Turner, Gebara, and Freij designed the study, reviewed the medical literature, participated in writing earlier drafts of the paper, and approve the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bishara J. Freij  https://orcid.org/0000-0002-5870-4356
https://orcid.org/0000-0002-5870-4356
References
- 1. Mehta NS, Mytton OT, Mullins EWS, et al. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis. 2020;71:2469-2479. doi: 10.1093/cid/ciaa556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882-889. doi: 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 5. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429-436. doi: 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 6. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608. doi: 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771-1778. doi: 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: a single center experience of 44 cases. Gastroenterology. 2020;159:1571-1574.e2. doi: 10.1053/j.gastro.2020.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawasaki T. Kawasaki disease. Proc Jpn Acad Ser B Phys Biol Sci. 2006;82:59-71. doi: 10.2183/pjab.82.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783-e789. doi: 10.1542/peds.2008-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health. 2020;4:e21-e23. doi: 10.1016/S2352-4642(20)30164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1930-1934. doi: 10.1093/cid/ciaa461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi: 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294-296. doi: 10.1001/jama.2020.10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 – COVID-NET, 14 states, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081-1088. doi: 10.15585/mmwr.mm6932e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45-54.e1. doi: 10.1016/j.jpeds.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790-794. doi: 10.1016/S2352-4642(20)30257-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsubara D, Kauffman HL, Wang Y, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. 2020;76:1947-1961. doi: 10.1016/j.jacc.2020.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297:E283-E288. doi: 10.1148/radiol.2020202288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20:453-454. doi: 10.1038/s41577-020-0367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701-1707. doi: 10.1038/s41591-020-1054-6 [DOI] [PubMed] [Google Scholar]


