Abstract
The purpose of the present review is to provide an update of the available recent scientific literature on the use of magnetic resonance imaging (MRI) in Alzheimer’s disease (AD). MRI is playing an increasingly important role in the characterization of the AD signatures, which can be useful in both the diagnostic process and monitoring of disease progression. Furthermore, this technique is unique in assessing brain structure and function and provides a deep understanding of in vivo evolution of cerebral pathology. In the reviewing process, we established a priori criteria and we thoroughly searched the very recent scientific literature (January 2018–March 2020) for relevant articles on this topic. In summary, we selected 73 articles out of 1654 publications retrieved from PubMed. Based on this selection, this review summarizes the recent application of MRI in clinical trials, defining the predementia stages of AD, the clinical utility of MRI, proposal of novel biomarkers and brain regions of interest, and assessing the relationship between MRI and cognitive features, risk and protective factors of AD. Finally, the value of a multiparametric approach in clinical and preclinical stages of AD is discussed.
Keywords: Alzheimer’s disease, biomarkers, mild cognitive impairment, MRI, neuroimaging
Introduction
In the past few years, with the increasing aging of the general population worldwide, there has been a strong consensus that an early diagnosis of dementia makes a difference in terms of health care and economic planning, familial organization, pharmacological and non-pharmacological treatment searching, especially in industrialized countries. In the Alzheimer’s disease (AD) field of research, the advent of reliable biomarkers able to detect signatures of the disease decades before the first clinical symptoms has anticipated the biological conception of AD. Recent molecular imaging techniques using amyloid and tau ligands have led to an accurate in vivo diagnosis, even at the preclinical stage, and have improved patient selection and monitoring for available clinical trials.1 As such, besides the recognized magnetic resonance imaging (MRI) role in excluding other causes of cerebral damage or in detecting the presence of atrophy, it is important to delineate its current and future role in the clinical setting.
Against this background, this review aims to provide an overview on very recent studies, published in the last 2 years, that have adopted MRI (alone or in combination with other non-MRI tools) to assess subjects within the AD spectrum, with a main focus on the powerful role of this instrument in increasing our knowledge in each phase of the disease.
Materials and methods
Inclusion and exclusion criteria
The following selection criteria were employed for our search. We selected articles: (a) targeting only humans; (b) on the use of MRI techniques, such as structural and functional MRI; (c) on syndromes along the AD spectrum [such as late- or early-onset AD, mild cognitive impairment (MCI), prodromal and preclinical AD, populations at risk of developing AD, including cases with known genetic mutations]; (d) available in English and in full text. We excluded articles which did not use MRI as the primary method of investigation, studies on animals, case reports, reviews or meta-analyses of the available literature.
Search strategy
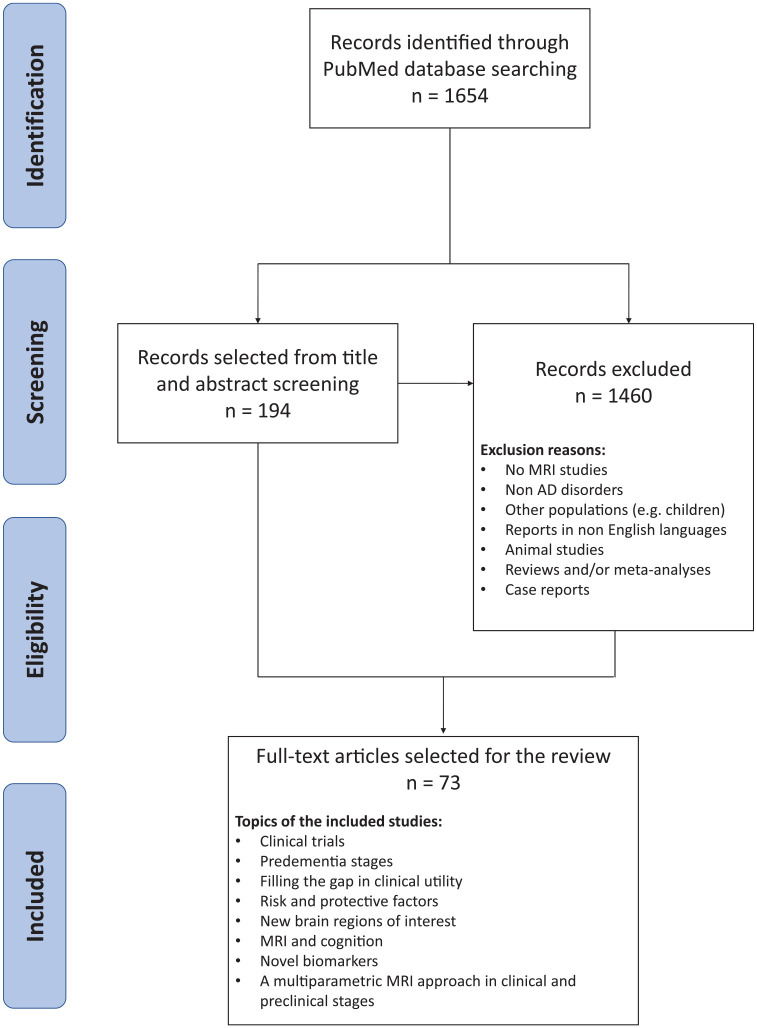
A formal literature review was performed using PubMed database on relevant articles recently published in peer-reviewed journals from 1 January 2018 to 2 March 2020 with the use of two macro areas, such as ‘MRI’ studies and ‘degenerative diseases in the AD spectrum’. The final search line was the following: [(((((((((((MRI) OR ‘magnetic resonance imaging’ (MeSH Terms)) OR MR) OR ‘magnetic resonance’) OR ‘structural magnetic resonance imaging’) OR ‘functional magnetic resonance imaging’) OR fMRI) OR sMRI)) AND (((((((((((((((((((((‘Alzheimer’s disease’) OR AD) OR Alzheimer) OR MCI) OR ‘mild cognitive impairment’) OR ‘prodromal AD’) OR ‘prodromal Alzheimer’s disease’) OR ‘preclinical AD’) OR ‘preclinical Alzheimer’s disease’) OR ‘posterior cortical atrophy’) OR PCA) OR PPA) OR ‘primary progressive aphasia’) OR LPA) OR lvPPA) OR ‘logopenic variant PPA’) OR ‘frontal AD’) OR ‘frontal Alzheimer’s disease’) OR ‘logopenic aphasia’) OR ‘logopenic variant of AD’) OR ‘frontal variant of AD’)]. We explicitly filtered our research on PubMed by excluding reviews, animal studies, articles not published in English language, and publications outside the established period. We imported our research string in Rayyan (http://rayyan.qcri.org)2 and, after duplicates removal, we obtained a total of 1654 articles available for a double-blinded title and abstract screening by two independent reviewers (EC, ML). Once each reviewer reached a decision for all articles, they shared their decisions and discussed each case with conflict until consensus was reached. Finally, the reviewers reached a consensus for the eligibility of 194 articles for a successive unblind full-text screening. After the full-text screening phase, only 73 articles were considered eligible for fulfilling the aim of the study and were included in the present review (Figure 1). These articles were clinical research papers, they have been reported in the text and in Supplementary Table 1.
Figure 1.
Flow chart of the reviewing process.
AD, Alzheimer’s disease; MRI, magnetic resonance imaging.
Results
Clinical trials
Numerous clinical trials have been performed to explore the efficacy of pharmacological and non-pharmacological treatments on cognitive and/or behavioral symptoms in AD and MCI patients. Structural and functional MRI have been shown as particularly useful for detecting early alterations in brain function and might be considered critical markers for the detection of physiological changes even over a short interval.
The amyloid hypothesis of AD suggests that the spread of tau-related neurofibrillary tangles, neuroinflammation and degeneration is triggered by the accumulation of beta-amyloid (Aβ) in the brain.3,4 Verubecestat is an inhibitor of beta-secretase 1 (BACE1) that blocks Aβ production. Unfortunately, the trial in mild-to-moderate AD patients5 was terminated since verubecestat did not improve clinical ratings of dementia, and some measures suggested that patients receiving this medication worsened in cognition and daily functioning. Hippocampal volumes were introduced among the secondary outcomes and have been observed as lower at week 104 compared with baseline by 6.1% in the placebo group and by 6.5–6.7% in the verubecestat group.
The cholinergic hypothesis of AD, suggesting that cholinergic augmentation should improve cognition, has led to the effective use of acetylcholinesterase inhibitors in AD patients. A recent trial6 examined resting-state functional MRI changes in patients with mild-to-moderate AD who were treated with donepezil for 6 months. After treatment, patients showed clinical and cognitive improvement and, compared with controls, decreased connectivity in the right gyrus rectus, right precentral gyrus and left superior temporal gyrus; however, no correlation between clinical and MRI findings were observed.
The risk to develop dementia is also associated with hypertension in mid and late life,7 and anti-hypertensive drugs have been considered as possible means for reducing the incidence of AD.8 One study confirmed the beneficial effects of anti-hypertensive treatments on cerebral autoregulation in AD, and reported that patients with mild-to-moderate AD who were treated with nilvadipine for 6 months showed reduced blood pressure, increased and stable cerebrovascular blood flow, assessed using MRI–arterial spin labeling, in the hippocampus and in the rest of the brain, respectively.9 Kehoe et al.10 recently proposed the study design of a new trial using losartan (an angiotensin-II-targeting drug), which should promote a reduced rate of brain volume loss and white matter hyperintensities (WMHs) in patients with mild-to-moderate AD with and without hypertension in 12 months.
Due to the lack of effective long-term treatments for AD, a new body of research started to target alternative approaches, such as nutrition, even though the results are controversial.11 In recent years, a few studies have established the effects of blueberry supplementation on diet, such as reversal of age-related decrements in cognitive and motor functioning.12 Boespflug et al.13 found that 16-week blueberry supplementation is associated with increased functional MRI activity during a working memory task in MCI patients in left pre-central gyrus, left middle–frontal gyrus, and left inferior parietal lobe, and only a trend of improvement in working memory performance, compared with placebo. On the contrary, a similar study14 failed to show cognitive, connectivity and microstructural changes after resveratrol (which is part of a group of polyphenols) supplementation in healthy elderlies.
In recent years, cognitive training has been one of the most important alternative methods in preventing and delaying cognitive dysfunctions in AD, and several studies showed that it could effectively improve activities of daily living as well as memory self-efficacy in MCI patients.15–17 Recent studies observed that cognitive trainings targeting multiple domains in patients with amnestic MCI (aMCI) improved cognition, increased brain functional connectivity (in the putamen, calcarine and inferior temporal gyrus), and reduced brain atrophy (in frontal, temporal and parietal volumes), with a consequent positive relationship between gray matter (GM) of parietal structures and improved memory recall and visuospatial features.18,19 Finally, using a 3-year computer-based cognitive training, a recently proposed trial20 has the aim of investigating the reduction of the conversion rate from MCI to AD, and brain structural and functional-related changes, in an expected population of 600–800 cases.
Summary
(1) Clinical trials on inhibition of BACE-1 in mild-to-moderate AD revealed clinical unsuccessful findings with reduced GM volumes in treated patients.
(2) Acetylcholinesterase inhibitors may have beneficial effects on brain structure and function in mild-to-moderate AD.
(3) To date, the effect of diet has led to controversial findings in AD.
(4) Anti-hypertensive treatments are promising in AD to preserve both clinical and brain features.
(5) Cognitive training, mainly targeting multiple domains, is associated with clinical improvement in MCI, and with a preserved integrity of crucial brain regions, such as the cortical parietal structures.
Predementia stages
During recent decades, a conceptual shift has occurred in the definition of AD, which is now conceived as a ‘continuum’ between healthy aging and neurodegeneration.21 MCI is considered an intermediate stage in the continuum from physiological aging to dementia and, during recent years, there has been a considerable effort to identify early indicators of pathological changes in this condition.
In MCI patients, the atrophy of the medial temporal lobe and its relationship with memory decline is a consistent finding using voxel-wise or volumetric approaches on T1-weighted images.22–24 However, the clinical complexity and heterogeneity of this condition is yet to be determined. Compared with a population of stable MCI, Qian and colleagues24 observed that MCI patients who were prone to progression to dementia 12 months later, showed a worse cognitive profile and reduced volumes of bilateral hippocampus, but also of left thalamus, and body and splenium of the corpus callosum. In these patients, while the baseline hippocampal volume was associated with a poorer memory performance, the left thalamus was related to non-memory deficits, such as language, executive and visual spatial abilities.
A recent study of Luo et al.23 showed that, compared with a population of single-domain MCI, multidomain MCI patients showed a worse cognitive profile, higher cerebrospinal fluid (CSF) total tau levels (but not lower Aβ levels), increased total WMH burden and decreased functional connectivity in the precuneus. These features were highly correlated with each other and suggested that multidomain MCI is a far more complex entity than was previously believed.
Research on the MCI condition has been also focused on white matter (WM) tissue.25–27 Using a graph analysis approach, Farrar et al.25 investigated WM tract connectivity within a reconstructed brain network in MCI patients with high or low executive abilities. Authors reported that high-performer MCI showed greater network size, density, clustering coefficient and inferior and superior longitudinal fasciculi integrity than low-performer MCI cases. Thus, according to the interpretation of authors, WM reserve may confer greater protection of executive abilities and restrict the progression to AD or to other dementing conditions. Using the same approach, another study showed that individuals with subjective cognitive decline (SCD; a condition with a perceived cognitive impairment but not objective neuropsychological deficits) showed less global and local WM connectivity efficiency compared with healthy controls.27 Lower regional efficiency was observed in the bilateral prefrontal WM and (again) left thalamus. The pattern of reduced structural connectivity in SCD versus controls showed a high accuracy in distinguishing these two populations and the reduced nodal strength in SCD was associated with their poorer memory performance.
Research on MCI and functional MRI has been focused on resting-state networks (RSNs) or task-based functional activity in relation with or beyond the default mode network (DMN).28–32 Zhang et al.31 provided an investigation on functional connectivity alterations across multiple RSNs in individuals with early and late (in terms of staging) MCI, and observed that dysfunctions behind the most commonly affected DMN, such as in sensorimotor networks, accompany the progression of the disease, and both intra- and inter-network functional alterations might be potential biomarkers for AD and MCI progression.31 Moreover, the relationship between the anticorrelated activity of the DMN with other brain networks, such as the dorsal attention network (DAN), has gained increasing interest, since their activity impacts on behavioural and cognitive functions and may represent a cerebral mechanism that switches the focus between internal channels (supported by the DMN) and external, attention-demanding events, supported by the DAN.33 Esposito et al.28 showed that this anticorrelation was significantly decreased with normal aging and MCI in most DMN–DAN connections, and that in MCI this decrease specifically involved the connection between the posterior cingulate cortex node of the DMN and the right inferior parietal node of the DAN.
Summary
(1) The MCI condition is a complex and heterogeneous clinical syndrome yet to be determined.
(2) Extra-hippocampal brain alterations in MCI are more associated with non-memory domains and likely with a progression to non-AD conditions.
(3) The involvement of WM connections in MCI and SCD reveals protection or vulnerability to neuropathological processes associated with AD.
(4) The study of resting-state functional connectivity and activity beyond and in relation to the DMN increased the understanding of the disease progression in the MCI condition.
Filling the gap in clinical utility
As largely reported in the previous section, MRI has a crucial role in detecting the early signs of neurodegeneration, and its contribution might increase the diagnostic reliability and accelerate therapeutic interventions in clinical settings.
Early signs of AD might include the presence of subtle clinical features, such as neuropsychiatric symptoms, which are difficult to recognize, and MRI could help in predicting their presence as part of the disease process. A recent investigation34 showed that functional connectivity MRI features of regions of interest, mostly from the fronto-limbic circuit, could predict the presence of neuropsychiatric symptoms with an average classification accuracy ranging from 70% to 80% in AD and MCI cases. In addition, the functional connectivity features of the regions of interest also predicted the severity of the neuropsychiatric symptoms, as well as the AD pathology (indexed by baseline and change of Aβ/phosphorylated tau ratio) with above 70% accuracy rate. Furthermore, measures of MRI at baseline (GM volumes of specific regions of interest, including the hippocampus) in combination with genetic [apolipoprotein E (APOE) ε4 allele and 19 single nucleotide polymorphisms (SNPs) significantly associated with AD]35 or hypometabolism (qualitatively investigated with 18F-fluoro-2-deoxyglucose positron emission tomography – FDG-PET)36 information could predict the progression to AD in MCI cases clinically followed up for 24 months, reaching accuracies higher than 80%.
Finally, MRI measures could help in disentangling misclassified cases during the diagnostic process. A recent study showed that cortical thickness alone could correctly classify AD and healthy controls with 90% accuracy, 96% sensitivity, and 76% specificity.37 However, about 10% subjects were misclassified. Although authors observed that sociodemographic (e.g. age) and cognitive information (e.g. Mini Mental State Evaluation, MMSE) contributed to understanding the misclassification, other MRI measures, such as increased WMH burden, were the most probable candidates for overestimating AD diagnosis in healthy controls, suggesting that vascular conditions investigated with MRI may further contribute to classification accuracy.
Summary
(1) MRI can significantly contribute to the detection of early clinical symptoms, which can indicate the presence of neurodegenerative processes.
(2) The combination of MRI features together with genetic and metabolism information can predict the MCI conversion about 2 years earlier.
(3) Additional MRI features, rather than demographic and cognitive information, may contribute to correctly classify AD and healthy individuals.
Risk and protective factors
Epidemiological evidence reports the presence of many risk factors linked to AD.38 Consequently, it is essential to develop strategies to identify those individuals who are at particular risk of developing AD in order to prevent or push forward the disease onset. In line with this, the Australian National University Alzheimer Disease Risk Index (ANU-ADRI) is a reliable instrument for the investigation of several known AD risk factors (such as age, education, diabetes, body mass index, etc.), which was proposed detecting individuals at risk of developing AD prior to clinical symptoms.39 In a large cohort of healthy subjects who were free of dementia at baseline and were followed up for 12 months, higher risk estimates by the index were associated with lower global cortical GM and, more interestingly, every additional risk point on the ANU-ADRI was associated with a 0.32% lower volume of DMN structures, and with the 8% increased risk of developing MCI or dementia over the follow-up period. A recent cross-sectional study in a large, healthy population 40 showed that, among the factors accounted by the ANU-ADRI index, the adherence to a Mediterranean diet and insulin sensitivity were the only variables that could explain cortical thickness abnormalities in key brain regions for AD [such as entorhinal cortex (EC) and posterior cingulate], and specifically that EC explained greater changes in subjects’ memory performance.
Cerebrovascular alterations can be considered risk factors for neurodegeneration. In older adults, increased total WMH volume was associated with poorer global cognition and memory.41 Interestingly, this relationship appeared to be mediated by global and medial temporal cortical thinning, suggesting that some of the observed associations of WMHs with cognition are, at least partially, attributable to their effect on atrophy. The severity of WMH assessed using visual rating scores and the presence of lacunes at baseline were independent predictors of incident cognitive decline over 2 years, and increased this risk by threefold.42 A longitudinal study43 on healthy controls, MCI and AD patients observed that both medial temporal lobe atrophy and periventricular WMH changes (mainly at the occipital lobe) were independently associated with 1-year cognitive decline, suggesting that treatment targeting WMH may play an important role in the prevention of AD. The research on MCI or SCD patients with hypertension has shown an association between episodic memory and amyloid binding, but no association44 or a circumscribed association between severe periventricular WMH and cognitive functions.45
Abnormal AD-like CSF biomarkers have been identified as high-risk factors for neurodegeneration. Recent studies on asymptomatic individuals showed that subjects with abnormal Aβ1-42 had cortical thinning of several AD-brain regions (such as precuneus, posterior cingulate cortex, hippocampus and parahippocampal gyrus) at baseline46 and over 2 years,47 or altered global cortical network organization (including decreased global efficiency and modularity) prior to cortical loss.48 However, the longitudinal analyses failed to be replicated in independent samples,47 with amyloid burden being one of the factors that could explain this incongruity. To this end, an elegant study49 reveals a dose–response relationship between increasing CSF Aβ1-42 burden and alterations in blood-oxygen-level-dependent activation during a challenging visuospatial task in cognitively normal individuals. During the task, the hypoactivation of precuneus and prefrontal cortices was related to good or bad performances depending on the grade of ‘Aβ’ elevation, demonstrating a transition over all the disease staging. On the other hand, subjects with both low CSF Aβ1-42 levels and high CSF phosphorylated tau values were characterized by reduced subiculum volume, lower microstructural integrity of the fornix, and also a trend towards cognitive impairment more than individuals who showed only reduction in CSF Aβ1-42.50 In addition, all subjects with (any) abnormal CSF measures presented with WM atrophy of the anterior and posterior cingulate bundle and more segregated cortical networks, with the Aβ-positive group showing heightened isolation of cingulate and temporal cortices.46 Recent results51 show that healthy subjects with APOE ε4ε4 genotype and AD first-degree family members have higher Aβ burden, assessed with amyloid PET, compared with ε3ε4 and ε3ε3 genotypes, and this burden was inversely associated with cortical GM mean volumes but not with episodic memory, likely suggesting that neurodegeneration occurs before manifest cognitive decline in these individuals.
By combining structural MRI and genome-wide genetic data from two independent young cohorts, another investigation52 highlighted that an elevated polygenic risk score (which combines loci with low effects to identify phenotypic associations) is associated with smaller precuneal volume even when accounting for APOE genotype in the analysis, suggesting an independent role of other genetic factors in AD.
Even though risk factors play an important role in the understanding of AD progression, recent investigations also focus their attention on the role of protective factors. One of the most debated protective factors in AD is cognitive reserve (CR), which relates to the discrepancy between the brain pathology and the severity of clinical manifestations, with subjects with higher CR tolerating a greater amount of AD pathology (in terms of more tau and Aβ aggregations) before cognitive impairments appear.53 Recent results showed that CR was associated with the capacity to process information efficiently in the brain (it was correlated with global efficiency, nodal clustering coefficient, and local efficiency of the right middle-temporal pole) in AD and MCI.54
Wolf et al.55 recently proposed a new explorative methodological approach to study resilience mechanisms of the brain, such as the well-known age and education (called general resilience factors which are independent of degree of pathology) in addition to hippocampal volume as a potential resilience factor (i.e. a dynamic resilience factor characterized by an increasing relevance with increasing levels of pathology). These analyses highlighted hippocampal volume as a promising resilience factor against age and AD-related brain pathology, particularly in case of elevated tau. Apart from age and education, another factor which may contribute to CR is multilingualism. A recent study56 investigated this aspect in MCI and AD. In areas related to language, both multilingual MCI and AD patients had thicker cortex than the monolinguals, and multilingual patients showed a positive correlation between cortical thickness in areas involved in language and performance on episodic memory tasks.
Finally, mounting evidence indicates that physical activity and cardiorespiratory fitness are positively associated with WM fiber integrity and cognitive performance in both healthy individuals and patients with MCI.57–59 Results provided by Ding et al.60 are in line with these assumptions, thus confirming that physical activity is a key factor to maintain WM fiber integrity in these subjects and also correlates with high cognitive performance, specifically in executive functioning.
Summary
(1) In healthy subjects, higher ANU-ADRI indices are associated with global and DMN GM atrophy.
(2) In healthy subjects, WMH burden is an independent predictor of cognitive decline.
(3) The relationship between abnormal CSF Aβ1-42 levels and MRI alterations in cognitively normal individuals seems to be mediated by Aβ burden. On the other hand, any abnormal CSF value in these subjects is related to a disruption of WM.
(4) In healthy, young populations, several genetic factors, other than APOEε4, contribute to smaller GM volumes in AD key regions such as the precuneus.
(5) Among the protective factors, in healthy individuals the CR is consistently associated with increased cortical thickness and greater functional and structural network efficiency.
New brain regions of interest
The differentiation of the early signs of degeneration from physiological aging represents a constant challenge in the neuroimaging field. Thus, identifying brain regions of interest, which are specifically hit in different phases of the AD-related neurodegeneration, has become more and more crucial.
AD has been widely associated with medial temporal atrophy involving the EC, the hippocampus and its subfields with cornu ammonis (CA) 1 and subiculum as the first regions involved.61,62 A recent cross-sectional study on AD, MCI, and healthy individuals revealed that atrophy of CA1 and subiculum were found in both AD and MCI compared with normal aging condition, while the stratum radiatum, lacunosum and moleculare (SRLM) integrity, which is intimately involved in the pathways between CA1 and CA3/EC, and between hippocampus and wider cortical areas, was only significantly reduced in AD compared with healthy controls, but not in MCI.63 However, SRLM integrity was correlated with clinical and cognitive measurements of disease severity in all subjects, highlighting this as a candidate biomarker for staging the progression of AD.
AD is associated with loss of cholinergic neurons in the nucleus basalis of Meynert, located in the posterior basal forebrain, and atrophy of this nucleus occurs early in the course of the disease. The role of anterior basal forebrain structures in AD, in particular the septal region, has been less studied. A recent study64 employed manual volumetric analysis of the septal nuclei in healthy subjects who developed MCI or AD. Healthy individuals converting to AD within an average of 2.8 years had enlarged septal nuclei as compared with the other groups. Further research is needed to determine if septal enlargement reflects neuroplastic compensation, amyloid deposition or inflammation, and whether it can serve as an early MRI biomarker of AD.
The pineal gland is another brain structure which has gained increasing interest in AD research because of its role in melatonin secretion and the regulation of the circadian rhythm.65,66 Previous studies pointed out that the suppression of the production of melatonin is frequently seen in patients with AD and MCI, with melatonin levels being lower in these patients compared with normal aging.67,68 A recent study69 reported that mean pineal gland volume was significantly smaller in patients affected by AD compared with healthy controls and MCI, but in all patients was related to cognitive decline.
Among the most investigated (new) brain regions of interest, basal ganglia (in particular the striatum) and thalamus are those that have gained the strongest attention in AD. Thalamic volume loss was observed in both AD and aMCI patients, and in these latter subjects it was an early sign associated with poor cognitive performance preceding the damage to other extra-hippocampal brain regions such as the amygdala.70 Compared with both MCI and SCD patients, a larger caudate nucleus volume, partially associated with age and female sex, was observed in AD patients and interpreted as result of pathology accumulation or mechanism of temporary compensation (i.e. to compensate for the reduced hippocampal volumes).71
Apart from brain atrophy, the study of disruption of resting-state functional brain connectivity beyond the DMN is gaining increasing interest. Using a sparse inverse covariance estimation approach on the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset, a recent study72 showed altered functional connectivity of frontal and parietal brain regions within the DMN but also in the frontoparietal and executive-control networks in initial AD patients. On the other hand, in aMCI patients with reduced DMN functional connectivity, executive-control and salience networks were found to be hyperconnected, as a possible sign of compensation.73
Summary
(1) Hippocampal subfields, such as SRLM, are candidate biomarkers for the disease staging.
(2) The role of the anterior basal forebrain is yet to be determined in AD.
(3) Brain structures involved in melatonin secretion and circadian rhythm regulation are gaining interest in the field.
(4) Among the new brain regions of interest, basal ganglia and thalamus have gained strong attention.
(5) In MCI, unaffected brain networks co-operate with the DMN likely for compensatory mechanisms. This co-operation is not possible later in the course of the disease when other networks become affected.
MRI and cognition
The investigation of specific cognitive and behavioral domains involved in AD is crucial for the detection of the early disease signs. Episodic memory is early impaired in AD patients, who have difficulties in encoding and recalling new information. One of the most diffuse paradigms to detect episodic memory disturbances is the Free and Cued Selective Reminding Test (FCSRT). In its ‘word’ (versus picture) version, FCSRT has been suggested as an indicator of hippocampal and temporal pole integrity due to its association with atrophy of these regions in AD and MCI.74,75 Asymptomatic autosomal-dominant carriers have been recently observed to make significantly more errors compared with non-carrier relatives at a test assessing memory generalization (i.e. the ability to transfer previous learning to new situations), with this impairment correlating with the left hippocampal volume loss.76 The importance of the integrity of the hippocampus has been highlighted also for the consolidation of arithmetic facts in memory during childhood and adolescence.77 However, a recent study78 demonstrated that the retrieval of arithmetic facts (such as multiplication) in AD and MCI patients are intact despite their hippocampal atrophy and episodic memory deficits, suggesting that the hippocampus might not be crucially involved in retrieving these facts when these are consolidated in long-term memory. It is important to underline that the memory process does not involve only temporal structures and that specific subcomponents of memory might be also subtended by extra-temporal brain regions. To this end, a longitudinal investigation on 233 SCD cases showed that a faster rate of memory impairment assessed as a global memory score was related to thinner cortex in frontal, temporal and occipital cortices.79
Another cognitive change that occurs in AD patients is the loss of visuo-constructional ability. Deficits in the well-known Pentagon Copying Task were related to GM volumes of the total parietal (for total score) and specific sub-regions of the parietal cortex (such as the posterior cingulate cortex and supramarginal gyrus, for intersection and the number of angle scores) in AD, MCI and SCD cases, conceptualizing the constructional apraxia as a failure in the integration of visual information from one fixation point to the next.80
Different studies are also targeting executive and frontal-lobe functioning in MCI, AD and healthy controls.81–83 Authors of a multimodal MRI study in healthy adults showed that low deactivation of the DMN, high activation of the executive-control network and high WMH burden were the most accurate predictors of executive functioning at baseline.82 In addition, greater AD pathology (assessed using CSF values), and poor WM structural connectivity within the DMN and the executive-control network predicted greater executive-dysfunction annual decline in asymptomatic subjects.
Another aspect which seems to be hugely affected in AD and has a great impact on daily living is mental orientation, which comprises orientation in time, space and person. Compared with the standard tests, mental orientation task reached the highest accuracy (nearly 95%) in the distinction between MCI and healthy controls, and this task preferentially recruited specific AD-related brain regions (e.g. precuneus, retrosplenial cortex, parahippocampal gyri),84 which, together with the posterior cingulate cortex, dorsal DMN and temporo-parietal junction, are the same with reduced resting state connectivity in AD patients with poor orientation in time compared with well-oriented AD and healthy controls.85
Several MRI studies also assessed structural and functional neural circuits related with neuropsychiatric symptoms in AD and MCI.86 Some of these studies showed that alterations of the functional or structural connectivity of the fronto-limbic network are associated with the most frequent behavioural aspects of AD (agitation, anxiety, depression, etc.) and premorbid personality traits (such as internal or externa locus of control) in addition to the severity of AD pathology assessed with CSF.34,87,88
Summary
(1) As a memory test assessing encoding and recall, FCSRT has been suggested as an indicator of hippocampal integrity in AD and MCI.
(2) The structural connectivity of the DMN and the executive-control network, together with CSF amyloid burden, contribute to the progressive executive dysfunction in healthy individuals.
(3) Structural and functional connectivity of fronto-limbic regions are linked to neuropsychiatric symptoms, premorbid personality traits and AD pathology.
Novel biomarkers
Recent studies in the treatment of AD have shown a failure to modify disease progression over time. The identification of novel biomarkers (beyond amyloid),89 specific in predicting the development of AD, is a primary concern in the field. Among fluid biomarkers, the most promising is the measurement of the neurofilament light chain (NfL) in the CSF and blood. In asymptomatic AD mutation carriers, the annual rate of serum NfL change was associated with cognitive impairment and cortical thinning of the precuneus at least a decade earlier than estimated symptom onset, and enabled researchers to discriminate AD mutation carriers from non-carriers almost a decade before disease onset.90 The measure of serum copper levels is also promising, since copper ions appear to modulate Aβ generation, aggregation and stabilization of the fibrillary form.91,92 However, although patients with MCI and AD presented with higher levels than healthy controls, these measures did not correlate with medial temporal lobe atrophy, nor with cognitive performance.93
Little focus has been concentrated on metabolites, such as sphingolipids and glycerophospholipids, which have been found associated with AD severity at autopsy, clinical and preclinical progression, low memory performance and typical pattern of brain atrophy in AD and MCI, and depletion of circulating progenitor cells, which are believed to promote angiogenesis and neurogenesis.94,95
Using different classification approaches, several studies demonstrated that brain structural MRI (particularly the medial temporal lobe), amyloid and FDG-PET, and genetic features (in particular the APOEε4 allele) better than other imaging and genetic features predicted the distinction between AD and healthy controls and the conversion from MCI to AD.35,36,37,96
One of the most promising and advanced biomarkers is the use of brain connectome to monitor AD spreading. A graph analysis study demonstrated that the progressive degeneration in the AD continuum is associated with an early breakdown of anatomical brain connections and follows the strongest connections with the disease epicentre (i.e. the most atrophic region), supporting the hypothesis that the topography of brain connectional architecture can modulate the spread of AD through the brain.97
Finally, the study of the WM microstructure (mainly of the hippocampal cingulum and fornix)98,99 using diffusion-tensor imaging (DTI), and of microbleeds (reflecting impaired small-vessel integrity), or iron accumulation using susceptibility-weighted imaging (SWI) and arterial spin labeling (ASL),100,101 are promising for the differential diagnosis of AD with other dementias, and for the detection of asymptomatic individuals at risk of developing AD.
Summary
(1) Among the fluid biomarkers, serum NfL change is promising, since it is associated with cognitive impairment and cortical thinning in presymptomatic individuals, decades before the disease onset.
(2) The most important predictors of AD from healthy controls’ distinction, and of MCI to AD conversion, are the APOEε4 allele, amyloid PET, qualitative FDG-PET, and the medial temporal volumes.
(3) SWI and ASL are promising tools for assessing small-vessel disease and iron deposition.
(4) Studying the topography of brain connectional architecture can allow prediction of the spread of AD pathology through the brain.
The study of the WM microstructure of hippocampal cingulum and fornix helps in the AD differential diagnosis and in the detection of healthy individuals at risk of AD.
A multiparametric MRI approach in clinical and preclinical stages
Multimodal MRI is proven to be a more valid approach than the single modality for investigating different aspects of neurodegeneration. To this end, recent reports showed that the evaluation of perfusion imaging and SWI are promising for detection of MCI converters,100 while functional MRI connectivity and DTI abnormalities enable the distinction of AD patients from other neurodegenerative conditions.98 Several studies demonstrated that T1-weighted images can detect specific patterns of brain atrophy for the differential diagnosis between AD and frontotemporal dementia, with accuracy reaching up to 84%.102–104 However, the employment of other advanced MRI techniques can further increase the diagnostic accuracy: several findings indicate that the assessment of ASL, perfusion patterns, and the contribution of DTI have an added value over mere atrophy measurements along the AD spectrum.105–108 Specifically, a recent study demonstrated an increased diagnostic accuracy in distinguishing AD from frontotemporal dementia, from 72% to 84% by adding DTI and ASL measures to the sole structural MRI.109
As previously mentioned here, the combination of MRI with other molecular imaging modalities, such as FDG-PET, is a valid approach for the accurate prediction of AD progression in MCI patients.36 In fact, hippocampal atrophy assessed with structural MRI might explain early symptoms of AD, whereas its hypometabolism, as seen with PET images, reflects early neuronal dysfunction. Furthermore, recent evidence highlights that multimodal imaging biomarkers (MRI, tau and amyloid PET) are pivotal for the AD detection and for the definition of distinct AD profiles.110 In addition, the combination of tau and FDG-PET with functional MRI would enable further insight into the consequences of pathological deposits on functional networks and to better understand the pathological course of AD.111 Future studies need to highlight the crucial role of combining different MRI techniques together with other imaging modalities, in order to study different aspects of neurodegeneration.
Summary
(1) Multimodal MRI rather than the single modality is a more valid approach for increasing the diagnostic accuracy and for predicting the MCI conversion.
(2) The combination of MRI and other imaging modalities, such as PET imaging, would be of utmost importance for reaching higher diagnostic accuracy and for understanding the consequences of the pathological processes.
(3) Multimodal MRI and/or imaging approaches are promising to define different aspects of neurodegeneration in the AD course.
Conclusion
This review comprehensively discusses the newest findings of structural and functional MRI in the study of AD, as published in the last 2 years. MRI measures were suggested as outcomes to be used for the earliest detection of the disease, for monitoring the disease progression, as well as the effect of treatments, and to unravel the complex interplay between upstream and downstream processes.
Among the most consistent findings, the study of extra-hippocampal structures, extra-DMN networks, and assessing the protective factors played by WM structural integrity offered new insights to explain the different trajectories of the heterogeneous MCI condition, and may help to predict conversion to AD or non-AD dementias. The use of MRI to test the efficacy of pharmacological and non-pharmacological treatments is mainly based on T1-weighted images and specifically, GM volumes. As such, non-conventional MRI sequences, such as DTI, SWI, ALS and functional MRI have only had a marginal role so far, typically related to small observational studies or non-pharmacological interventions (as in the case of functional MRI). While we should recognize that the longitudinal validity of functional MRI still deserves research to be proven, recent literature highlights that it may be a good candidate to detect even subtle changes. Among novel wet biomarkers, one the most promising is the measurement of the NfL in CSF and blood. A specific association of NfL with MRI-derived brain changes seems to occur only at the very early asymptomatic stages of the disease and become rather non-AD-specific in overt clinical stages. In general, from this review, it emerges that multiparametric MRI studies would be effective in holding a comprehensive view of the disease pathological processes. Furthermore, a combined approach using MRI and other imaging (e.g. molecular imaging) and non-imaging (e.g. genetic and clinical) modalities is gaining consideration, mainly in the preclinical stages of the disease, as a predictor of further decline.
Some limitations of the present review should be noted. The selected manuscripts reflect only a part of the huge work that has been done in the AD field using MRI in the last 2 years.
It is worth noting that our manuscript did not review in detail the scientific literature on how MRI is used with other imaging techniques, such as tau and amyloid molecular imaging. Even if we recognize its extreme importance, this aspect goes beyond the aim of our present investigation and needs to be deeply discussed in future dedicated reviews. Our search did not include research papers focused on atypical AD, specific advanced MRI sequences, nor methodological papers, since all of these require deep discussion. To this end, it is relevant to mention that methodological studies in the AD field, published in the same period of interest, are proposing relevant improvements for the automatic hippocampal segmentation, artificial-intelligence-based individual diagnosis, and for the MRI harmonization in multicenter projects, with all these studies providing promising findings for future research.
Despite these shortcomings, this manuscript has several strengths. We performed a formal literature search with a robust approach, and we reviewed the obtained manuscripts through a double-blinded screening by two independent reviewers. We finally provided a comprehensive view of state-of-the art MRI research, underlying the crucial role of MRI in diagnosing and monitoring the AD spectrum.
Supplemental Material
Supplemental material, Supplementary_Table_1 for An update on magnetic resonance imaging markers in AD by Michela Leocadi, Elisa Canu, Davide Calderaro, Davide Corbetta, Massimo Filippi and Federica Agosta in Therapeutic Advances in Neurological Disorders
Footnotes
Conflict of interest statement: M. Leocadi, D. Calderaro, D. Corbetta report no actual or potential conflicts of interest.
E. Canu has received research supports from the Italian Ministry of Health.
Prof. M. Filippi is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
F. Agosta is Section Editor of NeuroImage; Clinical: has received speaker honoraria from Philips, Novartis and Biogen Idec; and receives or has received research support from the Italian Ministry of Health, AriSLA, and the European Research Council.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Federica Agosta  https://orcid.org/0000-0003-3121-4979
https://orcid.org/0000-0003-3121-4979
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Michela Leocadi, Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Elisa Canu, Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Davide Calderaro, Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Davide Corbetta, Laboratory of Movement Analysis, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Massimo Filippi, Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, Neurology and Neurophysiology Units, IRCCS San Raffaele Scientific Institute, and Vita-Salute San Raffaele University, Milan, Italy.
Federica Agosta, Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, and Vita-Salute San Raffaele University, Via Olgettina 60, Milan 20132, Italy.
References
- 1. Kas A, Migliaccio R, Tavitian B. A future for PET imaging in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2020; 47: 231–234. [DOI] [PubMed] [Google Scholar]
- 2. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016; 8: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan R, Vassar R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol 2014; 13: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egan MF, Kost J, Voss T, et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med 2019; 380: 1408–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng J, Yang H, Zhang J. Donepezil’s effects on brain functions of patients with Alzheimer disease: a regional homogeneity study based on resting-state functional magnetic resonance imaging. Clin Neuropharmacol 2019; 42: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001; 322: 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies NM, Kehoe PG, Ben-Shlomo Y, et al. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis 2011; 26: 699–708. [DOI] [PubMed] [Google Scholar]
- 9. De Jong DLK, de Heus RAA, Rijpma A, et al. Effects of nilvadipine on cerebral blood flow in patients with Alzheimer disease. Hypertension 2019; 74: 413–420. [DOI] [PubMed] [Google Scholar]
- 10. Kehoe PG, Blair PS, Howden B, et al. The rationale and design of the reducing pathology in Alzheimer’s disease through angiotensin TaRgeting (RADAR) trial. J Alzheimers Dis 2018; 61: 803–814. [DOI] [PubMed] [Google Scholar]
- 11. Xu W, Tan L, Wang HF, et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2015; 86: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 12. Basu A, Du M, Leyva MJ, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr 2010; 140: 1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boespflug EL, Eliassen JC, Dudley JA, et al. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr Neurosci 2018; 21: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huhn S, Beyer F, Zhang R, et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults: a randomized controlled trial. Neuroimage 2018; 174: 177–190. [DOI] [PubMed] [Google Scholar]
- 15. Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol 2010; 67: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300: 1027–1037. [DOI] [PubMed] [Google Scholar]
- 17. Pieramico V, Esposito R, Cesinaro S, et al. Effects of non-pharmacological or pharmacological interventions on cognition and brain plasticity of aging individuals. Front Syst Neurosci 2014; 8: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng W, Wang D, Tang L, et al. Effects of different cognitive trainings on amnestic mild cognitive impairment in the elderly: a one-year longitudinal functional magnetic resonance imaging (MRI) study. Med Sci Monit 2018; 24: 5517–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang H, Wang Z, Wang J, et al. Computerized multi-domain cognitive training reduces brain atrophy in patients with amnestic mild cognitive impairment. Transl Psychiatry 2019; 9: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Y, Li B, Tang H, et al. Shanghai cognitive intervention of mild cognitive impairment for delaying progress with longitudinal evaluation-a prospective, randomized controlled study (SIMPLE): rationale, design, and methodology. BMC Neurol 2018; 18: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 2016; 12: 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang DW, Lim HK, Joo SH, et al. Alterations in intra- and interregional intrinsic brain connectivity are differentially associated with memory performance in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 2018; 46: 229–242. [DOI] [PubMed] [Google Scholar]
- 23. Luo X, Jiaerken Y, Huang P, et al. Alteration of regional homogeneity and white matter hyperintensities in amnestic mild cognitive impairment subtypes are related to cognition and CSF biomarkers. Brain Imaging Behav 2018; 12: 188–200. [DOI] [PubMed] [Google Scholar]
- 24. Qian L, Liu R, Qin R, et al. The associated volumes of sub-cortical structures and cognitive domain in patients of mild cognitive impairment. J Clin Neurosci 2018; 56: 56–62. [DOI] [PubMed] [Google Scholar]
- 25. Farrar DC, Mian AZ, Budson AE, et al. Retained executive abilities in mild cognitive impairment are associated with increased white matter network connectivity. Eur Radiol 2018; 28: 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heinrich J, Vidal JS, Simon A, et al. Relationships between lower olfaction and brain white matter lesions in elderly subjects with mild cognitive impairment. J Alzheimers Dis 2018; 61: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 27. Shu N, Wang X, Bi Q, et al. Disrupted topologic efficiency of white matter structural connectome in individuals with subjective cognitive decline. Radiology 2018; 286: 229–238. [DOI] [PubMed] [Google Scholar]
- 28. Esposito R, Cieri F, Chiacchiaretta P, et al. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav 2018; 12: 127–141. [DOI] [PubMed] [Google Scholar]
- 29. Liu X, Chen J, Shen B, et al. Altered intrinsic coupling between functional connectivity density and amplitude of low-frequency fluctuation in mild cognitive impairment with depressive symptoms. Neural Plast 2018; 2018: 1672708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Melrose RJ, Jimenez AM, Riskin-Jones H, et al. Alterations to task positive and task negative networks during executive functioning in mild cognitive impairment. Neuroimage Clin 2018; 19: 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Liu X, Zhao K, et al. Study of altered functional connectivity in individuals at risk for Alzheimer’s Disease. Technol Health Care 2018; 26: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng W, Su Z, Liu X, et al. Modulation of functional activity and connectivity by acupuncture in patients with Alzheimer disease as measured by resting-state. fMRI 2018; e0196933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gopinath K, Krishnamurthy V, Cabanban R, et al. Hubs of anticorrelation in high-resolution resting-state functional connectivity network architecture. Brain Connect 2015; 5: 267–275. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Ren P, Mapstone M, et al. Identify a shared neural circuit linking multiple neuropsychiatric symptoms with Alzheimer’s pathology. Brain Imaging Behav 2019; 13: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ning K, Chen B, Sun F, et al. Classifying Alzheimer’s disease with brain imaging and genetic data using a neural network framework. Neurobiol Aging 2018; 68: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrari BL, Neto GCC, Nucci MP, et al. The accuracy of hippocampal volumetry and glucose metabolism for the diagnosis of patients with suspected Alzheimer’s disease, using automatic quantitative clinical tools. Medicine (Baltimore) 2019; 98: e17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belathur Suresh M, Fischl B, Salat DH. Factors influencing accuracy of cortical thickness in the diagnosis of Alzheimer’s disease. Hum Brain Mapp 2018; 39: 1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nat Rev Neurol 2013; 9: 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cherbuin N, Shaw ME, Walsh E, et al. Validated Alzheimer’s Disease Risk Index (ANU-ADRI) is associated with smaller volumes in the default mode network in the early 60s. Brain Imaging Behav 2019; 13: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mosconi L, Walters M, Sterling J, et al. Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer’s disease: a cross-sectional study of middle-aged adults from the broader New York City area. BMJ Open 2018; 8: e019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizvi B, Narkhede A, Last BS, et al. The effect of white matter hyperintensities on cognition is mediated by cortical atrophy. Neurobiol Aging 2018; 64: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ding D, Xiong Y, Zhao Q, et al. White matter hyperintensity predicts the risk of incident cognitive decline in community dwelling elderly. J Alzheimers Dis 2018; 61: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 43. Chen YC, Tsao HH, Chu YC, et al. Exploring the spectrum of subcortical hyperintensities and cognitive decline. J Neuropsychiatry Clin Neurosci 2018; 30: 130–138. [DOI] [PubMed] [Google Scholar]
- 44. Smith EE, Muzikansky A, McCreary CR, et al. Impaired memory is more closely associated with brain beta-amyloid than leukoaraiosis in hypertensive patients with cognitive symptoms. PLoS One 2018; 13: e0191345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang S, Luo Y, Dong Z, et al. Impact of periventricular hyperintensities and cystatin C on different cognitive domains in the population of non-demented elderly Chinese. J Clin Neurosci 2019; 68: 201–210. [DOI] [PubMed] [Google Scholar]
- 46. Cantero JL, Atienza M, Sanchez-Juan P, et al. Cerebral changes and disrupted gray matter cortical networks in asymptomatic older adults at risk for Alzheimer’s disease. Neurobiol Aging 2018; 64: 58–67. [DOI] [PubMed] [Google Scholar]
- 47. Falcon C, Tucholka A, Monte-Rubio GC, et al. Longitudinal structural cerebral changes related to core CSF biomarkers in preclinical Alzheimer’s disease: a study of two independent datasets. Neuroimage Clin 2018; 19: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Voevodskaya O, Pereira JB, Volpe G, et al. Altered structural network organization in cognitively normal individuals with amyloid pathology. Neurobiol Aging 2018; 64: 15–24. [DOI] [PubMed] [Google Scholar]
- 49. Foster CM, Kennedy KM, Horn MM, et al. Both hyper- and hypo-activation to cognitive challenge are associated with increased beta-amyloid deposition in healthy aging: a nonlinear effect. Neuroimage 2018; 166: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tardif CL, Devenyi GA, Amaral RSC, et al. Regionally specific changes in the hippocampal circuitry accompany progression of cerebrospinal fluid biomarkers in preclinical Alzheimer’s disease. Hum Brain Mapp 2018; 39: 971–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mecca AP, Barcelos NM, Wang S, et al. Cortical beta-amyloid burden, gray matter, and memory in adults at varying APOE epsilon4 risk for Alzheimer’s disease. Neurobiol Aging 2018; 61: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li J, Zhang X, Li A, et al. Polygenic risk for Alzheimer’s disease influences precuneal volume in two independent general populations. Neurobiol Aging 2018; 64: 116–122. [DOI] [PubMed] [Google Scholar]
- 53. Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol 2008; 63: 112–118. [DOI] [PubMed] [Google Scholar]
- 54. Lee DH, Lee P, Seo SW, et al. Neural substrates of cognitive reserve in Alzheimer’s disease spectrum and normal aging. Neuroimage 2019; 186: 690–702. [DOI] [PubMed] [Google Scholar]
- 55. Wolf D, Fischer FU, Fellgiebel A; Alzheimer’s Disease Neuroimaging Initiative. A methodological approach to studying resilience mechanisms: demonstration of utility in age and Alzheimer’s disease-related brain pathology. Brain Imaging Behav 2019; 13: 162–171. [DOI] [PubMed] [Google Scholar]
- 56. Duncan HD, Nikelski J, Pilon R, et al. Structural brain differences between monolingual and multilingual patients with mild cognitive impairment and Alzheimer disease: evidence for cognitive reserve. Neuropsychologia 2018; 109: 270–282. [DOI] [PubMed] [Google Scholar]
- 57. Gons RA, Tuladhar AM, de Laat KF, et al. Physical activity is related to the structural integrity of cerebral white matter. Neurology 2013; 81: 971–976. [DOI] [PubMed] [Google Scholar]
- 58. Tseng BY, Gundapuneedi T, Khan MA, et al. White matter integrity in physically fit older adults. NeuroImage 2013; 82: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sattler C, Erickson KI, Toro P, et al. Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J Alzheimers Dis 2011; 26: 709–718. [DOI] [PubMed] [Google Scholar]
- 60. Ding K, Tarumi T, Zhu DC, et al. Cardiorespiratory fitness and white matter neuronal fiber integrity in mild cognitive impairment. J Alzheimers Dis 2018; 61: 729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–259. [DOI] [PubMed] [Google Scholar]
- 62. Liu L, Drouet V, Wu JW, et al. Trans-synaptic spread of tau pathology in vivo. PLoS One 2012; 7: e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Su L, Hayes L, Soteriades S, et al. Hippocampal stratum radiatum, lacunosum, and moleculare sparing in mild cognitive impairment. J Alzheimers Dis 2018; 61: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Butler T, Harvey P, Deshpande A, et al. Basal forebrain septal nuclei are enlarged in healthy subjects prior to the development of Alzheimer’s disease. Neurobiol Aging 2018; 65: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. He H, Dong W, Huang F. Anti-amyloidogenic and anti-apoptotic role of melatonin in Alzheimer disease. Curr Neuropharmacol 2010; 8: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosales-Corral SA, Acuna-Castroviejo D, Coto-Montes A, et al. Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res 2012; 52: 167–202. [DOI] [PubMed] [Google Scholar]
- 67. Srinivasan V, Kaur C, Pandi-Perumal S, et al. Melatonin and its agonist ramelteon in Alzheimer’s disease: possible therapeutic value. Int J Alzheimers Dis 2010; 2011: 741974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol 2003; 38: 199–206. [DOI] [PubMed] [Google Scholar]
- 69. Matsuoka T, Imai A, Fujimoto H, et al. Reduced pineal volume in Alzheimer disease: a retrospective cross-sectional MR imaging study. Radiology 2018; 286: 239–248. [DOI] [PubMed] [Google Scholar]
- 70. Zidan M, Boban J, Bjelan M, et al. Thalamic volume loss as an early sign of amnestic mild cognitive impairment. J Clin Neurosci 2019; 68: 168–173. [DOI] [PubMed] [Google Scholar]
- 71. Persson K, Bohbot VD, Bogdanovic N, et al. Finding of increased caudate nucleus in patients with Alzheimer’s disease. Acta Neurologica Scandinavica 2018; 137: 224–232. [DOI] [PubMed] [Google Scholar]
- 72. Zhao Q, Lu H, Metmer H, et al. Evaluating functional connectivity of executive control network and frontoparietal network in Alzheimer’s disease. Brain Res 2018; 1678: 262–272. [DOI] [PubMed] [Google Scholar]
- 73. Yu E, Liao Z, Tan Y, et al. High-sensitivity neuroimaging biomarkers for the identification of amnestic mild cognitive impairment based on resting-state fMRI and a triple network model. Brain Imaging Behav 2019; 13: 1–14. [DOI] [PubMed] [Google Scholar]
- 74. Arighi A, Carandini T, Mercurio M, et al. Word and picture version of the free and cued selective reminding test (FCSRT): is there any difference? J Alzheimers Dis 2018; 61: 47–52. [DOI] [PubMed] [Google Scholar]
- 75. Slachevsky A, Barraza P, Hornberger M, et al. Neuroanatomical comparison of the “word” and “picture” versions of the free and cued selective reminding test in Alzheimer’s disease. J Alzheimers Dis 2018; 61: 589–600. [DOI] [PubMed] [Google Scholar]
- 76. Petok JR, Myers CE, Pa J, et al. Impairment of memory generalization in preclinical autosomal dominant Alzheimer’s disease mutation carriers. Neurobiol Aging 2018; 65: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Menon V. Memory and cognitive control circuits in mathematical cognition and learning. Prog Brain Res 2016; 227: 159–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Delazer M, Zamarian L, Benke T, et al. Is an intact hippocampus necessary for answering 3x3? Evidence from Alzheimer’s disease. Brain Cogn 2019; 134: 1–8. [DOI] [PubMed] [Google Scholar]
- 79. Verfaillie SCJ, Slot RE, Tijms BM, et al. Thinner cortex in patients with subjective cognitive decline is associated with steeper decline of memory. Neurobiol Aging 2018; 61: 238–244. [DOI] [PubMed] [Google Scholar]
- 80. Van der Stigchel S, de Bresser J, Heinen R, et al. Parietal involvement in constructional apraxia as measured using the pentagon copying task. Dement Geriatr Cogn Disord 2018; 46: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Matsuoka T, Kato Y, Imai A, et al. Differences in the neural correlates of frontal lobe tests. Psychogeriatrics 2018; 18: 42–48. [DOI] [PubMed] [Google Scholar]
- 82. Brown CA, Schmitt FA, Smith CD, et al. Distinct patterns of default mode and executive control network circuitry contribute to present and future executive function in older adults. Neuroimage 2019; 195: 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fernaeus SE, Hellstrom A. Conceptual elaboration versus direct lexical access in WAIS-similarities: differential effects of white-matter lesions and gray matter volumes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2018; 25: 893–903. [DOI] [PubMed] [Google Scholar]
- 84. Peters-Founshtein G, Peer M, Rein Y, et al. Mental-orientation: a new approach to assessing patients across the Alzheimer’s disease spectrum. Neuropsychology 2018; 32: 690–699. [DOI] [PubMed] [Google Scholar]
- 85. Yamashita KI, Uehara T, Prawiroharjo P, et al. Functional connectivity change between posterior cingulate cortex and ventral attention network relates to the impairment of orientation for time in Alzheimer’s disease patients. Brain Imaging Behav 2019; 13: 154–161. [DOI] [PubMed] [Google Scholar]
- 86. McLachlan E, Bousfield J, Howard R, Reeves S. Reduced parahippocampal volume and psychosis symptoms in Alzheimer’s disease. Int J Geriatr Psychiatry 2018; 33: 389–395. [DOI] [PubMed] [Google Scholar]
- 87. Guo Z, Liu X, Xu S, et al. Abnormal changes in functional connectivity between the amygdala and frontal regions are associated with depression in Alzheimer’s disease. Neuroradiology 2018; 60: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 88. Ren P, Chapman B, Zhang Z, et al. Functional and structural connectivity of the amygdala underpins locus of control in mild cognitive impairment. Neuroimage Clin 2018; 20: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maeno N. Correlation between beta-amyloid deposits revealed by BF-227-PET imaging and brain atrophy detected by voxel-based morphometry-MR imaging: a pilot study. Nucl Med Commun 2019; 40: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 2019; 25: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hou P, Liu G, Zhao Y, et al. Role of copper and the copper-related protein CUTA in mediating APP processing and Abeta generation. Neurobiol Aging 2015; 36: 1310–1315. [DOI] [PubMed] [Google Scholar]
- 92. Singh I, Sagare AP, Coma M, et al. Low levels of copper disrupt brain amyloid-beta homeostasis by altering its production and clearance. Proc Natl Acad Sci U S A 2013; 110: 14771–14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rozzini L, Lanfranchi F, Pilotto A, et al. Serum non-ceruloplasmin non-albumin copper elevation in mild cognitive impairment and dementia due to Alzheimer’s disease: a case control study. J Alzheimers Dis 2018; 61: 907–912. [DOI] [PubMed] [Google Scholar]
- 94. Varma VR, Oommen AM, Varma S, et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med 2018; 15: e1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nation DA, Tan A, Dutt S, et al. Circulating progenitor cells correlate with memory, posterior cortical thickness, and hippocampal perfusion. J Alzheimers Dis 2018; 61: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Moreland J, Urhemaa T, van Gils M, et al. Validation of prognostic biomarker scores for predicting progression of dementia in patients with amnestic mild cognitive impairment. Nucl Med Commun 2018; 39: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Filippi M, Basaia S, Canu E, et al. Changes in functional and structural brain connectome along the Alzheimer’s disease continuum. Mol Psychiatry 2020; 25: 230–239. [DOI] [PubMed] [Google Scholar]
- 98. Meijboom R, Steketee RME, Ham LS, et al. Exploring quantitative group-wise differentiation of Alzheimer’s disease and behavioural variant frontotemporal dementia using tract-specific microstructural white matter and functional connectivity measures at multiple time points. Eur Radiol 2019; 29: 5148–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Song Z, Farrell ME, Chen X, et al. Longitudinal accrual of neocortical amyloid burden is associated with microstructural changes of the fornix in cognitively normal adults. Neurobiol Aging 2018; 68: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Huang Q, Cao X, Chai X, et al. Three-dimensional pseudocontinuous arterial spin labeling and susceptibility-weighted imaging associated with clinical progression in amnestic mild cognitive impairment and Alzheimer’s disease. Medicine (Baltimore) 2019; 98: e15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Park M, Moon Y, Han SH, et al. Motor cortex hypointensity on susceptibility-weighted imaging: a potential imaging marker of iron accumulation in patients with cognitive impairment. Neuroradiology 2019; 61: 675–683. [DOI] [PubMed] [Google Scholar]
- 102. Moller C, Pijnenburg YA, van der Flier WM, et al. Alzheimer disease and behavioral variant frontotemporal dementia: automatic classification based on cortical atrophy for single-subject diagnosis. Radiology 2016; 279: 838–848. [DOI] [PubMed] [Google Scholar]
- 103. Raamana PR, Rosen H, Miller B, et al. Three-class differential diagnosis among Alzheimer disease, frontotemporal dementia, and controls. Front Neurol 2014; 5: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Du AT, Schuff N, Kramer JH, et al. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain 2007; 130: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Du AT, Jahng GH, Hayasaka S, et al. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology 2006; 67: 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang Y, Schuff N, Ching C, et al. Joint assessment of structural, perfusion, and diffusion MRI in Alzheimer’s disease and frontotemporal dementia. Int J Alzheimers Dis 2011; 2011: 546871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Friese U, Meindl T, Herpertz SC, et al. Diagnostic utility of novel MRI-based biomarkers for Alzheimer’s disease: diffusion tensor imaging and deformation-based morphometry. J Alzheimers Dis 2010; 20: 477–490. [DOI] [PubMed] [Google Scholar]
- 108. Haller S, Missonnier P, Herrmann FR, et al. Individual classification of mild cognitive impairment subtypes by support vector machine analysis of white matter DTI. AJNR Am J Neuroradiol 2013; 34: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bron EE, Smits M, Papma JM, et al. Multiparametric computer-aided differential diagnosis of Alzheimer’s disease and frontotemporal dementia using structural and advanced MRI. Eur Radiol 2017; 27: 3372–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jeon S, Kang JM, Seo S, et al. Topographical heterogeneity of Alzheimer’s disease based on MR imaging, Tau PET, and Amyloid PET. Front Aging Neurosci 2019; 11: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Migliaccio R, Agosta F, Basaia S, et al. Functional brain connectome in posterior cortical atrophy. Neuroimage Clin 2020; 25: 102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_1 for An update on magnetic resonance imaging markers in AD by Michela Leocadi, Elisa Canu, Davide Calderaro, Davide Corbetta, Massimo Filippi and Federica Agosta in Therapeutic Advances in Neurological Disorders