Abstract
Clinicians frequently stress the importance of maintaining good oral health for multiple reasons, including its link to systemic health. Because periodontal treatment reduces inflammation in oral tissues, some hypothesize it may positively affect systemic outcomes by reducing inflammation in the body. A significant number of systematic reviews (SRs) and meta-analyses (MAs) have evaluated the effect of periodontal treatment on systemic outcomes. However, inconsistent findings and questionable methodological rigor make drawing conclusions difficult. We conducted a systematic review of reviews that studied the effect of nonsurgical periodontal treatment on systemic disease outcomes. We report on outcomes evaluated, categorizing them as biomarkers, and surrogate or clinical endpoints. In addition, we used A MeaSurement Tool to Access systematic Reviews 2 (AMSTAR 2) to evaluate the methodological quality of the reviews. Of the 52 studies included in our review, 21 focused on diabetes, 15 on adverse birth outcomes, 8 on cardiovascular disease, 3 each on obesity and rheumatoid arthritis, and 2 on chronic kidney disease. Across all studies, surrogate endpoints predominated as outcomes, followed by biomarkers and, rarely, actual disease endpoints. Ninety-two percent of studies had “low” or “critically low” AMSTAR 2 confidence ratings. Criteria not met most frequently included advance registration of the protocol, justification for excluding individual studies, risk of bias from individual studies being included in the review, and appropriateness of meta-analytical methods. There is a dearth of robust evidence on whether nonsurgical periodontal treatment improves systemic disease outcomes. Future reviews should adhere more closely to methodological guidelines for conducting and reporting SRs/MAs than has been the case to date. Beyond improved reviews, additional rigorous research on whether periodontal treatment affects systemic health is needed. We highlight the potential of large-scale databases containing matched medical and dental record data to inform and complement future clinical research studying the effect of periodontal treatment on systemic outcomes.
Keywords: periodontal disease, biomarkers, inflammation, oral-systemic disease, clinical outcomes, periodontal medicine
Introduction
Globally, the prevalence of periodontitis ranges from 20% to 50% among adults (Nazir et al. 2020). Periodontitis is associated with several chronic conditions, including cardiovascular disease, diabetes mellitus, chronic kidney disease, rheumatoid arthritis, and obesity (Martinez-Herrera et al. 2017; Sanz et al. 2018; Bui et al. 2019; Kapellas et al. 2019; Liccardo et al. 2019; Mankia et al. 2019; Rodríguez-Lozano et al. 2019). Inflammation plays a pivotal role in the initiation and progression of many of these diseases, and sustained low-grade inflammation in the body is suspected to be an antecedent of obesity (Wiebe et al. 2019), diabetes (Pradhan et al. 2001), and cardiovascular disease (Libby et al. 2002; Ridker et al. 2005; Ridker et al. 2017). Consequently, preventing and managing inflammatory processes may improve the prognosis of these diseases (Ridker et al. 2005; Ridker et al. 2017). Because periodontal treatment reduces inflammation in oral tissues, it may positively affect systemic disease outcomes by reducing inflammation in the body (Teeuw et al. 2014; Simpson et al. 2015; Calderaro et al. 2017). However, this hypothesis has yet to be validated.
In the past decade, a large number of systematic reviews (SRs) and meta-analyses (MAs) have examined the relationship between periodontal treatment and disease-specific systemic outcomes in over 175 primary studies. However, 3 major limitations across these SRs and MAs constrain the degree to which they can inform clinical care, policy, and research. First, their findings are inconsistent. While some SRs and MAs found positive systemic effects related to the periodontal treatment (Silvestre et al. 2016; Teshome and Yitayeh 2016; Calderaro et al. 2017), others have reported either no effect (Janket et al. 2003; Chambrone et al. 2011; Boutin et al. 2013) or insufficient evidence to make any claims (Mauri-Obradors et al. 2015; Pérez-Losada et al. 2016; Liu et al. 2019). Second, methodological issues such as the inclusion of studies with high risk of bias, differences in how periodontal disease is defined and measured, low sample sizes, and wide variation in study duration limit validity and generalizability (Faggion et al. 2016; Hasuike et al. 2017; Beck et al. 2019). Third and last, each review focused on only 1 systemic disease, making it difficult, if not impossible, to draw inferences regarding the relationship of periodontal treatment to overall systemic health across diseases. In consequence, it remains unclear whether periodontal treatment should be recommended as part of best practices in the management of systemic diseases and outcomes. Given that host inflammatory responses are similar in the pathogenesis of periodontitis and many chronic conditions, it is conceivable that reducing the systemic burden of inflammation in the body benefits the clinical management of these comorbidities. Since periodontal treatment is recommended to reduce the potential risk and complications associated with other chronic diseases, a comprehensive review of the effect of periodontal treatment on systemic health (clinical outcomes and biomarkers) is needed.
The purpose of this systematic review of reviews was to 1) summarize information about the effect of nonsurgical periodontal treatment on multiple systemic diseases and conditions, with emphasis on findings from high-quality SRs/MAs, and 2) assess the methodological quality of the underlying SRs and MAs. In addition, we offer recommendations for future research to address gaps identified by this review. Our findings are likely to be of interest to medical and dental providers who share in the care and management of patients, particularly those with chronic conditions, as well as policymakers and researchers interested in the link between oral and systemic health.
Methods
Protocol and Registration
We developed an a priori protocol for our study, which was registered in the International Prospective Register of Systematic Reviews (PROSPERO; #CRD42018087787). Furthermore, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for this article (Liberati et al. 2009).
Search Strategy
With the assistance of an academic librarian, we conducted a preliminary search in Ovid and EMBASE to identify SRs and MAs synthesizing primary literature on the relationship between periodontal treatment and systemic conditions. SRs and MAs synthesize the best available evidence from a body of work (typically randomized control trials [RCTs] and prospective cohort studies for intervention studies) to support evidence-based clinical decision making. The initial search focused on periodontal treatment and Alzheimer disease, asthma, cardiovascular disease, cerebrovascular disease, chronic kidney disease, diabetes mellitus, metabolic syndrome, obesity, pneumonia, pregnancy complications, respiratory tract diseases, and rheumatoid arthritis. We found SR and MAs for 6 diseases and conditions: adverse birth outcomes, cardiovascular disease, chronic kidney disease, diabetes mellitus, obesity, and rheumatoid arthritis, all of which were included in the study.
Medline via Ovid, EMBASE, and Cochrane Central databases were searched from inception to December 30, 2019. Keywords included in our search were periodontics, dental prophylaxis, pregnancy, cardiovascular diseases, kidney disease, obesity, rheumatoid arthritis, and diabetes mellitus. These search terms were modified and adapted based on the database searched (Appendix A). We did not search the gray literature because our focus was on high-quality, peer-reviewed evidence.
Inclusion Criteria
To be included, a study had to be 1) a peer-reviewed systematic review or meta-analysis, 2) written in English, and 3) focused on the effect of nonsurgical periodontal treatment on either adverse birth outcomes, cardiovascular disease, chronic kidney disease, diabetes mellitus, obesity, or rheumatoid arthritis. We included all outcomes associated with a systemic disease or condition, except for periodontal outcomes (such as probing depth or clinical attachment level). We excluded any study that evaluated the effect of nonsurgical periodontal treatment on only periodontal outcomes of patients with systemic diseases. For our intervention, we chose nonsurgical mechanical periodontal treatment, which uses scaling and root debridement to remove plaque, stain, and calculus. Nonsurgical periodontal treatment (root debridement) is the most common approach to treat periodontal disease (Sanz et al. 2012; Smiley et al. 2015). While there are other methods, such as surgical approaches, laser treatment, and antimicrobial therapy, they are less widely used than nonsurgical periodontal treatment. Studies were excluded if they only focused on the effect of antimicrobial therapy, laser treatment, or surgical periodontal treatments.
Study Selection
Article titles and abstracts were combined from all database searches, and duplicates were removed. Two reviewers (HLT and TKS) independently examined the title and abstract of all articles for potential inclusion. The full text of these articles was reviewed in detail against the inclusion/exclusion criteria. Discordant opinions on whether an article qualified for inclusion were discussed and resolved.
Quality Assessment
Three reviewers (HLT, SR, and TJT) independently appraised the methodological quality of each study using the A MeaSurement Tool to Access systematic Reviews 2 (AMSTAR 2) (Shea et al. 2017). This critical appraisal tool was developed specifically to evaluate the quality of reviews and meta-analyses that summarize RCTs, nonrandomized intervention studies, or both (Shea et al. 2017; Pieper et al. 2019). Since a significant number of SRs and MAs studying the effect of periodontal treatment on systemic outcomes draw on both RCTs and prospective cohort studies, AMSTAR 2 was deemed more appropriate to assess this body of work than AMSTAR, which should be used to assess quality of SRs and MAs based on RCTs only (Shea et al. 2017).
AMSTAR 2 provides a scheme for rating overall confidence in the quality of a SR or MA. The ratings range from “high” (indicating the study has no or 1 noncritical weakness) to “critically low” (indicating the study has more than 1 critical flaw with or without noncritical weaknesses). A full explanation of the criteria and rating scheme has been previously reported (Shea et al. 2017). AMSTAR 2 domains or items should not be used to calculate an overall numeric score, as this may hide critical weaknesses. Briefly, AMSTAR 2 lists 7 domains that can “critically affect the validity of a review and its conclusions” (Shea et al. 2017). These domains include consideration for a protocol being registered, adequacy of the literature search, justification for excluded studies, assessment of the risk of bias, the appropriateness of meta-analytical methods, whether risk of bias was considered in the interpretation of results, and whether publication bias was assessed (Shea et al. 2017). A study that is flawed in at least 1 of these critical 7 domains weakens confidence in the review. Thus, studies rated as having “low” or “critically low” confidence may not provide an accurate representation of the effect of periodontal treatment on systemic outcomes and should not be relied upon for clinical decision making or policy implications (Shea et al. 2017).
Data Extraction
Three reviewers (HLT, SR, and TJT) individually categorized the article’s systemic disease focus, the outcome(s) evaluated in each SR/MA, and the empirical result of each MA (negative, null, or positive). Empirical results were coded as “positive” if a study found a statistically significant positive association for positive outcomes (e.g., increase in birthweight) and negative association for negative outcomes (e.g., decline in HbA1c), “negative” if a study found a statistically negative association for positive outcomes (e.g., decline in birthweight) or positive associations for negative outcomes (e.g., increase in perinatal mortality), and “null” if no statistically significant associations were found. We also created a categorical variable to describe each outcome as a biomarker, surrogate endpoint, or clinical endpoint in accordance with a definition of chronic disease outcomes by the National Academy of Medicine (formerly the Institute of Medicine) (Institute of Medicine Committee on Qualification of Biomarkers and Surrogate Endpoints in Chronic Disease 2010). A biomarker is an “indicator of normal biological processes, pathogenic processes, or pharmacologic responses,” such as cholesterol level. A surrogate endpoint is an indicator believed to accurately predict a clinical endpoint. An example is blood pressure as a surrogate for myocardial infarction. Finally, a clinical endpoint is a variable that represents how a patient “feels, functions, or survives.” Clinical endpoints include mortality, improvements in quality of life, and relief of disease symptoms. All 3 reviewers met frequently to discuss findings and calibrate data extraction methods. Differences among reviewers were resolved by discussion.
Analyses
We used Covidence (2019) to combine search results, remove duplicates, and select articles for inclusion. Covidence is a SR automation software package that supports multiphase review workflow processes and includes features for data abstraction, screening, reviewer conflict resolution, and PRISMA flow diagrams (Kellermeyer et al. 2018). It is also the standard platform used in Cochrane reviews (Kellermeyer et al. 2018). Data extracted from the full text of studies were combined and prepared for analysis in Microsoft Excel (2016). Summary descriptive statistics were calculated for all meta-analyses and stratified by study quality using Stata/SE16 (2019). Overall conclusions for SR/MAs rated as having “moderate” or “high” confidence levels according to the AMSTAR 2 quality assessment were described.
Results
The initial search retrieved 926 articles (see Fig.). After duplicates were removed, 733 titles and abstracts were screened. Subsequently, 85 full-text articles were reviewed against inclusion/exclusion criteria. Articles excluded during full-text review and reasons for their exclusion are listed in Appendix B. In total, 52 articles met our inclusion criteria. Of these, 21 were focused on diabetes, 15 on adverse birth outcomes, 8 on cardiovascular disease, 3 each on obesity and rheumatoid arthritis, and 2 on chronic kidney disease. Most included studies defined the disease or condition of interest broadly, aiming to be more inclusive than exclusive of underlying studies. For instance, “CVD” encompassed heart disease, atherosclerosis, coronary artery disease, congestive heart failure, and hypertension. “Diabetes” was defined as type 1 diabetes mellitus, type 2 diabetes mellitus, hyperglycemia, and poor glycemic control. Last, “chronic kidney disease” definitions included renal failure and kidney damage.
Figure.
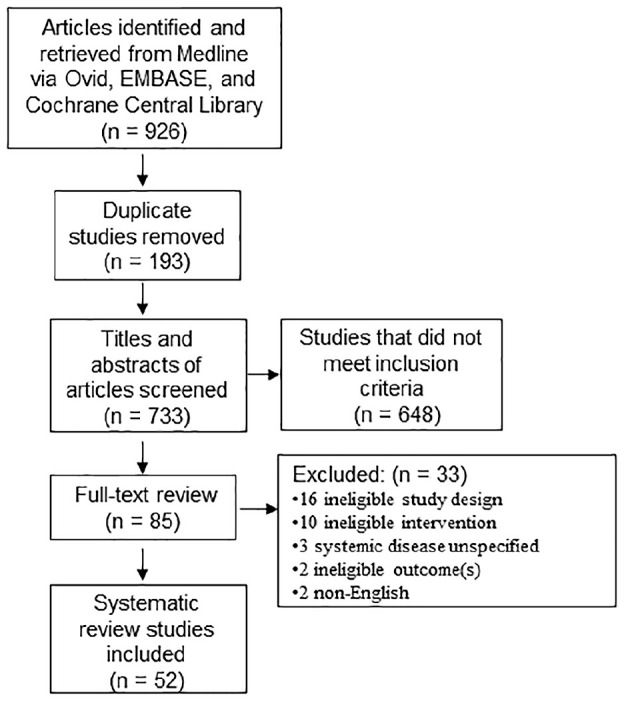
Study identification and selection process. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram illustrating article screening process. Of 926 articles retrieved from the search strategy, a total of 52 were selected for inclusion in this study.
Forty-four of the 52 studies were meta-analyses, and the remaining 8 systematic reviews did not include quantitative analyses. About half of the studies (53.8%; n = 28) restricted their inclusion criteria to RCTs only. The MAs comprised a total of 174 unique analyses of 41 outcomes. Table 1 displays outcomes evaluated in at least 3 separate analyses. Results of these analyses are grouped by study quality as determined by the AMSTAR 2 tool (see below). Across all studies, analyses of surrogate endpoints (n = 123; 70.7%) predominated by a large margin, followed by biomarkers (n = 40; 23.0%). True clinical endpoints, such as abortions/stillbirths and perinatal mortality, were evaluated only rarely (n = 11; 6.3%). The types of outcomes evaluated within the 174 unique analyses were also grouped by the condition or disease (Table 1). No meta-analysis has been conducted on clinical endpoints associated with cardiovascular disease, chronic kidney disease, diabetes, or obesity. Clinical endpoints evaluated thus far include adverse birth outcomes (such as perinatal mortality, abortion/stillbirth, and congenital malformations) and rheumatoid arthritis outcomes (such as tender joint count, swollen joint count, and disease activity score).
Table 1.
Outcomes Evaluated in Meta-Analyses Determining the Effect of Periodontal Treatment on Systemic Outcomes.
| High Qualitya | Low/Critically Low Qualityb | ||||||
|---|---|---|---|---|---|---|---|
| Systemic Outcome | Outcome Type | Total Number of Meta-Analyses | Number of Analyses with Positive Results | Numberof Analyseswith Null Results | Number of Analyses with Positive Results | Number of Analyses with Null Results | Chronic Condition Focus of Study |
| Preterm birth | Surrogate endpoint | 38 | 1 | 5 | 9 | 23 | ABO |
| HbA1c | Surrogate endpoint | 25 | 1 | 1 | 19 | 4 | Diabetes,c obesity |
| Low birthweight | Surrogate endpoint | 25 | 2 | 4 | 1 | 18 | ABO |
| C-reactive protein | Biomarker | 8 | 0 | 0 | 5 | 3 | CVD, diabetes,c obesity, RA |
| Fasting blood glucose | Surrogate endpoint | 7 | 0 | 0 | 3 | 4 | Diabetesc |
| High-density lipoprotein | Biomarker | 5 | 0 | 0 | 1 | 4 | CVD, diabetesc |
| Total cholesterol | Biomarker | 5 | 0 | 0 | 2 | 3 | CVD, diabetesc |
| Birthweight | Surrogate endpoint | 5 | 0 | 3 | 2 | 0 | ABO |
| Low-density lipoprotein | Biomarker | 4 | 0 | 0 | 0 | 4 | CVD, diabetesc |
| Triglycerides | Biomarker | 4 | 0 | 0 | 1 | 3 | CVD, diabetesc |
| Abortion/stillbirths | Clinical endpoint | 4 | 0 | 0 | 1 | 3 | ABO |
| Interleukin-6 | Biomarker | 3 | 0 | 0 | 1 | 2 | CVD, obesity |
| Perinatal mortality | Clinical endpoint | 3 | 0 | 1 | 1 | 1 | ABO |
| Tumor necrosis factor–α | Biomarker | 3 | 0 | 0 | 3 | 0 | CVD, diabetes,c obesity |
| Otherd | Biomarkers and surrogate endpoints | 35 | 0 | 1 | 7 | 27 | ABO, CKD, CVD, diabetes,c obesity, RA |
| Total | 174 | 4 | 15 | 56 | 99 | ||
ABO, adverse birth outcomes (perinatal outcomes); CKD, chronic kidney disease (renal failure, kidney damage); CVD, cardiovascular disease (heart disease, atherosclerosis, coronary heart disease, congestive heart failure, hypertension); RA, rheumatoid arthritis.
High quality rating as determined according to the AMSTAR 2 (Shea et al. 2017) tool.
Low/Critically low quality rating according to the AMSTAR 2 (Shea et al. 2017) tool.
Diabetes includes type 1, type 2, and hyperglycemia.
Includes all other biomarkers or surrogate endpoints evaluated in 2 or fewer unique meta-analyses. Examples include disease activity score, estimated globular filtration rate, creatinine, fibrinogen, leukocytes, flow-mediated dilation, adiponectin, body mass index, leptin, and so on.
As shown in Appendix C, 78.8% of studies were found to be of critically low quality (n = 41), 13.5% of low quality (n = 7), 0% of moderate quality (n = 0), and 7.7% of high quality (n = 4). Among studies that were rated as having “critically low” confidence (n = 41), 17 focused on diabetes, 10 on adverse birth outcomes, 7 on cardiovascular disease, 3 each on obesity and rheumatoid arthritis, and 1 on chronic kidney disease. Among studies that were rated as having “low” confidence (n = 7), 3 each focused adverse birth outcomes and diabetes and 1 on chronic kidney disease. Among studies identified as having a “high” overall confidence level (n = 4), 2 studies focused on adverse birth outcomes, 1 on cardiovascular disease, and 1 on diabetes.
Within the studies rated as having “high” confidence levels, 19 meta-analyses were conducted, with 4 analyses finding positive effects and 15 finding null effects. Simpson et al. (2015) reported that periodontal treatment reduced HbA1c levels within 3 to 4 mo of treatment but found insufficient evidence of reductions in months thereafter. Kim et al. (2012) reported a positive effect of periodontal treatment on low birthweight (<2,500 g) and preterm birth (<37 wk) among high-risk pregnant women and null effects for birthweight, low birthweight, and preterm birth among typical pregnancies. Iheozor-Ejiofor et al. (2017) reported periodontal treatment had a positive effect on low birthweight (<2,500 g) and null effects in other meta-analyses of birth outcomes, including preterm birth (<37 and <35 wk), perinatal mortality, preeclampsia, and low weight (<1,500 g). Liu et al. (2019) reported insufficient evidence for the effect of periodontal treatment on primary and secondary prevention of cardiovascular disease and were not able to conduct any meta-analyses.
Table 2 presents the number of SRs/MAs that adhere to each AMSTAR 2 critical domain. Critical domains in which the overwhelming majority of articles were in compliance included the adequacy of the literature search (n = 46; 88.5%), consideration of risk of bias when interpreting results (n = 36; 69.2%), the assessment of risk of bias from included studies (n = 35; 66.0%), the application of appropriate meta-analytical methods (n = 35; 66.0%), and assessing the presence and impact of publication bias (n = 31; 59.6%). The most frequent critical flaws included not registering a protocol prior to the review (n = 41; 78.8%) and not justifying excluded studies (n = 26; 50%). Detailed AMSTAR 2 ratings for each SR and MA can be found in Appendix D.
Table 2.
Number of Systematic Reviews and Meta-Analyses Evaluating the Effect of Periodontal Treatment on Systemic Disease That Did Not Meet AMSTAR 2 Critical Domains (n = 52).
| AMSTAR 2 Critical Domains | Criteria Not Met, n (%) |
|---|---|
| Protocol registered before commencement of the review (item 2) | 41 (78.8) |
| Justification for excluding individual studies (item 7) | 26 (50.0) |
| Assessment of presence and likely impact of publication bias (item 15) | 21 (40.4) |
| Risk of bias from individual studies being included in the review (item 9) | 18 (34.0) |
| Appropriateness of meta-analytical methods (item 11) | 18 (34.0) |
| Consideration of risk of bias when interpreting the results of the review (item 13) | 16 (30.8) |
| Adequacy of the literature search (item 4) | 6 (11.5) |
AMSTAR 2, A MeaSurement Tool to Access systematic Reviews 2 (Shea et al. 2017).
Discussion
Our systematic review of reviews evaluated the methodological quality of SRs/MAs that study the effect of periodontal treatment on systemic disease outcomes. The review yielded 2 major findings: 1) within the 4 (7.7%) high quality reviews, 4 analyses found positive effects and 15 analyses found null effects of periodontal treatment on a systemic outcome, and 2) the overwhelming majority of SRs/MAs (92.3%) have “low” or “critically low” confidence ratings as determined by the AMSTAR 2 instrument. Just over a half (53.8%) of the studies were based exclusively on RCTs, including the 4 studies rated as high quality. The remaining 46.2% of studies included prospective cohort studies, which, given their design, may contribute to bias and unreliable results within SRs/MAs. Among the 4 meta-analyses determined to be of high quality, periodontal treatment was found to have a positive effect on HbA1c among diabetics, low birthweight (<2,500 g), and preterm birth in high-risk pregnancies (<37 wk) (Kim et al. 2012; Simpson et al. 2015; Iheozor-Ejiofor et al. 2017). Yet, even the interpretations of these positive findings were framed with caution due to the low-quality studies that underpin the analyses (Kim et al. 2012; Simpson et al. 2015; Iheozor-Ejiofor et al. 2017). Given the paucity of positive effects as determined by high quality studies and the low quality of the remaining studies, there is a dearth of robust evidence as to whether periodontal interventions improve systemic outcomes.
Surrogate endpoints were, by far, the most frequently analyzed outcome, followed by biomarkers and, rarely, clinical endpoints. Many surrogate endpoints and biomarkers are variables that can be obtained fairly easily (for instance, through a laboratory test) and/or in a timely manner (for instance, low birthweight in the context of pregnancy). Surrogate endpoints and biomarkers may be useful proxies for clinical outcomes if they reliably predict clinical endpoints (FDA-NIH Biomarker Working Group 2016). For instance, HbA1c is considered a validated surrogate indicator for microvascular complications and can be used to demonstrate direct clinical benefits (FDA-NIH Biomarker Working Group 2016). Since a number of biomarkers may be indicators for several diseases, particularly those related to inflammation, we need to broadly understand how periodontal treatment affects these markers. For instance, many studies use C-reactive protein (CRP), a systemic indicator of inflammation in multiple diseases, as a primary outcome of interest (Koromantzos et al. 2012; Bharti et al. 2013; Okada et al. 2013). However, a change in biomarker levels following an intervention does not necessarily indicate a change in disease risk (Krumholz 2015). At the same time, clinical endpoints for many chronic diseases, such as myocardial infarction or death in cardiovascular disease, progression to end-stage renal disease in chronic kidney disease, and diabetic peripheral neuropathy in diabetes, may not be observable within typical prospective study periods and thus difficult to capture. In these cases, surrogate endpoints are useful study outcomes.
As a result of this review, we recommend 4 measures to help advance our knowledge on whether and how periodontal treatment affects systemic disease:
The quality of SRs and MAs should be improved. High standards in methods are a “pre-requisite for valid interpretation and application of review findings” (Shea et al. 2009). In 2008, the Cochrane Collaboration provided guidance on conducting high quality reviews (Higgins et al. 2019). While AMSTAR and AMSTAR 2 have been available for some time, poor quality in systematic reviews is common (Tian et al. 2017; Pieper et al. 2018). Regardless of the quality of the primary literature, methodologically sound SRs are expected to provide the highest level of evidence for research, policy, and clinical care decisions.
Future reviews should adhere more closely to methodological guidelines for conducting and reporting SRs/MAs, such as the Cochrane Collaboration Handbook (Higgins et al. 2019) and PRISMA (Moher et al. 2009), than has been the case to date. Requiring measures such as the use of an appropriate tool to assess the methodological quality of a review and protocol registration in advance may be practically implemented by journals to which reviews are submitted (Shea et al. 2017).
We need to understand the quality of the primary literature. While a poor-quality SR/MA does not necessarily mean that the included studies are also of low quality, concerns regarding their rigor have been raised (Faggion et al. 2016; Shaqman et al. 2020). Examining the methodological rigor of individual studies is critical to understanding the true nature of the quality of literature in the entire domain. Methodological issues that already have been identified include how periodontal disease is defined and measured, low sample sizes, and wide variation in study duration (Simpson et al. 2015; Faggion et al. 2016; Beck et al. 2019). These inconsistencies affect systematic review and meta-analyses outcomes, and they make comparisons across studies subject to bias (Beck et al. 2019). Quantifying these methodological issues may help guide future research on best practices for determining whether and how periodontal treatment affects systemic health.
Additional, rigorous research that examines the relationship between periodontal treatment and health outcomes longitudinally is needed. Multiple circumstances make it difficult to study disease endpoints through prospective clinical studies and RCTs, such as ethical implications and issues with feasibility, costs, and the statistical power needed to evaluate systemic effects of periodontal treatment. Furthermore, while RCTs often have high internal validity, they may suffer from low external validity, potentially limiting the generalizability of their findings. One notable opportunity for research complementing RCTs is studying medical and dental health record data of the same patients and using self-reported medical history information contained within an electronic dental record (EDR) (Song et al. 2013; Merchant and Virani 2017; Thyvalikakath et al. 2020). With the increasing use of EDR data to determine dental treatment effectiveness (Siddiqui et al. 2017; Thyvalikakath et al. 2020), researchers can conduct large-scale observational studies on available electronic health and dental data in a potentially ethical, feasible, and cost-efficient way (Merchant et al. 2016; Merchant and Virani 2017). Given the large sample sizes, high granularity, and high dimensionality of data obtained by linking of EHR and EDR data (St Sauver et al. 2017; Patel et al. 2018), robust analytic approaches and quasi-experimental designs can be used to generate high quality, causal evidence. Characterizing outcomes of the care provided in real-world clinical settings could produce more generalizable findings than RCTs.
This study is the first to assess the methodological quality of SRs/MAs evaluating the effect of periodontal treatment on systemic health outcomes across various diseases and conditions. Our study was limited to 6 conditions because we found no SRs or MAs focused on the effect of nonsurgical periodontal treatment on any others. Given the large number of diseases associated with periodontal disease (Monsarrat et al. 2016), we attempted to minimize the chance of missing relevant SRs/MAs focused on periodontal treatment by enlisting the aid of an academic librarian and by searching 3 different biomedical literature databases. Some primary studies have evaluated the effect of periodontal treatment on diseases such as asthma and chronic obstructive pulmonary disease. However, since no SRs/MAs were available for these diseases, they were not included in this review. Nonetheless, the 6 conditions included in this study constitute a significant burden of morbidity and as a result are relevant from a clinical care and public health perspective. Finally, we endeavored to reduce bias due to subjectivity in AMSTAR 2 ratings by obtaining consensus between 3 independent reviewers for the quality assessment.
Conclusion
Weak evidence and the limited number of methodologically rigorous SRs/MAs underscore how little we know about the effect of periodontal interventions on chronic disease outcomes. While consistent evidence shows periodontal disease is associated with chronic diseases, more research is needed to validate whether periodontal treatment improves systemic health. Given the quality of existing studies, the dental community should be cautious not to interpret associations of oral and systemic disease as causal (Pihlstrom et al. 2018).
Author Contributions
H.L. Taylor, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S. Rahurkar, contributed to conception, design, data acquisition, and interpretation, critically revised the manuscript; T.J. Treat, contributed to design, data acquisition, and interpretation, critically revised the manuscript; T.P. Thyvalikakath, contributed to design and data interpretation, critically revised the manuscript; T.K. Schleyer, contributed to data conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520965958 for Does Nonsurgical Periodontal Treatment Improve Systemic Health? by H.L. Taylor, S. Rahurkar, T.J. Treat, T.P. Thyvalikakath and T.K. Schleyer in Journal of Dental Research
Acknowledgments
We thank Rachel Hinrichs, a health sciences librarian, who provided support and assistance with the literature search strategy.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported in part by the National Library of Medicine of the National Institutes of Health under award number T15LM012502. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Library of Medicine. This publication was made possible in part by the Lilly Endowment, Inc. Physician Scientist Initiative.
ORCID iDs: H.L. Taylor  https://orcid.org/0000-0003-0958-9408
https://orcid.org/0000-0003-0958-9408
S. Rahurkar  https://orcid.org/0000-0001-8597-3087
https://orcid.org/0000-0001-8597-3087
T.J. Treat  https://orcid.org/0000-0001-5550-6867
https://orcid.org/0000-0001-5550-6867
T.P. Thyvalikakath  https://orcid.org/0000-0002-7294-2318
https://orcid.org/0000-0002-7294-2318
T.K. Schleyer  https://orcid.org/0000-0003-1829-971X
https://orcid.org/0000-0003-1829-971X
References
- Beck JD, Papapanou PN, Philips KH, Offenbacher S. 2019. Periodontal medicine: 100 years of progress. J Dent Res. 98(10):1053–1062. [DOI] [PubMed] [Google Scholar]
- Bharti P, Katagiri S, Nitta H, Nagasawa T, Kobayashi H, Takeuchi Y, Izumiyama H, Uchimura I, Inoue S, Izumi Y. 2013. Periodontal treatment with topical antibiotics improves glycemic control in association with elevated serum adiponectin in patients with type 2 diabetes mellitus. Obes Res Clin Pract. 7(2):e129–e138. [DOI] [PubMed] [Google Scholar]
- Boutin A, Demers S, Roberge S, Roy-Morency A, Chandad F, Bujold E. 2013. Treatment of periodontal disease and prevention of preterm birth: systematic review and meta-analysis. Am J Perinatol. 30(7):537–544. [DOI] [PubMed] [Google Scholar]
- Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, Asadi H, Ojcius DM. 2019. Association between periodontal pathogens and systemic disease. Biomed J. 42(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderaro DC, Corrêa JD, Ferreira GA, Barbosa IG, Martins CC, Silva TA, Teixeira AL. 2017. Influence of periodontal treatment on rheumatoid arthritis: a systematic review and meta-analysis. Rev Bras Reumatol Engl Ed. 57(3):238–244. [DOI] [PubMed] [Google Scholar]
- Chambrone L, Pannuti CM, Guglielmetti MR, Chambrone LA. 2011. Evidence grade associating periodontitis with preterm birth and/or low birth weight: II: a systematic review of randomized trials evaluating the effects of periodontal treatment. J Clin Periodontol. 38(10):902–914. [DOI] [PubMed] [Google Scholar]
- Covidence. 2019. Melbourne, Australia: Veritas Health Innovation; [accessed 2020. Sept 22]. www.covidence.org. [Google Scholar]
- Faggion CM, Jr, Cullinan MP, Atieh M. 2016. An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control. J Periodontal Res. 51(6):716–725. [DOI] [PubMed] [Google Scholar]
- FDA-NIH Biomarker Working Group. 2016. Best (biomarkers, endpoints, and other tools) resource. Bethesda (MD): Food and Drug Administration. [PubMed] [Google Scholar]
- Hasuike A, Iguchi S, Suzuki D, Kawano E, Sato S. 2017. Systematic review and assessment of systematic reviews examining the effect of periodontal treatment on glycemic control in patients with diabetes. Med Oral Patol Oral Cir Bucal. 22(2):e167–e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. 2019. Cochrane handbook for systematic reviews of interventions, version 6.0 ed [accessed 2020 Sept 22]. https://training.cochrane.org/handbook/current. [DOI] [PMC free article] [PubMed]
- Iheozor-Ejiofor Z, Middleton P, Esposito M, Glenny A-M. 2017. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst Rev. 6:CD005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Qualification of Biomarkers and Surrogate Endpoints in Chronic Disease. 2010. Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- Janket S-J, Baird AE, Chuang S-K, Jones JA. 2003. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 95(5):559–569. [DOI] [PubMed] [Google Scholar]
- Kapellas K, Singh A, Bertotti M, Nascimento GG, Jamieson LM; Perio-CKD Collaboration. 2019. Periodontal and chronic kidney disease association: a systematic review and meta-analysis. Nephrology. 24(2):202–212. [DOI] [PubMed] [Google Scholar]
- Kellermeyer L, Harnke B, Knight S. 2018. Covidence and Rayyan. J Med Libr Assoc. 106(4):580–583. [Google Scholar]
- Kim AJ, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY. 2012. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J Periodontol. 83(12):1508–1519. [DOI] [PubMed] [Google Scholar]
- Koromantzos PA, Makrilakis K, Dereka X, Offenbacher S, Katsilambros N, Vrotsos IA, Madianos PN. 2012. Effect of non-surgical periodontal therapy on C-reactive protein, oxidative stress, and matrix metalloproteinase (MMP)-9 and MMP-2 levels in patients with type 2 diabetes: a randomized controlled study. J Periodontol. 83(1):3–10. [DOI] [PubMed] [Google Scholar]
- Krumholz HM. 2015. Biomarkers, risk factors, and risk: clarifying the controversy about surrogate end points and clinical outcomes. Circ Cardiovasc Qual Outcomes. 8(5):457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. 2002. Inflammation and atherosclerosis. Circulation. 105(9):1135–1143. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, Rengo G. 2019. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 20(6):1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Cao Y, Dong L, Zhu Y, Wu Y, Lv Z, Iheozor-Ejiofor Z, Li C. 2019. Periodontal therapy for primary or secondary prevention of cardiovascular disease in people with periodontitis. Cochrane Database Syst Rev. 12:CD009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankia K, Cheng Z, Do T, Hunt L, Meade J, Kang J, Clerehugh V, Speirs A, Tugnait A, Hensor EMA, et al. 2019. Prevalence of periodontal disease and periodontopathic bacteria in anti-cyclic citrullinated protein antibody-positive at-risk adults without arthritis. JAMA Netw Open. 2(6):e195394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Herrera M, Silvestre-Rangil J, Silvestre FJ. 2017. Association between obesity and periodontal disease: a systematic review of epidemiological studies and controlled clinical trials. Med Oral Patol Oral Cir Bucal. 22(6):e708–e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri-Obradors E, Jané-Salas E, del Mar Sabater-Recolons M, Vinas M, López-López J. 2015. Effect of nonsurgical periodontal treatment on glycosylated hemoglobin in diabetic patients: a systematic review. Odontology. 103(3):301–313. [DOI] [PubMed] [Google Scholar]
- Merchant AT, Georgantopoulos P, Howe CJ, Virani SS, Morales DA, Haddock KS. 2016. Effect of long-term periodontal care on hemoglobin A1c in type 2 diabetes. J Dent Res. 95(4):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant AT, Virani SS. 2017. Evaluating periodontal treatment to prevent cardiovascular disease: challenges and possible solutions. Curr Atheroscler Rep. 19(1):4. [DOI] [PubMed] [Google Scholar]
- Microsoft Excel. (2016). Microsoft Corporation [accessed 2020 Sept 22]. https://office.microsoft.com/excel.
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsarrat P, Blaizot A, Kémoun P, Ravaud P, Nabet C, Sixou M, Vergnes J-N. 2016. Clinical research activity in periodontal medicine: a systematic mapping of trial registers. J Clin Periodontol. 43(5):390–400. [DOI] [PubMed] [Google Scholar]
- Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K. 2020. Global prevalence of periodontal disease and lack of its surveillance. ScientificWorldJournal. 2020:2146160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Kobayashi T, Ito S, Yokoyama T, Abe A, Murasawa A, Yoshie H. 2013. Periodontal treatment decreases levels of antibodies to Porphyromonas gingivalis and citrulline in patients with rheumatoid arthritis and periodontitis. J Periodontol. 84(12):e74–e84. [DOI] [PubMed] [Google Scholar]
- Patel J, Mowery D, Krishnan A, Thyvalikakath T. 2018. Assessing information congruence of documented cardiovascular disease between electronic dental and medical records. AMIA Annu Symp Proc. 2018:1442–1450. [PMC free article] [PubMed] [Google Scholar]
- Pérez-Losada FL, Jané-Salas E, Sabater-Recolons MM, Estrugo-Devesa A, Segura-Egea JJ, López-López J. 2016. Correlation between periodontal disease management and metabolic control of type 2 diabetes mellitus: a systematic literature review. Med Oral Patol Oral Cir Bucal. 21(4):e440–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper D, Koensgen N, Breuing J, Ge L, Wegewitz U. 2018. How is AMSTAR applied by authors—a call for better reporting. BMC Med Res Methodol. 18(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper D, Puljak L, González-Lorenzo M, Minozzi S. 2019. Minor differences were found between AMSTAR 2 and ROBIS in the assessment of systematic reviews including both randomized and nonrandomized studies. J Clin Epidemiol. 108:26–33. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Hodges JS, Michalowicz B, Wohlfahrt JC, Garcia RI. 2018. Promoting oral health care because of its possible effect on systemic disease is premature and may be misleading. J Am Dent Assoc. 149(6):401–403. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. 2001. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 286(3):327–334. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. 2005. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 352(1):20–28. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. 2017. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lozano B, González-Febles J, Garnier-Rodríguez JL, Dadlani S, Bustabad-Reyes S, Sanz M, Sánchez-Alonso F, Sánchez-Piedra C, González-Dávila E, Díaz-González F. 2019. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case-control study. Arthritis Res Ther. 21(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I, Alonso B, Carasol M, Herrera D, Sanz M. 2012. Nonsurgical treatment of periodontitis. J Evid Based Dent Pract. 12(3 Suppl):76–86. [DOI] [PubMed] [Google Scholar]
- Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, et al. 2018. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 45(2):138–149. [DOI] [PubMed] [Google Scholar]
- Shaqman M, Al-Abedalla K, Wagner J, Swede H, Gunsolley JC, Ioannidou E. 2020. Reporting quality and spin in abstracts of randomized clinical trials of periodontal therapy and cardiovascular disease outcomes. PLoS One. 15(4):e0230843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Henry DA, Boers M. 2009. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 62(10):1013–1020. [DOI] [PubMed] [Google Scholar]
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. 2017. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui Z, Wang Y, Makkad P, Thyvalikakath T. 2017. Characterizing restorative dental treatments of Sjögren’s syndrome patients using electronic dental records data. Stud Health Technol Inform. 245:1166–1169. [PubMed] [Google Scholar]
- Silvestre FJ, Silvestre-Rangil J, Bagan L, Bagan JV. 2016. Effect of nonsurgical periodontal treatment in patients with periodontitis and rheumatoid arthritis: a systematic review. Med Oral Patol Oral Cir Bucal. 21(3):e349–e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor-Ejiofor Z. 2015. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015(11):CD004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, Cobb CM, Rossmann J, Harrel SK, Forrest JL, et al. 2015. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 146(7):525–535. [DOI] [PubMed] [Google Scholar]
- Song M, Liu K, Abromitis R, Schleyer TL. 2013. Reusing electronic patient data for dental clinical research: a review of current status. J Dent. 41(12):1148–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata SE16. 2019. State base reference manual, release 16. StataCorp [accessed 2020 Sept 22]. https://www.stata.com/bookstore/base-reference-manual/.
- St Sauver JL, Carr AB, Yawn BP, Grossardt BR, Bock-Goodner CM, Klein LL, Pankratz JJ, Finney Rutten LJ, Rocca WA. 2017. Linking medical and dental health record data: a partnership with the Rochester Epidemiology Project. BMJ Open. 7(3):e012528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeuw WJ, Slot DE, Susanto H, Gerdes VEA, Abbas F, D’Aiuto F, Kastelein JJP, Loos BG. 2014. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 41(1):70–79. [DOI] [PubMed] [Google Scholar]
- Teshome A, Yitayeh A. 2016. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyvalikakath TP, Duncan WD, Siddiqui Z, LaPradd M, Eckert G, Schleyer T, Rindal DB, Jurkovich M, Shea T, Gilbert GH, et al. 2020. Leveraging electronic dental record data for clinical research in the national dental PBRN practices. Appl Clin Inform. 11(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Zhang J, Ge L, Yang K, Song F. 2017. The methodological and reporting quality of systematic reviews from china and the USA are similar. J Clin Epidemiol. 85:50–58. [DOI] [PubMed] [Google Scholar]
- Wiebe N, Stenvinkel P, Tonelli M. 2019. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw Open. 2(8):e1910456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520965958 for Does Nonsurgical Periodontal Treatment Improve Systemic Health? by H.L. Taylor, S. Rahurkar, T.J. Treat, T.P. Thyvalikakath and T.K. Schleyer in Journal of Dental Research


