Abstract
OBJECTIVE:
Kidney stones are painful and costly. Prevention guidelines emphasize a simple behavior change: increasing fluid intake and urine output. Unfortunately, adherence to those prevention guidelines is limited and patients report forgetting or not being thirsty enough. This study evaluated the acceptability of using semi-automated tracking of fluid consumption to trigger just-in-time reminders to drink and increase the experienced automaticity of fluid intake.
METHODS:
In a single-group trial, participants with a history of kidney stones (n = 31) used the sipIT digital tools (H2OPal connected water bottle, H2OPal mobile app for self-tracking, Fitbit smartwatch app for gesture detection) for three months.
RESULTS:
The semi-automated monitoring system detected 46,654 drinking events. From baseline to one-month follow-up, the experienced automaticity of fluid intake increased significantly (d = 0.50) and remained elevated at three-month follow-up (d = 0.64). A major barrier to adherence (lack of thirst) decreased from baseline to follow-ups. Retention rates and participant feedback indicated that this digital tool was acceptable to patients.
CONCLUSION:
Semi-automated tracking of fluid consumption can be used to trigger just-in-time reminders. Based on this demonstration, the sipIT tools are ready for testing in a rigorous Phase II trial to evaluate efficacy for increasing fluid consumption and urine output as recommended for preventing recurrence of kidney stones.
Keywords: mobile health, information technology, habits, secondary prevention, kidney calculi
Kidney stone disease is a chronic condition with high recurrence rates and acute episodes that are painful. This disease affects approximately 1 in 11 American adults (Scales et al., 2012). Annual medical costs for stones are estimated to exceed $2 billion, in part due to increased emergency department utilization (~$4500/visit) (Pearle et al., 2014). Approximately 50% of patients with stones are prescribed opioids to manage their pain so prevention is an important goal (Feinstein & Matlaga, 2018). Clinical guidelines for preventing a recurrence of stones are well established (Pearle et al., 2014; Qaseem et al., 2014). Standard guidelines for prevention are to increase fluid consumption enough to produce greater than 2.0–2.5 L of urine daily. Patients are familiar with this guideline and report a willingness to increase their fluid consumption (McCauley et al., 2012; Tarplin et al., 2016). Yet less than half of patients with stones adhere to this seemingly simple recommendation (Khambati et al., 2017; van Drongelen et al., 1998). Patients have reported interest in using digital tools to support adherence to this guideline (Streeper et al., 2018, 2019). This study presents the initial evaluation of a new digital tool called sipIT that combines consumer wearables, the science of behavior change, and informatics tools to support fluid intake in patients with a history of kidney stones.
Usual care for the prevention of kidney stones involves counseling patients to set goals for increasing fluid intake (Parks et al., 2003). Prior work has identified a variety of barriers to adherence to fluid consumption guidelines (McCauley et al., 2012; Streeper et al., 2019). Among the most common barriers are not being thirsty and forgetting to drink. One way for a behavioral intervention to address those barriers is to promote automaticity by strengthening habits for fluid consumption. Habits refer to learned behaviors that are contextually-cued and automatically performed without conscious effort (Wood & Neal, 2007). Habits can involve preparatory behaviors such as filling a water bottle in the morning or performance behaviors such as drinking from the water bottle (Gardner et al., 2016). Habit formation is an important goal for health behavior change because effortful goal pursuit is difficult to sustain. In the context of digital health, habit formation is an important clinical goal because it is unrealistic to assume that patients will sustain their engagement with digital tools indefinitely (Cole-Lewis et al., 2019).
Digital tools offer a noteworthy advantage when the goal is habit formation. As noted above, contextual consistency is important for habit formation during behavior change programs (Wood & Neal, 2007). Context is a broad concept with temporal, spatial, perceptual, physiological, and behavioral features. Although habit formation has often focused on visual or spatial cues (e.g., drink whenever you see your water bottle or enter the kitchen), interval timing is a well-established influence on learning and decision making that could be leveraged to increase the frequency of fluid intake (Marshall & Kirkpatrick, 2015; Staddon & Cerutti, 2003). Sensing technology can capture the timing of recent behavior to inform intervention delivery and shape behavioral habits. If context sensing is engaged appropriately, just-in-time interventions can be triggered for delivery at moments of vulnerability (Nahum-Shani et al., 2015, 2018). For example, a sensible goal for patients with a history of kidney stones would be to drink small volumes of fluids regularly (e.g., every 30 minutes) to avoid having to consume large volumes of fluids that could disrupt later activities because of an urgent need to urinate or cause discomfort from abdominal fullness (Streeper et al., 2019). A digital tool that monitored fluid intake could trigger reminders to drink when the patient has not consumed fluids recently. Incorporating information about recent behavior would avoid unnecessary reminders to drink that could be disruptive.
We developed the sipIT intervention to support patients with kidney stones in their efforts to develop a performance habit for regular fluid intake. Two key informatic components for designing this digital tool were the monitoring system and the intervention engine. The monitoring system involves specifying a tailoring variable and decision points. The intervention engine involves specifying a decision rule and intervention options.
Decision points in the initial implementation of sipIT were set for every 30 minutes outside of a user-defined Do Not Disturb period. The tailoring variable in sipIT is fluid intake since the last decision point. Several methods can be used to monitor fluid intake but no single method is likely to suffice by itself. For example, mobile apps commonly include self-tracking functions but users can find this technique burdensome and not record intake as a result (Conroy et al., 2016). Patients have expressed interest in connected water bottles that can sense fluid intake from the bottle and transmit it to a companion smartphone app but these devices are not sensitive to fluids consumed from other containers (e.g., coffee mugs, water fountains) (Streeper et al., 2018). Additionally, they often can only record water intake and may not be acceptable in certain social situations. Drinking gestures can be detected by wristworn inertial sensors (e.g., accelerometers, gyroscopes) but algorithms using data from these sensors can only sense gestures made by the wrist on which the sensors are worn and have a blind spot for some drinking events (e.g., via water fountains or straws) (Chun et al., 2019). In the absence of a single sufficient monitoring method, semi-automated tracking can be used to detect a diverse range of drinking behaviors (Choe et al., 2017). This method combines manual (e.g., self-monitoring via an app) with automated (e.g., connected water bottles, gesture detection) tracking methods to increase the scope of drinking events that can be detected.
The default intervention option in sipIT is to send the patient a notification message (via smartphone) reminding them to drink. Notifications are inherently disruptive. Sending too many notifications or notifications that are perceived as unnecessary or unhelpful contributes to notification fatigue which can reduce attention to alerts and long-term engagement (Cole-Lewis et al., 2019; Heckman et al., 2015; Wilken et al., 2017). Patients have indicated that lapse-contingent notifications would be valued as part of a fluid intake intervention (Streeper et al., 2018, 2019). The decision rule for sipIT notifications specifies that if the monitoring system indicates that the patient consumed a prespecified amount of fluids since the last decision point, then the reminder notification should be cancelled; otherwise (else) the reminder notification should be delivered. This decision rule employs negative reinforcement to shape behavior. Negative reinforcement is an operant conditioning technique used to create associations between a stimulus behavior (i.e., drinking) and a response consequence (i.e., not receiving disruptive reminder notifications) (Skinner, 1963). This negative reinforcement approach contrasts with the tendency in digital health to add more features to engage users. It assumes that reminder notifications are generally undesirable because they disrupt ongoing activities and can induce unpleasant affective states. By eliminating reminder notifications as a response consequence following desirable behavior (i.e., fluid intake), a patient’s fluid intake can be negatively reinforced.
The primary purpose of the present study was to evaluate the acceptability of the sipIT intervention for promoting fluid intake among patients with kidney stone disease. We also tested the hypothesis that the experienced automaticity of fluid intake would increase over time as pursuit of intake goals becomes automated and success is negatively reinforced throughout each day. Based on the nature of these questions, this research is an acceptability study within Phase I of the Obesity-Related Behavioral Intervention Trials framework (Czajkowski et al., 2015).
Methods
Participants
Participants (n = 31) were recruited using advertisements in community locations and urology clinics. Eligible participants were 18 years of age or older, had verbal and written fluency in English, were capable of giving informed consent, had a history of kidney stones, owned an iPhone (iOS v7 or later), and had no limitations impacting their ability to drink.
Intervention
The sipIT digital tools for participants included the H2OPal connected water bottle and its companion mobile app (Out of Galaxy LLC, San Francisco, CA), a Fitbit Versa smartwatch (San Francisco, CA) with a custom drinking gesture detection app and companion backend webservices operated by Fitabase (San Diego, CA), and a custom sipIT mobile application with companion backend webservices for message delivery.
The H2OPal mobile app provides an exceptional user interface for self-tracking fluid intake (relative to apps we evaluated previously; Conroy et al., 2016). The researcher paired the H2OPal water bottle with participant’s iPhone and calibrated the bottle per manufacturer’s instructions. The bottle uses a weight sensor in the base to estimate fluid intake and transmits data to the companion app via Bluetooth. The app transmitted data to the companion backend.
The sipIT drinking gesture detection app implemented an algorithm based on inertial sensor signals from the Fitbit Versa smartwatch (30 Hz sampling). This algorithm was based on our team’s prior work classifying drinking gestures using wrist-worn inertial sensors and adaptive window segmentation (Chun et al., 2019). Fluid intake from a container is characterized by a continuous sequence of movements performed by the hand, wrist and arm during the act of drinking: (1) grasping the container, (2) elevating it towards the mouth, (3) tilting the container such that the fluid flows into the mouth, (4) lowering the container back to its original position on the table, and (5) finally releasing the container. This motion pattern is reflected in the inertial sensor data captured by the smartwatch, and the algorithm is capable of discovering and segmenting this pattern irrespective of drinking duration. It outputs timestamped probabilities of drinking events and events with probability equal to 1.0 were accepted as drinking events for determining intervention delivery. The app caches classification data on the smartwatch for syncing with the Fitabase backend server.
The sipIT backend server used webhooks to receive data from the H2OPal server as it becomes available from users and an application programming interface (API) to pull data from Fitabase servers prior to every decision point. Using a proprietary decision rule, the server evaluated availability based on the participant’s Do Not Disturb window for that day, reviewed drinking data from the past 30 minutes, and determined whether to send a reminder notification to the participant. The sipIT mobile app delivered notifications as images on the smartphone (Supplementary File 1).
Measures
The experienced automaticity of fluid consumption in general was assessed at baseline and follow-up assessments using the four-item behavioral automaticity scale from the Self-Report Habit Index (Gardner et al., 2011, 2012; Verplanken & Orbell, 2003). Participants rated each item on a scale ranging from 1 (strongly disagree) to 7 (strongly agree). Higher scores indicate that fluid consumption was experienced as being more automatic across contexts (Hagger et al., 2015). Internal consistency (Cronbach’s alpha) estimates at the three time points ranged from .86 – .92.
Barriers to adherence were assessed at baseline and follow-up assessments using a checklist based on formative research (Streeper et al., 2018, 2019). This item read, “Which of the following have been barriers to meeting fluid intake guidelines for preventing stones (i.e., drinking enough to produce 2.5 L/day of urine)? Check all that apply.” Response options included “I am not thirsty enough,” “I forget to drink,” “It is a hassle to carry around a water bottle,” “I have to urinate too frequently if I drink that much,” “It’s hard to drink enough at work,” “I don’t like the taste of water,” “I am not aware of the need to drink more,” “It makes me feel bloated,” “Fluid is not easily available at work,” and “Other (specify).” The follow-up assessments added the stem, “Since using sipIT….”
The System Usability Scale was used to assess the usability (5 items; 3 items excluded based on content irrelevance for this use case) and learnability (2 items) of the sipIT system at both follow-up assessments (Bangor et al., 2008; Lewis & Sauro, 2009). Instructions indicated that, “The ‘sipIT tools’ in the items below refer to the connected water bottle, smartwatch and app you have been using to monitor your drinking and determine whether you should receive a notification reminding you to drink.” Participants rated each item on a scale ranging from 1 (strongly disagree) to 5 (strongly agree) with higher scores indicating higher usability. Internal consistency estimates for the usability and learnability scales were acceptable at 1 month (α ≥ .70) but unacceptable at 3 months (α < .50) so only 1-month scores are reported.
Satisfaction was measured with four custom items rated on a scale ranging from 1 (strongly disagree) to 5 (strongly agree). Items included, “I found the sipIT tools helpful in reaching my fluid consumption goals,” “I enjoyed using the sipIT tools to track my fluid consumption,” “I would strongly recommend using the sipIT tools,” and “I received notifications from sipIT too frequently.”
Additional user experience feedback was obtained with respect to perceptions of the optimal frequency for checking drinking data (tailoring variable). Participants rated the perceived value of the sipIT tools with two items: “How successful were you at increasing your fluid intake since you started sipIT?” (rated 0 [not successful], 1 [moderately successful], or 2 [very successful]) and “Over the past week, how successful have you been at meeting fluid intake guidelines for preventing stones (i.e., drinking enough to produce 2.5L/day of urine)?” (rated not at all successful [0 days], rarely successful [1–2 days], sometimes successful [3–4 days], often successful [5–6 days], always successful [7 days]).1
Procedures
All procedures were approved by the Penn State Institutional Review Board. A member of the research team completed a telephone screening with prospective participants to determine eligibility. During a laboratory visit, participants provided written informed consent. The researcher measured height with a wall-mounted stadiometer and weight with a digital scale in duplicate and participants completed questionnaires. The researcher then furnished the participant with a Fitbit Versa watch with the sipIT gesture detection app installed, and an H2OPal connected water bottle. The researcher installed the H2OPal app, the Fitbit app, and the sipIT mobile app on the participant’s smartphone and instructed the participant on app use. The researcher authenticated each component of the system using a project-specific email account. The system delivered notification reminders as needed from 8:00am until 9:00pm. Participants completed a battery of questionnaires, received information about fluid intake guidelines for prevention prepared by a board-certified urologist, and were assigned a goal to drink at least 0.12 L every 30 minutes during waking hours. Participants returned to the lab after using sipIT for 1 and 3 months to complete questionnaires and a semi-structured interview.
Data Analysis
Descriptive statistics were calculated to estimate acceptability. A repeated-measures analysis of variance was used to evaluate change in experienced automaticity scores from baseline to the two follow-up occasions. Chi-square tests of independence were used to evaluate changes in the proportion of participants reporting each perceived barrier to fluid intake. Interviews were digitally recorded and transcribed for analysis. Inductive content analysis was used to identify emergent themes.
Results
The baseline sample (n = 31) comprised women (58%) and men (42%) with an average age of 40.0 years (SD = 14.3, range = 20 – 71). Most participants were not Hispanic or Latino (97%), White (87%), and full-time employed (61%). Both the median and mode for highest level of education was a Bachelor’s degree. Approximately 48% were classified as overweight or obese based on BMI values greater than 25.
The median number of prior kidney stone episodes was 2 (mode = 1, M = 3.1, SD = 3.8, range = 1 – 18). Only 20% of the sample reported currently having a kidney stone but most perceived a moderate (35%) or high (35%) risk of developing another stone. Most participants (80%) had previously been advised by a health care provider to increase their fluid intake; however, few (23%) reported knowing that they should produce at least 2 L/day of urine.
Retention rates were high at 1-month (90%) and 3-month (87%) assessments, although participants opted to skip some measures in follow-up visits. Two participants dropped out because they did not believe that had enough flexibility with their time to engage with the intervention (e.g., due to occupational demands; n=2). Two other participants dropped out and were lost to follow-up despite multiple contact attempts. Over the 3-month study period, the semi-automated monitoring system detected 46,654 drinks (including drinking events outside of the notification window; self-tracking n = 6,797; connected water bottle n = 16,402; gesture detection app n = 23,455). The intervention engine delivered a total of 22,728 reminder notifications. The median number of daily notifications sent to each participant ranged from 2 to 14 (M = 7.1, SD = 2.8).
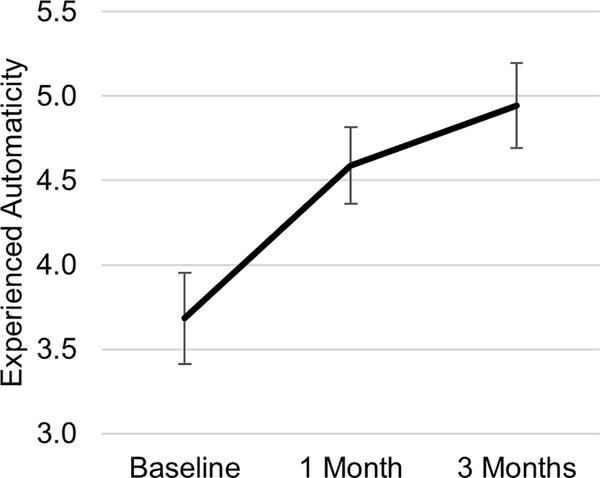
As seen in Figure 1, the experienced automaticity of fluid consumption increased from baseline, F (2, 24) = 8.70, p = .001. Post hoc contrasts revealed a significant increase from baseline to 1-month (mean difference = 0.76 [95% confidence interval {CI}, 0.20 – 1.32], p = .005, d = 0.50) and 3 months (mean difference = 1.14 [95% CI, 0.43 – 1.84], p = .001, d = 0.64) but no change from 1 month to 3 months (mean difference = 0.38 [95% CI, −0.17 – 0.92], p = .26, d = 0.23).
Figure 1.
Experienced automaticity of drinking increased over time while using sipIT
Table 1 presents the frequency of perceived barriers to adherence with fluid intake guidelines at each assessment point. After using the sipIT tools for one month, participants were significantly less likely to report lack of thirst as a barrier to meeting guidelines. No other barriers changed significantly; however, two non-significant trends were noteworthy: forgetting to drink was reported less frequently but the hassle of carrying a water bottle was reported more frequently.
Table 1.
Frequency of barriers to meeting fluid intake goals at each occasion.
| Follow-Up | χ2 | ||||
|---|---|---|---|---|---|
| Barrier | Baseline (B) n (%) | 1-Month (1M) n (%) | 3-Month (3M) n (%) | B-1M | 1M-3M |
| Lack of thirst | 16 (51.6) | 6 (23.1) | 7 (29.2) | 4.86* | 0.24 |
| Forgetting to drink | 22 (71) | 12 (46.2) | 12 (50) | 3.62 | 0.07 |
| Carrying water bottle is a hassle | 7 (22.6) | 7 (26.9) | 13 (54.2) | 0.14 | 3.86 |
| Urinating too frequently | 11 (35.5) | 12 (46.2) | 6 (25) | 0.67 | 2.42 |
| Difficult to drink at work | 10 (32.3) | 6 (23.1) | 7 (29.2) | 0.59 | 0.24 |
| Dislike the taste of water | 2 (6.5) | 0 (0) | 2 (8.3) | 1.45 | 0.74 |
| Unaware of the need to increase fluid consumption | 7 (22.6) | 4 (15.4) | 1 (4.2) | 0.47 | 1.75 |
| Feeling bloated | 1 (3.2) | 0 (0) | 1 (4.2) | 2.95 | 1.99 |
| Fluids not easily available at work | 2 (6.5) | 0 (0) | 0 (0) | 1.45 | 0.41 |
Note. Sample size varied from baseline (n=31) to 1-month (n=26) to 3-months (n=24) due to attrition and some participants opting to skip this questionnaire.
p < .05
User experience feedback from each follow-up assessment is summarized in Table 2. They felt moderately-to-very successful at increasing fluid intake (96%) and at least somewhat successful at meeting daily guidelines for urine output (82.6% at 1 month, 91.6% at 3 months). Although participants found sipIT tools helpful, enjoyed using them, and would recommend them, they were more ambivalent about the frequency of notifications. They described the optimal frequency for decision points as 51.6 minutes (1 month; SD = 23.5, median = 45) or 49.3 minutes (3 months; SD = 22.2, median = 45).
Table 2.
User experience ratings of sipIT at 1- and 3-month follow-up assessments.
| 1-Month Follow-Up | 3-Month Follow-Up | |||
|---|---|---|---|---|
| n (%) | M (SD) | n (%) | M (SD) | |
| Success at Increasing Fluid Intake | ||||
| Very successful | 13 (56.5%) | 12 (50%) | ||
| Moderately successful | 9 (39.1%) | 11 (45.8%) | ||
| Not successful | 1 (4.3%) | 1 (4.2%) | ||
| Success at Meeting Guidelines | ||||
| Always successful (7 days) | 5 (21.7%) | 5 (20.8%) | ||
| Often successful (5–6 days) | 10 (43.5%) | 9 (37.5%) | ||
| Somewhat successful (3–4 days) | 4 (17.4%) | 8 (33.3%) | ||
| Rarely successful (1–2 days) | 2 (8.7%) | 0 (0%) | ||
| Not at all successful (0 days) | 2 (8.7%) | 2 (8.3%) | ||
| Satisfaction | ||||
| Found sipIT tools helpful | 4.4 (0.7) | 4.0 (1.1) | ||
| Enjoyed using sipIT tools to track fluid consumption | 4.2 (0.9) | 4.0 (1.1) | ||
| Strongly recommend the sipIT tools | 4.4 (1.0) | 4.1 (1.0) | ||
| Received notifications from sipIT too frequently | 3.1 (1.3) | 3.1 (1.5) | ||
Open-ended feedback indicated that participants enjoyed using the H2OPal connected bottle and associated mobile app because they believed the bottle served as a visual cue for reminding them to drink, and the H2OPal mobile app had an easy user interface for self-tracking fluid consumption. Participants were receptive to the positive image notifications but suggested the negative image notifications be discarded. Participants noted that the sipIT app drained their smartphone battery and experienced frustration from difficulties in syncing the Fitbit. Participants expressed an interest in pairing the sipIT tools with other consumer-grade wearable devices (e.g., Apple Watch).
Discussion
This study evaluated the acceptability of sipIT, a new digital tool for promoting fluid intake in patients with kidney stones. The intervention combined a semi-automated fluid intake monitoring system with a just-in-time adaptive intervention framework. Overall, results were promising as the system detected drinking events, delivered lapse-contingent notifications, created a positive user experience, and created an experience of more automatic fluid intake.
One contribution of this study involved the application of semi-automated monitoring using three methods to detect fluid intake. Detecting drinking events is challenging because drinking behaviors can take a variety of forms (Chun et al., 2019). Patients can sip or gulp, drink from a variety of containers raised with the dominant or non-dominant hand, drink water or other types of fluids that count towards daily fluid intake goals, or avoid containers altogether by using straws or water fountains. No single method is sufficiently sensitive and specific when classifying the entire range of possible drinking behaviors. The sipIT tools addressed this challenge by integrating data from human, smartwatch, and water bottle sensors in a semi-automated tracking system to reduce the risk of missing drinking events and increase classification accuracy (Choe et al., 2017). The sensitivity and specificity of sipIT drinking event detections cannot be estimated from this study but should be examined in future research with a ground truth measure of drinking behavior in the wild (e.g., behavior observation or first-person video recording). It is possible that additional monitoring methods could increase the sensitivity and specificity of drinking event detection. Impact on patient burden and engagement should be also weighed before incorporating additional methods to detect drinking events.
The sipIT monitoring system prioritized specificity (i.e., reducing false positives) over sensitivity (i.e., reducing false negatives). False positives lead to reminder notifications (treatments) being withheld whereas false negatives lead to overtreatment. Withholding notifications due to false positives would likely lead to undertreatment and reduce adherence to fluid intake guidelines. In contrast, the greatest risk from delivering excessive reminder notifications due to false negative detections is likely notification fatigue (Cole-Lewis et al., 2019; Heckman et al., 2015). Notification fatigue may adversely impact engagement with behavioral interventions although this evidence is mixed (Conroy et al., 2020; Freyne et al., 2017; Morrison et al., 2017). It is notable that engagement was high throughout the present trial. Thus, the semi-automated monitoring approach in sipIT did not appear to inflate the false negative rate enough to compromise engagement. Provided engagement is not diminished, overtreatment has minimal cost and may be therapeutic for some as they will be reminded to drink more often.
A second contribution of this study involved the selection and implementation of behavior change techniques to increase the experienced automaticity of fluid intake. Although this finding did not provide direct evidence for habit formation, the approach was informed by habit formation principles and the result parallels one expected outcome if a habit was formed. Participants were assigned a fluid intake goal based on established clinical guidelines and instructed to distribute their drinking behavior in 30-minute increments across the day (i.e., implementation planning – if I did not drink within the past 30 minutes, then drink). This approach is the basis for habit formation because it contributes to contextual consistency of the desired behavior (Wood & Neal, 2007). Context is often assumed to be a spatial feature (e.g., when I am in the kitchen) or subjective experience (e.g., when I am thirsty) but was defined in temporal terms for sipIT. Temporal cues were selected because disruptions from frequent or urgent urination have been noted previously by patients (McCauley et al., 2012; Streeper et al., 2019). Distributing consumption across the day by increasing the frequency of lower-volume drinking events is one strategy for addressing those concerns. Participant feedback did not reflect any concerns about overactive bladders but clinicians can supplement treatment if that becomes a concern. Future research should evaluate experienced automaticity of fluid intake in specific contexts to draw stronger conclusions about habit formation as an outcome. Finally, although performance habits are most closely aligned with the clinical goal of increasing fluid consumption, this intervention could be enhanced by targeting the formation of preparatory habits for fluid consumption as well.
The design philosophy underlying sipIT was different from many digital health interventions. Rather than trying to sustain engagement by adding features to create friction, the underlying design principle was to minimize friction by eliminating as many touch points as possible. This approach appeared to be effective based on the high levels of retention and user satisfaction. Minimalist design approaches – in terms of user engagement – should be considered when developing other digital tools to contain costs and avoid over-engineering solutions to health problems.
The sipIT intervention provides a new digital tool to support a common treatment adherence problem for patients with kidney stones. Medication adherence is a related challenge but digital tools have yielded disappointing results to date in that work. For example, the recent REMIND trial used connected pillboxes to prompt patients to take medication but it did not increase adherence (Choudhry et al., 2017). Digital tools may be better suited for supporting behaviors that require more frequent enactment (e.g., drinking fluids, disrupting sedentary time). Less frequent behaviors may benefit more from social support (Nieuwlaat et al., 2014).
This study represents early-phase intervention development research and has several limitations that frame an agenda for future research. The sample was fairly homogeneous and it is unclear whether findings will generalize to more diverse populations, including patients who are less comfortable or interested in using technology to support pursuit of their health behavior goals. Eligibility criteria did not require nonadherence to fluid consumption guidelines but this inclusion criterion should be considered as intervention development enters Phase II. Second, the decision rule was based on clinical judgment and patient feedback from formative studies (Streeper et al., 2019). A micro-randomized trial could provide another form of evidence for a universal decision rule (Klasnja et al., 2015). Alternatively, open-loop system identification modeling could yield person-specific decision rules based on individual responses to reminder notifications (Conroy et al., 2019). It is also possible that notifications are not as onerous and disruptive as assumed in our design process. Future trials could compare the lapse-contingent notification approach with an approach that delivers reminder notifications at regular intervals. The technology used in sipIT was a minimum viable prototype. Future work will need to improve device syncing capabilities and system stability prior to deploying it in a larger study. Next, other than attrition rates, outcome measures were all self-reported. As this intervention progresses into the next phase of intervention development, it will be important to evaluate its efficacy for modifying behavioral and biobehavioral surrogate outcomes such as drinking event frequency, context-specific experienced automaticity, and 24-hour urine output. The duration of the intervention period was 3 months yet clinically-significant stone growth often takes years. Patients may have continued to form stones despite using sipIT. Imaging could provide a valuable clinical outcome for future studies. Finally, the study design was not experimental and did not include a comparator condition so causal inferences about the effects of the sipIT intervention are not possible. Randomized clinical trials comparing sipIT against usual care should be a priority for future research.
In closing, the sipIT digital tools were both technically feasible and acceptable for supporting adherence to fluid intake guidelines in a sample of patients with kidney stones. This intervention showed promise for reducing common barriers to adherence and increasing the experienced automaticity of fluid intake. Decision points should be extended to 45- or 60-minute intervals in future versions, and sipIT should be updated to allow for integration with other consumer wearables such as the Apple Watch. The sipIT intervention is ready for Phase II of intervention development focused on evaluating its efficacy for increasing fluid consumption and urine output as recommended in clinical guidelines for preventing recurrence of kidney stones.
Supplementary Material
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant UL1 TR002014, and the National Institute on Drug Abuse, NIH, through Grant T32 DA017629. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare that there are no competing interests.
This project was registered in clinicaltrials.gov (NCT03787615).
The User Burden Scale was utilized to assess the negative impact that the sipIT tools placed on users (Suh et al., 2016). Higher scores on this measure predict greater likelihood of abandoning the technology. At 1- and 3-month follow-ups, six items had no variance and all items had mean values less than 1. Due to these skewed distributions and restricted range indicative of little-to-no user burden, seven (out of 12) internal consistency estimates were not acceptable so scores from this measure will not be reported further.
References
- Bangor A, Kortum PT, & Miller JT (2008). An empirical evaluation of the System Usability Scale. International Journal of Human-Computer Interaction, 24(6), 574–594. 10.1080/10447310802205776 [DOI] [Google Scholar]
- Choe EK, Abdullah S, Rabbi M, Thomaz E, Epstein DA, Cordeiro F, Kay M, Abowd GD, Choudhury T, Fogarty J, Lee B, Matthews M, & Kientz JA (2017). Semi-automated tracking: A balanced approach for self-monitoring applications. IEEE Pervasive Computing, 16(1), 74–84. 10.1109/MPRV.2017.18 [DOI] [Google Scholar]
- Choudhry NK, Krumme AA, Ercole PM, Girdish C, Tong AY, Khan NF, Brennan TA, Matlin OS, Shrank WH, & Franklin JM (2017). Effect of reminder devices on medication adherence: The REMIND randomized clinical trial. JAMA Internal Medicine, 177(5), 624–631. 10.1001/jamainternmed.2016.9627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun KS, Sanders AB, Adaimi R, Streeper N, Conroy DE, & Thomaz E (2019). Towards a generalizable method for detecting fluid intake with wrist-mounted sensors and adaptive segmentation. Proceedings of the 24th International Conference on Intelligent User Interfaces, 80–85. 10.1145/3301275.3302315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole-Lewis H, Ezeanochie N, & Turgiss J (2019). Understanding health behavior technology engagement: Pathway to measuring digital behavior change interventions. JMIR Formative Research, 3(4), e14052 10.2196/14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DE, Dubansky A, Remillard J, Murray R, Pellegrini CA, Phillips SM, & Streeper NM (2016). Using behavior change techniques to guide selections of mobile applications to promote fluid consumption. Urology. 10.1016/j.urology.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Conroy DE, Hojjatinia S, Lagoa CM, Yang C-H, Lanza ST, & Smyth JM (2019). Personalized models of physical activity responses to text message micro-interventions: A proof-of-concept application of control systems engineering methods. Psychology of Sport and Exercise, 41, 172–180. 10.1016/j.psychsport.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DE, Yang C-H, Lanza ST, Smyth JM, & Lagoa CM (2020). Temporal dynamics of treatment receipt in a text message intervention for physical activity: Single-group, within-person trial. JMIR MHealth and UHealth, 8(4), e14270 10.2196/14270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, Laraia B, Olster DH, Perna FM, Peterson JC, Epel E, Boyington JE, & Charlson ME (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34(10), 971–982. 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein L, & Matlaga B (2018). Urologic Diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIH Publication No. 12–7865). US Government Printing Office; https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/urologic-diseases-in-america [Google Scholar]
- Freyne J, Yin J, Brindal E, Hendrie GA, Berkovsky S, & Noakes M (2017). Push notifications in diet apps: Influencing engagement times and tasks Int J Hum Comput Interact, 12, 1–3. Scopus. [Google Scholar]
- Gardner B, Abraham C, Lally P, & de Bruijn G-J (2012). Towards parsimony in habit measurement: Testing the convergent and predictive validity of an automaticity subscale of the Self-Report Habit Index. International Journal of Behavioral Nutrition and Physical Activity, 9, 102 10.1186/1479-5868-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B, de Bruijn G-J, & Lally P (2011). A systematic review and meta-analysis of applications of the Self-Report Habit Index to nutrition and physical activity behaviours. Annals of Behavioral Medicine, 42, 174–187. 10.1007/s12160-011-9282-0 [DOI] [PubMed] [Google Scholar]
- Gardner B, Phillips LA, & Judah G (2016). Habitual instigation and habitual execution: Definition, measurement, and effects on behaviour frequency. British Journal of Health Psychology, 21(3), 613–630. 10.1111/bjhp.12189 [DOI] [PubMed] [Google Scholar]
- Hagger MS, Rebar AL, Mullan B, Lipp OV, & Chatzisarantis NLD (2015). The subjective experience of habit captured by self-report indexes may lead to inaccuracies in the measurement of habitual action. Health Psychology Review, 9(3), 296–302. 10.1080/17437199.2014.959728 [DOI] [PubMed] [Google Scholar]
- Heckman BW, Mathew AR, & Carpenter MJ (2015). Treatment burden and treatment fatigue as barriers to health. Current Opinion in Psychology, 5, 31–36. 10.1016/j.copsyc.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambati A, Matulewicz RS, Perry KT, & Nadler RB (2017). Factors associated with compliance to increased fluid intake and urine volume following dietary counseling in first-time kidney stone patients. Journal of Endourology, 31(6), 605–610. 10.1089/end.2016.0836 [DOI] [PubMed] [Google Scholar]
- Klasnja P, Hekler EB, Shiffman S, Boruvka A, Almirall D, Tewari A, & Murphy SA (2015). Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychology, 34 Suppl, 1220–1228. 10.1037/hea0000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JR, & Sauro J (2009). The factor structure of the System Usability Scale. HCD 2009: International Conference on Human Centered Design, 94–103. 10.1007/978-3-642-02806-9_12 [DOI] [Google Scholar]
- Marshall AT, & Kirkpatrick K (2015). Everywhere and everything: The power and ubiquity of time. International Journal of Comparative Psychology, 28. [PMC free article] [PubMed] [Google Scholar]
- McCauley LR, Dyer AJ, Stern K, Hicks T, & Nguyen MM (2012). Factors influencing fluid intake behavior among kidney stone formers. The Journal of Urology, 187(4), 1282–1286. 10.1016/j.juro.2011.11.111 [DOI] [PubMed] [Google Scholar]
- Morrison LG, Hargood C, Pejovic V, Geraghty AWA, Lloyd S, Goodman N, Michaelides DT, Weston A, Musolesi M, Weal MJ, & Yardley L (2017). The effect of timing and frequency of push notifications on usage of a smartphone-based stress management intervention: An exploratory trial. PLOS ONE, 12(1), e0169162 10.1371/journal.pone.0169162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Hekler EB, & Spruijt-Metz D (2015). Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychology, 34, 1209–1219. 10.1037/hea0000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, & Murphy SA (2018). Just-in-time adaptive interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine, 52(6), 446–462. 10.1007/s12160-016-9830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi C, & Haynes RB (2014). Interventions for enhancing medication adherence. The Cochrane Database of Systematic Reviews, 11, CD000011 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks JH, Goldfischer ER, & Coe FL (2003). Changes in urine volume accomplished by physicians treating nephrolithiasis. The Journal of Urology, 169(3), 863–866. 10.1097/01.ju.0000044922.22478.32 [DOI] [PubMed] [Google Scholar]
- Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TMT, White JR, & American Urological Assocation. (2014). Medical management of kidney stones: AUA guideline. The Journal of Urology, 192(2), 316–324. 10.1016/j.juro.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, & Clinical Guidelines Committee of the American College of Physicians. (2014). Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 161(9), 659–667. 10.7326/M13-2908 [DOI] [PubMed] [Google Scholar]
- Scales CD, Smith AC, Hanley JM, Saigal CS, & Urologic Diseases in America Project. (2012). Prevalence of kidney stones in the United States. European Urology, 62(1), 160–165. 10.1016/j.eururo.2012.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF (1963). Operant behavior. American Psychologist, 18(8), 503–515. 10.1037/h0045185 [DOI] [Google Scholar]
- Staddon JER, & Cerutti DT (2003). Operant conditioning. Annual Review of Psychology, 54, 115–144. 10.1146/annurev.psych.54.101601.145124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeper NM, Dubnansky A, Sanders AB, Lehman K, Thomaz E, & Conroy DE (2019). Improving fluid intake behavior among patients with kidney stones: A focus group to understand patients’ experiences and acceptability of wearable sensors. Urology, 133, 57–66. 10.1016/j.urology.2019.05.056 [DOI] [PubMed] [Google Scholar]
- Streeper NM, Lehman K, & Conroy DE (2018). Acceptability of mobile health technology for promoting fluid consumption in patients with nephrolithiasis. Urology, 122, 64–69. 10.1016/j.urology.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Shahriaree N, Hekler EB, & Kientz JA (2016). Developing and validating the User Burden Scale: A tool for assessing user burden in computing systems. Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems, 3988–3999. 10.1145/2858036.2858448 [DOI] [Google Scholar]
- Tarplin S, Monga M, Stern KL, McCauley LR, Sarkissian C, & Nguyen MM (2016). Predictors of reporting success with increased fluid intake among kidney stone patients. Urology, 88, 49–56. 10.1016/j.urology.2015.10.024 [DOI] [PubMed] [Google Scholar]
- van Drongelen J, Kiemeney LA, Debruyne FM, & de la Rosette JJ (1998). Impact of urometabolic evaluation on prevention of urolithiasis: A retrospective study. Urology, 52(3), 384–391. 10.1016/s0090-4295(98)00201-5 [DOI] [PubMed] [Google Scholar]
- Verplanken B, & Orbell S (2003). Reflections on past behavior: A self-report index of habit strength. Journal of Applied Social Psychology, 33(6), 1313–1330. 10.1111/j.1559-1816.2003.tb01951.x [DOI] [Google Scholar]
- Wilken M, Hüske-Kraus D, Klausen A, Koch C, Schlauch W, & Röhrig R (2017). Alarm fatigue: Causes and effects. Studies in Health Technology and Informatics, 243, 107–111. [PubMed] [Google Scholar]
- Wood W, & Neal DT (2007). A new look at habits and the habit-goal interface. Psychological Review, 114, 843–863. 10.1037/0033-295X.114.4.843 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.