Abstract
Objective
There is a growing call to identify specific outcome predictors in real-world eating disorder (ED) treatment settings. Studies have implicated several ED treatment outcome predictors [rapid response (RR), weight suppression, illness duration, ED diagnosis, and psychiatric comorbidity] in inpatient settings or randomized controlled trials of individual outpatient therapy. However, research has not yet examined outcome predictors in intensive outpatient programs (IOP). The current study aimed to replicate findings from randomized controlled research trials and inpatient samples, identifying treatment outcome predictors in a transdiagnostic ED IOP sample.
Method
The current sample comprised 210 consecutive unique IOP patient admissions who received evidence-based ED treatment, M(SD)Duration = 15.82 (13.38) weeks. Weekly patient measures of ED symptoms and global functioning were obtained from patients’ medical charts.
Results
In relative weight analysis, RR was the only significant predictor of ED symptoms post treatment, uniquely accounting for 45.6% of the predicted variance in ED symptoms. In contrast, baseline ED pathology was the strongest unique predictor of end-of-treatment global functioning, accounting for 15.89% of predicted variance. Baseline factors did not differentiate patients who made RR from those who did not.
Conclusions
Consistent with findings in more controlled treatment settings, RR remains a robust predictor of outcome for patients receiving IOP-level treatment for EDs. Future work should evaluate factors that mediate and moderate RR, incorporating these findings into ED treatment design and implementation.
Level of evidence
Level IV, uncontrolled intervention.
Keywords: Eating disorder, Intensive outpatient program, Transdiagnostic, Rapid response
Introduction
Mounting consensus among stakeholders (e.g., researchers, clinicians, health insurance providers, and patients) calls for improved understanding of therapeutic processes and factors that influence favorable eating disorder (ED) treatment outcomes in real-world settings [1–3].
Predictors of outcome across levels of care
Intermediate levels of care
The structure of intermediate levels of care for EDs varies widely depending on the program; however, they are generally used by patients who may require frequent treatment, monitoring, and accountability, but who do not require 24-h care provided by inpatient and residential treatment settings [2]. In contrast to partial hospitalization programs, which typically involve 5–7 days a week for 6–8 h a day, intensive outpatient program (IOP) settings are less intensive, typically including 3–4 h a day of treatment 2–4 days per week [1]. Intermediate levels of care differ from higher levels of care (e.g., residential or inpatient settings) in that patients typically exhibit fair motivation to recover, are at least 80% of healthy body weight [4], and are expected to demonstrate increased self-sufficiency in regards to eating and weight gain or maintenance [5].
Preliminary findings demonstrate that compared to inpatient and residential treatment, intermediate levels of care, including IOPs, demonstrate comparable effectiveness and improved cost-effectiveness [1]. Thus, it is not surprising that intermediate levels of care for EDs are increasingly common [1, 6]. Furthermore, intermediate levels of care may be preferable to traditional intensive approaches for both economic and theoretical reasons [1, 6, 7]. Nonetheless, research supporting intermediate and high levels of care settings lags behind their proliferation [1]. Recent research has provided more nuanced understandings of ED outcome predictors in certain settings but is limited in treatment settings and sample characteristics. Specifically, in a recent meta-analysis of outcome predictors, 67% of included studies were from randomized controlled trials and reflected specific treatment settings: 51.5% inpatient, 32.5% outpatient, 1.6% in day programs, and 0.8% in residential settings; none were in IOPs [8]. Anderson and colleagues (2017) noted that there are numerous obstacles to research in intermediate and higher levels of care, including challenges both in translating empirically supported treatments to higher levels of care and in testing treatment effectiveness in these settings.
Previously reported ED treatment outcome predictors
Identifying treatment outcome predictors in real-world settings is necessary to improve therapeutic protocols, inform treatment planning, and identify patients at risk for poorer prognoses [8, 9]. To date, several baseline variables consistently predict favorable ED treatment outcome. A recent meta-analytic review of ED treatment outcome predictors indicated that higher body mass index (BMI) in anorexia nervosa, fewer binge/purge episodes, increased motivation to recover, lower shape/weight concern, fewer comorbidities, and better interpersonal functioning predict favorable treatment outcome [8]. Lower weight suppression has also been demonstrated to predict favorable ED outcome [10]. However, the outcome predictors identified across these studies are inconsistent and have yet to be adequately examined in intermediate level of care settings (e.g., day/partial hospitalization programs and IOPs).
Randomized controlled trial findings may not reflect findings in intermediate levels of care. First, participants in randomized controlled trial may have greater motivation to recover than IOP patients as a result of selection bias [11]. Relative to treatment in an IOP, prospective randomized controlled trial participants typically have many additional steps that they need to take to receive treatment (e.g., phone screens, batteries of assessments at baseline to determine whether they meet inclusion criteria, blood tests, urine screens, etc.), such that only individuals who are highly motivated for treatment would be randomized into a treatment arm. Because treatment motivation predicts outcome [8], this selection bias could alter sample characteristics and inflate remission rates in randomized controlled trials [11]. Alternately, real-world treatment settings may have more severe cases, as exclusion criteria in randomized control trails often select out for more severe symptoms that would be otherwise treated [11]. In addition to the possibility that treatment outcome predictors from intermediate levels of care potentially differ from those found in randomized controlled trial samples, treatment outcome predictors may also differ in IOPs compared to inpatient settings, where treatment compliance is required. As ED IOPs are proliferating [6], it is essential to determine if previously identified treatment outcome predictors are similar in transdiagnostic IOP samples [8]. One recent study examined moderators of change in a large (N = 1200) transdiagnostic ED sample of partial hospitalization programs and IOP patients and found that age, race, gender, and baseline depression scores moderated changes in quality of life and functional impairment [2]. Baseline depression scores also moderated ED outcome, such that participants with higher depression scores at intake exhibited greater improvement in ED outcome, quality of life, and decreased functional impairment [2]. However, given that additional ED treatment predictors have been identified in prior research in randomized controlled trial and residential/inpatient samples (e.g., duration of illness, weight suppression, rapid response (RR), personality pathology, and other comorbid diagnoses), additional research is needed to examine a broader range of ED treatment outcome predictors, mediators, and moderators in intermediate levels of care, specifically including RR, given it is a robust treatment predictor in randomized controlled trials [8].
Rapid response
Across ED diagnoses, RR is a well-established and consistently replicated mediator of treatment outcome [8, 12]. RR is typically defined as a clinically meaningful change in disorder-specific symptoms within the first half of treatment [12]. In two recent meta-analyses, RR was the strongest predictor of treatment success across diagnoses in several treatment settings [8, 13]. However, RR has not yet been examined as a predictor of outcome in IOP settings. Furthermore, growing attention has been paid to examining differences between those who do and do not make RR in ED treatment; however, data differentiating characteristics of rapid responders and those who fail to make RR have not yet been examined in intermediate levels of care. Given greater severity and comorbidity in community mental health samples compared to randomized controlled trial samples (for meta-analysis, see [11]), it is critical to examine factors that differentiate rapid responders from those who do not make RR in community mental health settings, to complement the body of evidence developed using randomized controlled trial methodology [11].
Current study
Given the large effect size in a recent meta-analysis of ED treatment outcome predictors [8] and consistent replication across samples from outpatient randomized controlled trials and higher levels of care [8, 13], the current study aims to replicate RR findings in a transdiagnostic sample receiving evidence-based treatment in an IOP. Planned follow-up analyses will examine treatment factors that differentiate those who achieved RR and those who did not, should the primary hypothesis be supported. The second aim of the current study is exploratory in nature and based on small to moderate meta-analytic effect sizes reported for all other significant predictors of ED treatment outcome [8], partially reflective of inconsistent findings across studies. We sought to examine other previously demonstrated ED treatment outcome predictors—weight suppression, illness duration, ED diagnosis, and psychiatric comorbidity—and a previously under-researched predictor, insurance supportiveness, on IOP treatment outcome. Although not previously examined in IOP settings, insurance supportiveness is associated with poorer outcomes at discharge and increased readmission in inpatient and residential programs [14], suggesting that it may also be an important predictor of ED outcome in IOPs. Insurance supportiveness is also a primary patient concern, with research suggesting the extent of patient care depended more on insurance supportiveness than patients’ health needs [15].
Method
Participants
Participants were 210 consecutive unique admissions to an ED IOP in the northeast US from May 2013–May 2017. Only the 181 (89.4% female) who attended at least one IOP treatment day following the intake assessment day were included in analysis of outcome predictors, with a M (SD) = 15.82 (13.38) weeks in treatment. Patients’ ages ranged from 13 to 62 years, M (SD) = 25.10 (10.43). Patients self-identified as White (94.2%), Hispanic/Latino (1.3%), Asian (3.9%), and Mixed Race (0.6%). Participant diagnosis and intake BMI are described in Table 1.
Table 1.
Primary and comorbid diagnoses
| Primary diagnosis | n (%) | MBMI | SDBMI | RangeBMI |
|---|---|---|---|---|
| AN-BP | 77 (36.7) | 19.65 | 3.03 | 14.52–29.07 |
| AN-R | 54 (25.7) | 19.85 | 3.50 | 15.45–32.53 |
| BN | 42 (20.0) | 24.39 | 6.03 | 17.16–41.59 |
| BED | 11 (5.2) | 35.65 | 7.84 | 19.08–46.72 |
| OSFED | 24 (11.4) | 21.56 | 2.75 | 15.76–25.80 |
| ARFID | 2 (1.0) | 21.80 | 4.12 | 18.97–24.80 |
| Comorbid diagnoses | n (%) | |||
| Unipolar mood disorder | 75 (37.69) | |||
| Anxiety disorder | 60 (30.15) | |||
| Borderline personality disorder | 18 (9.05) | |||
| Substance use disorder | 17 (8.54) | |||
| Post-traumatic stress disorder | 9 (4.52) | |||
| Bipolar disorder | 8 (4.02) | |||
| Body dysmorphic disorder | 5 (2.51) | |||
| Obsessive–compulsive disorder | 3 (1.51) | |||
| Attention-deficit hyperactivity disorder | 1 (0.50) | |||
| Excoriation disorder | 1 (0.50) | |||
| Dissociative disorder | 1 (0.50) | |||
| Autism spectrum disorder | 1 (0.50) | |||
Anxiety disorders, unipolar mood disorders, bipolar mood disorders, and substance use disorders were grouped together, and represent a range of specific diagnoses. Because patients were required to be medically stable, most with anorexia nervosa entered the intensive outpatient program after weight restoration. Comorbid diagnosis statistics present the number and percentage of each diagnostic category out of the total number of comorbid diagnoses represented, rather than as a percentage of the total sample
AN-BP anorexia nervosa-binge-eating/purging subtype, AN-R anorexia nervosa-restricting subtype, BN bulimia nervosa, BED binge eating disorder, OSFED other specified feeding or eating disorder, ARFID avoidant-restrictive food intake disorder
Measures
Eating attitudes test-26
The EAT-26 [16], a 26-item six-point Likert-type scale rated from “never” to “always,” assessed ED pathology at end-of-treatment. It has been shown to be a useful transdiagnostic measure of ED symptom severity [17]. Items are summed and higher scores indicate greater disordered eating (possible range 0–78). The EAT-26 distinguishes between ED cases and non-cases and exhibited good convergent validity [16] and internal consistency in prior work [Cronbach’s α = 0.87; 18]. Cronbach’s α for the current study was 0.91.
Outcome Rating Scale
The Outcome Rating Scale (ORS; [19]) includes four visual analog scales assessing individual, interpersonal, social, and overall well-being. Items are summed; higher scores reflect greater client functioning (possible range 0–40). The ORS demonstrated acceptable validity and reliability with overall Cronbach’s α of 0.93 in the validation sample [19]. In the current study, the ORS assessed global functioning at treatment completion, and Cronbach’s α = 0.84.
Clinical predictors
Clinicians diagnosed patients using the Diagnostic and Statistical Manual of Mental Disorders (5th ed.1; [20]). Self-reported age, ED duration, current height and weight were recorded during initial evaluation. Highest and lowest weight at the patient’s current height (excluding pregnancy) were also collected at initial evaluation; weight suppression was defined as the highest weight minus baseline weight at the patient’s current height. Previous work has not operationalized insurance supportiveness, with research limited to qualitative analyses [15]. Based on qualitative feedback from patients, IOP administrators, and case managers, the number of days required between insurance authorizations was averaged to measure insurance supportiveness, with greater values reflecting greater insurance supportiveness. For instance, an insurance company that required the IOP to justify a patient’s continuation of treatment after every three treatment days was considered less supportive than one that authorized a patient for ten treatment days at a time, between authorizations. The clinical predictors examined were ED diagnosis, number of comorbid diagnoses, age at intake, illness duration, weight suppression, weeks in treatment, and insurance supportiveness.
Rapid response
Because a primary aim of the present study was to examine RR in a transdiagnostic ED sample, an ED symptom measure demonstrated to be valid in transdiagnostic samples [21] was chosen over symptom specific indicators such as weight change or changes in frequency of binge eating or compensatory behaviors that have been used in some studies of RR (e.g., [22, 23]). To determine a definition of RR, a data-driven approach using ROC curves was selected.
Procedure
Clinicians conducted initial evaluations and provided DSM-5 diagnoses, as part of routine clinical practice. Each IOP day lasted four hours, including two treatment groups and one supervised meal. Patients attended up to three treatment days per week from 2013 to 2015 and up to 4 days per week from 2015 to 2017. Patients completed weekly EAT-26 and ORS measures; scores at treatment completion were dependent variables in the current analyses. Groups followed evidence-based treatment modalities, including cognitive behavioral therapy and dialectical behavior therapy. All groups were led by a licensed masters or doctoral level clinician or by a graduate student under the direct supervision of a licensed clinician. Data were collected as part of the IOP’s routine clinical practice and analyses received institutional review board approval.
Statistical analyses
A priori power analysis indicated that a sample size of 150 individuals were needed for an effect size (slope) of 0.2, α set at 0.05, and power set at 0.80.
Remission
We operationalized remission, based on prior work (e.g., [24–26]), as falling within 1 SD of community norms on the EAT-26 (9.65 ± 8.69) for each patient’s end-of-treatment EAT-26 score, such that patients whose scores fell at or below 18.34 were considered to be remitted [18].
Outcome predictors
Regression analyses were conducted in alignment with previous research examining similar predictors of outcome (e.g., [23, 24, 27–30]). However, regression models may yield misleading information due to multiple correlated independent variables [31]. To overcome the potential for misleading results, predictors were assessed using relative weight analysis [32] with RWA-Web [31], which provides relative weights that more accurately reflect the proportional contribution of the predictor variables in regression equations [31]. Confidence intervals for individual relative weights [32] and all corresponding significance tests were based on bootstrapping with 10,000 replications [31]. A predictor is statistically significant if the confidence interval does not include zero [31]. Raw relative weights are interpreted similar to R2, reflecting the proportion of unique variance accounted for by the specific predictor on the criterion variable. RS-RW % reflects the proportion of predicted variance in the criterion variable attributed to each predictor. Standard linear regression results were also conducted. Two models were examined: one examining predictors of last session EAT-26 scores and one examining predictors of last session ORS scores, using the following predictors: baseline scores on the criterion measure (EAT-26 and ORS, respectively), RR status, ED diagnosis, number of comorbid diagnoses, insurance supportiveness, weeks in IOP, illness duration at intake, age, and weight suppression.
Rapid response
Nonparametric ROC analyses were used to determine the definition of RR in the current sample that best predicted symptom remission at end-of-treatment (c.f., [24, 33, 34]). Similar to previous research [34], percent reduction in symptoms during the first ten sessions was assessed. Discrimination and accuracy of each potential RR definition (weeks 2–10) were assessed using the area under the curve (AUC) value. The AUC represents the likelihood that a randomly selected participant who has made RR will have a greater probability of being remitted at end-of-treatment than a randomly selected individual who did not make RR. The optimal cut-point was then selected as the percent reduction in EAT-26 scores with the highest sensitivity, where specificity was > 0.7 while minimizing misclassifications (based on [35]). To meaningfully account for early change in EAT-26 scores, RR analyses were conducted on patients who completed at least 4 weeks in IOP. Previous ROC analysis used to define RR in an ED treatment sample demonstrated no differences when data were imputed using maximum likelihood estimation, imputed using last observation carried forward, or left missing [34], so missing data were not imputed in the current sample.
Differentiation between rapid responders and non-rapid responders
Independent samples t tests and Chi-square test of independence evaluated statistical differences between rapid responders and non-rapid responders on the following factors: ED diagnosis, number of comorbid diagnoses, presence of substance use disorder, presence of personality disorder, BMI at intake, age, weight suppression, insurance supportiveness, weeks in IOP, illness duration at intake, and baseline EAT-26 and ORS scores.
Results
Clinical characteristics
The majority of patients (62.4%) were diagnosed with anorexia nervosa, with 36.7% diagnosed with binge eating/purging type, and 25.7% restricting type. The remaining participants were diagnosed with bulimia nervosa (20.0%), other specified feeding or ED (11.4%); binge ED (5.2%); and avoidant-restrictive food intake disorder (1.0%). For participant BMI by diagnosis, see Table 1. Most patients (66.6%) were diagnosed with one or more comorbid disorders, with 6.1% diagnosed with a comorbid personality disorder at intake, and 7.8% with comorbid substance use disorder diagnoses2 (comorbid diagnoses are described in Table 1).
Eating disorder pathology
Because IOP patients were required to be medically stable, many with anorexia nervosa entered IOP after weight restoration, with a small percentage of participants presenting with atypical AN.3 Week 1 EAT-26 scores did not differ significantly based on underweight status, t(148) = 1.02, p = 0.31. There was a significant difference in Week 1 ED symptom severity based on diagnosis, F(4147) = 4.66, p < 0.001, with patients with binge ED diagnoses having lower EAT-26 scores compared to those with anorexia nervosa restricting type (p = 0.002), anorexia nervosa binge eating/purging type (p = 0.001), and bulimia nervosa (p = 0.03). Week 1 EAT-26 scores were not significantly associated with age at intake, race/ethnicity, illness duration, number of comorbid diagnoses, presence of personality disorder or substance use disorder, intake BMI, or weight suppression.
Patients who completed four or more IOP weeks demonstrated statistically significant decreases in EAT-26 scores from Week 1, M(SD) = 35.37 (15.83), to discharge, M(SD) = 22.91 (18.14), t(146) = 8.61, p < 0.001. At IOP discharge, 43% of patients were defined as remitted from the intent-to-treat sample; 46.1% of patients who completed a minimum of 4 weeks in IOP were defined as remitted at end-of-treatment.
Global functioning
Patients (n = 154) who completed four or more IOP weeks demonstrated statistically significant increases in ORS scores from Week 1, M(SD) = 16.24 (8.01), to discharge, M(SD) = 21.09 (10.66), t(135) = − 5.67, p < 0.001. There were no significant differences in Week 1 ORS scores based on ED diagnosis, F(4,147) = 0.65, p = 0.63.
Outcome predictors
After controlling for baseline EAT-26 scores, only RR significantly predicted EAT-26 scores at discharge, accounting for 26.45% of the variance in final EAT-26 scores and a relative weight of 45.60% compared to all other predictors (Table 2). After controlling for baseline ORS scores, RR was not statistically significant in relative weights analysis, nor were any other significant predictor variables in the relative weights analysis (Table 2).
Table 2.
Relative weights of outcome predictors of end of treatment eating attitudes and global functioning
| Predictor | Standard linear regression results |
Relative weight analysis results |
|||
|---|---|---|---|---|---|
|
R2 = 0.575 |
R2 = 0.580 |
||||
| B (SEB) | β | RW | 95% CI | RS-RW% | |
| EAT-26 | |||||
| Week 1 EAT-26 | 0.46 (0.18) | 0.45* | 0.2345* | 0.1222 to 0.3387 | 40.43 |
| Week 1 ORS | 0.67 (0.31) | 0.34* | 0.0133 | − 0.0355 to 0.0286 | 2.29 |
| Rapid response | − 21.03 (4.34) | − 0.70*** | 0.2645* | 0.1391 to 0.3954 | 45.60 |
| Eating disorder diagnosis | − 1.86 (1.71) | − 0.16 | 0.0113 | − 0.0296 to 0.0341 | 1.95 |
| Comorbid diagnoses | 2.58 (2.74) | 0.15 | 0.0014 | − 0.0524 to 0.0073 | 0.25 |
| Insurance supportiveness | 1.77 (1.33) | 0.21 | 0.0124 | − 0.0364 to 0.0445 | 2.15 |
| Weeks in IOP | − 0.16 (0.20) | − 0.15 | 0.0361 | − 0.0096 to 0.0826 | 6.22 |
| Illness duration (in years) | − 0.05 (0.26) | − 0.03 | 0.0023 | − 0.0570 to 0.0110 | 0.39 |
| Age (in years) | 0.23 (0.26) | − 0.03 | 0.0028 | − 0.0525 to 0.0097 | 0.49 |
| Weight suppression (in lbs) | − 0.04 (0.07) | − 0.08 | 0.0013 | − 0.0572 to 0.0082 | 0.23 |
| Predictor | Standard linear regression results |
Relative weight analysis results |
|||
|
R2 = 0.154 |
R2 = .200 |
||||
| B (SEB) | β | RW | 95% CI | RS-RW% | |
| ORS | |||||
| Week 1 ORS | 0.08 (0.26) | 0.06 | 0.1314* | 0.0263 to 0.2420 | 65.83 |
| Week 1 EAT-26 | 0.06 (0.15) | 0.10 | 0.0317 | − 0.0341 to 0.0860 | 15.86 |
| Rapid response | 1.37 (3.70) | 0.08 | 0.0130 | − 0.0514 to 0.0564 | 6.53 |
| Eating disorder diagnosis | 1.14 (1.45) | 0.16 | 0.0009 | − 0.0707 to 0.0073 | 0.44 |
| Comorbid diagnoses | − 1.35 (2.33) | − 0.13 | 0.0048 | − 0.0631 to 0.0188 | 2.39 |
| Insurance supportiveness | − 0.85 (1.13) | − 0.17 | 0.00001 | − 0.0775 to 0.0124 | 0.49 |
| Weeks in IOP | − 0.11 (0.17) | − 0.16 | 0.0015 | − 0.0735 to 0.0111 | 0.74 |
| Illness duration (in years) | 0.10 (0.22) | 0,09 | 0.0030 | − 0.0767 to 0.0156 | 1.52 |
| Age (in years) | 0.04 (0.22) | 0.04 | 0.0121 | − 0.0521 to 0.0403 | 6.05 |
| Weight suppression (in lbs) | − 0.01 (0.06) | − 0.03 | 0.0003 | − 0.0745 to 0.0081 | 0.16 |
Insurance supportiveness measured as average number of days per authorization
EAT-26 Eating Attitudes Test-26, ORS Outcome Rating Scale, RWA relative weight analysis, RW raw relative weight (within rounding error, RW’s will sum to R2), RS-RW% relative weight rescaled as percentage of predicted variance in the criterion variable attributed to each predictor, rapid response defined as making a 16.52% reduction in EAT-26 scores by week 7, IOP intensive outpatient program
p < 0.05;
p < 0.01;
p< 0.001 for traditional regression analysis;
RWA* p value is set at 0.05
Rapid response
The highest AUC value, 0.777 (SE = 0.047; 95% CI = 0.686–0.869) was found using percent reduction of EAT-26 scores at Week 7, meaning that there is a 77.7% chance that a randomly selected participant who made RR at Week 7 would be remitted at end-of-treatment (Fig. 1). The cut-off which maximized both sensitivity and specificity after achieving a cut-point of 0.7 for sensitivity was a 16.52% reduction in EAT-26 scores, which yielded a sensitivity rate of 0.745 and specificity of 0.705, meaning that 74.5% are correctly identified as remitted, with 29.5% who would be incorrectly identified as remitted at Week 7, who did not actually meet remission criteria at end-of-treatment.
Fig. 1.
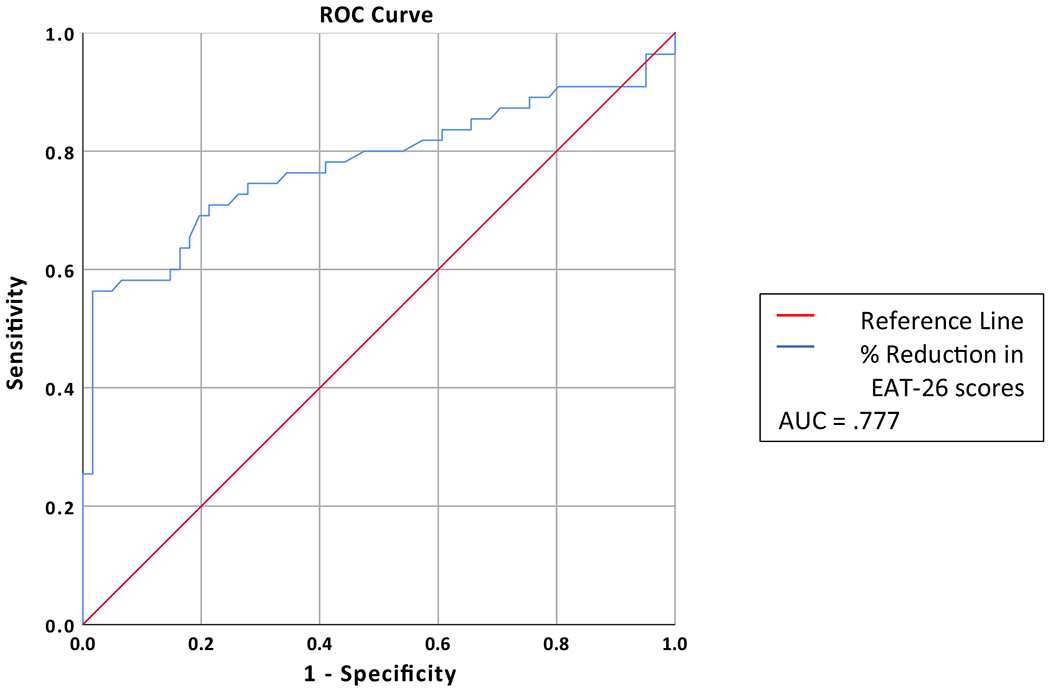
Week 7 ROC curve predicting remission at end of treatment. AUC area under the curve
Rapid Responders
Of the 116 patients who completed 7 weeks in IOP, 50.9% were defined as making RR. There were no baseline factors that differentiated between patients who did and did not make RR; those who demonstrated RR had significantly shorter treatment durations, t(114) = 2.10, p = 0.04 (Table 3).
Table 3.
Comparison between rapid and non-rapid response groups
|
M (SD)RR n = 59 |
M (SD)NRR n = 57 |
t | df | p | |
|---|---|---|---|---|---|
| Baseline BMI (in kg/m2) | 20.83 (4.25) | 22.25 (6.06) | 1.11 | 108 | 0.27 |
| Age (in years) | 23.11 (8.46) | 25.52 (11.37 | 1.31 | 117 | 0.19 |
| Weight suppression (in lbs) | 42.11 (28.07) | 49.50 (30.82) | 1.26 | 99 | 0.21 |
| Insurance supportiveness | 9.37 (1.35) | 8.81 (2.34) | − 1.32 | 75 | 0.19 |
| Weeks in IOP* | 18.46 (10.71) | 23.54 (15.12) | 2.10 | 114 | 0.04 |
| Illness duration at baseline (in years) | 7.86 (7.08) | 8.54 (8.18) | 0.40 | 78 | 0.69 |
| EAT-26 week 1 | 35.32 (16.14) | 36.89 (15.21) | 0.54 | 114 | 0.59 |
| ORS week 1 | 16.88 (8.09) | 15.40 (7.90) | − 0.95 | 106 | 0.34 |
| Chi square |
|||||
| t | df | p | |||
| Gender | 0.04 | 1 | 0.84 | ||
| Race/ethnicity | 1.04 | 2 | 0.60 | ||
| Primary ED diagnosis | 2.63 | 5 | 0.76 | ||
| Comorbid diagnoses | 3.32 | 4 | 0.51 | ||
| Substance use diagnosis | 1.98 | 1 | 0.20 | ||
| Personality disorder diagnosis | 0.60 | 1 | 0.68 | ||
Insurance supportiveness measured as average number of days per authorization
RR rapid responders, NRR non-rapid responders, IOP intensive outpatient program, ED eating disorder, EAT-26 Eating Attitudes Test-26, ORS Outcome Rating Scale
p < 0.05
Discussion
The current study was the first to examine RR as a mediator of outcome in a transdiagnostic ED sample in an IOP setting. Furthermore, we examined predictors of outcome that have been identified in previous research, predominantly conducted in randomized controlled trials and high level of care treatment settings, that have demonstrated inconsistent findings: weight suppression, illness duration, ED diagnosis, number of comorbid disorders, comorbid substance use disorder diagnosis, and comorbid personality disorder diagnosis. Finally, we examined whether an underexplored outcome predictor, insurance supportiveness, was predictive of treatment outcome. The remission rate at end-of-treatment in the current transdiagnostic IOP sample (43%) was similar to previous randomized controlled trial samples [33]. Supporting our hypotheses, RR was the best predictor of ED symptom reduction at the end of IOP treatment, but did not predict end-of-treatment global functioning. Replicating findings in randomized controlled trials and inpatient settings [8], RR was a robust outcome predictor in a real-world transdiagnostic IOP treatment setting, explaining 26.45% of the variance and uniquely accounting for 45.60% of the predicted variance in ED outcome. No other baseline variables predicted treatment outcome in this sample, in contrast to some prior research reporting less favorable outcomes among those with longer ED duration and comorbid personality pathology [9].
A lack of knowledge of precise mechanisms underlying RR or clinical profiles of clients who make RR are noted limitations in ED research [13] and have not previously been studied in intermediate level of care samples. In the current sample, there were no baseline differences between patients who did and did not make RR. However, patients who made RR had shorter IOP treatment durations than those who did not make RR. Shorter duration of IOP treatment among those who made RR compared to those who did not most likely resulted from faster improvement among those who made RR, leading to earlier discharge.
Prior research has shown mixed findings across studies regarding factors that distinguish those who make RR and those who do not. For instance, some studies have found that lower ED severity [36–39] or comorbid psychopathology such as depression/negative affect [36–38] and obsessive-compulsive symptoms [37] differentiated between those who made RR from those who did not. Although not examined as predictors in the current study, parental factors, such as lower parental educational attainment, criticism, and therapeutic alliance, have been shown to differentiate adolescents in family-based therapy for anorexia who make RR versus those who do not [25]. In contrast, similar to other studies that examined differences between those who do and do not make RR [20, 23, 30, 34], we did not find any baseline predictors of RR, suggesting that early change in the current sample was not a result of BMI, age, or baseline levels of ED severity or global functioning. Further research examining individualized trajectories of change and mediators of change in transdiagnostic intermediate level of care samples using routine outcome assessments (e.g., [40]) is critical to understanding these distinctions.
Our findings should be considered alongside certain limitations. First, in the current sample, participants with a range of ED diagnoses were considered together, and predictors and mediators of outcome that are commonly reported across diagnoses were examined (weight suppression, illness duration, psychiatric comorbidity, and RR). However, prognosis differs across ED diagnoses, most notably with less favorable prognosis for anorexia nervosa compared to other ED diagnoses and more favorable short-term prognosis for binge ED compared to other ED diagnoses [9]. Furthermore, prognostic indicators may also differ between diagnoses, with some prior research indicating that illness severity and duration predict anorexia nervosa outcome, whereas psychiatric comorbidity predicts bulimia nervosa outcome [9]. Given sample size limitations in the current study, we were unable to test interactions between each diagnosis and predictor, to examine whether these predictors were significant only within, but not across, diagnoses. Nonetheless, research evidence provides support for transdiagnostic models of ED pathology [41] and treatment [42]. Furthermore, ED diagnosis was included as a predictor of outcome in the current study and was not a significant predictor of eating pathology or global functioning at end-of-treatment. Future research in larger transdiagnostic IOP samples should examine interactions between predictors of outcome and specific ED diagnoses to further clarify this question.
Patients were predominantly White cisgender adult females with health insurance; findings may not generalize to more diverse samples and precluded our ability to conduct subgroup comparisons based on race/ethnicity, gender, age group, and insured status. Specifically, the small sample of adolescents (n = 32), precluded subgroup comparisons between adolescents and adults in the current sample. However, age was not predictive of treatment outcome, nor did it differentiate those who did and did not make RR in treatment. Future research should compare treatment outcome predictors in intermediate levels of care between adolescents and adults. Furthermore, research should also examine whether factors that distinguish those who make RR and those who do not make RR differ among adults and adolescents. Given recent findings indicating that parental factors predicted RR in adolescents with anorexia nervosa in family-based treatments [25, 37], this may be the case.
An additional limitation was the lack of a diagnostic tool at end-of-treatment. Although the EAT-26 reliably distinguishes those with ED diagnoses from those without ED diagnoses [16], it is not a diagnostic tool, so could not provide this specific status at discharge. Furthermore, the EAT-26 is both defining to the predictor variable of RR (from ROC analysis) and is also used as an outcome variable (based on falling within 1 SD of community norms at end-of-treatment). This methodology was chosen in the current study, because similar to previous research in transdiagnostic samples [20], our aim was to examine RR in a transdiagnostic sample. Thus, rather than using diagnosis-specific indicators (e.g., binge eating frequency, weight change, or compensatory behavior frequency), which are often used to define RR (e.g., [25, 36, 43], a general measure of ED pathology assessed at regular intervals was needed to capture transdiagnostic ED symptom change during treatment, such that the definition of RR would be comparable across diagnoses [20]. Nonetheless, it represents a potential limitation in the current study. To mitigate that potential limitation, baseline EAT-26 scores were included as a covariate and relative weights analysis was used to capture only the unique variance attributable by early symptom change in predicting overall ED symptom improvement. Furthermore, the definition of remission selected was conservative, requiring that IOP patients achieved EAT-26 scores at end-of-treatment that were within 1 SD of community norms [26], a cut-off which fell below the previously established clinical cut-off of 20 [16].
Because a requirement of IOP attendance was medical stability, patients diagnosed with anorexia nervosa in this sample had a higher BMI than is typically observed within this diagnostic classification—ranging from underweight (24.9% of the total sample) to obese BMIs. Participants diagnosed with anorexia nervosa who did not present with an underweight BMI were typically admitted after previous treatment or were diagnosed with atypical anorexia nervosa. Thus, our findings may not generalize to those with anorexia nervosa diagnoses who are classified by BMI as more severely underweight. However, prior research supports that early symptom change is predictive of remission at end-of-treatment in clinically underweight samples with anorexia nervosa [21, 24, 25].
While we consider our real-world transdiagnostic treatment setting a strength, some limitations of assessing treatment outcomes in routine clinical practice were that direct measures of therapist adherence were not measured; therapists were independently practicing within the clinic based on the DBT skills training manual [44] and enhanced CBT (CBT-E; [42]). Similarly, structured clinical interviews were not used at intake or discharge. In addition, patients in IOP were typically also meeting with psychiatrists and individual therapists in the community, whose interventions were not assessed and tracked. Finally, not all relevant predictors of ED treatment outcome were examined in the current study. For example, a recent study reported that poor sleep quality was predictive of treatment outcome in a sample of women with anorexia nervosa [45]. Future research should better examine and account for physiological predictors, such as sleep quality [45], heart rate variability [46], and microbiome [47], given findings suggesting their potential utility.
Despite these limitations, the current study extends prior examinations of treatment outcome predictors in single diagnostic categories [13] to transdiagnostic samples and extends previous examinations of ED treatment outcome predictors in intermediate levels of care [2] by examining additional predictors: RR, weight suppression, comorbid diagnoses, insurance supportiveness, treatment duration, and duration of illness.
Conclusion
To our knowledge, this is the first study to examine RR in addition to other outcome predictors in a transdiagnostic IOP sample. Furthermore, it is the only study specifically examining factors that may distinguish those who achieve RR from those who do not in a transdiagnostic IOP sample. Our findings highlight the importance of early, sustained improvement in transdiagnostic IOPs, as has been identified across other ED treatment settings [13]. Given the consistent prognostic utility of RR in predicting favorable ED treatment outcome, future research should systematically vary procedures to target RR (e.g., motivational enhancement methods) to determine their effectiveness. It is crucial to better understand outcome predictors in transdiagnostic real-world treatment settings to inform treatment planning, better target treatments, and narrow the widening “research-practice gap” [3].
What is already known on this subject?
A number of factors have been demonstrated to predict favorable ED treatment outcome: higher BMI in anorexia nervosa, fewer binge/purge episodes, greater motivation in treatment, lower shape/weight concern, fewer comorbidities, and less weight suppression, though support for some of these predictive factors is mixed. Early response to intervention, or RR, is a robust treatment mediator, which has been demonstrated predominantly in inpatient samples or randomized controlled treatment trials.
What this study adds?
These outcome predictors and the mediator of RR have had few replications in transdiagnostic samples and intermediate levels of care. Specifically, none of these outcome predictors have been examined in IOPs. The current study examined these predictors of outcome in a transdiagnostic intensive outpatient sample.
Acknowledgments
Funding Lisa Anderson is currently being supported through a National Institutes of Health T32 grant: MH082761 (PI: Scott J. Crow, MD) and Sasha Gorrell is currently being supported by the National Institutes of Health T32 grant: MH0118261-33 (PI: Linda Pfiffner, PhD).
Footnotes
The DSM-5 was published in May 2013, so clients evaluated for treatment from May to June 2013 were diagnosed using DSM-IV criteria. During data entry, patients’ initial evaluations were reviewed to ensure that the diagnosis was consistent with DSM-5 criteria. DSM-IV and DSM-5 diagnoses were consistent for all patients (n = 31) originally diagnosed using DSM-IV diagnostic criteria.
Individuals with substance use disorders were required to be in remission or concurrent treatment, with blood and urine screens required. Those with active moderate and severe substance use disorders were directed to first complete substance-specific treatment and then to demonstrate sobriety and concurrent ongoing substance abuse treatment.
Clinicians did not record specific reasons why clients were diagnosed with anorexia nervosa at BMIs higher than 18.5 or 85% ideal body weight alongside their diagnoses; however, review of the patients’ charts who were at the highest BMIs in AN-R and AN-BP diagnostic groups were cases in which clients self-reported extreme caloric restriction.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The procedures detailed in the current study were approved by Union College’s Human Subjects Review Committee.
Informed consent A waiver of informed consent was provided by the Human Subjects Review Committee for participants who began IOP treatment from May 2013 to May 2016 under the Department of Health and Human Services section §46.116 paragraph (d), with data used consisting solely of a retrospective chart review. Participants who participated in the study from May 2016 to May 2017 completed informed consent at their first clinical appointment, consenting to use all data provided for routine outcome monitoring during their course of IOP involvement to be used in research. Adult participants provided written informed consent; legal minor participants provided their written informed assent with one parent or legal guardian providing informed consent.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Anderson LK, Reilly EE, Berner L, Wierenga CE, Jones MD, Brown TA, Cusack A (2017) Treating eating disorders at higher levels of care: overview and challenges. Curr Psych Rep 19:48. 10.1007/s11920-017-0796-4 [DOI] [PubMed] [Google Scholar]
- 2.Hayes NA, Welty LJ, Slesinger N, Washburn JJ (2019) Moderators of treatment outcomes in a partial hospitalization and intensive outpatient program for eating disorders. Eat Disord 27(3):305–320. 10.1080/10640266.2018.1512302 [DOI] [PubMed] [Google Scholar]
- 3.Lilienfeld SO, Ritschel LA, Lynn SJ, Brown AP, Cautin RL, Latzman RD (2013) The research–practice gap: bridging the schism between eating disorder researchers and practitioners. Int J Eat Disord 46(5):386–394. 10.1002/eat.22090 [DOI] [PubMed] [Google Scholar]
- 4.Pritts SD, Susman J (2003) Diagnosis of eating disorders in primary care. Am Fam Physician 67(2):297–314 [PubMed] [Google Scholar]
- 5.Williams PM, Goodie J, Motsinger CD (2008) Treating eating disorders in primary care. Am Fam Physician 77(2):187–195 [PubMed] [Google Scholar]
- 6.Zipfel S, Reas DL, Thornton C et al. (2002) Day hospitalization programs for eating disorders: a systematic review of the literature. Int J Eat Disord 31(2):105–117. 10.1002/eat.10009 [DOI] [PubMed] [Google Scholar]
- 7.Dalle Grave R, Pasqualoni E, Calugi S (2008) Intensive outpatient cognitive behavior therapy for eating disorders. Psychol Topics 17(2):313–327 [Google Scholar]
- 8.Vall E, Wade TD (2015) Predictors of treatment outcome in individuals with eating disorders: a systematic review and meta-analysis. Int J Eat Disord 48(7):946–971. 10.1002/eat.22411 [DOI] [PubMed] [Google Scholar]
- 9.Keel PK, Brown TA (2010) Update on course and outcome in eating disorders. Int J Eat Disord 43(3):195–204. 10.1002/eat.20810 [DOI] [PubMed] [Google Scholar]
- 10.Gorrell S, Reilly EE, Schaumberg K, Anderson LM, Donahue JM (2018) Weight suppression and its relation to eating disorder and weight outcomes: a narrative review. Eat Disord. 10.1080/10640266.2018.1499297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J (2015) A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 16:495–509. 10.1186/s13063-015-1023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald DE, Trottier K, McFarlane T, Olmsted MP (2015) Empirically defining rapid response to intensive treatment to maximize prognostic utility for bulimia nervosa and purging disorder. Behav Res Ther 68:48–53. 10.1016/j.brat.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 13.Linardon J, Brennan L, de la Piedad Garcia X (2016) Rapid response to eating disorder treatment: a systematic review and meta-analysis. Int J Eat Disord 49(10):905–919. 10.1002/eat.22595 [DOI] [PubMed] [Google Scholar]
- 14.Treat TA, Gaskill JA, McCabe EB, Ghinassi FA, Luczak AD, Marcus MD (2005) Short-term outcome of psychiatric inpatients with anorexia nervosa in the current care environment. Int J Eat Disord 38(2):123–133. 10.1002/eat.20160 [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Koch T, Banker JD, Crow S et al. (2010) Service users’ views of eating disorder services: an international comparison. Int J Eat Disord 43(6):549–559. 10.1002/eat.20741 [DOI] [PubMed] [Google Scholar]
- 16.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE (1982) The eating attitudes test: psychometric features and clinical correlates. Psychol Med 12(4):871–878. 10.1017/S0033291700049163 [DOI] [PubMed] [Google Scholar]
- 17.Mintz LB, O’Halloran MS (2000) The eating attitudes test: validation with DSM-IV eating disorder criteria. J Pers Assess 74(3):489–503. 10.1207/S15327752JPA7403_11 [DOI] [PubMed] [Google Scholar]
- 18.Taylor MB, Daiss S, Krietsch K (2015) Associations among self-compassion, mindful eating, eating disorder symptomatology, and body mass index in college students. Transl Issues Psychol Sci 1(3):229–238. 10.1037/tps0000035 [DOI] [Google Scholar]
- 19.Miller SD, Duncan DL, Brown J, Sparks JA, Claud DA (2003) The outcome rating scale: a preliminary study of the reliability, validity, and feasibility of a brief visual analog measure. J Brief Ther 2(2):91–100 [Google Scholar]
- 20.American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th ed. Washington D.C. [Google Scholar]
- 21.Raykos BC, Watson HJ, Fursland A, Byrne SM, Nathan P (2013) Prognostic value of rapid response to enhanced cognitive behavioral therapy in a routine clinical sample of eating disorder out-patients. Int J Eat Disord 46:764–770. 10.1002/eat.22169 [DOI] [PubMed] [Google Scholar]
- 22.Doyle PM, Le Grange D, Loeb K, Doyle AC, Crosby RD (2010) Early response to family-based treatment for adolescent anorexia nervosa. Int J Eat Disord 43(7):659–662. 10.1002/eat.20764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrone S, Mitchell JE, Crosby R, Wonderlich S, Jollie-Trottier T (2009) Predictors of response to cognitive behavioral treatment for bulimia nervosa delivered via telemedicine versus face-to-face. Int J Eat Disord 42(3):222–227. 10.1002/eat.20603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden S, Miskovic-Wheatley J, Wallis A, Kohn M, Hay P, Touyz S (2015) Early weight gain in family-based treatment predicts greater weight gain and remission at the end of treatment and remission at 12-month follow-up in adolescent anorexia nervosa. Int J Eat Disord 48(7):919–922. 10.1002/eat.22414 [DOI] [PubMed] [Google Scholar]
- 25.Lock J, Couturier J, Bryson S, Agras S (2006) Predictors of dropout and remission in family therapy for adolescent anorexia nervosa in a randomized clinical trial. Int J Eat Disord 39(8):639–647. 10.1002/eat.20328 [DOI] [PubMed] [Google Scholar]
- 26.Le Grange D, Accurso EC, Lock J, Agras S, Bryson SW (2014) Early weight gain predicts outcome in two treatments for adolescent anorexia nervosa. Int J Eat Disord 47(2):124–129. 10.1002/eat.22221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schebendach J, Mayer LES, Devlin MJ, Attia E, Walsh BT (2012) Dietary energy density and diet variety as risk factors for relapse in anorexia nervosa: a replication. Int J Eat Disord. 45:79–84. 10.1002/eat.20922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wales J, Brewin N, Cashmore R, Haycraft E, Baggott J, Cooper A, Arcelus J (2016) Predictors of positive treatment outcome in people with anorexia nervosa treated in a specialized inpatient unit: the role of early response to treatment. Eur Eat Disorders Rev 24:417–424. 10.1002/erv.2443 [DOI] [PubMed] [Google Scholar]
- 29.Neubauer K, Weigel A, Daubmann A, Wendt H, Rossi M, Löwe B, Gumz A (2014) Paths to first treatment and duration of untreated illness in anorexia nervosa: are there differences according to age of onset? Eur Eat Disorder Rev 22:292–298. 10.1002/erv.2300 [DOI] [PubMed] [Google Scholar]
- 30.Vaz AR, Conceição E, Machado PPP (2014) Early response as a predictor of success in guided self-help treatment for bulimic disorders. Eur Eat Disord Rev 22(1):59–65. 10.1002/erv.2262 [DOI] [PubMed] [Google Scholar]
- 31.Tonidandel S, LeBreton JM (2015) A free, comprehensive, web-based and user-friendly tool for relative weight analyses. J Bus Psychol 30:207–216. 10.1007/s10869-014-9351-z [DOI] [Google Scholar]
- 32.Johnson JW (2003) A heuristic method for estimating the relative weight of predictor variables in multiple regression. Multivar Behav Res 35:1–19. 10.1207/S15327906MBR3501_1 [DOI] [PubMed] [Google Scholar]
- 33.Thompson-Brenner H, Shingleton RM, Sauer-Zavala S, Richards LK, Pratt EM (2015) Multiple measures of rapid response as predictors of remission in cognitive behavior therapy for bulimia nervosa. Behav Res Ther 64:9–14. 10.1016/j.brat.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 34.Zunker C, Peterson CB, Cao L et al. (2010) A receiver operator characteristics analysis of treatment outcome in binge eating disorder to identify patterns of rapid response. Behav Res Ther 48(12):1227–1231. 10.1016/j.brat.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartmann A, Wirth C, Zeeck A (2007) Prediction of failure of inpatient treatment of anorexia nervosa from early weight gain. Psychother Res 17(2):218–229. 10.1080/10503300600702315 [DOI] [Google Scholar]
- 36.Grilo CM, White MA, Masheb RM, Gueorguieva R (2015) Predicting meaningful outcomes to medication and self-help treatments for binge-eating disorder in primary care: the significance of early rapid response. J Consult Clin Psycholy 83(2):387–394. 10.1037/a0038635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes EK, Sawyer SM, Accurso EC, Singh S, Le Grange D (2019) Predictors of early response in conjoint and separated models of family-based treatment for adolescent anorexia nervosa. Eur Eat Disord Rev 27(3):283–294. 10.1002/erv.2668 [DOI] [PubMed] [Google Scholar]
- 38.Masheb RM, Grilo CM (2007) Rapid response predicts treatment outcomes in binge eating disorder. J Consult Clin Psycholy 75(4):639–644. 10.1037/0022-006X.75.4.639 [DOI] [PubMed] [Google Scholar]
- 39.Schlup B, Meyer AH, Munsch S (2010) A non-randomized direct comparison of cognitive-behavioral short- and long-term treatment for binge eating disorders. Obes Facts 3:261–266. 10.1159/000319538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barkham M, Connell J, Stiles WB et al. (2006) Dose-effect relations and responsive regulation of treatment duration. J Consult Clin Psycholy 74(1):160–167. 10.1037/0022-006X.74.1.160 [DOI] [PubMed] [Google Scholar]
- 41.Solmi M, Colantoni E, Meneguzzo P, Degortes D, Tenconi E, Favaro A (2018) Network analysis of specific psychopathology and psychiatric symptoms in patients with eating disorders. Int J Eat Disord 51(7):680–692. 10.1002/eat.22884 [DOI] [PubMed] [Google Scholar]
- 42.Fairburn CG (2008) Cognitive behavioral therapy for eating disorders. Guilford Press, New York [Google Scholar]
- 43.Safer DL, Joyce EE (2011) Does rapid response to two group psychotherapies for binge eating disorder predict abstinence? Behav Res Ther 49(5):339–345. 10.1016/j.brat.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linehan MM (2015) DBT skills training manual, 2nd edn. Guilford Press, New York [Google Scholar]
- 45.Sauchelli S, Jiménez-Murcia S, Sánchez I, Riesco N, Custal N, Fernández-García JC, Fernández-Aranda et al. (2016) Orexin and sleep quality in anorexia nervosa: clinical relevance and influence on treatment outcome. Psychoneuroendocrinology 65:102–108. 10.1016/j.psyneuen.2015.12.0140306-4530 [DOI] [PubMed] [Google Scholar]
- 46.Peschel SKV, Feeling NR, Vögele C, Kaess Thaeyr JF, Koenig J (2016) A systematic review on heart rate variability in bulimia nervosa. Neurosci Biobehav Rev 63:78–97. 10.1016/j.neubiorev.2016.01.0120149-7634 [DOI] [PubMed] [Google Scholar]
- 47.Lam YY, Maguire S, Palacios T, Caterson ID (2017) Are the gut bacteria telling us to eat or not to eat? Reviewing the rolse of gut microbiota in the etiology, disease progression and treatment of eating disorders. Nutrients 9:602. 10.3390/nu9060602 [DOI] [PMC free article] [PubMed] [Google Scholar]


