Abstract
Choroid plexus tumors are rare pediatric neoplasms ranging from low-grade papillomas to overtly malignant carcinomas. They are commonly associated with Li–Fraumeni syndrome and germline TP53 mutations. Choroid plexus carcinomas associated with Li–Fraumeni syndrome are less responsive to chemotherapy, and there is a need to avoid radiation therapy leading to poorer outcomes and survival. Malignant progression from choroid plexus papillomas to carcinomas is exceedingly rare with only a handful of cases reported, and the molecular mechanisms of this progression remain elusive. We report a case of malignant transformation of choroid plexus papilloma to carcinoma in a 7-yr-old male with a germline TP53 mutation in which we present an analysis of molecular changes that might have led to the progression based on the next-generation genetic sequencing of both the original choroid plexus papilloma and the subsequent choroid plexus carcinoma. Chromosomal aneuploidy was significant in both lesions with mostly gains present in the papilloma and additional significant losses in the carcinoma. The chromosomal loss that occurred, in particular loss of Chromosome 13, resulted in the losses of two critical tumor suppressor genes, RB1 and BRCA2, which might play a possible role in the observed malignant transformation.
Keywords: choroid plexus papilloma, neoplasm of the nervous system
INTRODUCTION
Choroid plexus tumors are rare central nervous system (CNS) neoplasms with varying malignant potential ranging from low-grade papillomas to overtly malignant carcinomas. Atypical choroid plexus papilloma (CPP) is an intermediate lesion characterized by increased mitotic rate but still clearly distinguishable from carcinoma (Safaee et al. 2013). The Li–Fraumeni syndrome, associated with germline mutations of the TP53 tumor suppressor gene, is known to increase the risk of choroid plexus tumors (Tabori et al. 2010; Orr et al. 2020). Malignant transformation of choroid plexus papillomas is extremely rare with less than a handful of cases reported (Chow et al. 1999; Jeibmann et al. 2007; Ruggeri et al. 2018). The most comprehensive retrospective report on transformation of papilloma to carcinoma followed 124 children with CPP, 12 of whom developed recurrences with two of 124 (1.6%) cases of confirmed transformation (Jeibmann et al. 2007). Because of the uncommon nature of these cases, the molecular mechanisms that trigger this malignant progression remain elusive. Here we report a child with histologically confirmed progression of papilloma into carcinoma 7 yr after initial presentation for whom we were able to perform comprehensive molecular sequencing analyses of both tumor samples from initial diagnosis of papilloma and ultimate transformation to carcinoma.
RESULTS
Clinical Presentation
The patient is a Caucasian male, 9 yr old at the time of this report. He had a history of poor weight gain, fussiness, and macrocephaly since birth and magnetic resonance imaging (MRI) at 3 wk of age demonstrated a large lobulated and hypervascular mass in the left cerebral hemisphere causing mass effect and midline shift. The patient underwent staged gross total resection of the mass that required three separate procedures. Pathology was diagnostic of atypical choroid plexus papilloma based on the increased mitotic activity. Despite surgical removal of a significant part of the left hemisphere, the patient had only mild right-sided hemiparesis and was developing well, showing no developmental delays and good school performance.
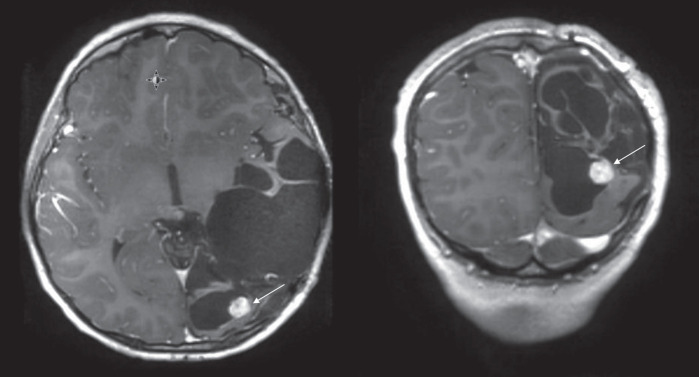
During his scheduled interval brain MRI evaluation 7 yr and 2 mo after the original surgery the patient was found to have a 2-cm nodular recurrent mass at the resection cavity (Fig. 1) and underwent an uncomplicated gross total resection with pathology demonstrating clear progression to a higher grade choroid plexus carcinoma (Fig. 2), with pathology showing highly cellular tumor invading brain parenchyma with a high nuclear to cytoplasmic ratio, marked nuclear pleomorphism, and high mitotic activity (up to eight mitoses per high power field compared to less than one in the original sample). Staining with Ki-67 labeled at least 40%–50% of tumor cell nuclei compared to 20%–30% in the original lower grade tumor. The papillary architecture noted in the original papilloma specimen was not evident at this time. The p53 immunostain was positive in nearly 100% of the tumor cells. The original and recurrent pathology specimens were reviewed at both Children's Hospital of Michigan and Nationwide Children's Hospital, Columbus and the progression from atypical CPP into choroid plexus carcinoma (CPC) was confirmed by two independent pathologists.
Figure 1.
Recurrent mass in the resection cavity found on a routine surveillance MRI 7 yr and 2 mo after initial surgery.
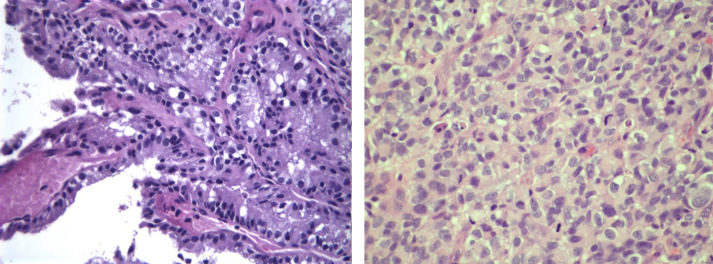
Figure 2.
(Left) Atypical CPP, 2009, hematoxylin and eosin (H&E), 400×, tumor cells in a papillary configuration; (right) CPC, 2017, H&E, 400×, patternless sheets of densely packed cells with atypia and high mitotic activity.
The patient's initial germline TP53 gene polymerase chain reaction (PCR)-based sequencing at an outside genetics laboratory that was done during initial diagnosis and treatment planning for the recurrent tumor was reported to be negative for TP53 mutations; however, the subsequent repeat test by the same laboratory and at the University of Michigan did demonstrate TP53 germline mutation. The patient received chemotherapy according to a modified “HeadStart II” protocol including three cycles of induction with high-dose methotrexate, vincristine, cyclophosphamide, etoposide, and cisplatin, followed by three cycles of tandem high-dose thiotepa and carboplatin with autologous hematopoietic cell support (Chi et al. 2004; Cohen et al. 2015).
Molecular Studies
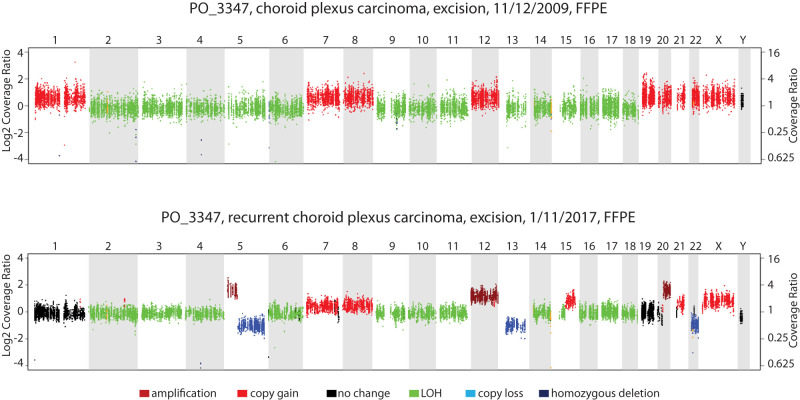
During his recurrence treatment, the patient was enrolled on a clinical integrative sequencing study consisting of targeted 1711 gene panel and tumor whole transcriptome (RNA-seq) along with matched germline DNA sequencing, performed on the patient's original papilloma, recurrent carcinoma and the patient's blood DNA samples using the PEDS-MI-Oncoseq clinical sequencing protocol (Mody et al. 2015). Chromosomal copy-number alterations were evaluated as well by analyzing the distribution of the sequencing reads from the patient's sample aligned to the reference genome. The sequencing analysis revealed a pathogenic germline variant of TP53 p.R248W with loss of heterozygosity (LOH) by uniparental disomy (UPD) in the tumor in both the papilloma and the carcinoma samples. As noted above, the TP53 variant was not detected in the initial test; interestingly the results at the University of Michigan showed a variant allele frequency of 39% in the germline sample, suggesting a possible mosaicism state. No notable gene expression outliers were detected by RNA-seq. Analysis of copy-number alterations in the two samples identified extensive aneuploidy and similar pattern of gains in Chromosomes 7, 8, 12, 20, 21, and X, and uniparental disomy of Chromosomes 2, 3, 4, 6, 9, 10, 11, 14, 16, 17, and 18 in both the samples. Striking copy-number aberrations newly acquired in the carcinoma included copy gain of Chromosomes 5p, 12, 15q, and 20, and copy loss of Chromosomes 5q, 13, and 22 that were not present in the papilloma sample (Fig. 3). In terms of somatic mutations, the original papilloma was found to harbor three missense mutations with low allelic fractions (4%–21%), including HDAC9 p.R248Q, and two different NCOR1 mutations, p.G5V and p.Y20S; notably, these were not detected in the subsequent carcinoma, which was found to harbor a subclonal somatic indel NCOA3 p.Q1263dup insertion with an allelic fraction of 6% (Table 1).
Figure 3.
Copy-number profile of the choroid plexus carcinoma sample, primary excision on 11/12/2009 (top) and that of the recurrent CPC sample excised on 1/11/2017 (bottom). The color code corresponding to different aberrations is indicated below. (FFPE) Formalin-fixed, paraffin-embedded, (LOH) loss of heterozygosity.
Table 1.
List of mutations in atypical choroid plexus papilloma (aCPP) and choroid plexus carcinoma (CPC) samples
| Gene | Chromosome | HGVS DNA reference | HGVS protein reference | Variant type | Predicted effect | dbSNP/dbVar ID | Genotype | Tumor allelic fraction |
|---|---|---|---|---|---|---|---|---|
| aCPP, 2009 sample | ||||||||
| NCOR1 | 17, somatic | NM_006311.3: c.14G > T | NP_006302.2: p. Gly5Val | Single nucleotide | Missense | rs76145228 | LOH by UPD | 21% |
| NCOR1 | 17, somatic | NM_006311.3: c.59A > C | NP_006302.2: p. Tyr20Ser | Single nucleotide | Missense | rs73281920 | LOH by UPD | 14% |
| HDAC9 | 7, somatic | NM_178425.3: c.743G > A | NP_848512.1: p. Arg248Gln | Single nucleotide | Missense | rs759089852 | Heterozygous | 4% |
| TP53 | 17, germline | NM_000546.6: c.742C > T | NP_000537.3: p. Arg248Trp | Single nucleotide | Missense | rs121912651 | LOH by UPD | |
| CPC, 2017 sample | ||||||||
| NCOA3 | 20, somatic | NM_181659.2: c.3788_3789ins ACA | NP_858045.1: p.Gln1263 | Indel | Frameshift | rs753491875 | Heterozygous | 6% |
| TP53 | 17, germline | NM_000546.6: c.742C > T | NP_000537.3: p. Arg248Trp | Single nucleotide | Missense | rs121912651 | LOH by UPD | |
(LOH) Loss of heterozygosity; (UPD) uniparental disomy.
Treatment Outcome
The patient was in uneventful remission for two and a half years off therapy, but at 2 yr and 8 mo off therapy he presented with worsening cytopenias and was found to have acute myeloid leukemia (AML). His AML cytogenetics revealed trisomy 21, deletion 5q, monosomy 7, deletion 9q, gain 5p consistent with therapy-related myelodysplastic syndrome/AML.
DISCUSSION
The common molecular defect in the patient's papilloma and carcinoma tumors was R248W mutation of the TP53 gene. This particular mutation is one of the most common TP53 alterations in choroid plexus tumors and is associated with the loss of an apoptotic role of TP53 as well as a gain of tumorigenic function (Willis et al. 2004; Song et al. 2007; Thompson and Compton 2010).
Although some of the other genes found to be altered by somatic mutations in both the patient's papilloma and subsequent carcinoma samples are known to play a role in epigenetic regulation of gene expression (Gil et al. 2016), most of the specific mutations detected here represent nonrecurrent mutations of unknown significance, and their allelic fractions were low and unlikely responsible for the observed malignant transformation (Table 1).
More pertinently, chromosomal aneuploidy was significant in both lesions with mostly gains present in the papilloma and additional significant losses in the carcinoma. It has been shown by Thomas et al. (2016) that for supratentorial choroid plexus tumors in young patients, mainly losses in DNA copy-number alterations were prevalent and thus potentially relevant to CPC oncogenesis in this group of patients (Thomas et al. 2016). Aneuploidy is typically a result of chromosomal instability phenotype (CIN) (Sansregret et al. 2018). A functional TP53 pathway has been shown to prevent CIN and aneuploidy via accumulation of nuclear TP53 during chromosome missegregation and subsequent elimination of aneuploid cells (Thompson and Compton 2010). The chromosomal loss that occurred—in particular loss of Chromosome 13—resulted in the losses of two critical tumor suppressor genes, RB1 and BRCA2, which might play a possible role in the observed malignant transformation in our case (Dick and Rubin 2013; Zámborszky et al. 2017).
Although we cannot entirely rule out a possibility that the CPC did not arise from the CPP and these represent two distinct tumors, development of two distinct rare tumors in the same location is very unlikely. Also, the pattern of chromosomal number alterations has striking similarities (Fig. 3) and is suggestive of the common origin.
Consistent with the observations in this case, Li–Fraumeni syndrome (LFS)-associated choroid plexus tumors (LFS-CPC) have been noted to harbor a significantly higher burden of chromosomal structural variations as well as significant risk of progression (Tabori et al. 2010). Furthermore, LFS-CPC patients were found to display marginally detrimental response to radiation therapy as compared to those with wild-type TP53 (Bahar et al. 2015). Consistent with this observation, a case report of a 3-yr-old girl with LFS-CPC described a complete remission upon treatment with surgery and chemotherapy (without radiation therapy) (McEvoy et al. 2017). Similarly, the “Head Start” Consortium experience suggested that with intensive myeloablative-chemotherapy containing regimens, the prognosis of TP53 mutant CPC may be improved in the absence of radiation therapy (Zaky et al. 2015). It is worth noting that both OncoSeq and repeat TP53 testing results that confirmed TP53 mutation were received when the patient was undergoing therapy and did not influence our decisions over therapy choices. Our patient was treated using an etoposide-containing regimen, and it has been shown that BRCA-deficient cancers are sensitive to topoisomerase II inhibitors (Treszezamsky et al. 2007); however, the risk of secondary malignancies after etoposide should not be underestimated in patients with germline TP53 mutation as demonstrated by our patient's case. Chemotherapy agents used to treat CPCs including etoposide, alkylating, and platinum-based drugs have an increased risk of secondary cancers and their use versus avoidance should always be carefully considered in children with LFS-associated CPCs who carry predisposition to multiple primary cancers and increased sensitivity to radiation and chemotherapy induced carcinogenesis.
METHOD
The patient was consented and enrolled on a prospective, institutional review board–approved (HUM00056496), integrative clinical sequencing trial (PEDS-MI-OncoSeq), in which patient samples undergo paired tumor/normal DNA sequencing, tumor RNA sequencing, and bioinformatics analyses, details of which have been previously described (Mody et al. 2015).
ADDITIONAL INFORMATION
Database and Deposition
Coding variants identified in the PEDS-MI-OncoSeq analysis are reported in Table 1. The patient did not provide consent for public deposition of all raw sequencing data for all genes included in the assay. The variants were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession number VCV000012347.13.
Ethics Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The patient's parents provided informed consent to participate in PEDS-MI-OncoSeq and received mandatory preenrollment genetic counseling in which no family history of cancer was noted. In addition, written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
We acknowledge the patient and his family.
Author Contributions
M.Y., J.L.F., and H.G. were responsible for patient care and clinical decision-making. M.Y., J.L.F., C.J.K., and R.M. were responsible for the concept and design. W.K. and D.R.B. provided pathologic diagnosis and figures. C.J.K., C.K.-S., M.Y., and R.M. obtained, analyzed, and interpreted molecular data. M.Y. wrote the manuscript. All authors performed a final review of the manuscript.
Funding
We acknowledge the support by the National Institutes of Health (NIH) Clinical Sequencing Exploratory Research (CSER) Award NIH 1UMIH006508. We acknowledge the Om Foundation for the support of this publication (M.Y.). R.M. is a Hyundai Hope on Wheels Scholar. None of the sponsors played a role in the design; data collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Competing Interest Statement
The authors have declared no competing interest.
REFERENCES
- Bahar M, Kordes U, Tekautz T, Wolff J. 2015. Radiation therapy for choroid plexus carcinoma patients with Li–Fraumeni syndrome: advantageous or detrimental? Anticancer Res 35: 3013–3017. [PubMed] [Google Scholar]
- Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, Wisoff JH, Weiner HL, Finlay JL. 2004. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol 22: 4881–4887. 10.1200/JCO.2004.12.126 [DOI] [PubMed] [Google Scholar]
- Chow E, Jenkins JJ, Burger PC, Reardon DA, Langston JW, Sanford RA, Heideman RL, Kun LE, Merchant TE. 1999. Malignant evolution of choroid plexus papilloma. Pediatr Neurosurg 31: 127–130. 10.1159/000028847 [DOI] [PubMed] [Google Scholar]
- Cohen BH, Geyer JR, Miller DC, Curran JG, Zhou T, Holmes E, Ingles SA, Dunkel IJ, Hilden J, Packer RJ, et al. 2015. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the children's oncology group. Pediatr Neurol 53: 31–46. 10.1016/j.pediatrneurol.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FA, Rubin SM. 2013. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol 14: 297–306. 10.1038/nrm3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil VS, Bhagat G, Howell L, Zhang J, Kim CH, Stengel S, Vega F, Zelent A, Petrie K. 2016. Deregulated expression of HDAC9 in B cells promotes development of lymphoproliferative disease and lymphoma in mice. Dis Model Mech 9: 1483–1495. 10.1242/dmm.023366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeibmann A, Wrede B, Peters O, Wolff JE, Paulus W, Hasselblatt M. 2007. Malignant progression in choroid plexus papillomas. J Neurosurg 107: 199–202. 10.3171/PED-07/09/199 [DOI] [PubMed] [Google Scholar]
- McEvoy M, Robison N, Manley P, Yock T, Konopka K, Brown RE, Wolff J, Green AL. 2017. Successful treatment of recurrent Li–Fraumeni syndrome–related choroid plexus carcinoma. J Pediatr Hematol Oncol 39: e473–e475. 10.1097/MPH.0000000000000965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody RJ, Wu YM, Lonigro RJ, Cao X, Roychowdhury S, Vats P, Frank KM, Prensner JR, Asangani I, Palanisamy N, et al. 2015. Integrative clinical sequencing in the management of refractory or relapsed cancer in youth. J Am Med Assoc 314: 913–925. 10.1001/jama.2015.10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr BA, Clay MR, Pinto EM, Kesserwan C. 2020. An update on the central nervous system manifestations of Li–Fraumeni syndrome. Acta Neuropathol 139: 669–687. 10.1007/s00401-019-02055-3 [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Alberio N, Alessandrello R, Cinquemani G, Gambadoro C, Lipani R, Maugeri R, Nobile F, Iacopino DG, Urrico G, et al. 2018. Rapid malignant progression of an intraparenchymal choroid plexus papillomas. Surg Neurol Int 5: 131 10.4103/sni.sni_434_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaee M, Oh MC, Bloch O, Sun MZ, Kaur G, Auguste KI, Tihan T, Parsa AT. 2013. Choroid plexus papillomas: advances in molecular biology and understanding of tumorigenesis. Neuro Oncol 15: 255–267. 10.1093/neuonc/nos289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansregret L, Vanhaesebroeck B, Swanton C. 2018. Determinants and clinical implications of chromosomal instability in cancer. Nat Rev Clin Oncol 15: 139–150. 10.1038/nrclinonc.2017.198 [DOI] [PubMed] [Google Scholar]
- Song H, Hollstein M, Xu Y. 2007. P53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol 9: 573–580. 10.1038/ncb1571 [DOI] [PubMed] [Google Scholar]
- Tabori U, Shlien A, Baskin B, Levitt S, Ray P, Alon N, Hawkins C, Bouffet E, Pienkowska M, Lafay-Cousin L, et al. 2010. TP53 Alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol 28: 1995–2001. 10.1200/JCO.2009.26.8169 [DOI] [PubMed] [Google Scholar]
- Thomas C, Sill M, Ruland V, Witten A, Hartung S, Kordes U, Jeibmann A, Beschorner R, Keyvani K, Bergmann M. 2016. Methylation profiling of choroid plexus tumors reveals 3 clinically distinct subgroups. Neuro Oncol 18: 790–796. 10.1093/neuonc/nov322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, Compton DA. 2010. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol 188: 369–381. 10.1083/jcb.200905057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treszezamsky AD, Kachnic LA, Feng Z, Zhang J, Tokadjian C, Powell SN. 2007. BRCA1- and BRCA2-deficient cells are sensitive to etoposide-induced DNA double-strand breaks via topoisomerase II. Cancer Res 67: 7078–7081. 10.1158/0008-5472.CAN-07-0601 [DOI] [PubMed] [Google Scholar]
- Willis A, Jung EJ, Wakefield T, Chen X. 2004. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene 23: 2330–2338. 10.1038/sj.onc.1207396 [DOI] [PubMed] [Google Scholar]
- Zaky W, Dhall G, Khatua S, Brown RJ, Ginn KF, Gardner SL, Yildiz VO, Yankelevich M, Finlay J. 2015. Choroid plexus carcinoma in children: the Head Start experience. Pediatr Blood Cancer 62: 784–789. 10.1002/pbc.25436 [DOI] [PubMed] [Google Scholar]
- Zámborszky J, Szikriszt B, Gervai JZ, Pipek O, Póti A, Krystanek M, Ribli D, Szalai-Gindl JM, Csabai I, Szallasi Z, et al. 2017. Loss of BRCA1 or BRCA2 markedly increases the rate of base substitution mutagenesis and has distinct effects on genomic deletions. Oncogene 36: 746–755. 10.1038/onc.2016.243 [DOI] [PMC free article] [PubMed] [Google Scholar]