Abstract
Diagnosis of B-cell chronic lymphocytic leukemia (B-CLL) is usually straightforward, involving clinical, immunophenotypic (Matutes score), and (immuno)genetic analyses (to refine patient prognosis for treatment). CLL cases with atypical presentation (e.g., Matutes ≤ 3) are also encountered, and for these diseases, biology and prognostic impact are less clear. Here we report the genomic characterization of a case of atypical B-CLL in a 70-yr-old male patient; B-CLL cells showed a Matutes score of 3, chromosomal translocation t(14;18)(q32;q21) (BCL2/IGH), mutated IGHV, deletion 17p, and mutations in BCL2, NOTCH1 (subclonal), and TP53 (subclonal). Quite strikingly, a novel PAX5 mutation that was predicted to be loss of function was also seen. Exome sequencing identified, in addition, a potentially actionable BRAF mutation, together with novel somatic mutations affecting the homeobox transcription factor NKX2-3, known to control B-lymphocyte development and homing, and the epigenetic regulator LRIF1, which is implicated in chromatin compaction and gene silencing. Neither NKX2-3 nor LRIF1 mutations, predicted to be loss of function, have previously been reported in B-CLL. Sequencing confirmed the presence of these mutations together with BCL2, NOTCH1, and BRAF mutations, with the t(14;18)(q32;q21) translocation, in the initial diagnostic sample obtained 12 yr prior. This is suggestive of a role for these novel mutations in B-CLL initiation and stable clonal evolution, including upon treatment withdrawal. This case extends the spectrum of atypical B-CLL with t(14;18)(q32;q21) and highlights the value of more global precision genomics for patient follow-up and treatment in these patients.
Keywords: hematological neoplasm
CASE PRESENTATION
A male patient was diagnosed with Binet stage A B-cell chronic lymphocytic leukemia (B-CLL) at the age of 58. At diagnosis, the lymphocyte count was 30 × 109/l and the following atypical immunophenotype was found: CD5+, CD23−, CD22+ (weak), FMC7+ (weak), and immunoglobulin lambda light chain positive (weak). CD79B was negative (Matutes score of 3). CD43 was positive. Cytogenetics (conventional and fluorescence in situ hybridization [FISH]) revealed a karyotype as follows; 46,XY[18].nuc ish(ATM × 2)[200],(D12Z1 × 3)[97/200],[D13S319 × 0][10/200],(TP53 × 2)[200], thus revealing presence of trisomy 12 in 50% of interphase nuclei, deletion 13q14.3 in 5% of nuclei, and absence of ATM (11q22) or TP53 loss. The patient presented a clonal FR1 rearrangement with mutated IGHV gene (91% identity to nearest germline VH gene), at a CDR3 junction as follows: VH4-34*01-DH5-24*01-JH3*02 (Supplemental Fig. S1A,B). At that time, the patient obtained a very good partial response to six cycles of fludarabine (70 mg per day on days 1, 2, and 3, respectively, per treatment cycle) and cyclophosphamide (400 mg per day on days 1, 2, and 3, respectively, per treatment cycle) (in 2007) and remained in stable response for 8 yr when he presented with adenopathy, splenomegaly, a lymphocyte count of 47.1 × 109/l, and thrombocytopenia at 122 × 109/l (Binet stage B). Immunochemotherapy with rituximab and bendamustine was commenced (one cycle) and then stopped because the patient showed non-treatment-related acute coronary syndrome that was subsequently successfully treated by coronary artery bypass. Against medical advice, the patient refused any further treatment for CLL and was proposed monthly laboratory-based surveillance with clinical consultation every 6 mo. Three years later, at the age of 70 yr (12 yr after initial diagnosis of stage A B-CLL), blood work showed progression to Binet stage C with a lymphocytosis at 30.5 × 109/l, anemia (Hb 116 × 109/l), and thrombocytopenia at 97 × 109/l. Immunophenotyping, cytogenetics and CLL/lymphoma gene panel sequencing were performed for restaging. Clonal B cells presented an identical atypical immunophenotype, IGH rearrangement and IGHV somatic mutation status, to that seen at initial diagnosis 12 yr earlier. Morphology was typical. Cytogenetics revealed clonal evolution with the following karyotype: 47,XY,+12,t(14;18)(q32;q21)[17]/47,idem,dic(3;4)(p10;q10),add(17)(p11)[2]/46,XY[1].nuc ish(TP53 × 1,D17Z1 × 2)[9/200]; thus showing acquisition of deletion 17p and detection of a t(14;18)(q32;q21) (IGH/BCL2) that was found by polymerase chain reaction (PCR) to have been present in the initial diagnostic sample 12 yr prior (Supplemental Fig. S1C). Gene panel sequencing revealed mutations in BCL2, NOTCH1 (subclonal), TP53 mutation (subclonal), and unexpectedly PAX5 (Supplemental Table S1). Rescreening of the initial diagnostic DNA sample by gene panel next-generation sequencing (NGS) showed that these variants were already present at diagnosis 12 yr earlier, except for the subclonal TP53 variant (Supplemental Table S1). In view of the clinical and genetic (TP53 alteration) evidence of progression to high-risk CLL, in the setting of an atypical presentation, exploratory theranostic exome sequencing was performed. This led to discovery of a class II BRAF somatic variant (Table 1). The patient unfortunately died from complications related to his previous heart surgery before new treatment options could be started.
Table 1.
Variants of interest by exome sequencing in an atypical case of B-cell chronic lymphocytic leukemia (B-CLL) with t(14;18)(q32;q21)
| Gene | Chromo some | Exon | Accession ID | mRNA change | Predicted protein effect | Variant type | COSMIC | Sequenc ing depth | Ref depth | Alt depth | Variant allele frequency | GERP++ RS scorea | CADD phred scoreb | PolyPhen-2 HDIV scorec | SIFT scored | ExAC pLI scoree | Metadome tolerance scoref | ClinVar signifi canceg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCL2 | 18q21.33 | 2/3 | NM_000633.2 | c.17G > C | p.A6T | Missense | COSV61 374585 | 35 | 28 | 7 | 20.0% | 3.61 | 16.37 | 0.534 (possibly damaging) | 0.015 (damaging) | 0.2965 | 0.51 (intolerant) | / |
| BRAF | 7q34 | 11/18 | NM_004333.4 | c.1406G > C | p.G469A | Missense | COSV56 061424 | 34 | 24 | 10 | 29.4% | 5.62 | 27.50 | / | 0.000 (damaging) | 0.9968 (intolerant) | 0.48 (intolerant) | Pathogenic |
| LRIF1 | 1p13.3 | 2/4 | NM_018372.3 | c.793dupA | p.T265fs | Frameshift | N/A | 49 | 30 | 19 | 38.8% | / | / | / | / | 0.0906 (tolerant) | 0.63 (slightly intolerant) | / |
| NKX2-3 | 10q24.2 | 2/2 | NM_145285.2 | c.547A > G | p.S183G | Missense | COSV10 0755073 | 61 | 33 | 28 | 45.9% | 5.45 | 27.10 | 0.217 (benign) | 0.159 (tolerated) | 0.9505 (intolerant) | 0.32 (intolerant) | / |
| PAX5 | 9p13.2 | 5/10 | NM_016734.2 | c.570_582 delCAGCGC CGACACC | p.S191fs | Frameshift | N/A | 36 | 27 | 9 | 25.0% | / | / | / | / | 0.6463 | 0.37 (intolerant) | / |
See text for reference publications on pathogenicity prediction scores.
(COSMIC) Catalog Of Somatic Mutations In Cancer, (GERP++RS) Genomic Evolutionary Rate Profiling (GERP)—Rejected Substitutions (RS) score, (CADD) Combined Annotation Dependent Depletion, (PolyPhen-2) Polymorphism Phenotyping v2, (SIFT) Sorting Intolerant From Tolerant, (N/A) not applicable, (/) not determined.
aFrom −12.3 to 6.17. The higher the score, the more conserved the site.
b<20: variant is in the top 1% to 0.1% most damaging variations; <30: variant is in the top 0.1 to 0.01% most damaging variations.
cFrom 0 to 1. The higher the score, the higher the probability for the mutation to be damaging for the protein.
dPredicts whether an amino acid substitution affects protein function.
eDescribes the probability of a gene being loss of function intolerant. >0.9: extremely intolerant; <0.1: tolerant.
fThe MetaDome tolerance is based on the tolerance colors of the web server MetaDome (https://stuart.radboudumc.nl/metadome/).
TECHNICAL ANALYSIS
Morphology, Immunophenotyping, and Conventional Cytogenetics
Blood examinations, immunophenotyping, and conventional cytogenetic analysis were performed according to guidelines established in French National Cooperative networks on hemato-immunology and cytogenetics, respectively (Emadali et al. 2016).
Targeted Gene Panel, Exome Sequencing, and Bioinformatics
Targeted and whole-exome capture sequencing was performed on blood-derived DNA obtained at progression, 12 yr after initial diagnosis of B-CLL. Targeted gene panel sequencing was performed, in both the initial diagnostic and progression samples, by custom capture sequencing of a consensus 51 gene panel (IDT probes and reagent kits) on an Illumina NextSeq 500 sequencer, according to manufacturer's instructions. Paired-end exome sequencing was performed by using the TWIST exome capture protocol, according to the manufacturer's protocol. Bioinformatics for alignment, somatic variant calling and annotation in targeted panel and exome sequencing were performed according to the “Best Practices Workflow” from GATK (see supplemental materials, for details). Insufficient material was available for RNA-seq. Germline DNA was not available.
VARIANT INTERPRETATION
Variants discovered in the above pipeline were curated for biological relevance and for somatic origin in cancer (COSMIC). In first line filtering, common and rare variants with annotations in dbSNP, gnomAD were removed from the patient variant list, except for exceptionally rare variants that were also listed in the COSMIC database (Tate et al. 2018). The latter were manually checked. Variants outside of exons and splice sites were removed, as were synonymous variants. Further filtering was performed using functional impact scores (see Table 1 for details and results) and manual curation based on potential clinical (therapy) and/or biological relevance (focus on known CLL variants and on B-cell transcription factors and epigenetic regulators with potential to drive atypical or “extreme” phenotypes in blood cancers) in this atypical case of B-CLL. To enter the final short list of novel variants for this work, absence from control gnomAD and ExAC databases was a prerequisite, because germline DNA was not available for further validation. These were validated by Sanger resequencing in both the sample obtained at progression (and used for exome sequencing) and the initial diagnostic sample obtained 12 yr prior.
RESULTS
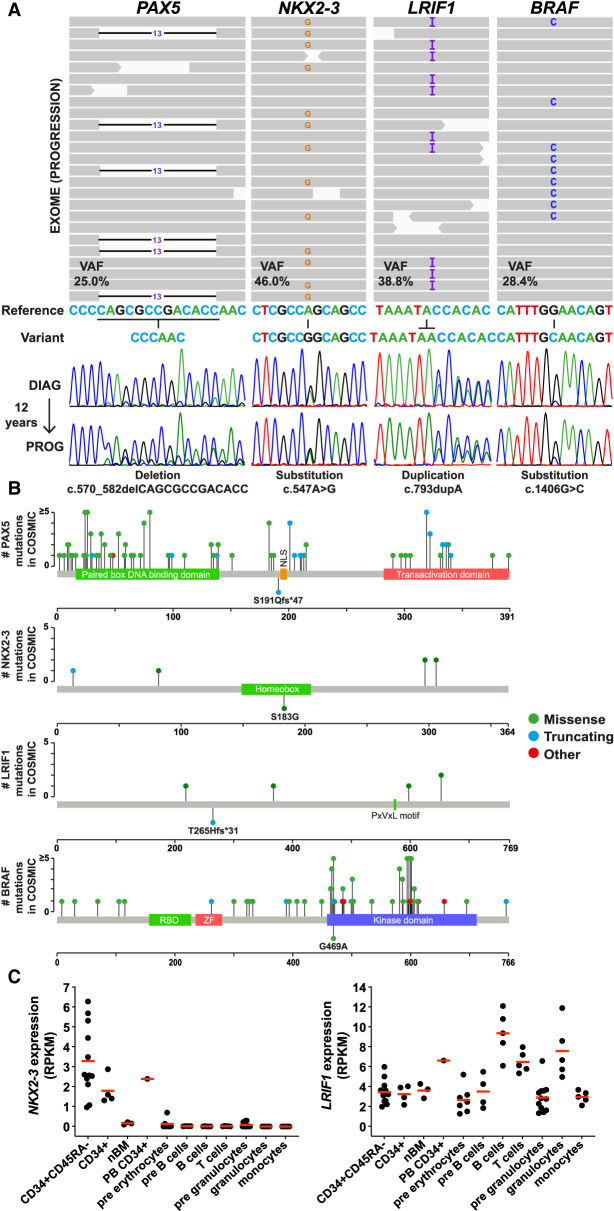
After filtering as described above, we retained a total of 238 candidate somatic variants (195 genes) from the exome analysis of the DNA sample obtained at progression 12 yr after the initial diagnosis of B-CLL, in our patient (Table 1). Our objective was to first screen for variants of potential direct therapeutic value for our patient and then to look for additional known B-CLL variants. In a second step we scored for novel clonal, somatic variants that target transcription factors and/or epigenetic factors of potential relevance to B-CLL pathogenesis, particularly in view of the atypical clinicobiological presentation of this case. Following this logic, one BRAF somatic variant was retained for its theranostic interest (p.G469A; variant allele frequency [VAF] = 29.4%) (Table 1). This variant has previously been reported in B-CLL, but not in the setting of IGH/BCL2 translocation-positive B-CLL cases. BRAF p.G469A is a known class II BRAF mutation (characterized by homodimeric signaling) (Yaeger and Corcoran 2019). Although our patient died before therapy could be started, our analysis indicates the utility of deploying clinical exome sequencing in selected B-CLL cases, as described here. No other known B-CLL somatic variants were uncovered, aside from the BCL2 variant already identified by targeted gene panel sequencing (Table 1; Supplemental Table S1). It can be noted that the subclonal mutations affecting NOTCH1 and TP53 were not detected by exome analysis, because of insufficient sequencing depth (Table 1; Supplemental Table S1). Our next step was to search for novel variants affecting B-cell transcription factors or epigenetic regulatory pathways of potential relevance to B-CLL and that could underpin the unusual features of our case. This led us to focus on three variants affecting the PAX5, NKX2-3, and LRIF1 genes, respectively. None of these variants had previously been linked to B-CLL (in either published series or COSMIC submissions) (Table 1; Fig. 1A). Their absence from control databases was an indicator that they were bona fide novel somatic variants. We thus proceeded to assessment of pathogenicity (Table 1). The PAX5 variant (initially seen in gene panel sequencing, Supplemental Table S1) consisted of a frame shift deletion [c.570_582delCAGCGCCGACACC (VAF = 25%)] in exon 5 that was predicted to lead to a premature stop codon (p.S191fs*) downstream from the paired box DNA binding domain and upstream of the nuclear localization signal (NLS) (Table 1; Fig. 1B), predictive of possible haploinsufficiency for PAX5 function in this case. Interestingly, the Ser191 amino acid is not conserved in PAX2 and PAX8, the closest paralogues of PAX5. Moreover, the NM_016734: c.570_582delCAGCGCCGACACC (p.S191fs*) is not described in COSMIC database (Tate et al. 2018).
Figure 1.
(A) Integrated Genomic Viewer (IGV) display (at progression) and Sanger validation (at diagnosis [DIAG] and progression [PROG]) of the PAX5, NKX2-3, LRIF1, and BRAF mutations identified in the patient's blood. (B) Representation of PAX5, NKX2-3, LRIF1, and BRAF proteins with annotated domains and amino acids numbered underneath. Plots were generated (https://www.cbioportal.org/mutation_mapper) with recurrent mutations (green, missense; blue, truncating; red, other) identified in COSMIC (hematopoietic and lymphoid tissues). Mutations seen in the patient are shown beneath the protein map. (C) Gene expression of NKX2-3 and LRIF1 in hematopoietic populations isolated from cord blood, bone marrow, and peripheral blood of healthy donors (nBM, normal bone marrow; PB, peripheral blood). RNA-seq data (GSE51984 and GSE48846) were downloaded from the Gene Expression Omnibus (GEO).
The NKX2-3 gene underwent a single-nucleotide substitution (c.547A > G; VAF = 45.9%) leading to a missense mutation (p.S183G) in the highly conserved homeobox domain (Table 1; Fig. 1B). Although the NKX2-3 gene is not constrained for missense variation by PolyPhen22 (Adzhubei et al. 2010) and SIFT (Kumar et al. 2009) algorithms, this variant had GERP++RS (Cooper et al. 2005) and CADD (Kircher et al. 2014) scores of 5.45 and 27.1, respectively, indicative of a high degree of conservation and potential deleterious impact, respectively (Table 1). Additionally, MetaDome analysis predicted this missense mutation to be intolerant (MetaDome quantifies genetic tolerance by calculating a missense-over-synonymous ratio based on the variations reported in gnomAD database) (Table 1; Supplemental Fig. S2A; Wiel et al. 2019). We identified a second case of somatic mutation at this site (COSV100755073), reported in a patient with gastric cancer. Pathogenicity prediction by FATHMM performed at COSMIC scores this variant (identical to ours) at 0.97 (pathogenic). Thus, by FATHMM and MetaDome, the NKX2-3 gene at the nucleotide position identified in our study is constrained for missense mutation. Based on these pathogenicity assessments, the NKX2-3 p.S183G variant is predicted to be somatic and functionally deleterious, in particular for interaction of NKX2-3 with cognate binding sites. For LRIF1, a duplication (c.793dupA; VAF = 38.8%) in exon 2 was seen that was predicted to lead to a frameshift mutation and premature stop codon (p.T265fs*), possibly leading to expression of a truncated variant (potentially mislocalized because the NLS is lost) and thus haploinsufficiency for this factor, at least in the nucleus (Table 1; Fig. 1B). Alternatively, this premature stop codon might lead to degradation of the mutant transcript by nonsense-mediated decay. Although not constrained for loss of function by pLI score (0.09) (Table 1), MetaDome analysis predicts LRIF1 p.T265 to be slightly intolerant to missense variants (Supplemental Fig. S2B). Targeted NGS and Sanger sequencing in the initial diagnostic sample obtained 12 yr prior revealed the presence of these PAX5, LRIF1, and NKX2-3 likely somatic variants, indicating that they occurred early in clonal evolution and that they were stably propagated thereafter. By VAF, it is likely that the PAX5 variant occurred later that the LRIF1 and NKX2-3 variants (Table 1). Data mining confirmed that RNA expression levels of NKX2-3 and LRIF1 are highest in the hematopoietic stem cell (HSC) and mature B-lymphocyte compartments, respectively (Fig. 1C).
SUMMARY
This case extends our knowledge on atypical B-CLL presenting t(14;18)(q32;q21), a relatively rare entity for which disease mechanisms and prognostic significance remain unclear (Nguyen-Khac et al. 2011; Fang et al. 2019; Pérez-Carretero et al. 2020). Indeed, only nine cases have been examined thus far by WGS/exome and RNA-seq with a major conclusion being that BCL2 mutations are frequent and that these occur on the translocated BCL2 allele, thus leading to overexpression on the translocated allele (Puente et al. 2015).
A further study (gene panel sequencing) of 46 B-CLL cases with IGH translocation revealed a lower mutation frequency and enrichment for BCL2 and IGLL5 mutations in IGH/BCL2-translocated compared to non-IGH/BCL2-rearranged cases. The latter were seen to present mutations in genes related to poor prognosis (NOTCH1, SF3B1, and TP53) and to have a shorter time to first treatment (Pérez-Carretero et al. 2020). The present case, however, falls outside of this dichotomy; even if BCL2 and subclonal NOTCH1 and TP53 mutations were seen, unique molecular features were also present. For example, a non-V600E BRAF mutation (p.G469A) that was present 12 yr prior to progression to high-risk B-CLL, was observed. CLL with mutations in the RAS-BRAF-MAPK-ERK pathway is proposed to define a specific subgroup of patients with adverse clinical features (Giménez et al. 2018). Preclinical testing has shown sensitivity to BVD-523 (Giménez et al. 2018), a pan-ERK inhibitor that shows activity in non-V600E BRAF mutant cancers (Yaeger and Corcoran 2019), thus highlighting the clinical interest of precision genomics in atypical B-CLL, particularly with IGH/BCL2 translocation.
Our case also displays novel mutations likely to impede B-cell development and homing (PAX5 and NKX2-3, respectively) (Choukrallah and Matthias 2014; Nagel and Drexler 2019). PAX5 encodes a key factor in B-cell differentiation with a dual role in that it enables the expression of B-cell-specific genes while repressing inappropriate expression of genes involved in the commitment to other lineages (Cobaleda et al. 2007). PAX5 loss-of-function mutations affecting a noncoding regulatory element have been described in CLL, but not PAX5-coding mutations (Puente et al. 2015). As such, our case is unique and raises the possibility that PAX5-coding regions may be a direct target for mutation in rare entities such as IGH/BCL2-translocated B-CLL. Although the consequences of this variant for PAX5 expression are not as yet known, haploinsufficiency for PAX5 function, as seen with PAX5 locus-regulatory sequence mutations (Puente et al. 2015), may occur.
In sporadic BCP-ALL (B-cell precursor acute lymphoblastic leukemia), PAX5 is the target of a wide diversity of alterations both in children and adult cases (Mullighan et al. 2007; Familiades et al. 2009). The impact of these alterations is not equivalent in disease progression, whereas loss of function by non-sense mutations or deletions is considered as a secondary event, translocation and some point mutations are described as primary events in the disease (Coyaud et al. 2010). PAX5 loss of function is known to be a predisposition to BCP-ALL (Dang et al. 2015; Duployez et al. 2020).
The presence in our case of a potentially damaging mutation in the homeobox-encoding region of the NKX2-3 gene is of interest for disease pathogenesis in B-CLL, particularly in light of the role of NKX2-3 in B-cell homing, B-cell development (marginal zone B cells), B-cell receptor signaling, and lymphomagenesis (at least in mouse models of overexpression) (Robles et al. 2016). High expression of NKX2-3 in CD34+ HSC is also worth highlighting in our case, because this is a compartment known to harbor somatic mutations in B-CLL (Damm et al. 2014). Judging by VAF frequency (45.9%) for this variant, a concern was that it might be of germline origin. However, pathogenicity assessments tend to rule this out. We suspect that NKX2-3 mutation is a comparatively early event in the natural history of CLL development in our case. Whether it might be present in a preneoplastic clone remains to be determined and will require sequencing and functional analyses in additional B-CLL cases.
LRIF1 (also known as HBiX1 and encoding ligand-dependent nuclear receptor-interacting factor 1), was a novel target for mutation (truncating) in our case. LRIF1 has been established as a key factor mediating interaction of the chromatin protein SMCHD1 with HP1γ at trimethylated histone H3 lysine 9 (H3K9me3)-modified chromatin sites on chromosome arms, a process which is essential for X-chromosome inactivation (Nozawa et al. 2013; Brideau et al. 2015). Other studies implicate LRIF1 in the shutdown of nuclear hormone receptor signaling (Li et al. 2007), specifically retinoic acid, and in as-yet-undefined functions at centromeric repeats (Buxton et al. 2017) and at telomeres (Grolimund et al. 2013), respectively. The predicted consequence of the mutation observed in our B-CLL (premature stop codon upstream of the chromatin and SMCHD1-interacting domain and NLS) case is haploinsufficiency for LRIF1 chromatin compaction functions in the nucleus (Brideau et al. 2015). Whether a mutant LRIF1 protein is expressed or whether mutant transcripts are targeted for nonsense-mediated decay remains to be established. In favor of this hypothesis, functional analysis of a similar (germline) homozygous LRIF1 variant (p.T291*) to ours (p.T265fs*), reported in a patient with a clinical phenotype consistent with FSHD (fascioscapulohumeral muscular dystrophy), has shown that homozygous mutation in this region leads to absence of the long isoform of LRIF1 protein, whereas a short isoform of unknown function and that retains the carboxy-terminal chromatin/SMCHD1-interacting domain and NLS remains expressed (Hamanaka et al. 2020). The functional consequence of loss of the LRIF1 long-form expression was found to be chromatin relaxation (depletion of DNA methylation and H3K9me3) at D4Z4 repeats, which is an epigenetic hallmark of FSHD.
Regarding clonal evolution in this atypical B-CLL case, we can only speculate. We propose that the t(14;18)(q32;q21) was present in a stem line clone that also harbored at least the NKX2-3 and LRIF1 mutations (VAFs of 45.9% and 38.8%, respectively at progression and equivalent peak height at variant nucleotide positions between diagnosis and progression at 12 yr) (Fig. 1A). It is then reasonable to assume that further evolution occurred with acquisition of the BRAF variant followed by PAX5, and BCL2 mutations, possibly in the same subclone. Indeed, the PAX5 variant was detected at a VAF of 17.1% in the progression sample compared to a VAF of 8.4% in the initial diagnostic sample (Supplemental Table S1), suggestive of slow clonal expansion over time (immunophenotyping and molecular clonality analysis at the FR1 locus indicated similar B-CLL clone infiltration in both samples [Supplemental Fig. S1]). The BCL2 mutation showed similar kinetics with a VAF of 21.3% observed at progression compared to 10.7% at diagnosis, 12 yr prior. The NOTCH1 mutant subclone remained relatively stable over time, detectable at 1.1% VAF compared to 3.9% VAF between the time of diagnosis and progression, respectively. A TP53 mutant clone detected at 0.7% at the time of progression was not detectable at diagnosis, suggestive of late acquisition possibly concomitant to deletion 17(p) in a subclone.
Taken together this case extends our knowledge of atypical CLL with BCL2/IGH translocation and highlights the clinical and cognitive value of performing global genomics characterization of these cases early in disease evolution.
ADDITIONAL INFORMATION
Database Deposition and Access
All interpreted variants have been deposited in COSMIC (submission number COSP48664).
Ethics Statement
All testing was done as part of routine clinical laboratory workup. Consent was thus implicit.
Acknowledgments
M.B.C. wishes to acknowledge institutional support.
Author Contributions
M.B.C. designed and coordinated the study and wrote the paper. R.A. coordinated genetic analyses, interpreted data, and co-wrote the paper. B.B. and S. Ram. collected and interpreted samples and data and co-wrote the paper. B.B., S.Rag., and C.Bu. performed sequencing analyses. C.F. performed bioinformatics analysis. Diagnostic findings were reviewed by M.B.C., M.-L.C., F.B., J.R., J.G., N.N., L.M., and M.M. J.A. performed cardiogenetic analysis and interpretation. Clinical findings were provided and reviewed by J.-N.B., C.R., O.C., C.T., and D.C. C.C. supervised panel sequencing and interpreted data. B.B., B.T., C.F., Y.D., R.A., and M.B.C. analyzed and interpreted exome data. M.J.A.-R., C.S., and L.D. contributed scientific discussion of novel variants in hematopoiesis and epigenetic control. C.Br. provided expert analysis on the PAX5 variant. All authors reviewed the paper. M.B.C. and R.A. were responsible for final approval of the paper.
Funding
Grants from the Bourgogne-Franche-Comté region, the FEDER programme, the Foundation ARC, and INCa (epigenetics and cancer program).
Competing Interest Statement
The authors have declared no competing interest.
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. 2010. A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau NJ, Coker H, Gendrel A-V, Siebert CA, Bezstarosti K, Demmers J, Poot RA, Nesterova TB, Brockdorff N. 2015. Independent mechanisms target SMCHD1 to trimethylated histone H3 lysine 9-modified chromatin and the inactive X chromosome. Mol Cell Biol 35: 4053–4068. 10.1128/MCB.00432-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton KE, Kennedy-Darling J, Shortreed MR, Zaidan NZ, Olivier M, Scalf M, Sridharan R, Smith LM. 2017. Elucidating protein–DNA interactions in human alphoid chromatin via hybridization capture and mass spectrometry. J Proteome Res 16: 3433–3442. 10.1021/acs.jproteome.7b00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukrallah MA, Matthias P. 2014. The interplay between chromatin and transcription factor networks during B cell development: who pulls the trigger first? Front Immunol 5: 156 10.3389/fimmu.2014.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobaleda C, Schebesta A, Delogu A, Busslinger M. 2007. Pax5: the guardian of B cell identity and function. Nat Immunol 8: 463–470. 10.1038/ni1454 [DOI] [PubMed] [Google Scholar]
- Cooper GM, Stone EA, Asimenos G, Green ED, Batzoglou S, Sidow A. 2005. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15: 901–913. 10.1101/gr.3577405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyaud E, Struski S, Prade N, Familiades J, Eichner R, Quelen C, Bousquet M, Mugneret F, Talmant P, Pages M-P, et al. 2010. Wide diversity of PAX5 alterations in B-ALL: a Groupe Francophone de Cytogénétique Hématologique study. Blood 115: 3089–3097. 10.1182/blood-2009-07-234229 [DOI] [PubMed] [Google Scholar]
- Damm F, Mylonas E, Cosson A, Yoshida K, Valle VD, Mouly E, Diop M, Scourzic L, Shiraishi Y, Chiba K, et al. 2014. Acquired initiating mutations in early hematopoietic cells of CLL patients. Cancer Discov 4: 1088–1101. 10.1158/2159-8290.CD-14-0104 [DOI] [PubMed] [Google Scholar]
- Dang J, Wei L, de Ridder J, Su X, Rust AG, Roberts KG, Payne-Turner D, Cheng J, Ma J, Qu C, et al. 2015. PAX5 is a tumor suppressor in mouse mutagenesis models of acute lymphoblastic leukemia. Blood 125: 3609–3617. 10.1182/blood-2015-02-626127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duployez N, Jamrog LA, Fregona V, Hamelle C, Fenwarth L, Lejeune S, Helevaut N, Geffroy S, Venet AC, Marceau-Renaut A, et al. 2020. Germline PAX5 mutation predisposes to familial B acute lymphoblastic leukemia. Blood 10.1182/blood.2020005756. [DOI] [PubMed] [Google Scholar]
- Emadali A, Hoghoughi N, Duley S, Hajmirza A, Verhoeyen E, Cosset F-L, Bertrand P, Roumier C, Roggy A, Suchaud-Martin C, et al. 2016. Haploinsufficiency for NR3C1, the gene encoding the glucocorticoid receptor, in blastic plasmacytoid dendritic cell neoplasms. Blood 127: 3040–3053. 10.1182/blood-2015-09-671040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familiades J, Bousquet M, Lafage-Pochitaloff M, Béné M-C, Beldjord K, De Vos J, Dastugue N, Coyaud E, Struski S, Quelen C, et al. 2009. PAX5 mutations occur frequently in adult B-cell progenitor acute lymphoblastic leukemia and PAX5 haploinsufficiency is associated with BCR-ABL1 and TCF3-PBX1 fusion genes: a GRAALL study. Leukemia 23: 1989–1998. 10.1038/leu.2009.135 [DOI] [PubMed] [Google Scholar]
- Fang H, Reichard KK, Rabe KG, Hanson CA, Call TG, Ding W, Kenderian SS, Muchtar E, Schwager SM, Leis JF, et al. 2019. IGH translocations in chronic lymphocytic leukemia: clinicopathologic features and clinical outcomes. Am J Hematol 94: 338–345. 10.1002/ajh.25385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez N, Martínez-Trillos A, Montraveta A, Lopez-Guerra M, Rosich L, Nadeu F, Valero JG, Aymerich M, Magnano L, Rozman M, et al. 2018. Mutations in RAS-BRAF-MAPK-ERK pathway define a specific subgroup of patients with adverse clinical features and provide new therapeutic options in chronic lymphocytic leukemia. Haematologica 104: haematol.2018.196931 10.3324/haematol.2018.196931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolimund L, Aeby E, Hamelin R, Armand F, Chiappe D, Moniatte M, Lingner J. 2013. A quantitative telomeric chromatin isolation protocol identifies different telomeric states. Nat Commun 4: 2848 10.1038/ncomms3848 [DOI] [PubMed] [Google Scholar]
- Hamanaka K, Šikrová D, Mitsuhashi S, Masuda H, Sekiguchi Y, Sugiyama A, Shibuya K, Lemmers RJLF, Goossens R, Ogawa M, et al. 2020. Homozygous nonsense variant in LRIF1 associated with facioscapulohumeral muscular dystrophy. Neurology 94: e2441–e2447. 10.1212/WNL.0000000000009617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. 2014. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46: 310–315. 10.1038/ng.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4: 1073–1081. 10.1038/nprot.2009.86 [DOI] [PubMed] [Google Scholar]
- Li HJ, Haque ZK, Chen A, Mendelsohn M. 2007. RIF-1, a novel nuclear receptor corepressor that associates with the nuclear matrix. J Cell Biochem 102: 1021–1035. 10.1002/jcb.21340 [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, et al. 2007. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 446: 758–764. 10.1038/nature05690 [DOI] [PubMed] [Google Scholar]
- Nagel S, Drexler HG. 2019. Deregulated NKL homeobox genes in B-cell lymphoma. Cancers (Basel) 11: 1874 10.3390/cancers11121874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Khac F, Chapiro E, Lesty C, Grelier A, Luquet I, Radford-Weiss I, Lefebvre C, Fert-Ferrer S, Callet-Bauchu E, Lippert E, et al. 2011. Specific chromosomal IG translocations have different prognoses in chronic lymphocytic leukemia. Am J Blood Res 1: 13–21. [PMC free article] [PubMed] [Google Scholar]
- Nozawa R-S, Nagao K, Igami K-T, Shibata S, Shirai N, Nozaki N, Sado T, Kimura H, Obuse C. 2013. Human inactive X chromosome is compacted through a PRC2-independent SMCHD1-HBiX1 pathway. Nat Struct Mol Biol 20: 566–573. 10.1038/nsmb.2532 [DOI] [PubMed] [Google Scholar]
- Pérez-Carretero C, Hernández-Sánchez M, González T, Quijada-Álamo M, Martín-Izquierdo M, Hernández-Sánchez J, Vidal M, Coca AG, Aguilar C, Vargas-Pabón M, et al. 2020. Chronic lymphocytic leukemia patients with IGH translocations are characterized by a distinct genetic landscape with prognostic implications. Int J Cancer 147: 2780–2792. 10.1002/ijc.33235 [DOI] [PubMed] [Google Scholar]
- Puente XS, Beà S, Valdés-Mas R, Villamor N, Gutiérrez-Abril J, Martín-Subero JI, Munar M, Rubio-Pérez C, Jares P, Aymerich M, et al. 2015. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 526: 519–524. 10.1038/nature14666 [DOI] [PubMed] [Google Scholar]
- Robles EF, Mena-Varas M, Barrio L, Merino-Cortes SV, Balogh P, Du M-Q, Akasaka T, Parker A, Roa S, Panizo C, et al. 2016. Homeobox NKX2-3 promotes marginal-zone lymphomagenesis by activating B-cell receptor signalling and shaping lymphocyte dynamics. Nat Commun 7: 11889 10.1038/ncomms11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E, et al. 2018. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res 47: gky1015 10.1093/nar/gky1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiel L, Baakman C, Gilissen D, Veltman JA, Vriend G, Gilissen C. 2019. MetaDome: pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum Mutat 40: 1030–1038. 10.1002/humu.23892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger R, Corcoran RB. 2019. Targeting alterations in the RAF–MEK pathway. Cancer Discov 9: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.