Abstract
Rewarming following accidental hypothermia is associated with circulatory collapse due primarily to impaired cardiac contractile (systolic) function. Previously, we found that reduced myofilament Ca2+ sensitivity underlies hypothermia/rewarming (H/R)-induced cardiac contractile dysfunction. This reduced Ca2+ sensitivity is associated with troponin I (cTnI) phosphorylation. We hypothesize that H/R induces reactive oxygen species (ROS) formation in cardiomyocytes, which leads to cTnI phosphorylation and reduced myofilament Ca2+ sensitivity. To test this hypothesis, we exposed isolated rat cardiomyocytes to a 2-h period of severe hypothermia (15°C) followed by rewarming (35°C) with and without antioxidant (TEMPOL) treatment. Simultaneous measurements of cytosolic Ca2+ ([Ca2+]cyto) and contractile (sarcomere shortening) responses indicated that H/R-induced contractile dysfunction and reduced Ca2+ sensitivity was prevented in cardiomyocytes treated with TEMPOL. In addition, TEMPOL treatment blunted H/R-induced cTnI phosphorylation. These results support our overall hypothesis and suggest that H/R disrupts excitation-contraction coupling of the myocardium through a cascade of event triggered by excessive ROS formation during hypothermia. Antioxidant treatment may improve successful rescue of accidental hypothermia victims.
Keywords: hypothermia, rewarming, rewarming shock, cardiomyocyte, reactive oxygen species, antioxidant, TEMPOL
1. Introduction
Guidelines for rescue of accidental hypothermia victims have not been standardized due to an incomplete understanding of the key physiological responses to hypothermia and rewarming (H/R) [6]. Clinical evidence indicates high mortality of accidental hypothermia victims related to “rewarming shock”, defined as circulatory failure occurring during rewarming [26].
Experimental models of H/R demonstrate that rewarming shock is associated with insufficient cardiac contractile function during systole attributed by reduced myofilament Ca2+ sensitivity rather than altered [Ca2+]cyto levels per se [16, 36].
In rat papillary muscle [16] and isolated cardiomyocytes [36], Ca2+ sensitivity of force generation is reduced following H/R, and is associated with increased phosphorylation of cardiac troponin I (cTnI). In other models of cardiac contractile failure, excessive formation of reactive oxygen species (ROS) has been suggested to play an important role in reducing Ca2+ sensitivity [32, 40, 44]. Although there is in vitro evidence of hypothermia-induced ROS formation in a variety of mammalian cells [1, 8, 33], it is unknown whether increased ROS formation plays a role in the H/R-induced reduction in Ca2+ sensitivity and contractile dysfunction in cardiomyocytes.
We hypothesize that H/R induces excessive ROS formation in cardiomyocytes, which underlies cTnI phosphorylation and reduced Ca2+ sensitivity. To test this hypothesis, we measured ROS formation in isolated ventricular cardiomyocytes during H/R. We also determined whether treatment of cardiomyocytes with an antioxidant, TEMPOL, mitigates H/R-induced changes in cardiomyocyte contraction, cTnI phosphorylation and Ca2+ sensitivity.
Materials and Methods
2.1. Animals
The use and handling of rats for this study was in accordance with the Mayo Clinic Institutional Animal Care and Use Committee (IACUC). A total of 24 male Sprague-Dawley rats (250–350 g) were used in this study. These rats were equally assigned to 4 experimental conditions: 1) control (CTL), 2) CTL + TEMPOL, 3) hypothermia/rewarming (H/R), and 4) H/R + TEMPOL. The animals were anesthetized by intramuscular injection of 90 mg/kg ketamine with 10 mg/kg xylazine, and the hearts were excised.
2.2. Cardiomyocyte isolation
The method for isolating cardiomyocytes has been described previously [36]. Briefly, the freshly-excised heart was cannulated via the aorta, connected to a modified Langendorff apparatus and perfused with warmed (37°C) enzyme solution containing collagenase type II (0.6 mg/ml, Worthington) to isolate cardiomyocytes from the myocardium. Isolated cardiomyocytes were washed by resuspending cells following centrifugation. Finally, cardiomyocytes were resuspended in a M199 culture media containing 5% fetal bovine serum (FBS) and incubated at 35°C for 30 min before evoking contractions.
With this isolation technique, most of freshly-isolated cardiomyocytes maintained a straight, rod-shaped morphology with clear sarcomere patterns throughout the cell. Only rod-shaped cells with a clear contractile response to stimulation (see below) were included in this study. Using this technique, robust cardiomyocyte contractile responses can be maintained for up to 5 h at 100% survival during continuous pacing at 0.5 Hz.
2.3. Hypothermia/Rewarming protocol and contractile and [Ca2+]cyto measurements
After cardiomyocyte isolation was complete, cells were placed in a glass coverslip based cell chamber. The cell chamber allowed for continuous perfusion of cells with oxygenated Tyrode solution (95% O2 and 5% CO2). A pair of platinum electrodes were attached to the cell chamber and stimulated at 0.5 Hz using the MyoPacer (IonOptix) to evoke contractile responses over time. Electrical pacing during continuous solution perfusion was done for 30 min before measurement to allow sufficient time for stabilization of contractile responses.
The cell chamber and perfusing solutions were surrounded by water flowing through a precision digital circulating water bath. Using a feedback circuit, the temperature of the perfusion solution was either maintained at 35°C (time-matched control) or in the H/R group changed as follows (illustrated in Figure 1): cooled from 35°C to 15°C over a 30-min period, then maintained at 15°C for 2 h, followed by rewarming to 35°C over a 30-min period (overall experiment completed in 3 h).
Fig. 1.
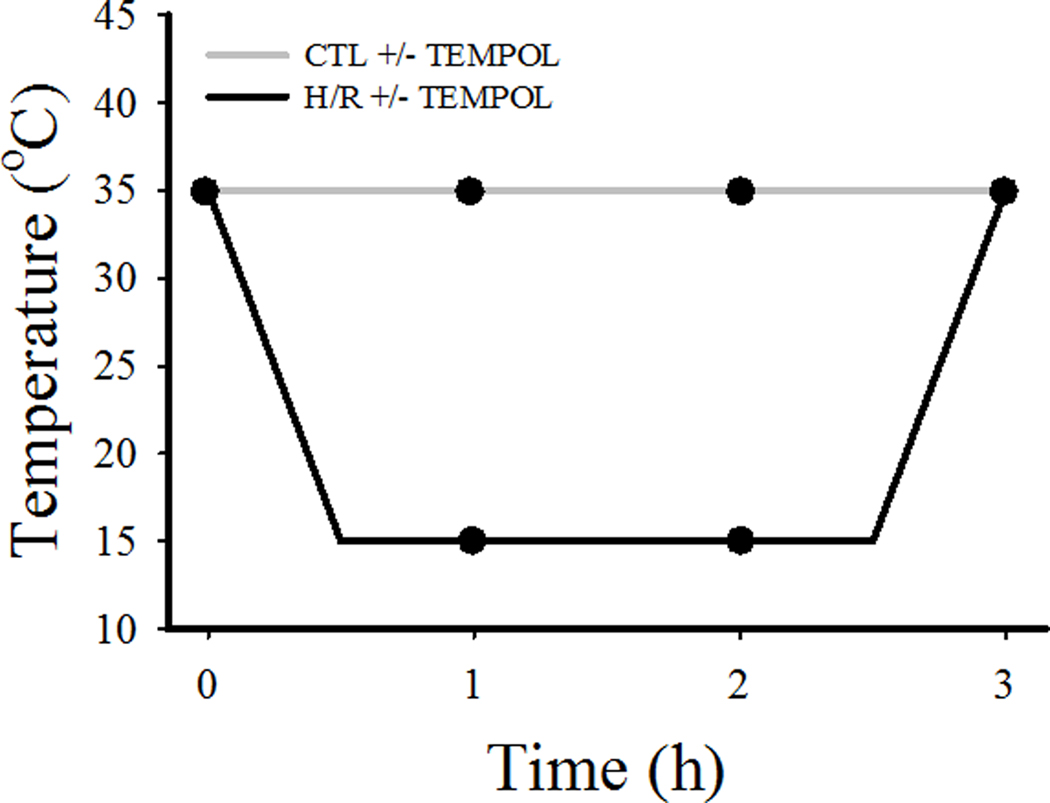
Temperature over time of H/R and CTL groups. As shown, After an initial measurement (time 0), the CTL group was maintained at a constant temperature of 35°C (±TEMPOL). On the other hand, after an initial measurement, the H/R group was cooled from 35°C to 15°C within 0.5 h, maintained at 15°C for 2 h, and finally rewarmed from 15°C to 35°C within 0.5 h (±TEMPOL). Evoked [Ca2+]cyto and contractile responses in cardiomyocytes in both CTL and H/R groups were measured at 0, 1, 2, and 3 h (solid circles).
In the TEMPOL-treated group, 200 μM of TEMPOL (4-Hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) was added to the perfusion solution at the onset of hypothermia and treatment (or time CTL) and continued throughout the remaining protocol. TEMPOL is a widely used superoxide dismutase (SOD) mimetic and a pleiotropic intracellular antioxidant. Importantly, TEMPOL is cell membrane permeable. We tested the effects of TEMPOL in time-matched control myocytes, and if anything, TEMPOL stabilized contractility over the 3-h time period of the experiment. This is likely due to the production of ROS over time, which may account for some rundown of the preparation.
In isolated cardiomyocytes, evoked [Ca2+]cyto and contractile responses were measured simultaneously using an IonOptix system, as previously described [16, 36]. Briefly, cardiomyocytes were incubated with 0.5 μM Fura-2 AM for 10 min at 35°C. Fura-2 fluorescence was excited using alternate wavelengths of 340 and 380 nm and fluorescence emission was measured at 510 nm. Contractility was assessed by measuring sarcomere length based on fast Fourier analysis of sarcomeric striation pattern (IonOptix). Cardiomyocytes were stimulated using electrical field stimulation (5 ms pulse width, 0.5 Hz), Only cardiomyocytes with robust [Ca2+]cyto (peak > 500 nM) and contractile responses (>5% shortening from resting sarcomere length) were included for further analysis. Approximately 75% of isolated cardiomyocytes met these inclusion criteria.
2.4. Measurements of intracellular superoxide anions
Two complementary techniques were used to determine ROS generation in cardiomyocytes following H/R exposure. HPLC-based measurement of oxy-ethidium was performed as described in [10, 15] with minor modification for use in isolated cardiomyocytes. Briefly, the accumulation of oxy-ethidium is used to indicate the conversion of dihydroethidium (DHE) by superoxide anions. Using fluorescence detection, oxy-ethidium is quantified as the area under the curve at a unique retention time distinct from other fluorescent products such as ethidium [51]. Cells were incubated in phosphate based saline (PBS) solution containing 10 μM of dihydroethidium (Molecular Probes) at 37° C for 15 min. The cells were washed to remove free dihydroethidium in Krebs-HEPES buffer for 1 h at 37°C. After the wash, the cells were centrifuged at 12,000 g for 10 min at 4°C. The supernatant was eluted and analyzed to quantify oxy-ethidium from the reaction of DHE and superoxide anions using high-performance liquid chromatography (HPLC)/fluorescence assay. [10]. The pellets in 2% SDS were saved for protein assay (Bio-Rad DC protein assay). Intracellular superoxide anion levels were determined by normalizing the quantity of oxy-ethidium for protein concentration.
Changes in fluorescence of MitoSOX Red (Invitrogen) was used as a second technique for ROS detection in individual cardiomyocytes. Using this technique, cardiomyocytes were incubated with 5 μM MitoSOX for 10 min at 35°C, and subsequently imaged using a Nikon A1R confocal system equipped with a Plan-Apo 60x/1.4 numerical aperture oil objective. MitoSox Red was excited at 488 nm and resulting emission was measured at 590 nm. MitoSox Red is targeted to the mitochondria and fluoresces after reaction with superoxide anions.
2.5. Western blot
Cardiomyocytes samples were lysed and protein extracted in radio-immunoprecipitation assay (RIPA) buffer supplemented with 2% sodium dodecyl sulfate (SDS) and phenylmethane sulfonyl fluoride (PMSF). Protein lysates were collected by centrifuging at 10,000 g for 15 min at 4°C. To prepare the sample for Western blot, total protein content was measured using a Lowry assay (Bio-Rad DC protein assay) in order to achieve equal loading of protein sample during SDS-PAGE. Samples were denatured by boiling at 100°C for 3 min. Using SDS-PAGE, protein samples were fractionated over a gel and then electrically transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The protein-containing PVDF membranes were blocked using 5% dry milk and then incubated with antibodies for the protein of interest. After incubating with horseradish peroxidase (HRP) conjugated secondary antibodies, PVDF membranes were treated with an enhanced chemiluminescence HRP substrate according the manufacturer’s instructions (Thermo Scientific SuperSignal West Dura Extended Duration Substrate). Finally, the PVDF membranes were imaged and analyzed using a Kodak Imaging System. Actin was used as a loading control and the extent of TnI phosphorylation was presented as the ratio of p-TnI to TnI.
2.6. Chemicals and solutions
Solutions used for these experiments have been defined in our previous study [36]. The enzyme used for cell isolation was Collagenase Type II (Worthington). Antibodies used for Western blot include: actin (Cytoskeleton, Inc.), cTnI (Fitzgerald), and p-TnI at Ser23/24 (Cell Signaling).
2.7. Statistical methods
In the experimental design, each isolated cardiomyocyte served as its own control, and changes were assessed relative to the initial condition before H/R or addition of TEMPOL. A total of 6 rats were assigned to each of the 4 experimental groups and 3–5 cardiomyocytes were studied per heart. The final number of cardiomyocytes studied for each experimental group varied from 22 to 30. Based on a power analysis to detect a 20% change in primary outcome measured (power = 80%, α = 0.05) a total number of at least 15 cardiomyocytes was required, so the experimental design exceeded this number for each of the major outcome measures. The results shown in the figures are the overall mean and standard deviation of the individual cardiomyocyte responses. The data was statistically analyzed using a two-way repeated measure ANOVA. If a primary effect of treatment or time was observed, a post-hoc Bonferroni Student t-test was used for further analysis. Statistical significance at P<0.05 is indicated using an asterisk in the figures.
3. Results
3.1. Reactive oxygen species formation increases in cardiomyocytes exposed to H/R
In cardiomyocytes loaded with DHE, H/R induced an increase in intracellular ROS formation as reflected by an increase in oxy-ethidium (Fig. 2). A disadvantage of this technique is that it only allows end-point measurements. In this respect, it should be noted that the time controls displayed an increase in oxy-ethidium over the 3-h period, indicating ROS formation. However, the H/R-induced change in oxy-ethidium in cardiomyocytes was 36% greater than the time controls (P<0.05).
Fig. 2.
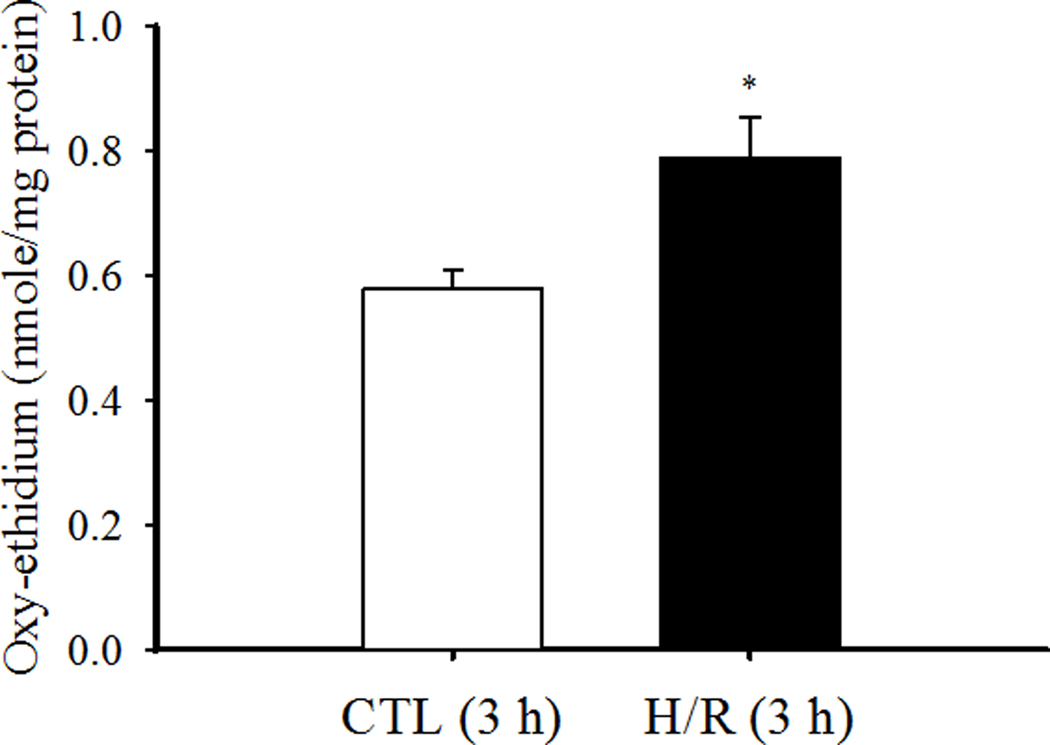
Superoxide anion production in a population of cardiomyocytes was measured using an HPLC/fluorescence assay to detect oxy-ethidium. Data are presented as means ± standard deviations (n=6 animals/hearts). * P<0.05 vs CTL.
In cardiomyocytes loaded with the mitochondrially-targeted fluorescent indicator MitoSOX Red, H/R also induced an increase in intracellular ROS formation, as indicated by an increase in MitoSOX fluorescence (Fig. 3). Using this technique, it was apparent that intracellular ROS formation began to increase during hypothermia, plateauing before rewarming. MitoSOX fluorescence also increased in the time controls, indicating ROS formation. However, the H/R-induced increase in MitoSOX fluorescence was ~60% greater at 2 h and ~30% at 3 h compared to time-matched CTLs (P<0.05; Fig. 3).
Fig. 3.
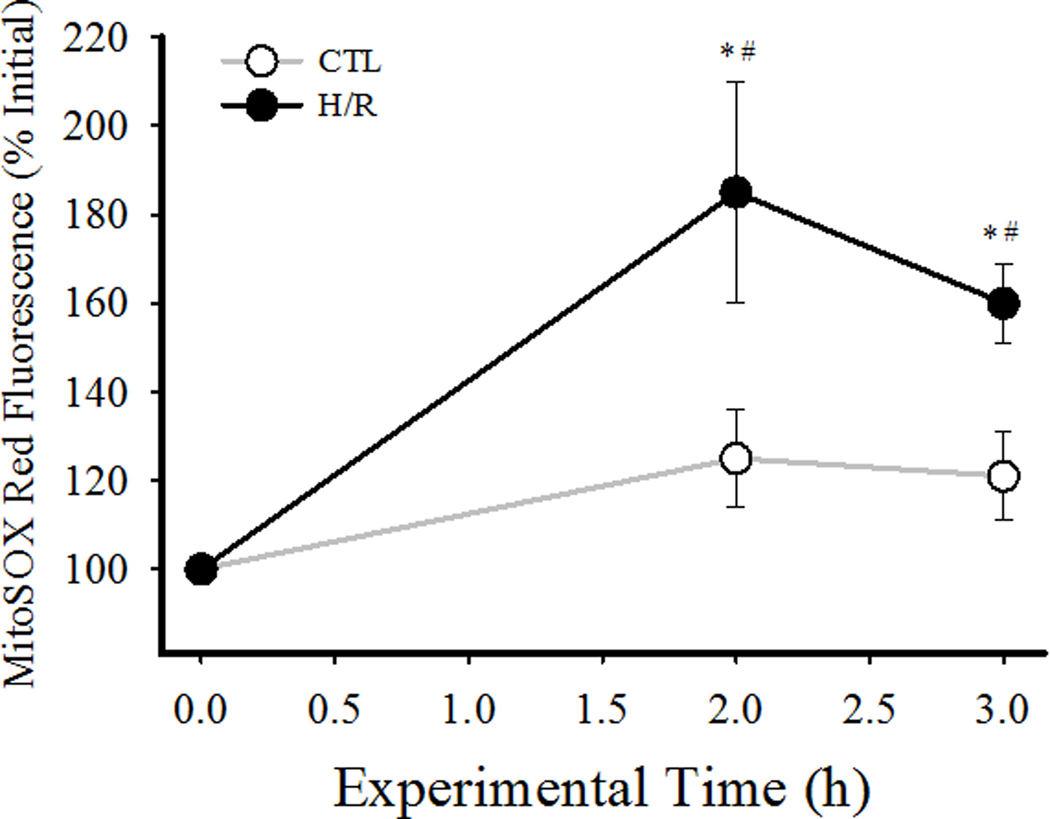
Superoxide anion production in individual cardiomyocytes was measured using MitoSOX Red fluorescence and confocal microscopy. After measuring initial MitoSox Red fluorescence measurements were obtained at 2 h, and 3 h under normothermic (35°C, time matched controls) and H/R conditions. Data are presented as means ± standard deviations (n=30 cells per condition). * P<0.05 vs initial measure; # P<0.05 vs CTL.
3.2. Contractile dysfunction following H/R is mitigated by TEMPOL
Evoked contractile responses were compared in four groups of cardiomyocytes (CTL with/without TEMPOL and H/R with/without TEMPOL) over time (Fig 4). In CTL cells with/without TEMPOL (Fig. 4A, C), there were no time-dependent differences in resting sarcomere length or the extent of sarcomere shortening for the entire 3-h protocol. In contrast, during hypothermia in the H/R group, there was a significant time-dependent decrease in resting sarcomere length that was not mitigated by TEMPOL (Fig. 4A, C.). In the H/R group, there was a significant time-dependent increase in the extent of sarcomere shortening (P<0.05; Fig. 4A, C), which was only slightly mitigated by TEMPOL treatment. Following rewarming in the H/R group, the extent of sarcomere shortening was reduced compared to initial values (P<0.05; Fig. 4A, C). With TEMPOL treatment in the H/R group, the extent of sarcomere shortening returned to CTL values after rewarming (Fig. 4A, C).
Fig. 4.
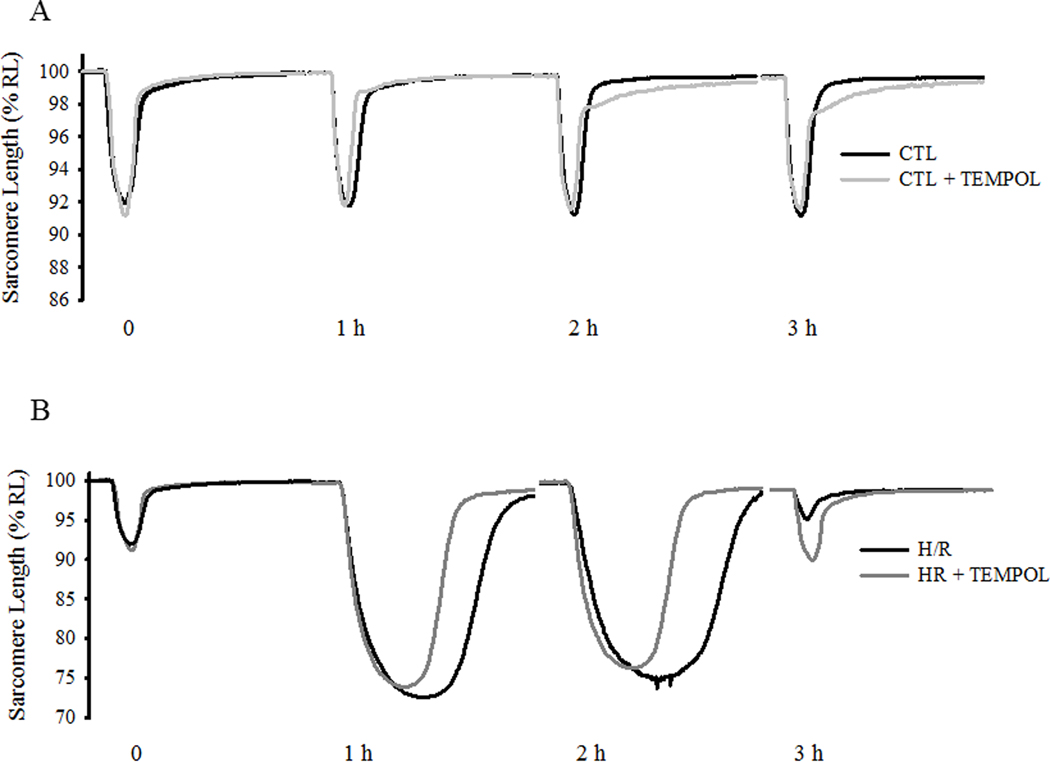
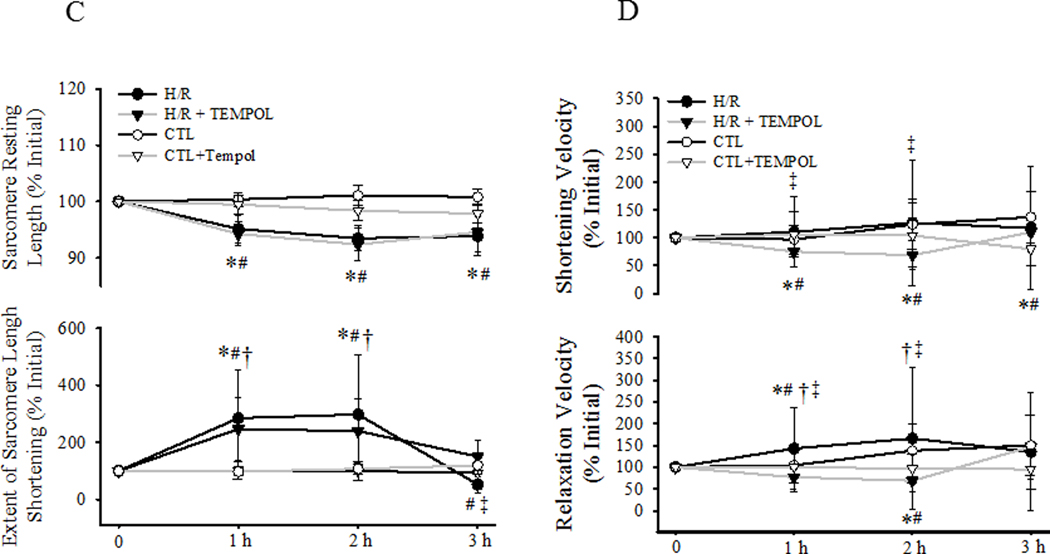
Contractile responses to electric field stimulation (0.5 Hz) of individual cardiomyocytes were measured under CTL (normothermic) and H/R conditions with and without concurrent TEMPOL treatment. Representative contractile responses of cardiomyocytes from CTL (±TEMPOL; A) and H/R (±TEMPOL; B) groups are shown and compared over time. Changes in initial resting sarcomere length (top) and the extent of sarcomere shortening (bottom) are shown in C. Changes in sarcomere shortening velocity (top) and relaxation velocity (bottom) are shown in D. Data are presented as means ± standard deviations. The number of cells used for each condition varied: CTL, n=22; CTL+TEMPOL, n=25; H/R, n=21; and H/R+TEMPOL, n=25. * P<0.05 vs initial value. # P<0.05 vs CTL, † P<0.05 vs CTL + TEMPOL, ‡ P<0.05 vs H/R + TEMPOL.
In the CTL group, the velocity of sarcomere shortening increased with time, which was mitigated by TEMPOL treatment (P<0.05; Fig. 4B, D). In the H/R group, the velocity of sarcomere shortening also increased with time, similar to CTLs (P<0.05; Fig. 4B, D). This effect on sarcomere shortening velocity was reversed by TEMPOL treatment throughout the 3-h protocol in the H/R group (Fig. 4B, D). In both CTL and H/R groups, the velocity of relaxation increased progressively with time (P<0.05; Fig. 4B, D). This effect was mitigated by TEMPOL treatment in both CTL and H/R groups.
3.3. [Ca2+]cyto changes during hypothermia blunted by TEMPOL
Evoked [Ca2+]cyto responses were compared in four groups of cardiomyocytes (CTL with/without TEMPOL and H/R with/without TEMPOL) over time (Fig. 5). In CTL cardiomyocytes, there was a slight time-dependent increase in basal [Ca2+]cyto levels that was mitigated by TEMPOL treatment (P<0.05; Fig. 5A, C). In the H/R group, the time-dependent increase in basal [Ca2+]cyto levels was much more pronounced(P<0.05; Fig. 5B, C), and this effect was mitigated by TEMPOL treatment (P<0.05; Fig. 5B, C).
Fig. 5.
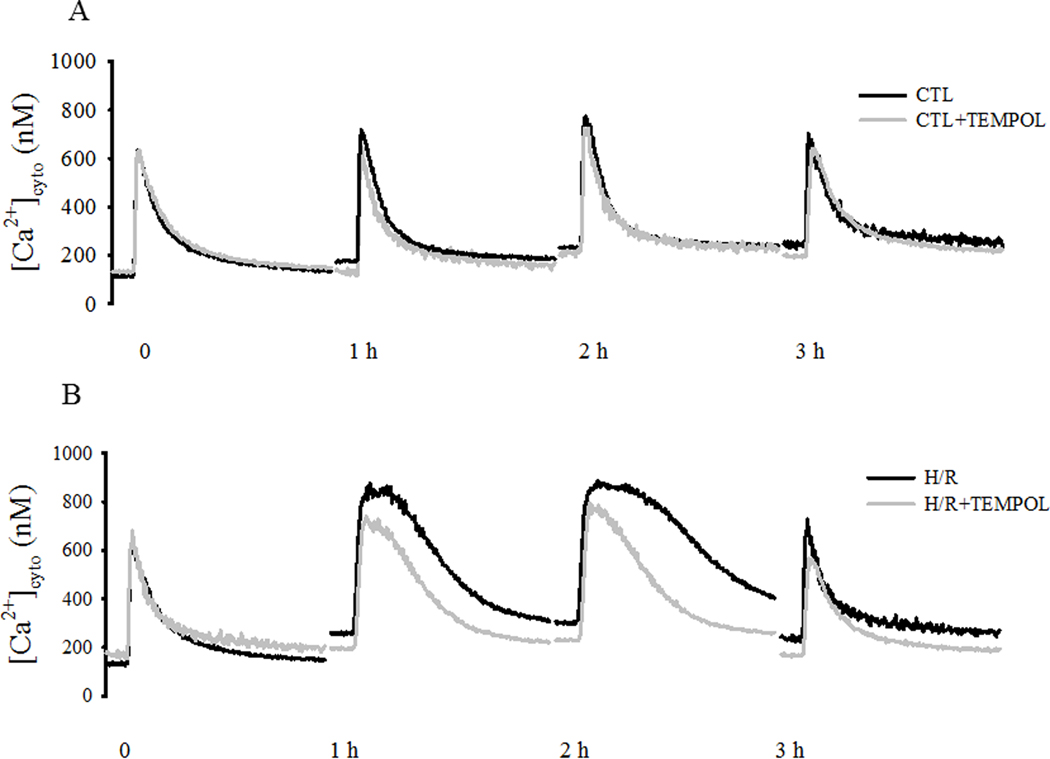
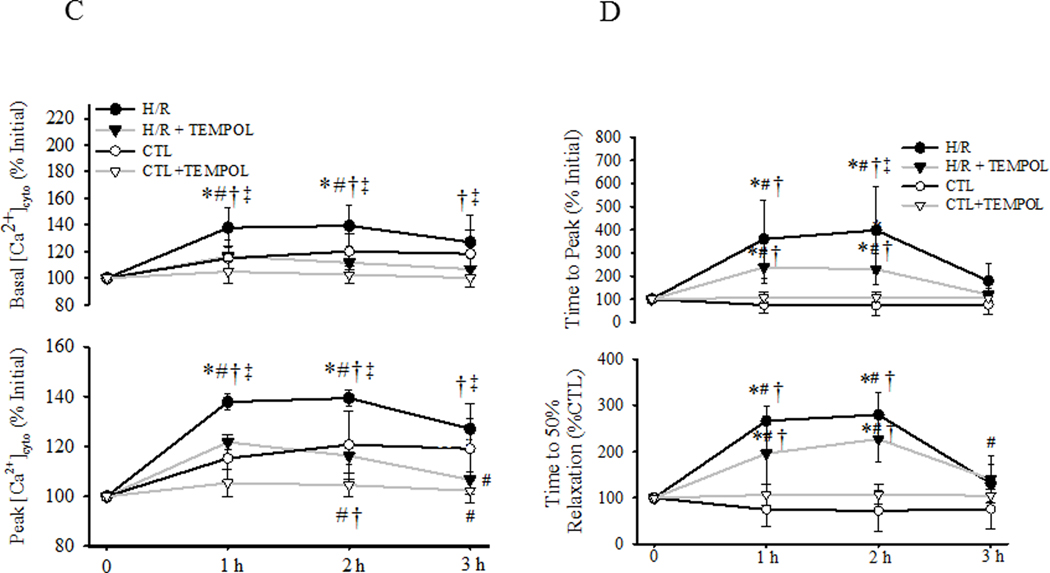
[Ca2+]cyto responses to electric field stimulation (0.5 Hz) of individual cardiomyocytes were measured under CTL (normothermic) and H/R conditions with and without concurrent TEMPOL treatment. . Representative [Ca2+]cyto responses of cardiomyocytes from CTL ±TEMPOL (A) and H/R ±TEMPOL (B) groups are shown and compared over time. After determining initial basal [Ca2+]cyto levels and evoked [Ca2+]cyto responses for each cell, changes in basal [Ca2+]cyto and peak evoked [Ca2+]cyto responses were measured over time (C). Similarly, after measuring initial transient properties of the evoked [Ca2+]cyto responses to stimulation, changes in the time to peak (top) and time to 50% relaxation (bottom) of the [Ca2+]cyto responses were measured over time (D). Data are presented as means ± standard deviations. The number of cells used for each condition varied: CTL, n=22; CTL+TEMPOL, n=25; H/R, n=21; and H/R+TEMPOL, n=25. * P<0.05 vs initial value. # P<0.05 vs CTL, † P<0.05 vs CTL + TEMPOL, ‡ P<0.05 vs H/R + TEMPOL.
In CTL, there was a time-dependent increase in the peak evoked [Ca2+]cyto responses; an effect mitigated by TEMPOL treatment (Fig. 5A, C). . In contrast, in the H/R group, there was a much more pronounced increase in the peak evoked [Ca2+]cyto response (p<0.05; Fig. 5B, C), which was partially mitigated by TEMPOL treatment.
In CTL cardiomyocytes with or without TEMPOL, there were no time-dependent changes in the duration of the [Ca2+]cyto transient, either the time to peak and 50% relaxation time (Fig. 5A, D). In contrast, in the H/R group, there was a marked increase in the duration of the [Ca2+]cyto transient with an increase in both time to peak and 50% relaxation time (P<0.05; Fig. 5B, D). This effect was partially mitigated by TEMPOL treatment in the H/R group.
3.4. H/R-induced increased myofilament Ca2+ sensitivity prevented by TEMPOL
Changes in myofilament Ca2+ sensitivity were evaluated by comparing initial phase-loop plots after 3 h in the 4 experimental groups (CTL with or without TEMPOL and H/R, with or without TEMPOL; Fig 6). Compared to initial responses, there were no significant shifts in the phase-loop plots of CTL and CTL+TEMPOL groups after 3 h (Fig. 6A, B and C). In the H/R group, there was a pronounced rightward shift in the phase-loop plots indicating a decrease in myofilament Ca2+ sensitivity, which was mitigated by TEMPOL treatmet (P<0.05; Fig. 6A, B, and C). In the H/R group, the rightward shift of the phase-loop was reflected by an increase in both the [Ca2+]cyto required for 50% shortening and the [Ca2+]cyto level at which 50% relaxation occurred (P<0.05; Fig. 6B and C).
Fig. 6.
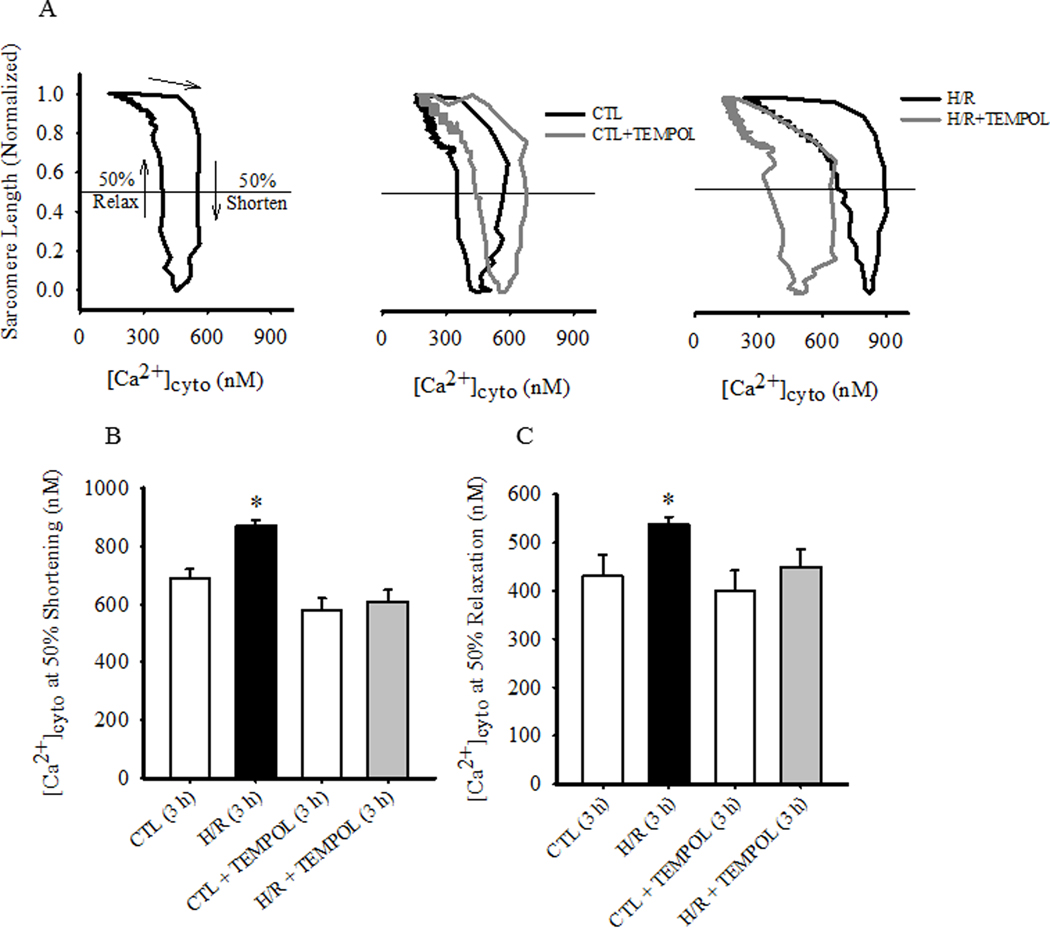
To assess the sensitivity of the contractile responses of cardiomyocyte to [Ca2+]cyto, simultaneous measurements of sarcomere shortening and [Ca2+]cyto were compared in a phase loop plot. (A). Compared to CTL, the phase-loop plots in time-matched CTL cardiomyocytes treated with TEMPOL were shifted rightward. Similarly, compared to CTL, the phase-loop plots in cardiomyocytes exposed to H/R were shifted rightward, but this shift was partially mitigated by TEMPOL treatment. These changes in phase-loop plots reflecting myofilament Ca2+ sensitivity were quantified using two metrics: B) the [Ca2+]cyto at which 50% sarcomere shortening was observed, and C) the [Ca2+]cyto at which 50% sarcomere relaxation was observed. Data are presented as means ± standard deviations. . The number of cells used for each condition varied: CTL, n=22; CTL+TEMPOL, n=25; H/R, n=21; and H/R+TEMPOL, n=25. * P < 0.05 vs control.
3.5. H/R-induced TnI phosphorylation prevented by TEMPOL
Phosphorylation of TnI in cardiomyocytes was measured by comparing the relative changes of a phospho-specific marker to Ser 23/24 residues (p-TnI) to total TnI protein. In CTL groups, there were no time-dependent changes in the ratio of p-TnI/TnI in cardiomyocytes. In contrast, in the H/R group, the ratio of p-TnI/TnI in cardiomyocytes increased progressively during hypothermia (P<0.05; Fig. 7), which was prevented by TEMPOL treatment (Fig. 7).
Fig. 7.
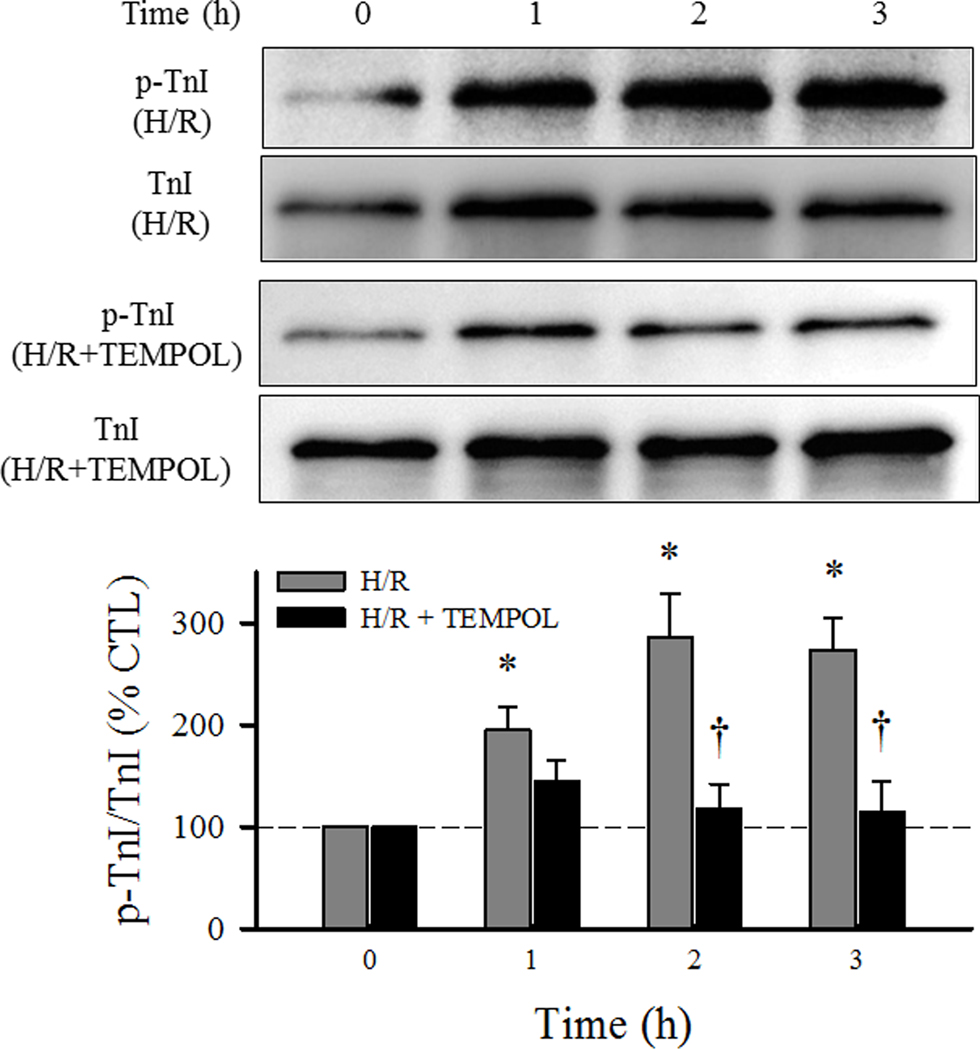
Changes in TnI phosphorylation were measured over time using Western blot in cardiomyocytes exposed to H/R with and without TEMPOL. Data are presented as means ± standard deviations (n = 6 animals/hearts). * P<0.05 vs CTL, † P<0.05 vs H/R + TEMPOL.
4. Discussion
In support of our hypothesis, the results of the present study show that ROS formation increases in cardiomyocytes during hypothermia and may contribute to contractile dysfunction and reduced myofilament Ca2+ sensitivity. In support, we found that scavenging excess ROS formation using the antioxidant TEMPOL largely mitigates the effects of H/R. TEMPOL treatment prevented contractile dysfunction following H/R and prevented hypothermia-induced cTnI phosphorylation, a key molecular determinant of myofilament Ca2+ sensitivity and a key player in H/R-induced contractile dysfunction. In addition, TEMPOL treatment did not have significant consequences on either [Ca2+]cyto, contractile function, or myofilament Ca2+ sensitivity during normothermia controls (CTL and CTL + TEMPOL). These results support antioxidant treatment as a viable therapeutic strategy for rescue of accidental hypothermia victims.
4.1. The role of increased ROS formation during H/R
The term “ROS” refers to a variety of chemical species with unique properties including superoxide anions, hydrogen peroxide, hydroxyl, etc. Furthermore, ROS can damage biological systems, yet ROS can also play a wide variety of signaling roles that are adaptive or protective [7, 18, 37]. Likewise, antioxidant treatment has not always had beneficial clinical outcomes [41]. Due to the ambiguous nature of ROS in terms of both structure and function, this study was designed to specifically focused on superoxide formation from the mitochondria after H/R exposure based on supporting evidence discussed below. Furthermore, we hypothesized that increased superoxide formation contributes to the signaling cascade culminating in contractile failure following H/R. Our results using the superoxide dismutase mimetic, TEMPOL, support this hypothesis by preventing H/R-induced contractile function.
The mitochondrial electron transport chain (ETC) is a major site of superoxide anion formation. Cardiomyocytes have a particularly high capacity for excessive ROS generation given a relatively high mitochondrial density and therefore an abundance of site for ROS formation [30]. Accordingly, mitochondrial ROS plays a key role in the pathophysiology of many cardiac-specific conditions such as ischemia-reperfusion injury and heart failure [29, 45].
Superoxide formation increases when the mitochondrial ETC is stimulated, e.g., with an increase in [Ca2+]cyto [3, 5, 17], an increase in ATP hydrolysis (increased [ADP/ATP]cyto) [35], and/or an increase in mitochondrial uncoupling [5, 19]. All three factors may occur during H/R. First of all, our group has previously reported that hypothermia increases basal [Ca2+]cyto levels in cardiomyocytes and markedly prolongs the duration of evoked [Ca2+]cyto responses [36]. This hypothermia-induced [Ca2+]cyto overload may underlie the increase in ROS generation. Secondly, hypothermia also has a positive inotropic effect on cardiomyocyte contraction [38], reflected by an increase in contractile force of rat papillary muscle [16] and work performed by the left ventricle [28]. This suggests an increase in ATP hydrolysis [39] and a corresponding increase in the [ADP/ATP]cyto that would stimulate the ETC and oxidative phosphorylation, leading to an increase in ROS formation. As a third point, cellular thermogenesis is achieved in part by activation of mitochondrial uncoupling proteins (UCPs) resulting in thermodynamic inefficiency and heat production but also an associated increase in ROS formation [9, 13].
4.2. The limitations of superoxide anion measurement
In this study, two methods to detect superoxide anion were used: 1) an HPLC-based superoxide assay and 2) MitoSOX live-cell imaging. Although HPLC-based methods show superior superoxide-specific detection [20, 21, 48, 52], the HPLC measurement requires lysis of cells and therefore limited to a single measurement in time per sample. Both methods were included to provide converging evidence that ROS formation increases during H/R. We found that H/R was associated with an increase in ROS, specifically superoxide, formation in cardiomyocytes. These results are consistent with previous reports showing an association between hypothermia exposure and increased ROS formation in various mammalian cells types [1, 8, 33].
4.3. Hypothermia affects basal [Ca2+]cyto and evoked [Ca2+]cyto responses
The hypothermia-induced increase in basal [Ca2+]cyto in cardiomyocytes has been previously reported by our group[36] and others [4, 24, 25, 42, 49] and has been referred to as a [Ca2+]cyto overload [47]. Treatment with TEMPOL mitigated this hypothermia-induced increase in basal [Ca2+]cyto in cardiomyocytes suggesting that ROS formation plays a role. Several important Ca2+ handling proteins are functionally sensitive to increased ROS levels. For example, cardiac ryanodine receptors (RyR2), the major Ca2+ release channels in the sarcoplasmic reticulum, contain multiple reactive thiol groups, which make RyR2 particularly vulnerable to increased ROS formation [27]. In response to ROS, RyR2 open probability increases leading to increased spontaneous Ca2+ release from the SR [2, 22]. In addition, sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), the major Ca2+ reuptake channel, is inhibited by increased ROS formation, slowing Ca2+ reuptake into the SR [53]. Thus, the combined response of hypothermia-induced activation of RyR2 and inhibition of SERCA would contribute to an increase in basal [Ca2+]cyto.
Consistent with our previous report [36], we found that hypothermia induces a prolongation of evoked [Ca2+]cyto responses in cardiomyocytes that return to normal after rewarming. Treatment with TEMPOL did not affect the hypothermia-induced slowing of the kinetics of the evoked [Ca2+]cyto responses in cardiomyocytes. The slowing of the evoked [Ca2+]cyto responses could reflect the primary effects of temperature on RyR2 open probability or SERCA activity, independent of ROS modification.
4.4. ROS-sensitive targets underlie contractile dysfunction following H/R
The contractile dysfunction of cardiomyocytes induced by H/R and prevented by TEMPOL does not involve altered [Ca2+]cyto at rewarming, but does involve Ca2+-sensitivity of the myofilaments or sarcomere [36]. As we previously reported, H/R induces PKA activation and cTnI phosphorylation at Ser 23/24 [36]. Similarly, β-adrenergic stimulation also induces PKA activation and cTnI phosphorylation at Ser 23/24 [43, 50]. In the present study, we found that TEMPOL did prevent cTnI phosphorylation, supporting the central role that increased ROS formation and cTnI phosphorylation plays in contractile dysfunction induced by H/R.
Other than cTnI phosphorylation resulting from ROS-sensitive kinases, other sarcomere-targeted modifications include direct oxidation of reactive cysteine residues. We have shown previously that oxidative stress can lead to reduced myofilament Ca2+ sensitivity via oxidative modification of reactive thiols on the myosin head [32].
4.5. Antioxidants as a potential therapy for accidental hypothermia victim rescue
Unfortunately, treatment options for accidental victims are limited. Previous research has focused on either catecholamine or Ca2+ sensitizer treatment to stimulate contractile function during rewarming. However, epinephrine treatment, which typically promotes a positive inotropic response in cardiomyocytes at normothermia, is ineffective in hypothermia-exposed cardiomyocytes. [12, 23, 46] On the other hand, the use of the Ca2+ sensitizer, levosimendan, appears to improve cardiac function after hypothermia exposure in an acute animal model [11, 34]. Levosimendan does not directly target cTnI, but instead targets Ca2+-TnC interactions as well as other targets including phosphodiesterase 3 (PDE3) inhibition [14, 31]. Levosimendan is currently in use in other countries, but it is not available for use in the U.S. The current results support antioxidant treatment as an effective alternative to either catecholamine or Ca2+ sensitizer treatment during H/R, and potentially novel therapeutic strategy to rescue accidental hypothermia victims.
In conclusion, although H/R impairs cardiomyocyte contractile function during rewarming, treatment with antioxidant TEMPOL during hypothermia preserves contractile function during rewarming. The protective effect of TEMPOL is associated with prevention of decreased myofilament Ca2+ sensitivity as well as prevention of cTnI phosphorylation.
Acknowledgments
The authors would like to thank Dr. Zvonimir Katusic and Dr. Livius d’Uscio for generously allowing use of lab equipment and expertise regarding HPLC-based oxy-ethidium measurement.
Statement of Funding
This work was supported by the Mayo Foundation (GCS), an NIH training grant (5T32HL105355) (NSS) and the Norwegian Air Ambulance Foundation (YSH).
Abbreviations:
- [Ca2+]cyto
cytosolic Ca2+ concentration
- CTL
time-matched controls
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- DHE
dihydroethidium
- ETC
mitochondrial electron transport chain
- H/R
hypothermia/rewarming
- H/R + TEMPOL
hypothermia/rewarming with TEMPOL treatment
- HPLC
high-performance liquid chromatography
- PDE3
phosphodiesterase 3
- ROS
reactive oxidant species
- RyR
ryanodine receptors
- SERCA
sarco/endoplasmic reticulum Ca2+ ATPase
- UCP
mitochondrial uncoupling protein
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Author Agreement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from sieck.gary@mayo.edu.
References
- [1].Ali SS, Marcondes MC, Bajova H, Dugan LL, Conti B, Metabolic depression and increased reactive oxygen species production by isolated mitochondria at moderately lower temperatures, J Biol Chem, 285 (2010) 32522–32528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anzai K, Ogawa K, Kuniyasu A, Ozawa T, Yamamoto H, Nakayama H, Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers, Biochem Biophys Res Commun, 249 (1998) 938–942. [DOI] [PubMed] [Google Scholar]
- [3].Balaban RS, Cardiac energy metabolism homeostasis: role of cytosolic calcium, J Mol Cell Cardiol, 34 (2002) 1259–1271. [DOI] [PubMed] [Google Scholar]
- [4].Bers DM, SR Ca loading in cardiac muscle preparations based on rapid-cooling contractures, Am J Physiol, 256 (1989) C109–120. [DOI] [PubMed] [Google Scholar]
- [5].Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS, Calcium ATP, and ROS: a mitochondrial love-hate triangle, Am J Physiol Cell Physiol, 287 (2004) C817–833. [DOI] [PubMed] [Google Scholar]
- [6].Brown DJ, Brugger H, Boyd J, Paal P, Accidental hypothermia N Engl J Med, 367 (2012) 1930–1938. [DOI] [PubMed] [Google Scholar]
- [7].Burgoyne JR, Mongue-Din H, Eaton P, Shah AM, Redox signaling in cardiac physiology and pathology, Circ Res, 111 (2012) 1091–1106. [DOI] [PubMed] [Google Scholar]
- [8].Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF, Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart, American journal of physiology. Heart and circulatory physiology, 286 (2004) H1289–1299. [DOI] [PubMed] [Google Scholar]
- [9].Cannon B, Nedergaard J, Brown adipose tissue: function and physiological significance, Physiol Rev, 84 (2004) 277–359. [DOI] [PubMed] [Google Scholar]
- [10].d’Uscio LV, Smith LA, Katusic ZS, Erythropoietin increases expression and function of vascular copper- and zinc-containing superoxide dismutase, Hypertension, 55 (2010) 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dietrichs ES, Haheim B, Kondratiev T, Sieck GC, Tveita T, Cardiovascular effects of levosimendan during rewarming from hypothermia in rat, Cryobiology, 69 (2014) 402–410. [DOI] [PubMed] [Google Scholar]
- [12].Dietrichs ES, Schanche T, Kondratiev T, Gaustad SE, Sager G, Tveita T, Negative inotropic effects of epinephrine in the presence of increased beta-adrenoceptor sensitivity during hypothermia in a rat model, Cryobiology, 70 (2015) 9–16. [DOI] [PubMed] [Google Scholar]
- [13].Divakaruni AS, Brand MD, The regulation and physiology of mitochondrial proton leak, Physiology (Bethesda), 26 (2011) 192–205. [DOI] [PubMed] [Google Scholar]
- [14].Figgitt DP, Gillies PS, Goa KL, Levosimendan, Drugs, 61 (2001) 613–627; discussion 628–619. [DOI] [PubMed] [Google Scholar]
- [15].Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S, Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay, Am J Physiol Cell Physiol, 287 (2004) C895–902. [DOI] [PubMed] [Google Scholar]
- [16].Han YS, Tveita T, Prakash YS, Sieck GC, Mechanisms underlying hypothermia-induced cardiac contractile dysfunction, American journal of physiology. Heart and circulatory physiology, 298 (2010) H890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harrison SM, Bers DM, Influence of temperature on the calcium sensitivity of the myofilaments of skinned ventricular muscle from the rabbit, The Journal of general physiology, 93 (1989) 411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Holmstrom KM, Finkel T, Cellular mechanisms and physiological consequences of redox-dependent signalling, Nat Rev Mol Cell Biol, 15 (2014) 411–421. [DOI] [PubMed] [Google Scholar]
- [19].Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD, Mitochondrial proton and electron leaks, Essays Biochem, 47 (2010) 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, H. Ischiropoulos, Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations, Free Radic Biol Med, 52 (2012) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kalyanaraman B, Hardy M, Podsiadly R, Cheng G, Zielonka J, Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling, Arch Biochem Biophys, 617 (2017) 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kawakami M, Okabe E, Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel, Mol Pharmacol, 53 (1998) 497–503. [DOI] [PubMed] [Google Scholar]
- [23].Kondratiev TV, Myhre ES, Simonsen O, Nymark TB, Tveita T, Cardiovascular effects of epinephrine during rewarming from hypothermia in an intact animal model, J Appl Physiol (1985), 100 (2006) 457–464. [DOI] [PubMed] [Google Scholar]
- [24].Kondratiev TV, Wold RM, Aasum E, Tveita T, Myocardial mechanical dysfunction and calcium overload following rewarming from experimental hypothermia in vivo, Cryobiology, 56 (2008) 15–21. [DOI] [PubMed] [Google Scholar]
- [25].Liu B, Wang LC, Belke DD, Effect of low temperature on the cytosolic free Ca2+ in rat ventricular myocytes, Cell calcium, 12 (1991) 11–18. [DOI] [PubMed] [Google Scholar]
- [26].Maclean D, Emslie-Smith D, Accidental hypothermia, Blackwell Scientific, Oxford, 1977. [Google Scholar]
- [27].Meissner G, Regulation of Ryanodine Receptor Ion Channels Through Posttranslational Modifications, Curr Top Membr, 66 (2010) 91–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Monroe RG, Strang RH, Lafarge CG, Levy J, Ventricular Performance, Pressure-Volume Relationships, and O2 Consumption during Hypothermia, Am J Physiol, 206 (1964) 67–73. [DOI] [PubMed] [Google Scholar]
- [29].Murphy E, Steenbergen C, Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury, Physiol Rev, 88 (2008) 581–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nickel A, Kohlhaas M, Maack C, Mitochondrial reactive oxygen species production and elimination, J Mol Cell Cardiol, 73 (2014) 26–33. [DOI] [PubMed] [Google Scholar]
- [31].Papp Z, Edes I, Fruhwald S, De Hert SG, Salmenpera M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikstrom BG, Jorgensen K, Filippatos G, Parissis JT, Gonzalez MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F, Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan, Int J Cardiol, 159 (2012) 82–87. [DOI] [PubMed] [Google Scholar]
- [32].Perkins WJ, Han YS, Sieck GC, Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor, J Appl Physiol (1985), 83 (1997) 1326–1332. [DOI] [PubMed] [Google Scholar]
- [33].Rauen U, de Groot H, Mammalian cell injury induced by hypothermia- the emerging role for reactive oxygen species, Biol Chem, 383 (2002) 477–488. [DOI] [PubMed] [Google Scholar]
- [34].Rungatscher A, Hallstrom S, Giacomazzi A, Linardi D, Milani E, Tessari M, Luciani GB, Scarabelli TM, Mazzucco A, Faggian G, Role of calcium desensitization in the treatment of myocardial dysfunction after deep hypothermic circulatory arrest, Crit Care, 17 (2013) R245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T, Cardiac system bioenergetics: metabolic basis of the Frank-Starling law, J Physiol, 571 (2006) 253–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schaible N, Han YS, Hoang T, Arteaga GM, Tveita T, Sieck GC, Hypothermia/Rewarming Disrupts Excitation-Contraction Coupling in Cardiomyocytes, Am J Physiol Heart Circ Physiol, DOI 10.1152/ajpheart.00840.2015(2016) ajpheart 00840 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schieber M, Chandel NS, ROS function in redox signaling and oxidative stress, Curr Biol, 24 (2014) R453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shattock MJ, Bers DM, Inotropic response to hypothermia and the temperature-dependence of ryanodine action in isolated rabbit and rat ventricular muscle: implications for excitation-contraction coupling, Circ Res, 61 (1987) 761–771. [DOI] [PubMed] [Google Scholar]
- [39].Sieck GC, Regnier M, Invited Review: plasticity and energetic demands of contraction in skeletal and cardiac muscle, J Appl Physiol (1985), 90 (2001) 1158–1164. [DOI] [PubMed] [Google Scholar]
- [40].Steinberg SF, Oxidative stress and sarcomeric proteins, Circ Res, 112 (2013) 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Steinhubl SR, Why have antioxidants failed in clinical trials?, Am J Cardiol, 101 (2008) 14D–19D. [DOI] [PubMed] [Google Scholar]
- [42].Stowe DF, Fujita S, An J, Paulsen RA, Varadarajan SG, Smart SC, Modulation of myocardial function and [Ca2+] sensitivity by moderate hypothermia in guinea pig isolated hearts, Am J Physiol, 277 (1999) H2321–2332. [DOI] [PubMed] [Google Scholar]
- [43].Strang KT, Sweitzer NK, Greaser ML, Moss RL, Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats, Circ Res, 74 (1994) 542–549. [DOI] [PubMed] [Google Scholar]
- [44].Sumandea MP, Steinberg SF, Redox signaling and cardiac sarcomeres, J Biol Chem, 286 (2011) 9921–9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Takano H, Zou Y, Hasegawa H, Akazawa H, Nagai T, Komuro I, Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases, Antioxid Redox Signal, 5 (2003) 789–794. [DOI] [PubMed] [Google Scholar]
- [46].Tveita T, Sieck GC, The physiologic responses to epinephrine during cooling and after rewarming in vivo, Crit Care, 15 (2011) R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vassalle M, Lin CI, Calcium overload and cardiac function, J Biomed Sci, 11 (2004) 542–565. [DOI] [PubMed] [Google Scholar]
- [48].Winterbourn CC, The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells, Biochim Biophys Acta, 1840 (2014) 730–738. [DOI] [PubMed] [Google Scholar]
- [49].Wold RM, Kondratiev T, Tveita T, Myocardial calcium overload during graded hypothermia and after rewarming in an in vivo rat model, Acta physiologica, 207 (2013) 460–469. [DOI] [PubMed] [Google Scholar]
- [50].Zhang R, Zhao J, Mandveno A, Potter JD, Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation, Circ Res, 76 (1995) 1028–1035. [DOI] [PubMed] [Google Scholar]
- [51].Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B, Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide, Free Radic Biol Med, 34 (2003) 1359–1368. [DOI] [PubMed] [Google Scholar]
- [52].Zielonka J, Kalyanaraman B, Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth, Free Radic Biol Med, 48 (2010) 983–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zima AV, Blatter LA, Redox regulation of cardiac calcium channels and transporters, Cardiovasc Res, 71 (2006) 310–321. [DOI] [PubMed] [Google Scholar]


