Abstract
Uterine sarcomas are a rare group of malignancies that account for less than 10% of all uterine malignancies. They are histologically diverse and fall into two broad groups: mesenchymal and epithelial tumors. The treatment in both these groups is marked by high failure rates and quick progression of disease. Patients with stage I to II with resectable disease benefit from operative cytoreduction. Those with advanced stages, benefit from chemotherapy with or without external beam radiation therapy. Our research in this paper looks at the number of LMS cases at our institution, Wyckoff Heights Medical Center in Brooklyn, NY for a period of 20 years from 1996 until 2015 and assesses our cohort’s age at diagnosis and their survival in accordance to grade and stage of diagnosis. Our findings suggest that disease stage is a strong prognostic factor with good survival rates in stage I and II, with higher incidence in African-American women. All LMS patients with distant metastasis died within five years.
Keywords: Uterine sarcoma, Uterine inversion, Stromal sarcoma, Epithelioid leiomyosarcoma, Fibrosarcoma, Myxoid leiomyosarcoma, Undifferentiated uterine sarcoma, Uterine tumor
INTRODUCTION
Uterine sarcomas are a heterologous group of rare malignancies accounting for 8%–10% of all uterine malignancies but are significantly more aggressive and have worse prognoses. [1] Uterine sarcomas fall into two broad categories histolgoically: Mesenchymal tumors (including mixed mesenchymal) and epithelial tumors. Mesenchymal tumors include leiomyosarcomas (LMS), endometrial stromal sarcomas, and smooth muscle tumors of uncertain potential (STUMP), as well as mixed endometrial stromal and smooth muscle tumors. [2] The two most common subtypes of these gynecological sarcomas are leiomyosarcomas (LMS) and endometrial stromal sarcoma (ESS). LMS accounts for approximately 2% of all uterine malignancies. [1] This type of neoplasm has an annual incidence of 0.64 per 100,000 women and it spreads hematogeneously early in the presentation, which leads to high local and distant failure rates [2–6]. Compared to endometrial carcinomas of the uterus, leiomyosarcomas are more aggressive and have poorer prognosis.
Mesenchymal tumors, in which a significant epithelial component is also noted, include carcinosarcomas, adenosarcomas, carcinofibroma, adenofibroma and adenomyomas. According to the International Federation of Gynecology and Obstetrics (FIGO) 2009 staging, carcinosarcomas are now considered metaplastic epithelial carcinomas and are treated similarly to high grade epithelial carcinomas, rather than based on their sarcomatous elements [6]. These pathologies therefore have not been included in the data analysis of this paper.
The patient’s age, tumor size, mitotic count and stage are important factors that impact the clinical prognosis [7]. Treatment options for patients with these gynecologic sarcomas have been limited in the past, and currently, there are few agents that provide reasonable response rates and none that provide a cure. Unfortunately, the staging system for LMS of the uterus does not provide an adequate prediction for clinical relapse or even death, therefore there is wide disagreement on whether to treat with radiotherapy alone, or combine with adjuvant chemotherapy [6]. Many studies suggest chemotherapy does not improve survival [8]. While others suggest that certain chemotherapeutics may benefit disease spread and extra-pelvic recurrence, leading to possible disease stabilization [9]. LMS extra-pelvic recurrence is most commonly in the lungs, abdomen and liver with relapse in 45%–80% of cases [10,11]. The treatment of choice for high grade stage I-II LMS is surgical resection that includes hysterectomy and bilateral salpingooophorectomy.
Our study aims at looking at the number of LMS cases at our institution, Wyckoff Heights Medical Center (WHMC) in Brooklyn, NY for a period of 20 years from 1996 until 2015. Medical records before 1996 are difficult to obtain due to lack of digitalization rendering us unable to perform databank searches for the neoplasm. Our primary objective is to identify the disease-specific survival of patients with the LMS from the time of diagnosis and compare the clinical outcome between patients who received different treatments. Furthermore, we want to assess the age of our cohorts at diagnosis and their subsequent survival in accordance to their grade and stage of diagnosis. In addition, since the hospital serves a very diverse neighborhood, the authors wanted to assess the variance of disease diagnosis and progression in different ethnicities as identified by the patients themselves.
MATERIAL AND METHODS
Data Collection
The WHMC Institutional Review Board approved the research that was conducted and allowed for patient digital and physical paper files to be reviewed by the investigators. The board did not require informed consent by the patients, provided it was solely for the purposes of medical analysis. Given that this paper was written for the purposes of a retrospective medical analysis of the cases in the hospital, the authors conducted a review of all the LMS cases and all the histopathological variants from 1996 to 2015. This was conducted by the medical records department of the institution on behalf of the investigators to preserve unnecessary exposure of patient data. A medical record search was conducted using International Classification of Disease, Ninth Revision (ICD-9) codes of LMS and other malignant uterine sarcomas. A list of patients was generated that had the required specifications and given to the principal investigator. Due to FIGO 2009 guideline change regarding carcinosarcomas, we had to drop 50% the size of our cohort from our study.
The patient files were researched according to the dates of diagnosis. The time of diagnosis was the date of diagnosis by a pathologist which includes WHMC staff pathologists or pathologist that diagnosed the patient at a different institution but came to our hospital for treatment. For those patients, whose date of diagnosis fell in 2004 and after we used the hospital medical records system Meditech to research pathology reports and other relevant information. In cases that this yielded no results, we conducted research on the hospital outpatient database E-Clinicals. For the patients, whose date of diagnosis was before 1996, we requested retrieval of paper medical records and manual investigation of the files was performed. The department of radiation oncology has separate record system. Their records were consulted regarding the use of radiotherapy for those patients on our list that we could not confirm if they had had radiotherapy along with their surgery allowing us to fill in the gaps of information. It is important to note that several patient names that matched the diagnosis of LMS or its subtypes had incomplete medical records and many were missing important pathology reports rendering their use in statistical analysis impossible. Nevertheless, even though they cannot be used, it shows that there were more patients diagnosed with LMS in the community than we can report. The list of patient names was known only to the principal investigators, who subsequently encrypted the names of the patients using numbers.
Grading
The National Cancer Institute (NCI) grading was retrieved in each pathology report. To make the diagnosis of LMS, the biopsy specimen needs to have cytological atypia, coagulative necrosis and mitotic activity. Grade 1 tumors show diffuse, mild cytological atypia. Grade 2 has more nuclear irregularity with greater variation is sizes and shapes. Grade 3 and 4 has moderate or majority nuclear atypia. Of these, Grade 1 is considered low grade LMS and Grades 2, 3, 4 are considered high grade LMS [7].
RESULTS
Demographics
From 1996 to 2005, a period of 20 years, the hospital had a total of 17 patients (Table 1) who were diagnosed with LMS or the following subtypes: endometrial stromal sarcoma, spindle cell sarcoma or sarcoma NOS (not otherwise specified). Ten out of seventeen patients or 58.8% of the diagnoses were LMS. Five patients or 29.4% were endometrial stromal sarcoma and spindle cell sarcoma and sarcoma NOS had 1 patient each or 5.9%. There were a total of 12 patients that we identified as carcinosarcoma and 2 other ones identified as Mullerian adenosarcoma which were originally included in the study due to previous guidelines. Leiomyosarcomas and carcinosarcomas are treated in the same manner; with total abdominal hysterectomy and potential radiation therapy thus we wanted to include them in the study since they would add to statistical significance and also contribute to a comparison in clinical outcomes of the disease. Nevertheless, due to the FIGO 2009 staging guidelines, the data had to be excluded.
Table 1:
Patient characteristics.
| Characteristic (n = 17) | n | % |
|---|---|---|
| Mean Age of Patients | 52.1 | |
| Median Age | 50 | |
| Age Range | 41–92 | |
| SD | 10.99 | |
| Race | ||
| White | 8 | 47.1 |
| Black | 4 | 23.5 |
| Hispanic | 3 | 17.6 |
| Indian | 1 | 5.9 |
| Asian | 1 | 5.9 |
| Pathology | ||
| Leiomyosarcoma | 10 | 58.8 |
| Endometrial Stromal Sarcoma | 5 | 29.4 |
| Spindle Cell Sarcoma | 1 | 5.9 |
| Sarcoma NOS | 1 | 5.9 |
| Treatment | ||
| Surgery | 11 | 64.7 |
| Surgery + Radiotherapy | 3 | 17.6 |
| Surgery + Chemotherapy | 2 | 11.8 |
| No Treatment | 1 | 5.9 |
| NCI Tumor Grade | ||
| No Grading Assigned | 2 | 11.8 |
| Grade 1 | 1 | 5.9 |
| Grade 2 | 3 | 17.6 |
| Grade 3 | 11 | 64.7 |
| TNM Tumor Staging | ||
| Stage 1 | 4 | 23.5 |
| Stage 4 | 8 | 47.1 |
| No Staging Assigned | 5 | 29.4 |
The cohort of 17 patients identified had an average age of 52 years old with median age of 50 years. The age ranges from 41 years to 92 years with 94.1% of the patients being diagnosed with the disease in the 4th and 5th decade of life (Figure 1). We did not have any patients with LMS or any subtypes in the 6th, 7th or 8th decade of life. The only outlier is a single 92-years old patient. There were several patients however who fell in the category of carcinosarcoma who were in the age range of 60 years – 89 years.
Figure 1:
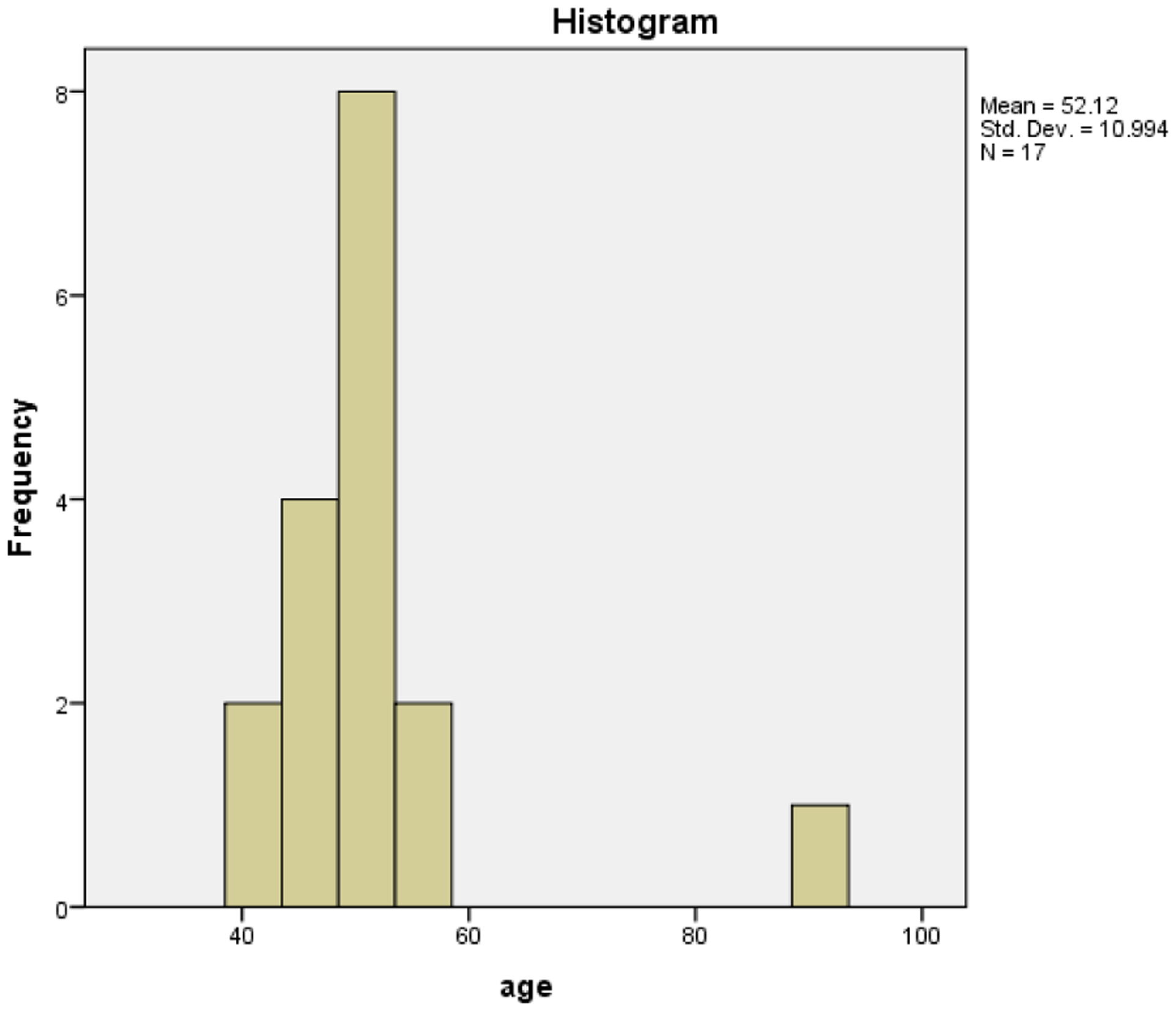
With the exception of one patient, the whole cohort got diagnosed with LMS or a subtype of LMS in the 4th and 5th decade of life, highlighting the occurrence age of the malignancy.
The race of the patient as identified by the patient themselves were the following: Eight patients (47.1%) White, four (23.5%) black, three (17.6%) Hispanic, one (5.9%) Indian and one (5.9%) Asian. Almost of half of the patients identified as white. It is important to note that the neighborhood which the hospital serves, per the government 2010 population census has a population of 8% White, 70% Hispanic and 17% Black [12].
Grading
Pathologists categorize leiomyosarcomas as high grade or low grade. Grade 1 tumors correspond to low-grade leiomyosarcomas. Grade 2, 3 and 4 tumors are equivalent to high-grade leiomyosarcomas. [7] Nevertheless grading maybe difficult to obtain in certain circumstances thus two of our patients had no grading assigned. From our cohort of 17 therefore only 15 were graded and of these fifteen, one patient had NCI grade of 1. Three patients or 17.6% were Grade 2 and the remaining eleven patients or 64.7% were Grade 3. Therefore, most our patients had high-grade LMS at presentation (Figure 2).
Figure 2:
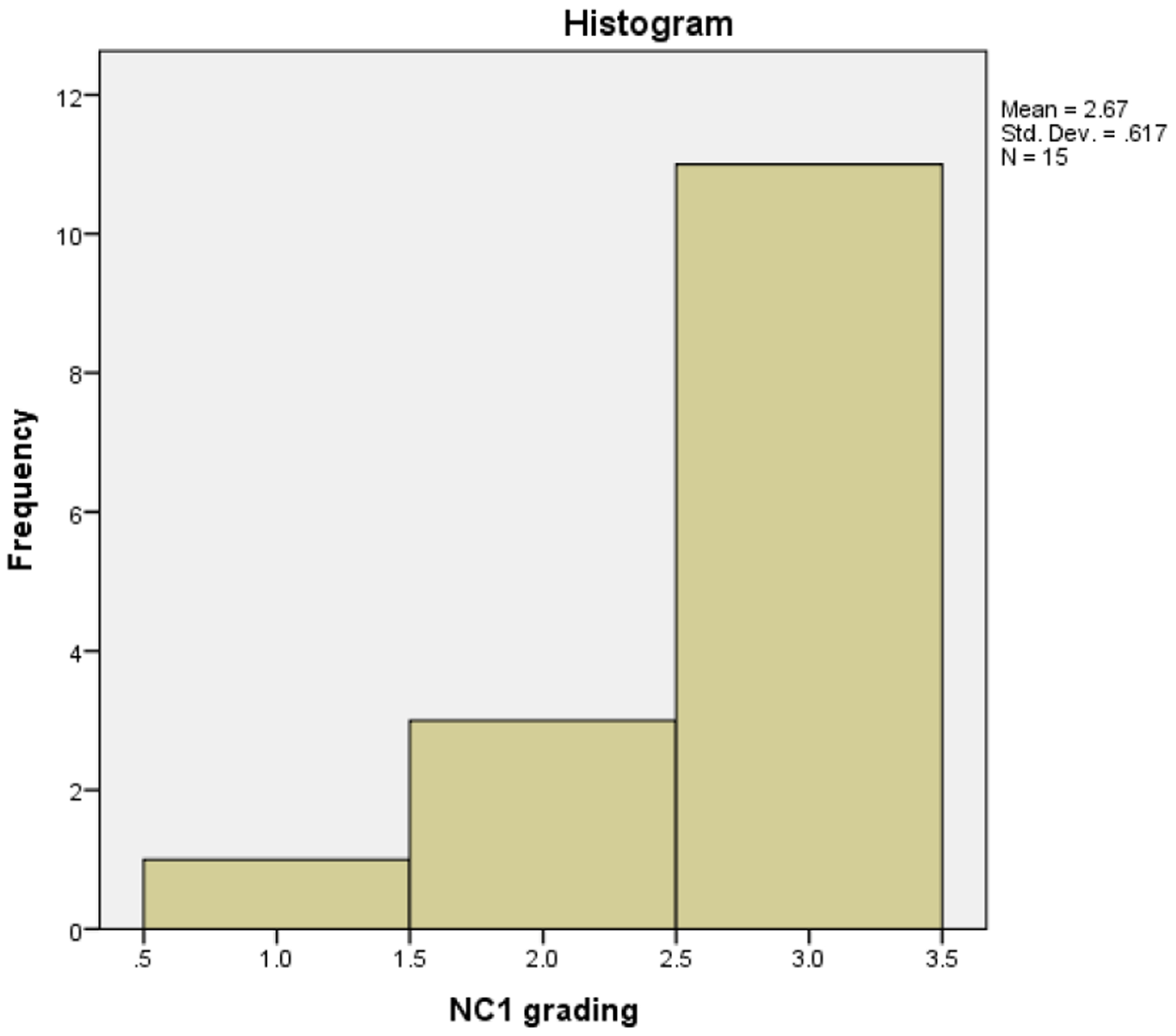
Two thirds of the cohort presented with NCI Grade 3. The high grade of the malignancy at presentation correlates with the poor prognosis of the overall disease and difficulty of treatment.
Staging
TNM staging of the tumors were present in most the pathology reports of our cohort. Of those that did not have staging, we used pathology and radiation oncology records to stage the disease with areas that were not evaluated considered negative. Four of the patients or 23.5% were Stage 1. Eight patients or 47.1% were Stage 4. Due to incomplete file preservation, unfortunately almost ⅓ of our patients did not have staging assigned to them.
TREATMENT
The recommended treatment for LMS is Total Abdominal Hysterectomy and Bilateral Salpingo-Oophorectomy (TBH-BSO) with subsequent radiotherapy depending on clinical condition of the patient. From our cohort of 17 patients, sixteen received treatment except one patient. This patient was admitted and diagnosed in the very late stages of disease progression and was sent to hospice with no treatment as per her wishes. Eleven patients (64.7%) received the treatment TBH-BSO alone with no radiotherapy or chemotherapy. Three other patients (17.6%) received radiotherapy in addition to surgery and only two patients (11.8%) received the surgery with subsequent use of chemotherapy.
SURVIVAL
Kaplan-meir curves (Figure 3) were generated to compare the overall survival of patients of the patients who were diagnosed with different histological subtypes. The curves demonstrated that in our cohort the subtype of endometrial stromal sarcoma (EMS) has the longest survival of the other subtypes. The second longest was LMS. The difference of almost 100 days or more than 3 months existed between LMS and EMS. Spindle cell sarcoma and Sarcoma NOS had a very short survival curve with less than a month.
Figure 3:
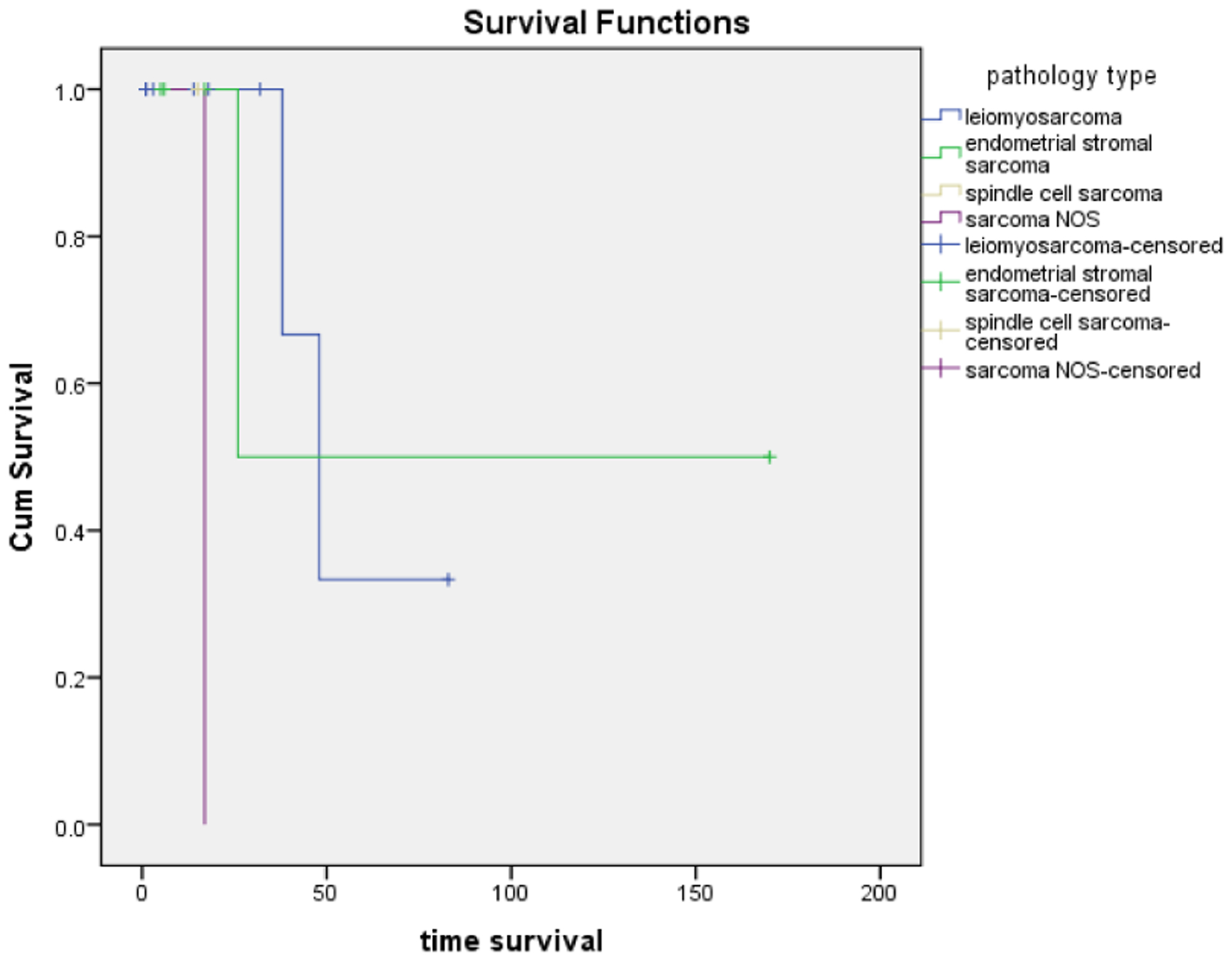
Kaplan-meir curves: Overall survival of patients according to different subtypes. Endometrial stromal sarcoma has the highest overall survival. Sarcoma NOS has the lowest survival.
DISCUSSION
Sarcomas of the uterus are an uncommon heterogeneous group of malignancies that comprise approximately 8% of all uterine malignancies in adults [13]. In 2017, an estimated 4910 cases of uterine sarcomas were anticipated [14]. Uterine sarcomas are malignant mesenchymal tumors that include ESS, UUS, and uLMS. According to a 2012 systematic review of data from 1970 to 2011, uLMS was the most common subtype (63%), followed by (ESS) endometrial stromal sarcoma (21%) and less common subtypes such as UUS undifferentiated endometrial/uterine sarcoma [15]. The series examining similar groups are small; however, surgery seems to be the treatment of choice for those with first time recurrent disease in selected patients. Specifically, those with resectable disease from initial low stage disease and low-grade tumors might benefit the most with operative cytoreduction in this setting [1,7,16,17]. Patients with advanced uterine leiomyosarcoma are treated with systemic therapy and/or external beam radiation therapy [18]. Furthermore, uterine LMS is a separate biologic entity with a different prognosis from the other uterine sarcomas and it must be uniquely separated from other primary uterine sarcomas.
Some of the prognostic factors that have been identified in the literature include lack of residual tumor following primary surgery. Five-year crude survival was 51% for patients with stage I LMS, 25% for those with stage II LMS and 32% for all patients combined. All LMS patients with distant metastasis died within five years [19–23]. Tumor size was the second most important independent prognostic factor for survival. When the tumor diameter was less than 5 cm, the overall survival was 86%, compared to 18% when the tumor diameter was larger than 10 cm. Race has also been identified as an independent prognostic factor for DSS disease specific survival. An analysis of the SEER data from 1989 to 1999 confirmed the higher incidence of LMS in African Americans (1.51 per 100,000 African Americans vs 0.91 per 100,000 Whites vs 0.89 per 100,000 women of other races; P <.01). [24] These values were not corrected for the impact of racial differences in hysterectomy rates, which may reduce the differences in the incidence between whites and African Americans.
The results of this small series are in accordance with other studies in which disease stage was found to be a strong prognostic factor. Relatively good survival rates were noted only for patients with stage I or stage II disease. Five-years survival rates ranging from approximately 66% to 74% have been attained by other investigators in the U.S. [12]. Most patients in our institution presented with metastatic disease, eight patients in our cohort had metastatic disease and had a similar OS 50%–60%. Metastasis rate (initial or at recurrence) was 50% in our series. Previous analyses from other institutions reported rates that range from 29.4% to 44.7%. [25,26]. The relationship between stage at diagnosis and population is complex and additional studies are needed in uterine sarcoma to determine the racial, demographic and socioeconomic disparities, and its association with overall survival.
One weaknesses of the current study include a small series, the lack of information regarding the extent of residual disease after surgery, details concerning tumor size, and mitotic count. However, given the results of the current study and our review of the literature on the treatment of LMS, we find similar results in terms of overall survival among patients with LMS with metastatic disease.
CONCLUSION
The treatment of leiomyosarcoma is challenging and thus early recognition and diagnosis are critical to improve patient outcomes. Patients should be referred to sarcoma centers, ideally before planned surgery so that multimodal measures may be considered as well as entry into appropriate clinical trials. The treatment of patients with uterine leiomyosarcoma will continue to improve now in the era of biomarker analysis, targeted therapy and immunotherapy. Ongoing efforts to increase our understanding of the biologic underpinnings of the disease are critical to continued progress in improving the lives of patients with this disease.
REFERENCES
- 1.Gadducci A, Landoni F, Sartori E, et al. (1996) Uterine leiomyosarcoma: Analysis of treatment failures and survival. Gynecologic Oncology 62(1): 25–32. [DOI] [PubMed] [Google Scholar]
- 2.Harlow BL, Weiss NS, Lofton S (1986) The epidemiology of sarcomas of the uterus. Journal of National Cancer Institute 76(3): 399–402. [PubMed] [Google Scholar]
- 3.Echt G, Jepson J, Steel J, et al. (1990) Treatment of uterine sarcomas. Cancer 66(1): 35–39. [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Piver MS, Tsukada Y, et al. (1989) Patterns of metastasis in uterine sarcoma. An autopsy study. Cancer 63: 935–938. [DOI] [PubMed] [Google Scholar]
- 5.Ueda SM, Kapp DS, Cheung MK, et al. (2008) Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. American Journal of Obstetrics & Gynecology 198(2): 218.e1–218.e6. [DOI] [PubMed] [Google Scholar]
- 6.Zivanovic O, Leitao MM, Iasonos A, et al. (2009) Stage-specific outcomes of patients with uterine leiomyosarcoma: A comparison of the International federation of gynecology and obstetrics and American joint committee on cancer staging systems. Journal of Clinical Oncology 27(12): 2066–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuntoli RL 2nd, Metzinger DS, DiMarco CS, et al. (2003) Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecologic Oncology 89(3): 460–469. [DOI] [PubMed] [Google Scholar]
- 8.Mancari R, Signorelli M, Gadducci A, et al. (2014) Adjuvant chemotherapy in stage I-II uterine leiomyosarcoma: A multicentric retrospective study of 140 patients. Gynecologic Oncology 133(3): 531–536. [DOI] [PubMed] [Google Scholar]
- 9.Ricci S, Giuntoli R, Eisenhauer E, et al. (2013) Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecologic Oncology 131(3): 629–633. [DOI] [PubMed] [Google Scholar]
- 10.Dinh TA, Oliva EA, Fuller AF Jr, et al. (2004) The treatment of uterine leiomyosarcoma: Results from a 10-year experience (1990–1999) at the Massachusetts General Hospital. Gynecologic Oncology 92(2): 648–652. [DOI] [PubMed] [Google Scholar]
- 11.Mayerhofer K, Obermair A, Windbichler G, et al. (1999) Leiomyosarcoma of the uterus: Aclinicopathologic multicenter study of 71 cases. Gynecologic Oncology 74(2): 196–201. [DOI] [PubMed] [Google Scholar]
- 12. https://www1.nyc.gov/assets/planning/download/pdf/data-maps/nyc-population/census2010/t_pl_p3a_nta.pdf .
- 13.Ueda SM, Cheung MK, Osann K, et al. (2007) Factors responsible for the increase in death rate of women diagnosed with uterine corpus cancer. Proceedings of the Society of Gynecologic Oncologists 38th Annual Meeting: 644. [Google Scholar]
- 14.Cancer facts and figures (2017) Atlanta, GA: American Cancer Society. [Google Scholar]
- 15.Trope CG, Abeler VM, Kristensen GB (2012) Diagnosis and treatment of sarcoma of the uterus. A review. Acta Oncologica 51(6): 694–705. [DOI] [PubMed] [Google Scholar]
- 16.Kapp DS, Shin JY, Chan JK (2008) Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: Emphasis on impact of lymphadenectomy and oophorectomy. Cancer 112(4): 820–830. [DOI] [PubMed] [Google Scholar]
- 17.Loizzi V, Cormio G, Nestola D, et al. (2011) Prognostic factors and outcomes in 28 cases of uterine leiomyosarcoma. Oncology 81(2): 91–97. [DOI] [PubMed] [Google Scholar]
- 18.Amant F, Coosemans A, Deblec-Rychter M, et al. (2009) Clinical management of uterine sarcomas. Lancet Oncology 10(12): 1188–1198. [DOI] [PubMed] [Google Scholar]
- 19.Abeler VM, Røyne O, Thoresen S, et al. (2009) Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 54(3): 355–364. [DOI] [PubMed] [Google Scholar]
- 20.Nam JH (2011) Surgical treatment of uterine sarcoma. Best Practice & Research Clinical Obstetrics & Gynaecology 25(6): 751–760. [DOI] [PubMed] [Google Scholar]
- 21.Koivisto-Korander R, Butzow R, Koivisto AM, et al. (2008) Clinical outcome and prognostic factors in 100 cases of uterine sarcoma: Experience in Helsinki university central hospital 1990–2001. Gynecologic Oncology 111(1): 74–81. [DOI] [PubMed] [Google Scholar]
- 22.Denschlag D, Masoud I, Stanimir G, et al. (2007) Prognostic factors and outcome in women with uterine sarcoma. European Journal of Surgical Oncology 33(1): 91–95. [DOI] [PubMed] [Google Scholar]
- 23.Larson B, Silfverswärd C, Nilsson B, et al. (1990) Prognostic factors in uterine leiomyosarcoma. A clinical and histopathological study of 143 cases. The Radiumhemmet series 1936–1981. Acta Oncologica 29(2): 185–191. [DOI] [PubMed] [Google Scholar]
- 24.Brooks SE, Zhan M, Cote T, et al. (2004) Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecologic Oncology 93(1): 204–208. [DOI] [PubMed] [Google Scholar]
- 25.Gladdy RA, Qin LX, Moraco N, et al. (2013) Predictors of survival and recurrence in primary leiomyosarcoma. Annals of Surgical Oncology 20(6): 1851–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svarvar C, Bohling T, Berlin O, et al. (2007) Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the Scandinavian sarcoma group. Cancer 109(2): 282–291. [DOI] [PubMed] [Google Scholar]


