Abstract
Age is a key risk factor associated with the severity of symptoms caused by SARS-CoV-2, and there is an urgent need to reduce COVID-19 morbidity and mortality in elderly individuals. We discuss evidence suggesting that trained immunity elicited by BCG vaccination may improve immune responses and can serve as a strategy to combat COVID-19 in this population.
Age is a key risk factor associated with the severity of symptoms caused by SARS-CoV-2, and there is an urgent need to reduce COVID-19 morbidity and mortality in elderly individuals. We discuss evidence suggesting that trained immunity elicited by BCG vaccination may improve immune responses and can serve as a strategy to combat COVID-19 in this population.
Main text
COVID-19 is a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease spreads very quickly and became a pandemic short after it emerged China in December 2019. Depending on the patients’ health conditions and age, COVID-19 clinical symptoms can range from an asymptomatic or mild disease to a very severe form. Pre-existing underlying health conditions such as diabetes, atherosclerosis, hypertension, cancer, immunocompromised states, chronic kidney disease, and overweight/obesity substantially increase COVID-19 mortality and morbidity.1 In addition, COVID-19 severity drastically increases with advancing age.2 , 3 In patients with severe disease, COVID-19 induces a complex dysregulation of both innate and adaptive immune responses, that sometimes result in a combination of inappropriate systemic inflammation (leading to pulmonary edema and respiratory insufficiency), as well as defects in specific components of the immune responses (e.g., interferon [IFN] pathway defects, lymphopenia), contributing to disease severity.1 , 4 Older people have a higher chance of developing a systemic inflammation when encountering COVID-19.4
Aging as a risk factor of COVID-19 progression
Aging is one of the main risk factors for increased susceptibility to many infectious diseases. Systematic analysis of more than 140 databases on 19 viral and 13 bacterial diseases demonstrated that severity increases with age and clinical symptoms markedly worsen in older ages.5 , 6 Although the COVID-19 pandemic affects people across all ages, children and young adults 10–19 years old show very mild or no symptoms. In contrast, the elderly are the most vulnerable group to the disease and are often hospitalized and require intensive care unit support.3 , 6 Subsequently, the mortality rate among this age group is significantly higher than among younger or middle-aged groups. Individuals under 20 years of age are almost half as susceptible to COVID-19 as those over 20. Clinical symptoms increased dramatically to 57%–82% in adults aged over 65–70 years.6
Increased susceptibility to disease is a complex and multifactorial process. Physiologic alterations affect many organs such as the skin, epithelium, and mucosal tissue. In addition, poor disease outcome is closely associated with the immune system weakening during aging2 (Figure 1 ). Age-associated changes in both lymphocytes functions and proportions have been linked to chronic diseases. Lymphoid tissues deteriorate with rising age and fail to support lymphocyte hemostasis, which result in less T and B cells and finally less robust cellular and humoral immune responses. Evidence also shows that aging causes hematopoiesis shift from lymphoid toward myeloid progenitors. This may contribute to lower lymphoid cell numbers and a higher number of myeloid-derived cells such as monocytes/macrophages.2 , 5 With aging, the frequencies of naive CD4+ or CD8+ T cells decrease but the counts of memory CD4+ or CD8+ T cells increase or remain the same.2 , 5 , 7 Moreover, the normal function of T cell receptor (TCR) and cytokine production by T cells is also impaired.7 All changes in adaptive immunity parameters lead to a decline in their ability to build a proper immune response against new antigens, and thus poor response to infection or vaccination. This is referred to as immunosenescence (reduced proliferation) and exhaustion (reduced inflammatory mediators such as cytokine production).3 , 7 Exhausted T cells continuously produce small amounts of proinflammatory cytokines such as IFN-γ and tumor necrosis factor alpha (TNF-α) that induce low-grade inflammation in older people.3
Figure 1.
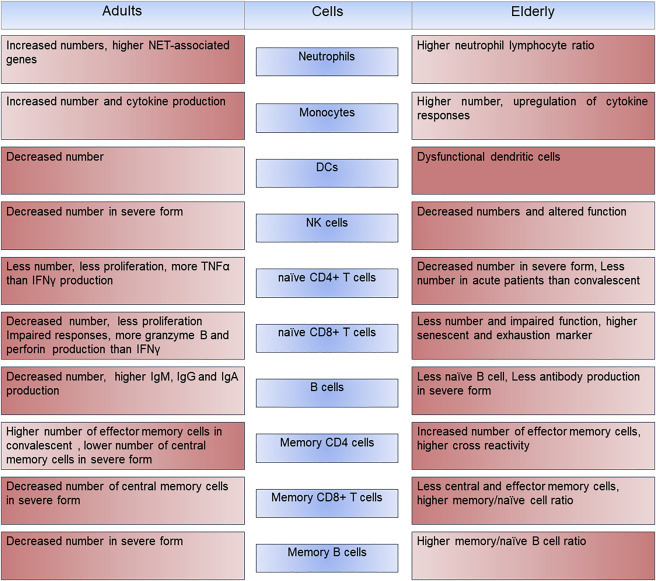
COVID-19 alters innate and adaptive immune systems in the adult and the elderly
Age-related alteration in immune responses increases susceptibility to SARS-CoV-2 infection. Changes in innate and adaptive immune systems are indicated in adults and older COVID-19 patients. Intensity of the color indicates direction of changes where lighter color displays decrease in phenotypic value.
Age-related defects in the adaptive immune system against COVID-19
Recently, Moderbacher et al. showed that scarcity of all three arms of adaptive immunity, such as B cells (antibody-producing cells), CD4+T cells (helper T cells), and CD8+T cells (cytotoxic, or killer, T cells) play a crucial role in the severity of COVID-19 in the elderly.2 SARS-CoV-2-specific CD4+ and CD8+ T cell levels were significantly lower in acute COVID-19 samples in comparison to convalescent COVID-19 cases.2 The serological tests showed that many types of circulating antibodies against receptor-binding domain (RBD), Spike protein (S), and Nucleocapsid protein (N) as well as neutralizing antibodies against full-length SARS-Cov-2 S protein were pronounced in both acute and convalescent COVID-19.2 These neutralizing antibodies were associated with a protective response against COVID-19 and were tested as vaccine candidates in non-human primates.2 Conversely, strong SARS-CoV-2-specific CD4+ or CD8+ T cell responses were associated with protection against the disease and milder symptoms in the acute phase. With aging, frequencies of naive CD8+ and CD4+ T cells are dramatically reduced and their responses against diseases including COVID-19 are significantly altered.2 In order to mount a well-defined immune response against a pathogen, the immune system uses highly coordinated processes involving many cells and mediators. Moderbacher and her colleagues demonstrated that the alteration in coordination of the immune system in older people is a reason why they cannot compete with the novel coronavirus.2 Loss of the ability to coordinate CD4+ and CD8+ T cell responses, and lack of adequate coordination between the CD4+ T cell and antibody responses, lead to a severe form of COVID-19 in the elderly. Interestingly, there was a strong association between low frequencies of naive CD8+ and CD4+ T cells, severity of COVID-19, and age. On the other hand, acute cases that had high IFN-γ-producing CD8+ T cells exhibited mild symptoms.2 Intriguingly, pairwise analysis revealed a positive association between disease severity, antigen specific antibodies, and CD4+ and CD8+ T cells responses, but neutralizing antibodies alone were not a factor to reduce disease symptoms. This suggests that an efficient coordination between all three arms of the adaptive immunity is needed for protection against COVID-19, which is almost lacking in the elderly.2
Age-associated defects in the innate immune system
Innate immunity is the first line of defense that responds immediately upon encountering a threat. It also plays a crucial role in initiating an effective adaptive immunity. The innate immune system is affected by age to a lesser degree. Adaptive immune cells respond to inflammatory stimuli in an antigen-specific manner, whereas innate immune cells provide non-specific defense against invading agents. Neutrophils, monocytes, and natural killer (NK) numbers are preserved, but the number of dendritic cells (DCs) is reduced with increasing age. However, function of monocytes, NKs, and neutrophils such as chemotaxis and signal transduction are impaired in older individuals.8 Induction of adaptive immune responses require presentation of antigens to T cells by antigen-presenting cells (APCs) mainly DCs. Higher number of DCs not only enhances the innate immune response but also can mediate activation of adaptive immune response against pathogens. On the other hand, impaired DCs function causes delayed T cell responses and ultimately lower anti-viral responses.
Age-associated decrease of Toll-like receptors (TLRs) such as TLR1, TLR3 expression could be another reason for weakening immune responses.7 Moreover, the expression of TLR7 in the lung is depleted with age, which may contribute to a poor immune response to COVID-19 in elderly patients.3 In addition, immune-response-related gene expression analysis revealed an age-related upregulation of innate immunity and downregulation of adaptive immunity, which was associated with viral replication and severity of COVID-19.3
Dysregulation of innate immune responses plays a central role in elevation of peripheral cytokines and chemokines levels such as interleukin-1 beta (IL-1β), IL-6, IL-8, and CXCL10 that are associated with immunopathogenesis and serious clinical signs in the late stages of severe SARS-CoV-2 infection.2 , 4 Elderly individuals might experience a sustained activation of innate immunity and increased circulating inflammatory mediators, which together with low level of cytokines that are constantly produced by T cells induce a chronic low-grade inflammation. It was noted that concentrations of inflammatory cytokines were increased with aging in the acute respiratory syndrome.4 Interestingly, Moderbacher et al. also showed that CXCL10, IL-8, and IL-6 production was increased in the acute phase of COVID-19 and positively associated with the severity of the disease. CXCL10 also showed a strong negative correlation to SARS-CoV-2-specific CD4+ and CD8+ T cells, suggesting that CXCL10 may serve as a surrogate marker in predicting poor CD4+ and CD8+ T cell response against COVID-19.2
In elderly patients, failure to fight against SARS-CoV-2 infection is a complex process, and in addition to a less efficient immune system, several other factors can play a role:
-
•
Angiotensin-converting enzyme 2 (ACE2) is a highly specific receptor that facilitates SARS-CoV-2 entry into cells such as epithelial or alveolar cells.5 The expression of ACE2 in the lung, nasal epithelium, and other organs increases with age. Heightened receptor availability enhances the viral entry into the host cells and increases the number of infected cells. Therefore, the level of ACE2 expression can aggravate COVID-19 severity.3 , 9 Sajuthi et al. found that IL-4, IL-5, and IL-13 production significantly reduces ACE2, which correlated with a less severe COVID-19, whereas induction of type 1 IFN increases ACE2 expression.9
-
•
Many elderly individuals suffer from underlying medical conditions and are therefore at a greater risk for a more severe disease progression.
-
•
Shifting lymphoid cell fractions to predominantly memory cells increases the chances that these cells may cross react with new antigens, which can induce an unwanted immune reaction and lead to a severe form of the disease.2 , 3
Trained immunity against COVID-19 in the elderly
In early life, children receive many vaccines against infectious diseases. Randomized trials have shown that vaccines such as the measles vaccine or Bacillus Calmette-Guérin (BCG) vaccine non-specifically reduced overall child mortality due to acute respiratory tract infections.1 Non-specific cross protection of vaccines against unrelated infectious diseases is mediated by epigenetic and metabolic changes in innate immune cells and is termed trained immunity or innate immune memory.1 Trained immunity can confer broader aspect of therapeutic strategies against infectious and non-infectious diseases such as atherosclerosis, the bladder cancer and destruction of cancer cells.1 , 10 Several investigations have reported a significant correlation between BCG vaccination and decreasing COVID-19 morbidity and mortality in countries where BCG vaccination is a part of the immunization program (reviewed by Sohrabi et al.1).10 , 11 These ecological studies are prone to bias because of several confounding factors such as genetic background, geographical regions, diagnostic tests, type of data collection, and control regulations.1 , 10 Moreover, non-specific protection induced by BCG vaccination varies depending on BCG strains, dose, administration route, and manufacturing, which can explain a lack of or less efficient protection in some populations.1 , 10 Escobar et al. found that after mitigating multiple confounding factors, every 10% increase in the BCG index was associated with a 10.4% reduction in COVID-19 mortality.10 These data warrant the further investigation of the concept of non-specific effects of live attenuated vaccines in prospective randomized trials.
Vaccination is the most effective way to prevent infectious diseases. However, in general, vaccines are less effective in older people. For example, the influenza vaccine efficiency is reduced to about 20%–60% in older population.6, 7, 8 It is therefore an emerging task to protect this vulnerable group from the diseases. Development of an effective vaccine against COVID-19 has resulted in exciting news regarding several potential candidates with a good effectiveness and safety profile, at least in young individuals, but it is an additional concern to increase efficacy of the potential vaccines in the elderly. Immune system and vaccine effectiveness in the elderly can be boosted by several ways. Changing the administration rout or the use of adjuvants can increase the efficiency of vaccines.7 Another strategy is using a trained immunity approach to boost the immune system and protect the older population against COVID-19. Administration of microbial compounds such as BCG or β-glucan induces a long-term memory and enhanced immune responses in monocytes/macrophages, DCs, and NK cells in older people8 (Figure 2 ). A randomized controlled trial showed that BCG vaccination was able to reprogram the bone marrow hematopoietic stem cells (HSCs) and upregulate myeloid and granulocytic lineage associated transcripts 90 days after vaccination.12 Interestingly, BCG vaccination induced persistent epigenetic changes in circulating CD14+ monocytes 90 days post-administration. This suggests that BCG induced transcriptomic changes in hematopoietic stem/progenitor cells (HSPCs) passed to the major effector cells of peripheral blood mononuclear cells (PBMCs) such as monocytes.12
Figure 2.
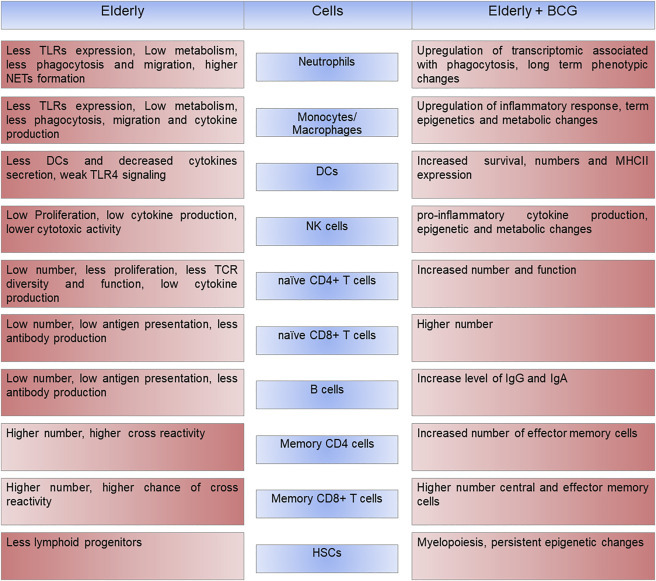
BCG vaccination modulates innate and adaptive immune system in the elderly
Age-related alterations in the immune system contribute to the increased susceptibility to diseases in the elderly. BCG vaccination improves immune systems in terms of cell numbers and functions in both innate and adaptive immune systems. Changes in immune cells are indicated before and after BCG vaccination in the elderly. Intensity of the color indicates direction of changes where lighter color displays decrease in phenotypic value.
A recent paper by Giamarellos-Bourboulis et al. demonstrated the impact of BCG vaccination on the modulation of immune responses and prevention of respiratory infections in the older patients.11 In this randomized trial, elderly hospitalized patients were vaccinated on the day of their hospital discharge with a single dose of a placebo or BCG. Surprisingly, vaccination drastically decreased respiratory infection incidence by 80% in the BCG-vaccinated group in comparison with the placebo group during the 12 months of follow up, suggesting a long-term non-specific memory effect of BCG.11 Thus, while the defective adaptive immune memory might lead to lower responses to vaccination, gain of innate immune memory could be a key to the survival of the elderly. As expected, the PBMCs of the immunized group showed a significant increase in IL-6 and TNF-α cytokine production after Pam3Cys restimulation. These results were coupled with the increased levels of H3K27ac at the regions of IL-6 and TNF-α promotors, suggesting sustained histone modification upon BCG administration.11 The mechanism behind this effect can be explained by transcriptional and functional changes at the level of myeloid bone marrow progenitors. BCG vaccination upregulates expression of genes associated with neutrophils and mononuclear phagocytic cells within HSPCs 90 days post-vaccination.12 The number of neutrophils increases dramatically in COVID-19, but their functions in older patients are impaired. Thus, BCG vaccination might improve neutrophil functions and their antiviral activity. BCG vaccination also induced IFN-γ production after M. tuberculosis restimulation, which indicates the activation of NK cells or adaptive immune responses.11 NK cells are crucial for an efficient antiviral immunity. These cells identify and eliminate infected cells and produce cytokines or chemokines such as IFN. Interestingly, recent studies have implicated that BCG vaccination improves NK cell functions.13 This suggests that BCG treatment can revive their antiviral ability, which was altered due to aging.
BCG induced IL-1β production and enrichment of pathways related to IFN-α and IFN-γ response signalling in PBMCs, 90 days after administration.12 In many viral infections, T cells play an important role in the control and clearance of an acute infection. Coordination and synergistic effect of the innate and the adaptive immune responses is the most efficient way to fight against SARS-CoV-2. Interestingly, data showed that there were no remarkable changes in circulating monocyte, granulocyte, or lymphocyte counts. Moreover, there was no significant increase in the level of inflammatory mediators such as IL-6 and IL-18. This indicates that in fact there was no systemic inflammation after BCG vaccination.11 Previously, it was shown that BCG decreases systemic chronic inflammation by improving cell responsiveness.14 Lowering systemic inflammation and enhancing innate and adaptive immunity during COVID-19 might be an important step to reduce mortality rate due to cytokine storm syndrome.
Conclusions
Considering the impairment of the elderly immune system, these data suggest that BCG is able to recover both innate immune system and the T cell immune responses and significantly imporoves the level of immune responsiveness. BCG is able to induce both adaptive and innate immune responses and have a beneficial effect on coordination of both systems, which can result in more efficient and effective protection against COVID-19 in the older age group. Moreover, the prevalence of cancer diseases such as non-muscle-invasive bladder cancer and melanoma are significantly higher in older people, and BCG administration against COVID-19 might have protective effects against those diseases, which is beyond its intended scope. It is important to study whether BCG administration can also restore hematopoietic progenitor cells function and frequency in aged humans. It has been shown that trained immune memory can also be induced in non-immune cells, such as stromal and epithelial cells.1 Epithelial cells are the first host cells to be infected by SARS-CoV-2, which is why they are particularly important target cells for the induction of innate immune memory.
In conclusion, BCG-induced innate immune memory can improve antiviral-host defense in older people. Several epidemiological and proof-of-principle studies have shown that BCG vaccination prior to other vaccines boosts the efficiency.15 This suggests that building a pre-existing immunological memory not only could be a strategy to protect the elderly against new infection, but also be a tool to strength their immune system capabilities and increase the efficacy of vaccines. It is very important to consider that currently, BCG is used as a routine neonatal vaccination policy in 152 countries and also is used as an immunotherapy for non-muscle-invasive bladder cancer and melanoma worldwide.1 , 10 If BCG vaccination is considered as an approach to prevent COVID-19, the new application should not interfere with the routine infant vaccination program or immunotherapy of adult patients. A well-defined measure and careful evaluation are required to manage any possible shortage in the vaccine supply. Altogether, BCG vaccine is safe and can protect the elderly against infection without inducing serious adverse effects and systemic inflammation. Considering the effect of BCG on the reduction of COVID-19 severity and death, non-specific boosting of immune responses by BCG or other microbial compounds could be used as a transient but a powerful approach to protect the vulnerable populations. Likewise, this strategy can decrease mortality and morbidity rates of COVID-19 and other infectious diseases as a “bridge vaccination” between the occurrence of a new pathogen and the development and deployment of novel specific vaccines.
Acknowledgments
M.G.N. was supported by a European Research Council (ERC) Advanced Grant (ERC 833247) and a Netherlands Organization for Scientific Research Spinoza Grant (NWOSPI 94-212).
Declaration of interests
M.G.N. and L.A.B.J. are scientific founders of Trained Therapeutics and Discoveries (TTxD). The other authors declare no competing interests.
References
- 1.Sohrabi Y., Dos Santos J.C., Dorenkamp M., Findeisen H., Godfrey R., Netea M.G., Joosten L.A. Trained immunity as a novel approach against COVID-19 with a focus on Bacillus Calmette-Guérin vaccine: mechanisms, challenges and perspectives. Clin. Transl. Immunology. 2020;9:e1228. doi: 10.1002/cti2.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santesmasses D., Castro J.P., Zenin A.A., Shindyapina A.V., Gerashchenko M.V., Zhang B., Kerepesi C., Yim S.H., Fedichev P.O., Gladyshev V.N. COVID-19 is an emergent disease of aging. Aging Cell. 2020;19:e13230. doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn K.M., Fox A., Harland K.L., Russ B.E., Li J., Nguyen T.H.O., Loh L., Olshanksy M., Naeem H., Tsyganov K., et al. Age-Related Decline in Primary CD8+ T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8+ T Cells. Cell Rep. 2018;23:3512–3524. doi: 10.1016/j.celrep.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 6.Glynn J.R., Moss P.A.H. Systematic analysis of infectious disease outcomes by age shows lowest severity in school-age children. Sci. Data. 2020;7:329. doi: 10.1038/s41597-020-00668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Giudice G., Goronzy J.J., Grubeck-Loebenstein B., Lambert P.H., Mrkvan T., Stoddard J.J., Doherty T.M. Fighting against a protean enemy: immunosenescence, vaccines, and healthy aging. NPJ Aging Mech. Dis. 2017;4:1. doi: 10.1038/s41514-017-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulut O., Kilic G., Domínguez-Andrés J., Netea M.G. Overcoming immune dysfunction in the elderly: trained immunity as a novel approach. Int. Immunol. 2020;32:741–753. doi: 10.1093/intimm/dxaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman J.B., Lum F.M., Ho P.P., Kaminski N., Steinman L. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc. Natl. Acad. Sci. USA. 2020;117:24620–24626. doi: 10.1073/pnas.2012358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc. Natl. Acad. Sci. USA. 2020;117:17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Dominguez-Andres J., Kyriazopoulou E., Gkavogianni T., Adami M.E., Damoraki G., et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020;183:315–323. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirovic B., de Bree L.C.J., Groh L., Blok B.A., Chan J., van der Velden W., Bremmers M.E.J., van Crevel R., Handler K., Picelli S., et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe. 2020;28:322–334. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Jacobs C., Xavier R.J., van der Meer J.W., van Crevel R., Netea M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014;155:213–219. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koeken V.A., de Bree L.C.J., Mourits V.P., Moorlag S.J., Walk J., Cirovic B., Arts R.J., Jaeger M., Dijkstra H., Lemmers H., et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J. Clin. Invest. 2020;130:5591–5602. doi: 10.1172/JCI133935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann P., Donath S., Perrett K.P., Messina N.L., Ritz N., Netea M.G., Flanagan K.L., van der Klis F.R.M., Curtis N., MIS BAIR group The influence of neonatal Bacille Calmette-Guérin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine. 2019;37:3735–3744. doi: 10.1016/j.vaccine.2019.03.016. [DOI] [PubMed] [Google Scholar]


