Abstract
Background
We investigated the dynamic changes in lipid profiles and their correlations with disease severity and clinical outcome in patients with severe COVID-19.
Methods
We retrospectively reviewed 519 severe COVID-19 patients with confirmed outcomes (discharged or deceased), admitted to the West Court of Union Hospital in Wuhan, China, between 29 January and 8 April 2020.
Results
Altogether, 424 severe COVID-19 patients, including 34 non-survivors and 390 survivors, were included in the final analyses. During hospitalization, low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A-I (apoA-I) showed an increasing trend in survivors, but showed a downward trend in non-survivors. The serum concentrations of HDL-C and apoA-I were inversely correlated with C-reactive protein (CRP), length of hospital stay of survivors, and disease severity scores. For in-hospital deaths, the areas under the receiver operating characteristic curves (AUCs) of the ratios of CRP/HDL-C and CRP/apoA-I at admission were 0.84 and 0.83, respectively. Moreover, patients with high ratios of CRP/HDL-C (>77.39) or CRP/apoA-I (>72.37) had higher mortality rates during hospitalization (log-rank p < 0.001). Logistic regression analysis demonstrated that hypertension, lactate dehydrogenase, SOFA score, and High CRP/HDL-C ratio were independent predictors of in-hospital mortality.
Conclusions
During severe COVID-19, HDL-C and apoA-I concentrations are dramatically decreased in non-survivors. Moreover, High CRP/HDL-C ratio is significantly associated with an increase in mortality and a poor prognosis.
Keywords: Lipid metabolism, CRP/ HDL-C ratio, COVID-19, SARS-CoV-2
1. Introduction
The coronavirus disease-2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has seriously threatened global public health security. By 27 January 2021, this brings the cumulative numbers to over 98.2 million reported cases and over 2.1 million deaths globally since the start of the pandemic [1]. Although most patients with mild COVID-19 are asymptomatic and/or have mild clinical symptoms and a good prognosis, some severe COVID-19 patients, especially those with old age and several pre-existing comorbidities, could develop severe illnesses including acute respiratory distress syndrome, septic shock, multiple organ failure or even death in a short period of time [2], [3], [4]. Therefore, it is necessary to determine effective indicators to predict the disease severity and clinical outcome, and to help reduce the mortality of severe and critical patients with COVID-19.
Dyslipidemia plays an important role in the pathogenesis and evolution of atherosclerosis, and it is always at the forefront of medical research. Significant alterations in metabolic regulation, including lipids and lipoproteins, have been reported to occur during bacterial, viral, and parasitic infections [5]. HDL and apoA-I, which are the major protein components of HDL, display pleiotropic characteristics, including cholesterol traffic, lipopolysaccharide (LPS) and lipoteichoic acid (LTA) neutralisation, anti-inflammatory, anti-thrombotic, anti-oxidative, anti-apoptotic and endotheliocyte protective effects [6], [7]. Decreased HDL and apoA-I concentrations have been reported to be closely associated with poor prognosis in patients with sepsis, pneumonia, and other infections [8], [9], [10]. Recently, LDL-C, TC and HDL-C concentrations significantly decreased in COVID-19 patients [11], [12], while the changes and effects of lipoprotein and apolipoprotein concentrations and dynamic changes in lipid profiles in severe COVID-19 patients have rarely been reported. In this retrospective study, we aimed to describe the lipid profile characteristics and dynamic changes in severe COVID-19 patients, and to evaluate the associations between lipid profile features, new markers (CRP/HDL-C and CRP/Apo-AI), disease severity, and prognosis.
2. Materials and methods
2.1. Study design and participants
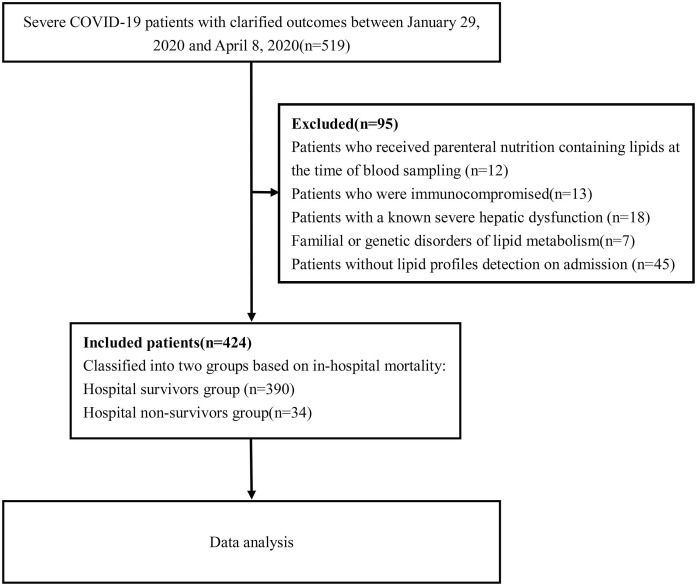
This was a retrospective, single-center, observational study among patients with severe COVID-19 who were admitted to the West Court of Union Hospital, Huazhong University of Science and Technology during the management by a national medical team from 29 January to 8 April 2020. All participants were diagnosed with COVID-19 based on the WHO interim guidance. Classification of the COVID-19 clinical types was based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), published by the National Health Commission and National Administration of Traditional Chinese Medicine of China [13]. Patients were classified as severe COVID-19 of they met any of the following criteria: 1) respiratory rate ≥ 30 breaths/min; (2) finger oxygen saturation ≤ 93% at rest and (3) PaO2/FiO2 ≤ 300 mmHg. We excluded patients who received parenteral nutrition containing lipids at the time of blood sampling, immunocompromised patients, patients with known severe hepatic dysfunction, familial or genetic disorders of lipid metabolism, and those without lipid profile detection on admission. By 8 April 2020, a total of 424 severe COVID-19 patients with a confirmed clinical outcome (died or recovered) were recruited in this study, and a flow diagram is shown in Fig. 1 . The primary outcome of the study was defined as in-hospital death. The study was approved by the Ethics Committee of the Union Hospital of Huazhong University of Science and Technology and ethics board of Xiangya Hospital, Central South University (No. 202003049). Written informed consent was waived by the Ethics Commission of the designated hospital because the disease was an emerging infectious disease.
Fig. 1.
Flow diagram of the study population.
2.2. Data collection
The clinical records and laboratory data of each patient were obtained from the electronic medical system. A group of experienced respiratory clinicians reviewed and refined the data. The demographic data, comorbidities, clinical symptoms, signs, and clinical outcomes (death or recovery) were extracted from their electronic medical records. Laboratory results at admission, including routine blood tests, liver function, kidney function, coagulation function, and C-reactive protein, were collected and evaluated. Furthermore, disease severity was evaluated using the CURB-65 score and Sequential Organ Failure Assessment (SOFA) score.
2.3. Laboratory tests
When measuring the concentration of plasma lipids, venous blood samples were collected from each subject after at least 12 h of fasting. A BC-6800 automatic hematology analyzer was used for routine blood parameter analysis. The blood biochemistry indexes and lipid profiles were measured using the Labospect AS chemistry analyzer. All laboratory data were tested in the same laboratory with standardization and certification procedures.
2.4. Statistical analysis
Categorical variables were presented as numbers (percentages, %) and compared using the χ2 test or Fisher’s exact test. Continuous variables with normal distribution are shown as mean ± SD and compared with the Student’s t test; otherwise, continuous variables with skewed distribution were presented with medians [interquartile range (IQR)], and compared with the Mann-Whitney U test. Correlations between variables were analyzed using Spearman’s coefficients. Receiver operating characteristic (ROC) curve analyses were performed to determine the cut-off values, sensitivity, and specificity of new markers (apoA-I, HDL-C, CRP/HDL-C ratio and CRP/apoA-I ratio) for predicting in-hospital mortality. Meanwhile, the best Youden index (sensitivity + specificity − 1) was obtained to calculate the appropriate cut-off point of the potential mediators (CRP/HDL-C ratio and CRP/Apo-AI ratio) to predict in-hospital death. In addition, risk factors were evaluated by univariate analysis, and variables with statistical significance in univariate analysis were selected in the multivariate logistic regression by using a forward stepwise regression to calculate independent risk factors. Survival differences among groups were compared by Kaplan-Meier analysis using the log-rank test. For all analyses, P < 0.05 (2-tailed) was considered statistically significant. GraphPad Prism 8.0 and SPSS 22.0 software were used for statistical analyses.
3. Results
3.1. Demographics and baseline clinical characteristics of severe COVID-19 patients
A total of 424 severe COVID-19 patients were included in the final analysis based on the selection criteria (Fig. 1). The demographic and baseline clinical characteristics of the severe COVID-19 patients are summarized in Table 1 . Thirty-four patients died during hospitalization, and 390 were discharged from the hospital. The mean age of the 424 patients was 60.7 years, and 220 (51.9%) were men. Hypertension was the most common comorbidity, followed by diabetes, coronary heart disease, chronic obstructive lung disease, and malignancy. Compared with the survivor group, the non-survivor group had a higher prevalence of men (82.4% vs. 49.2%, p < 0.001), hypertension (76.5% vs. 44.6%, p < 0.001), and lower SpO2 [85.0 (81.8–86.0) vs. 92 (90.0–93.0), p < 0.001]. The respiration rate and temperature were significantly higher in non-survivors than in survivors. Compared with the survivors, the non-survivors had greater disease severity, as evidenced by higher SOFA scores and CURB-65 scores (p<0.001), accompanied by significantly higher white blood cell and neutrophil count, CRP, procalcitonin, lactate dehydrogenase, total bilirubin, blood urea nitrogen, high-sensitive cardiac troponin I and D-dimer and lower lymphocyte, albumin, and platelet count (Table 2 ).
Table1.
Baseline Characteristics of Patients with severe COVID-19.
| Variable | Total (n = 424) | Non-survivor (n = 34) | Survivor (n = 390) | p Value |
|---|---|---|---|---|
| Age, mean ± SD, y | 60.7 ± 12.2 | 66.5 ± 11.1 | 60.2 ± 12.1 | 0.003 |
| Male, n [%] | 220(51.9%) | 28(82.4%) | 192(49.2%) | <0.001 |
| Comorbidities, n [%] | ||||
| Hypertension | 200(47.2%) | 26(76.5%) | 174(44.6%) | <0.001 |
| Diabetes | 92(21.7%) | 6(17.6%) | 86(22.1%) | NS |
| Coronary heart disease | 52(12.3%) | 9(26.5%) | 43(11.0%) | 0.018 |
| Chronic obstructive lung disease | 34(8.0%) | 6(17.6%) | 28(7.1%) | NS |
| Malignancy | 28(6.6%) | 4(11.8%) | 24(6.2%) | NS |
| Vital signs | ||||
| Respiration rate, median (IQR), breaths per minute | 22.0(20.0–25.0) | 24.5(20.0–30.0) | 22(20.0–25.0) | 0.011 |
| SpO2, median (IQR),% | 92.0(89.0–93.0) | 85.0(81.8–86.0) | 92(90.0–93.0) | <0.001 |
| Pulse, median (IQR), beats per minute | 88.0(79.0–101.0) | 91.5(80.0–104.0) | 88.0(79.0–101.0) | NS |
| MAP, median (IQR), mm Hg | 95.8(90.0–105.7) | 95.7(90.3–106.4) | 96.0(89.9–105.4) | NS |
| Temperature, median (IQR), ℃ | 36.8(36.4–37.4) | 37.2(36.5–37.9) | 36.8(36.4–37.3) | 0.033 |
| Disease severity scores | ||||
| SOFA score, median (IQR) | 2.0(2.0–3.0) | 4.0(3.0–5.0) | 2.0(2.0–3.0) | <0.001 |
| CURB-65 score, median (IQR) | 1.0(0.0–1.0) | 1.0(1.0–2.0) | 1.0(0.0–1.0) | <0.001 |
Data are presented as mean ± SD, medians (IQR) and n (%). P values were calculated by Student’s t test, Mann–Whitney U test, χ2 test or Fisher’s exact test, as appropriate. P values indicate differences between non-survivors and survivors. Abbreviations: IQR, interquartile range; SpO2,percutaneous oxygen saturation; MAP, mean arterial pressure; SOFA score, Sequential Organ Failure Assessment score.
Table 2.
Laboratory findings of severe COVID-19 patients on admission.
| Laboratory findings | Normal Range | Total (n = 424) | Non-survivor (n = 34) | Survivor (n = 390) | p Value |
|---|---|---|---|---|---|
| Blood Routine | |||||
| White blood cell count, ×109/l | 3.5–9.5 | 6.0(4.4–7.9) | 8.1(4.5–11.2) | 5.9(4.4–7.5) | 0.004 |
| Platelet count, ×109/l | 125–350 | 222.5(169.0–294.8) | 153.5(95.0–216.8) | 230.5(173.0–300.3) | <0.001 |
| Neutrophil count, ×109/l | 1.8–6.3 | 4.0(2.9–6.2) | 6.9(3.6–9.5) | 3.9(2.9–5.8) | <0.001 |
| Lymphocyte count, ×109/l | 1.1–3.2 | 1.0(0.7–1.4) | 0.6(0.5–0.8) | 1.1(0.8–1.4) | <0.001 |
| Hemoglobin, g/l | 115–150 | 125.0(114.0–136.0) | 127.5(114.8–143.5) | 125.0(113.0–135.3) | 0.037 |
| Blood Biochemistry | |||||
| Aspartate aminotransferase, U/l | 8–40 | 29.0(22.0–42.0) | 42.0(28.0–62.5) | 28.0(21.0–40.3) | 0.001 |
| Alanine aminotransferase, U/l | 5–35 | 32.0(20.3–51.8) | 35.4(17.5–55.0) | 32.0(21.0–51.3) | NS |
| Lactate dehydrogenase, U/l | 109–245 | 254.0(195.0–354.8) | 492.0(338.3–617.3) | 243.5(192.0–329.8) | <0.001 |
| Total bilirubin, μmol/l | 3.0–20 | 10.9(8.2–15.3) | 14.1(9.7–22.9) | 10.8(8.0–15.1) | 0.007 |
| Albumin, g/l | 33–55 | 31.1(27.1–34.4) | 28.1(25.1–31.7) | 31.4(27.6–34.8) | 0.001 |
| Blood urea nitrogen, mmol/l | 2.9–8.2 | 4.8(3.7–6.6) | 6.5(4.2–9.2) | 4.7(3.6–6.2) | <0.001 |
| Creatinine, μmol/l | 41–81 | 67.7(56.8–79.7) | 75.1(65.0–85.2) | 66.1(56.6–79.3) | 0.017 |
| Myocardial Injury Mediators | |||||
| Creatine kinase, U/l | 24–170 | 69.5(43.0–125.0) | 137.5(55.8–263.0) | 68.0(42.0–117.0) | <0.001 |
| High-sensitive cardiac troponin I, ng/l | <26.2 | 4.1(2.1–10.3) | 19.5(8.1–196.3) | 3.7(1.9–8.0) | <0.001 |
| Inflammatory Mediators | |||||
| C-reactive protein, mg/l | 0–8 | 24.1(3.9–66.2) | 83.4(62.3–129.3) | 19.3(3.6–58.9) | <0.001 |
| Blood Coagulation | |||||
| D-dimer, μg/ml | 0–0.5 | 0.7(0.3–2.1) | 6.5(0.9–8.0) | 0.6(0.3–1.7) | <0.001 |
| Prothrombin time, s | 11.0–16.0 | 13.1(12.5–14.1) | 14.1(13.0–15.1) | 13.1(12.5–13.9) | <0.001 |
| International normalized ratio | 0.83–1.36 | 1.0(1.0–1.1) | 1.1(1.0–1.2) | 1.0(1.0–1.1) | <0.001 |
| Bacterial Infection Mediators | |||||
| Procalcitonin, µg/l | <0.05 | 0.07(0.05–0.14) | 0.21(0.14–0.41) | 0.07(0.05–0.12) | <0.001 |
| Lipids | |||||
| TC, mmol/l | 0–5.2 | 4.1(3.5–4.7) | 3.7(3.2–4.6) | 4.1(3.5–4.7) | 0.041 |
| TG , mmol/l | 0–1.7 | 1.3(1.0–1.8) | 1.2(1.1–1.7) | 1.3(1.0–1.8) | NS |
| HDL-C, mmol/l | 1.1–1.74 | 0.9(0.8–1.1) | 0.8(0.6–0.9) | 0.9(0.8–1.1) | 0.001 |
| LDL-C, mmol/l | 0–3.12 | 2.4(1.9–2.9) | 2.2(1.8–2.8) | 2.4(1.9–2.9) | NS |
| apoA-I, g/l | 1–1.6 | 0.8(0.7–1.0) | 0.7(0.6–0.7) | 0.8(0.7–1.0) | <0.001 |
| apoB, g/l | 0.6–1.2 | 1.0(0.8–1.1) | 0.9(0.8–1.2) | 1.0(0.8–1.1) | NS |
| Lp(a), mg/dl | 0–30 | 14.2(6.5–25.8) | 14.4(7.0–30.2) | 14.2(6.3–25.4) | NS |
Data are presented as medians (IQR). P values were calculated by Student’s t test, Mann–Whitney U test, as appropriate. P values indicate differences between non-survivors and survivors. Abbreviations: apoA-I, apolipoprotein A-I; apo-B, apolipoprotein B; Lp(a), lipoprotein A; CRP,C-reactive protein.
3.2. Lipid parameters on admission and dynamic alterations in lipid profiles
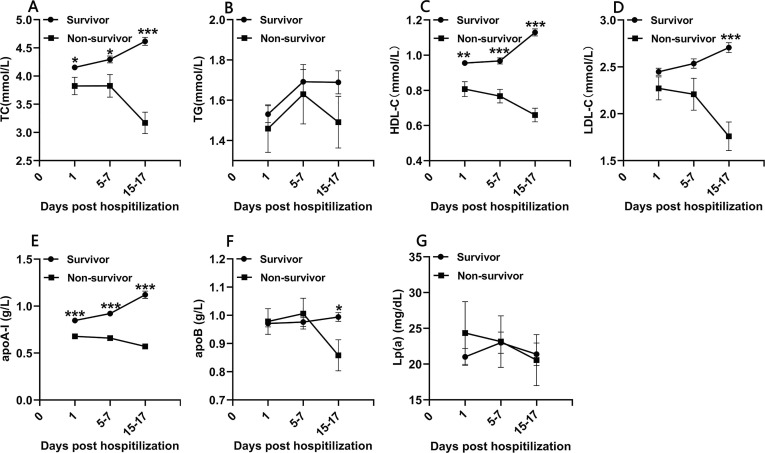
Lipid profiles were analyzed on admission (day 1), on days 5–7 and days 15–17 after admission (Fig. 2 ). On admission, the serum concentrations of total cholesterol (TC), HDL-C and apolipoprotein A-I (apoA-I) were significantly lower in non-survivors, whereas there was no difference in triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), apoB and lipoprotein A [LP(a)] (Table 2). Correlation analysis was performed to detect inflammatory and disease severity related to admission lipid profiles. As shown in Table S1, both HDL-C and apoA-I were inversely correlated with SOFA score, CURB-65 score, length of hospital stay of survivors, and CRP concentration (p < 0.001). Of these, apoA-I had the strongest inverse correlation with SOFA score (rs = -0.457, p < 0.001), length of stay among survivors (rs = -0.479, p < 0.001), and CRP (rs = -0.549, p < 0.001) (Table S1). Furthermore, TC, HDL-C, and Apo-AI concentrations were significantly lower in non-survivors at all time points (p < 0.05, Fig. 2). The LDL-C and apoB concentrations were only significantly lower in non-survivors on days 15–17 (p < 0.05). From day 1 to day 15–17, TC, LDL-C, HDL-C and apoA-I showed a slow upward trend in survivors, but maintained lower concentrations or showed a rapid downward trend in non-survivors (Fig. 2).
Fig. 2.
Presentation of total cholesterol(TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol(LDL-C), apolipoprotein A-I (apo-AI) and lipoprotein A [Lp(a)] concentrations for survivors and non-survivors on admission (day 1, n = 424), day 5–7 (n = 285) and day 15–17 (n = 279). Data are presented as mean ± SEM. Statistical significance was calculated by Mann-Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001 survivors vs. non-survivors.
3.3. Novel serological indicators (CRP/HDL-C and CRP/ apoA-I) on admission
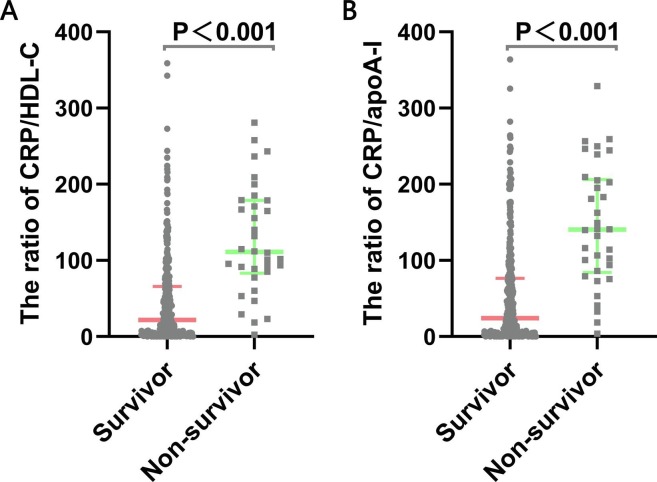
The concentrations of novel serological biomarkers, including the ratio of CRP/HDL-C [111.1 (83.3–178.9) vs 22.0 (3.5–66.0), p < 0.001] and CRP/apoA-I [140.4 (84.3–206.1) vs. 24.3(3.5–76.6), p < 0.001] were higher in non-survivors than in survivors (Fig. 3 ). In correlation analysis, the ratio of CRP/HDL-C had the strongest positive correlation with the SOFA score (rs = 0.611, p < 0.001) and length of hospital stay of survivors (rs = 0.551, p < 0.001) (Table S1). Additionally, the ratio of CRP/apoA-I was also positively correlated with the SOFA score (rs = 0.613, p < 0.001) and length of hospital stay of survivors (rs = 0.550, p < 0.001) (Table S1).
Fig. 3.
The ratios of CRP/HDL-C and CRP/ apoA-I in severe COVID-19 patients. Data are presented as medians (IQR). Statistical significance was calculated by Mann-Whitney U test. P values indicate differences between non-survivors and survivors. Abbreviations: apoA-I, apolipoprotein A-I; CRP,C-reactive protein.
3.4. Association of novel biomarkers with adverse clinical outcomes
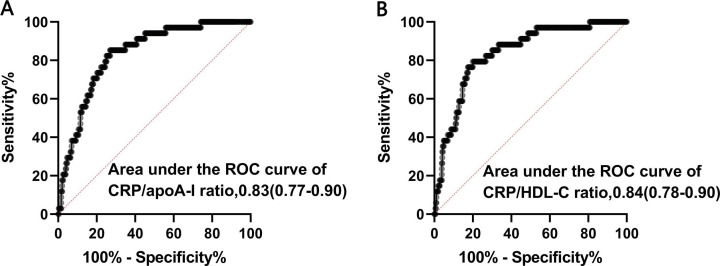
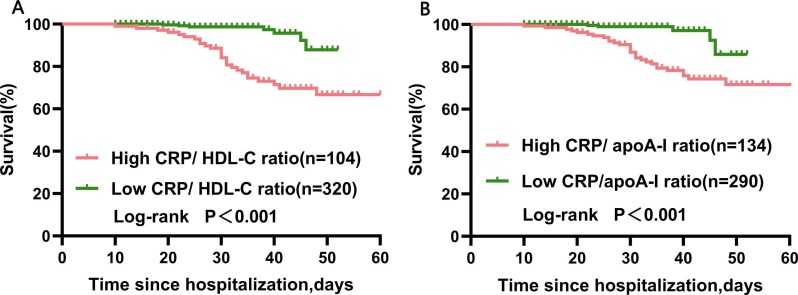
In order to evaluate the prognostic value and determine the best cut-off of the CRP/HDL-C ratio for predicting in-hospital mortality among severe COVID-19 patients, receiver operating characteristic (ROC) curves were obtained (Fig. 4 ).The AUCs was 0.28 (95% CI: 0.20–0.36, p<0.001) for apoA-I, 0.33 (95% CI:0.23–0.43, p = 0.001) for HDL-C, 0.84 (95% CI: 0.78–0.90, p<0.001) for CRP/ HDL-C ratio and 0.83(95% CI:0.77–0.90, p<0.001) for CRP/ apoA-I ratio (Table 3 ). The best cut-off points of the CRP/HDL-C ratio for predicting in-hospital mortality was 77.39 with the best Youden index, sensitivity of 79.4%, and specificity of 80.0% (Fig. 4). Similarly, the best cut-off points of CRP/apoA-I ratio were 72.37 for predicting in-hospital death, with a sensitivity of 85.3% and specificity of 73.1%. Then, we defined a high CRP/HDL-C ratio (>77.39) and a high CRP/apoA-I ratio (>72.37). Furthermore, the Kaplan-Meier survival curves confirmed that severe COVID-19 patients with high CRP/HDL-C or CRP/apoA-I ratios had a higher rate of in-hospital mortality (Fig. 5 ).
Fig. 4.
ROC curves of the ratios of CRP/apoA-I(A) and CRP/HDL-C(B) in prediction of in-hospital mortality. Abbreviations: apoA-I, apolipoprotein A-I; CRP,C-reactive protein.
Table 3.
ROC Curve of HDL-C, apoA-I, CRP/ HDL-C ratio and CRP/ apoA-I ratio to predict in-hospital mortality.
| Parameter | AUC (95% CI) | SE | P-value |
|---|---|---|---|
| apoA-I | 0.28 (0.20–0.36) | 0.04 | <0.001 |
| HDL-C | 0.33 (0.23–0.43) | 0.05 | 0.001 |
| CRP/ HDL-C ratio | 0.84 (0.78–0.90) | 0.03 | <0.001 |
| CRP/ apoA-I ratio | 0.83(0.77–0.90) | 0.03 | <0.001 |
Abbreviations: apoA-I, apolipoprotein A-I; CRP,C-reactive protein.
Fig. 5.
Kaplan-Meier survival curves in severe COVID-19 patients with high and low CRP/ HDL-C or CRP/apoA-I ratios at admission. A log-rank test was used to evaluate differences between groups. The survival rates were 97.8% (313 of 320) and 74.0% (76 of 104) for the low CRP/ HDL-C ratio (≤77.39) group and high CRP/ HDL-C ratio (>77.39) group at the observed endpoint (death or discharge), respectively(p<0.001). The survival rates were 98.3% (285 of 290) and 78.4% (105 of 134) for the low CRP/ apoA-I ratio (≤72.4) group and high CRP/ apoA-I ratio (>72.4) group at the observed endpoint (death or discharge), respectively (p<0.001).
We performed logistic regression analysis to explore the most efficient combination of parameters to predict in-hospital mortality. Multivariate logistic regression analysis showed that Hypertension (OR: 4.641, 95% CI: 1.646–13.081, p = 0.004), Lactate dehydrogenase (OR: 1.004, 95% CI: 1.002–1.007, p = 0.002), SOFA score (OR: 2.554, 95% CI: 1.738–3.751, p<0.001) and High CRP/ HDL-C ratio (OR: 6.599, 95% CI: 2.324–18.742, p<0.001) in the final model for the prediction of in-hospital mortality (Table 4 ). The clinical characteristics and outcomes in severe COVID-19 patient subgroups stratified by the CRP/HDL-C ratio are listed in Table S2. Patients in the high CRP/HDL-C ratio group had higher in-hospital death, ICU admission, invasive mechanical ventilation, rate of progression to critical illness, disease severity scores and longer hospital stay (Table S2).
Table 4.
Logistic regression of the final model.
| Parameter | β Coefficient | Standard error | Odds ratios (95%CI) | p |
|---|---|---|---|---|
| Hypertension | 1.535 | 0.529 | 4.641(1.646–13.081) | 0.004 |
| Lactate dehydrogenase | 0.004 | 0.001 | 1.004(1.002–1.007) | 0.002 |
| SOFA score | 0.938 | 0.196 | 2.554(1.738–3.751) | <0.001 |
| High CRP/ HDL-C ratio a | 1.887 | 0.533 | 6.599(2.324–18.742) | <0.001 |
Abbreviations: SOFA score, Sequential Organ Failure Assessment score; CRP,C-reactive protein.
CRP/ HDL-C ratio higher than 77.39.
4. Discussion
In this study, we found dyslipidemia in patients with severe COVID-19 and demonstrated that low concentrations of HDL-C and apoA-I at admission were significantly associated with high concentrations of CRP, prolonged hospital stay and increased disease severity. Additionally, the analysis of the longitudinal changes in lipid profiles showed that non-survival COVID-19 patients had persistent hypolipidemia, including TC, HDL-C, LDL-C and apoA-I, than the survival patients during the early period of hospitalization. Furthermore, Hypertension, Lactate dehydrogenase, SOFA score, and High CRP/HDL-C ratio could serve as independent factors to predict in-hospital mortality; in particular, a higher CRP/HDL-C ratio was closely associated with higher hospital mortality, ICU admission, invasive mechanical ventilation and longer hospital stay.
Acute inflammation caused by viral infection may result in dyslipidemia in patients, and lipid metabolism is known to play an important role in the host immune response. Clinical observations have shown that patients with acute Epstein-Barr virus (EBV) infection had lower concentrations of apoA-I, HDL-C, TC, apoB, LDL-C and Lp (a) compared with their controls [14]. Another study showed that cytomegalovirus (CMV) infection was associated with lower HDL-C in normal-weight females [15]. Compared with other febrile patients, dengue-positive patients had lower HDL-C and LDL-C concentrations [16]. In addition, SARS patients had lower concentrations of apoA-I compared to their normal controls from the results of plasma proteomics [17]. Similarly, our study showed that non-survivors with severe COVID-19 showed lower HDL-C and apoA-I concentrations at admission compared to those survivors. Moreover, the analysis of longitudinal changes of lipid profiles demonstrated that LDL-C, HDL-C, TC and apoA-I remained persistent at low concentrations, or even further sharply decreased during disease progression in non-survivors, while in survivors, although initially decreased, aforementioned lipid profiles were shown to increase steadily during recovery.
Several possible hypotheses might explain the dynamic changes during the course of the COVID-19. First, the liver plays a critical role in lipid metabolism, and liver dysfunction caused by SARS-CoV2 infection or potential drugs affect lipid synthesis. It was reported that 14%-53% of patients with COVID-19 had hepatic dysfunction, especially in severe and critical patients [18]. Therefore, the synthesis of apolipoproteins and lipoproteins would be affected by hepatic dysfunction in patients with severe COVID-19. Second, acute inflammation caused by SARS-CoV-2 might alter lipid metabolism as well. Severe and critical patients with COVID-19 were commonly accompanied with largely excessive release of pro-inflammatory cytokines, such as IL-1, IL-6, IL-12, IFN-γ and TNF-α, as the disease progresses over time and gradually worsens [19], [20]. It was shown that tumour necrosis TNF-α, IL-1β and IL-6 could decrease the synthesis and/or secretion of apolipoproteins in hepatic cell lines in a dose-dependent manner [21]. Furthermore, a severe inflammatory response could also cause capillary leakage, thus resulting in the leakage of lipoproteins and apolipoproteins particles from intravascular to extravascular compartments [22]. In our study, we found that HDL-C and apoA-I were closely associated with the inflammatory marker CRP, which might partially explain the association between hypolipidemia and inflammatory response in severe COVID-19 patients. Finally, a recent study has shown that a rare missense variant in the cholesteryl ester transfer protein gene (CETP, rs1800777-A) is associated with marked reduction in HDL-C concentrations and adverse clinical outcome during sepsis [23]. COVID-19 patients who carry the A allele may have a lower HDL concentration and worse prognosis compared with non-carriers. At present, the genetic variation of the CETP gene in patients with COVID-19 has not been reported, and it may be a promising research direction in the treatment and evaluation of prognosis among patients with COVID-19.
Since COVID-19 is a global pandemic with a high mortality rate, it will be helpful to determine several early markers to predict the disease severity and prognosis of COVID-19. Previous studies have shown that low concentrations of apoA-I and HDL-C have been used as prognostic biomarkers in patients with bacterial and viral infections. An observational study indicated that a low concentration of apoA-I was an indicator of poor prognosis in cirrhotic patients with severe sepsis [9]. Similarly, gradually declined HDL-C concentrations from day 1 to day 7 after admission could serve as a poor prognostic indicator among patients with severe community-acquired pneumonia [24]. Consistently, our data suggested that both HDL-C and apoA-I concentrations were inversely correlated with disease severity scores (SOFA score and CURB-65 score), length of stay of survivors and CRP in patients with severe COVID-19. However, our data reported the low power of HDL-C and apoA-I concentrations at admission to predict in-hospital mortality. HDL and apoA-I display pleiotropic properties, including antioxidant and anti-inflammatory functions [7]. CRP is a common inflammatory marker. Thus, the ratio of CRP/HDL-C or CRP/apoA-I may reflect the balance between pro-inflammatory and anti-inflammatory factors. It is noteworthy that severe COVID-19 patients usually have an imbalance between anti-inflammatory and pro-inflammatory processes [25]. Clinical and experimental studies have shown that patients with severe COVID-19 may exhibit features of systemic hyper-inflammation and inflammatory cytokine storm, which releases pro-inflammatory cytokines excessively and uncontrollably, including IL-6 and TNF-α [20], [25], [26]. Clinical reports have shown that anti-inflammatory therapies (such as glucocorticoids, immunosuppressant and inflammatory cytokine antagonists), which may help in preventing further injury in severe and critical COVID-19 patients, is an effective treatment to improve the clinical outcome [27]. Similarly, we found that the ratios of CRP/HDL-C and CRP/apoA-I were significantly higher in survival COVID-19 patients than in non-survivors, and these ratios had strong negative correlation with SOFA score, length of stay of survivors. Moreover, a high CRP/HDL-C ratio was shown to be an independent predictor of in-hospital mortality among patients with severe COVID-19. Based on these findings, a high CRCR/HDL-C ratio not only shows an imbalance of inflammation in patients with severe COVID-19, but also correlates with deteriorating disease severity and worsening prognosis, and might serve as a potential indicator of poor outcomes among severe COVID-19.
HDL and its major protein, apoA-I, display pleiotropic protective functions, including anti-infectious, anti-inflammatory, anti-oxidative, anti-thrombotic, and anti-diabetic properties [6], [7]. An increasing amount of evidence has shown that HDL, particularly its major protein, apoA-I, has protective effects in a variety of lung diseases, including acute lung injury (ALI), chronic obstructive pulmonary disease (COPD), asthma, pulmonary fibrosis, and viral pneumonia [28]. However, there are no clinical and experimental studies on the protective effect of HDL and apoA-I in COVID-19. In severe and critical COVID-19 patients, the clinical outcome can be significantly worsened by the excessive release of pro-inflammatory cytokines [19]. HDL and apoA-I may help in preventing inflammatory injury and improving clinical outcomes with anti-inflammatory and anti-oxidative properties. A systematic review and meta-analysis showed that bacterial co-infection occurred in 7% of hospitalized COVID-19 patients and 14% of ICU patients, and bacterial co-infection would lead to a higher mortality of COVID-19 [29]. Studies showed that HDL was capable to bind and neutralize Gram-negative LPS and Gram-positive lipoteichoic acid (LTA), thus reducing LTA- and LPS-induced inflammatory injury [7], and providing the conception that HDL-based therapies might be promising in severe COVID-19 patients with bacterial co-infection. Diabetes is a common comorbidity in patients with COVID-19, and is associated with greater disease severity and higher mortality of COVID-19 [30], especially in those population with poorly controlled glycaemia [31]. Several experimental studies have demonstrated that HDL particles display anti-diabetic properties by improving insulin sensitivity and β-cell insulin secretion [7]. The evidence suggested that HDL or apoA-I might improve glycaemic control and promote a better prognosis in patients with severe COVID-19. Although no clinical and experimental studies have been conducted to determine the role of HDL-and Apo-AI-based therapy in COVID-19, it would be a promising direction in searching for novel treatments for severe patients with COVID-19.
Our study was subject to a few limitations. First, this was a retrospective study, and a large cohort study would be required to further confirm our conclusion. Second, asymptomatic patients and those with mild symptoms were not enrolled; thus, the conclusions drawn by the study might not be applicable to asymptomatic and mild patients. Third, Lp (a) is given in mg/dL. The results of Lp (a) can be under- or overestimated in mass-based methods instead of molar-concentration-based methods. Finally, a large number of factors could affect lipid metabolism in COVID-19, the specific mechanism of dyslipidemia could not be concluded, and further investigation is required.
In conclusion, our study demonstrated that dyslipidemia was associated with the inflammatory response, disease severity and poor prognosis of COVID-19. A high CRCR/HDL-C ratio may serve as an independent potential predictor for hospital mortality among patients with severe COVID-19. The persistent hypolipidemia in COVID-19 patients would raise an urgent awareness to clinical physicians in the frontline to fight against this global pandemic. Whether HDL-based therapies have potential therapeutic effects in patients with severe COVID-19 deserves further exploration.
Acknowledgments
We thank all the patients and their families involved in the study. We thank all the medical staff who are fighting against this public crisis. This study was supported by National Key R&D Program of China (No. 2016YFC1304204) ; National Natural Science Foundation of China (No. 82000089, No. 81770080) ; Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant No. 2020LNJJ05); Emergency Project of Prevention and Control for COVID-19 of Central South University(No.160260004) ; China Postdoctoral Science Foundation (No. 2020M670104ZX).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2021.02.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update. https://www.who.int/publications/m/item/weekly-epidemiological-update---27-january-2021. Accessed January 27, 2021.
- 2.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L., Zhang B., Ti M.N., Yang K., Zou Y., Zhang S. Clinical course of severe and critically ill patients with coronavirus disease 2019 (COVID-19): A comparative study. J. Infect. 2020;81:e82–e84. doi: 10.1016/j.jinf.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippas-Ntekouan S., Liberopoulos E., Elisaf M. Lipid testing in infectious diseases: possible role in diagnosis and prognosis. Infection. 2017;45:575–588. doi: 10.1007/s15010-017-1022-3. [DOI] [PubMed] [Google Scholar]
- 6.Georgila K., Vyrla D., Drakos E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers (Basel) 2019:11. doi: 10.3390/cancers11081097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka S., Couret D., Tran-Dinh A., et al. High-density lipoproteins during sepsis: from bench to bedside. Crit. Care. 2020;24:134. doi: 10.1186/s13054-020-02860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka S., Labreuche J., Drumez E., et al. Low HDL concentrations in sepsis versus trauma patients in intensive care unit. Ann. Intensive Care. 2017;7:60. doi: 10.1186/s13613-017-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai M.H., Peng Y.S., Chen Y.C., et al. Low serum concentration of apolipoprotein A-I is an indicator of poor prognosis in cirrhotic patients with severe sepsis. J. Hepatol. 2009;50:906–915. doi: 10.1016/j.jhep.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Morin E.E., Guo L., Schwendeman A., Li X.A. HDL in sepsis - risk factor and therapeutic approach. Front. Pharmacol. 2015;6:244. doi: 10.3389/fphar.2015.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X., Chen D., Wu L., He G., Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin. Chim. Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G., Zhang Q., Zhao X., et al. Low high-density lipoprotein concentration is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. 2020;19:204. doi: 10.1186/s12944-020-01382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), Chin. Med. J. (Engl.), (133) (2020) 1087–1095. [DOI] [PMC free article] [PubMed]
- 14.Apostolou F., Gazi I.F., Lagos K., et al. Acute infection with Epstein-Barr virus is associated with atherogenic lipid changes. Atherosclerosis. 2010;212:607–613. doi: 10.1016/j.atherosclerosis.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Fleck-Derderian S., McClellan W., Wojcicki J.M. The association between cytomegalovirus infection, obesity, and metabolic syndrome in U.S. adult females. Obesity (Silver Spring) 2017;25:626–633. doi: 10.1002/oby.21764. [DOI] [PubMed] [Google Scholar]
- 16.Biswas H.H., Gordon A., Nuñez A., Perez M.A., Balmaseda A., Harris E. Lower Low-Density Lipoprotein Cholesterol Concentrations Are Associated with Severe Dengue Outcome. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan J., Sun W., Li X., et al. Inflammation inhibitors were remarkably up-regulated in plasma of severe acute respiratory syndrome patients at progressive phase. Proteomics. 2006;6:2886–2894. doi: 10.1002/pmic.200500638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jothimani D., Venugopal R., Abedin M.F., Kaliamoorthy I., Rela M. COVID-19 and the liver. J. Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ettinger W.H., Varma V.K., Sorci-Thomas M., et al. Cytokines decrease apolipoprotein accumulation in medium from Hep G2 cells. Arterioscler. Thromb. 1994;14:8–13. doi: 10.1161/01.atv.14.1.8. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen H.J., Heezius E.C., Dallinga G.M., van Strijp J.A., Verhoef J., van Kessel K.P. Lipoprotein metabolism in patients with severe sepsis. Crit. Care Med. 2003;31:1359–1366. doi: 10.1097/01.CCM.0000059724.08290.51. [DOI] [PubMed] [Google Scholar]
- 23.Trinder M., Genga K.R., Kong H.J., et al. Cholesteryl Ester Transfer Protein Influences High-Density Lipoprotein Concentrations and Survival in Sepsis. Am. J. Respir. Crit. Care Med. 2019;199:854–862. doi: 10.1164/rccm.201806-1157OC. [DOI] [PubMed] [Google Scholar]
- 24.Chien Y.F., Chen C.Y., Hsu C.L., Chen K.Y., Yu C.J. Decreased serum concentration of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. J. Crit. Care. 2015;30:506–510. doi: 10.1016/j.jcrc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W., Zhao Y., Zhang F., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon E.M., Figueroa D.M., Barochia A.V., Yao X., Levine S.J. High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease. Front. Pharmacol. 2016;7:323. doi: 10.3389/fphar.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Cui Y., Shen M., et al. Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diabetes Res. Clin. Pract. 2020;165 doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A.K., Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: A narrative review. Diabetes Res. Clin. Pract. 2020;165 doi: 10.1016/j.diabres.2020.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.