Abstract
Purpose
Pulmonary embolism (PE) in COVID-19 patients can play a key role in precipitating clinical conditions. We aimed to evaluate PE distribution on CTA and to investigate any possible association with D-dimer (DD), pulmonary stage of disease and prognosis.
Method
COVID-19 patients of two affiliated Hospitals, undergone a CTA examination for PE suspicion, were retrospectively enrolled. Comorbidities, laboratory tests and clinical outcomes (hospitalization, discharge, death) were assessed. A parenchymal stage (early, progressive, peak, absorption) for lung involvement was assigned.
Results
A cohort of 114 patients (mean age 61 years; 26.3 % females) with severe COVID-19 pneumonia were evaluated. At last follow-up 25 (21.9 %) were hospitalized, 72 (63.2 %) discharged, 17 (14.9 %) dead. Eighty-eight patients (77.2 %) had at least one comorbidity, being cardiovascular ones the most frequent (44.7 %). CTA revealed PE in 65 patients (57 %), with concomitant pulmonary trunk and/or main arteries involvement in 16.9 %. PE defects were ubiquitous in 18.5 % of cases. The predominant parenchymal stages were the progressive (24.6 %) and peak (67.7 %). DD levels showed a significant correlation with PE occurrence and extent in pulmonary branches, despite anticoagulant therapies; trend of correlation with pulmonary stages was also noted.
Conclusions
PE is a frequent complication in severe COVID-19 patients, particularly during central parenchymal stages and despite ongoing anticoagulant therapy. CTA and DD levels play a crucial role in the assessment of suspected PE, despite anticoagulant therapies, along with proper information about lung involvement extent.
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; DD, D-dimer; DIC, disseminated tomography angiography; ER, emergency room; GGO, ground-glass opacities; ICU, intensive care unit; IQR, interquartile range; PE, pulmonary embolism; VTE, venous thromboembolism
Keywords: COVID-19, SARS-CoV-2, Pneumonia, Pulmonary embolism
1. Introduction
In the Coronavirus disease 2019 (COVID-19) work up at emergency room (ER) admission, imaging plays a complementary role to detect lung abnormalities in each suspected patient, either by plain X ray or computed tomography (CT). Additionally, CT allows better estimating lung involvement severity [1], being more sensitive for early parenchymal disease, progression, and alternative diagnoses [2]. In patients with remarkable clinical conditions worsening, the possible role of pulmonary embolism (PE) in precipitating symptoms and blood gas values (despite the prophylactic anticoagulant therapy) has been highlighted. PE has emerged as a common complication in COVID-19 patients with a reported frequency of 16.5 %, rising up to 24.7 % in severe cases admitted to the Intensive Care Unit (ICU) [3]. However, the paucity of data regarding frequency of PE in COVID-19 patients, as well as the effects of PE on their prognosis, lead to uncertainties in the management, particularly in terms of anticoagulant therapy. Computed tomography angiography (CTA) is an established tool to confirm the PE clinical suspicion, even in COVID-19 [4].
The aims of our work are to assess PE distribution on CTA and PE impact on patients affected by severe COVID-19 pneumonia, and to investigate any possible association between PE and lung involvement, d-dimer (DD) levels in blood and short-term prognosis. The originality of the study lies in correlating the occurrence and extent of PE to parenchymal lung stages. The final reference to corresponding pathological data raises questions about phenomenon occurring at a microscopic level and not visible at imaging.
2. Materials and methods
The ethical approval was obtained from the local committee of Papa Giovanni XXIII Hospital, Bergamo (protocol number COVIDPGXXIIIRAD001) for this retrospective study.
Inclusion criteria and clinical data. Among admissions in two affiliated Medical Centers (Papa Giovanni XXIII Hospital and San Giovanni Bianco Hospital, Bergamo), we retrospectively evaluated 114 patients (mean age 61 years, range 23–89), with probable (9) or confirmed (105) severe COVID-19 pneumonia, according to World Health Organization interim guidance documents and radiological data (positive imaging examinations and clinical signs of pneumonia plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; SpO2 < 90 % on room air) [5]. A negative real-time polymerase chain reaction (RT-PCR) in throat swab samples was not an exclusion criterion because of the known reported possibility of contemporary negative RT-PCR and positive imaging results [6]. We enrolled patients who performed a CTA (March 10th - April 2nd 2020) for clinical suspicion of PE and distinguished them in two groups: in-patients at the time of CTA (ICU or non-ICU) and ER patients. The clinical suspicion of PE included one or more of the following acute symptoms together with increased DD levels: worsening dyspnea, pleuritic pain, hemoptysis, circulatory collapse. All in-patients were on anticoagulant regimen since the beginning of the hospitalization (at least standard doses thromboprophylaxis); ER patients were not on any anticoagulant treatment. Patients were followed until two sequential observation times: April 8th 2020 and May 26th 2020. Thus, the first follow-up period ranged between 6–29 days and the second one between 54–77 days after CTA. Outcomes were assessed in terms of current hospitalization (ICU/non-ICU), discharge or death.
Data about patients’ comorbidities on admission were recorded. DD, C-reactive protein (CRP), international normalized ratio (INR), activated partial thromboplastin time (aPTT), platelets count and fibrinogen levels obtained as close as possible to CTA date (maximum interval 4 days) were also available.
2.1. CT acquisition
Chest CTA were performed on a 64-detector CT scanner (Revolution EVO; GE Medical Systems), with an automated tube current modulation at 100 kV. Collimation, pitch, rotation time and CT dose index volume (CTDIvol) were respectively 64 × 0.625 mm, 1.531:1, 0.4 s and 32 mGy (mean value). Slice thickness was 1.25 mm. All but one patient were examined supine. The enhanced scanning was performed during the intravenous bolus injection of a non-ionic contrast medium (∼50−70 mL; flow rate at least 3.5 mL/s; iomeprol 350 mg/mL; Bracco Imaging), followed by a rapid saline solution flush (∼30 mL, flow rate at least 3 mL/s). The acquisition of the arterial pulmonary phase relied on bolus tracking technique: a circular region of interest was manually set on the main pulmonary trunk, with a standard threshold of 100HU and a minimum scanning delay guaranteed by the CT scanner (7 s).
2.2. Image analysis
Image analysis was independently performed by two radiologists (D. C. M. and F. P. N., 10 and 3 years of chest imaging experience, respectively). For the final diagnosis of pulmonary embolism or parenchymal stage we considered the evaluation of the most experienced reader (D.C.M.). In confirmed PE cases on CTA, the thromboembolic distribution in pulmonary lobes (total up to 5) and the eventual pulmonary trunk and/or main pulmonary arteries involvement were annotated. Then for pulmonary involvement a stage was assigned, as reported by Pan F et al. [7]: early (ground-glass opacities (GGO), partial crazy paving pattern, low number of involved lobes), progressive (increase in GGO and crazy paving pattern extension, initial consolidations), peak (prevalent consolidations), absorption (gradual resolution).
2.3. Statistics
Usual descriptive statistics were employed on our sample: median and interquartile range (IQR) for quantitative variables, frequency tables for nominal or ordinal variables; distributions of quantitative variables with or without PE were compared via the Wilcoxon-Mann-Whitney test for independent samples, whereas frequency distributions of nominal or ordinal variables were compared via the Fisher exact test. Differences with two-sided p-values <0.001 were deemed as statistically strongly significant, <0.05 as mildly significant, <0.1 as weakly significant and >0.1 as non-significant. The Kruskal-Wallis test was used to test any statistically significant difference in DD levels for number of lobes involved by PE. To strengthen the analysis on the relationship among DD levels in blood, PE and parenchymal stage, the Scheirer-Ray-Hare test has been employed. Finally, the Cohen’s Kappa index of interobserver agreement among readers with different experiences has been used to evaluate CT reproducibility.
3. Results
3.1. Patients and outcomes
Population included 114 severe COVID-19 pneumonia cases: 84 males (73.7 %) and 30 females (26.3 %), median age of 61 years (IQR 51.2–66, range 23–89). No significant differences were found in terms of gender or age between PE and non-PE groups. A negative RT-PCR in throat swab samples was found in 9 cases, subsequently confirmed as COVID-19 by serological analysis and/or suggestive imaging features. The median time from symptoms’ onset and admission was 7 days (IQR 5–10) and from symptoms’ onset and CTA execution 15 days (IQR 10.2–20), with no significant differences in 2 groups. Patients’ outcomes (ICU/non-ICU, discharged, dead) at first and second follow-up are listed in Table 1 ).
Table 1.
Overview of study population.
| Total (n = 114) | PE (n = 65) | Non-PE (n = 49) | p-value (p < 0.001) | |
|---|---|---|---|---|
| Variables | ||||
| Gender (n) | 0.830 | |||
| - Male | 84 (73.7 %) | 47 (72.3 %) | 37 (75.5 %) | |
| - Female | 30 (26.3 %) | 18 (27.7 %) | 12 (24.5 %) | |
| Age (years) | 61 (51.2–66) | 60 (51–68) | 62 (53–66) | 0.817 |
| Comorbidity (n) | ||||
| - At least one | 88 (77.2 %) | 47 (83.6 %) | 41 (72.3 %) | 0.181 |
| - Cardiovascular | 51 (44.7 %) | 24 (36.9 %) | 27 (55.1 %) | 0.060 |
| - Arterial hypertension | 41 (35.9 %) | 19 (29.2 %) | 22 (44.9 %) | 0.115 |
| - Metabolic | 32 (28.1 %) | 16 (24.6 %) | 16 (32.7 %) | 0.402 |
| - Diabetes mellitus | 17 (14.9 %) | 6 (9.2 %) | 11 (22.4 %) | 0.064 |
| - Overweight or obesity | 13 (11.4 %) | 10 (15.4 %) | 3 (6%) | 0.147 |
| - Dyslipidemia | 11 (9.6 %) | 6 (10 %) | 5 (9.2 %) | 0.999 |
| - Any other (e.g. neoplastic) | 42 (36.8 %) | 24 (36.9 %) | 18 (36.7 %) | 0.999 |
| Time to admission (days) | 7 (5–10) | 7 (5–10) | 7 (4–10) | 0.683 |
| Time to computed tomography angiography (days) | 15 (10.2–20) | 16 (12–20) | 14 (10–20) | 0.409 |
| Thromboprophylaxis (n) | 0.353 | |||
| - At least standard doses | 91 (79.9 %) | 54 (83.1 %) | 37 (75.5 %) | |
| - None | 23 (20.1 %) | 11 (16.9 %) | 12 (24.5 %) | |
| Outcome at first follow-up (n) | 0.032 | |||
| - Hospitalized | ||||
| - Intensive care units | 28 (24.5 %) | 22 (33.8 %) | 6 (12.2 %) | |
| - Non-intensive care units | 49 (43 %) | 26 (40 %) | 23 (47 %) | |
| - Discharged | 25 (22 %) | 10 (15.4 %) | 15 (30.6 %) | |
| - Died | 12 (10.5 %) | 7 (10.8 %) | 5 (10.2 %) | |
| Outcome at second follow-up (n) | 0.179 | |||
| - Hospitalized | ||||
| - Intensive care units | 15 (13.1 %) | 12 (18.5 %) | 3 (6.1 %) | |
| - Non-intensive care units | 10 (8.8 %) | 5 (7.7 %) | 5 (10.2 %) | |
| - Discharged | 72 (63.2 %) | 37 (56.9 %) | 35 (71.5 %) | |
| - Died | 17 (14.9 %) | 11 (16.9 %) | 6 (12.2 %) | |
Data presented as median (interquartile range) or n (%). Differences with two-sided p-values above 0.001 not deemed as statistically strongly significant. PE: pulmonary embolism.
3.2. Comorbidities
Eighty-eight patients (77.2 %) had at least one comorbidity. Cardiovascular comorbidities were prominent (51, 44.7 %) with arterial hypertension being the most frequent disease (41, 35.9 %), followed by metabolic ones (32, 28.1 %), more frequently diabetes mellitus (17, 14.9 %) and overweight/obesity (13, 11.4 %).
3.3. Laboratory tests
Laboratory abnormal results regarded DD (median 4.47 ug/mL, IQR, 1.83–13), CRP (median 11.5 mg/dL, IQR, 2.65–25.5) and fibrinogen (median 534 mg/dL, IQR, 396–750). INR, aPTT and platelets count were in their normal range. Only DD levels demonstrated significant correlation with PE at CTA. CRP demonstrated a higher trend in non-PE patients, but a statistically significant difference was not achieved (Table 2 ).
Table 2.
Laboratory tests’ results and correlation with presence or absence of pulmonary embolism.
| Median (IQR) | PE (n = 65) | Non-PE (n = 49) | p-value (p < 0.001) | |
|---|---|---|---|---|
| Variables | ||||
| DD (ug/mL) | 4.47 (1.83–13) | 8.39 (3.19–23.2) | 2.61 (1.17–4.97) | <0.001* |
| CRP (mg/dL) | 11.5 (2.65–25.5) | 6.2 (3.7–27.4) | 13.2 (2–22.2) | 0.096 |
| INR | 1.08 (1.02–1.16) | 1.06 (1.05–1.19) | 1.10 (1–1.13) | 0.056 |
| aPTT | 1.03 (0.92–1.17) | 1.03 (0.91–1.2) | 1.03 (0.94–1.15) | 0.978 |
| Platelets count (109/L) | 261 (176–361) | 258 (172–361) | 265 (178–348) | 0.702 |
| Fibrinogen (mg/dL)^ | 534 (396–750) | 612 (402–609) | 506 (384–838) | 0.163 |
Data presented as median (IQR). Differences with two-sided p-values below 0.001 deemed as statistically strongly significant and marked with *. PE: pulmonary embolism; DD: d-dimer; CRP: C-reactive protein; INR: international normalized ratio; aPTT: activated partial thromboplastin time; ^: plasma levels.
3.4. Imaging findings and relevant correlations
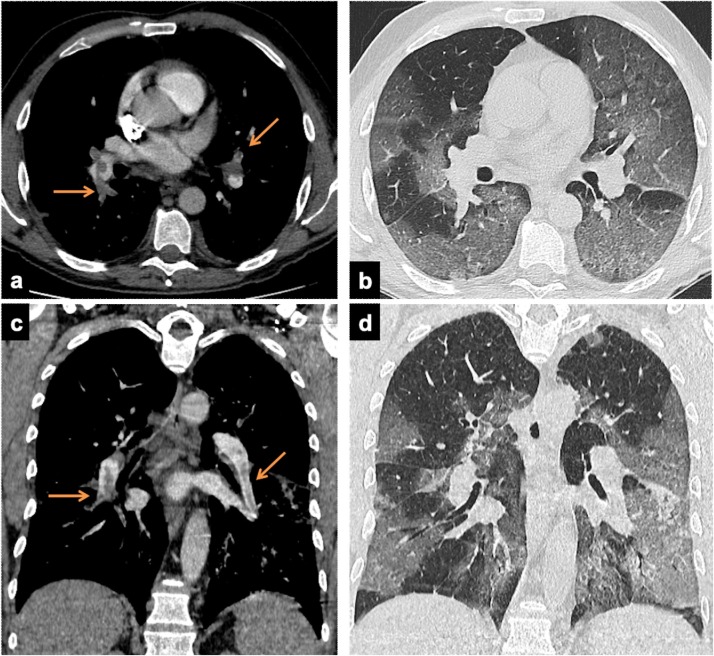
The clinical PE suspicion relayed on a sudden worsening breathing status during the hospitalization (91 patients, 79.9 %) or on ER arrival (23 patients, 20.1 %). CTA revealed PE in 65/114 patients (57 %): arteries of one pulmonary lobe in 40 %, ubiquitous in 18.5 %, main trunk and/or main pulmonary arteries in 11 cases (16.9 %) (Fig. 1 ). The lobes where most frequently PE occurred were the right lower (49, 75.3 %), the left (37, 56.9 %) and the right upper (31, 47.7 %).
Fig. 1.
Axial (a and c) and paracoronal (b and d) CT images in two 56 years old males, with d-dimer levels of 13.16 ug/mL and 4.99 ug/mL respectively. CTA demonstrate thromboembolic defects involving the pulmonary trunk and main arteries (arrows, a and b). The lung window demonstrates bilateral patchy consolidations and GGO (progressive stage).
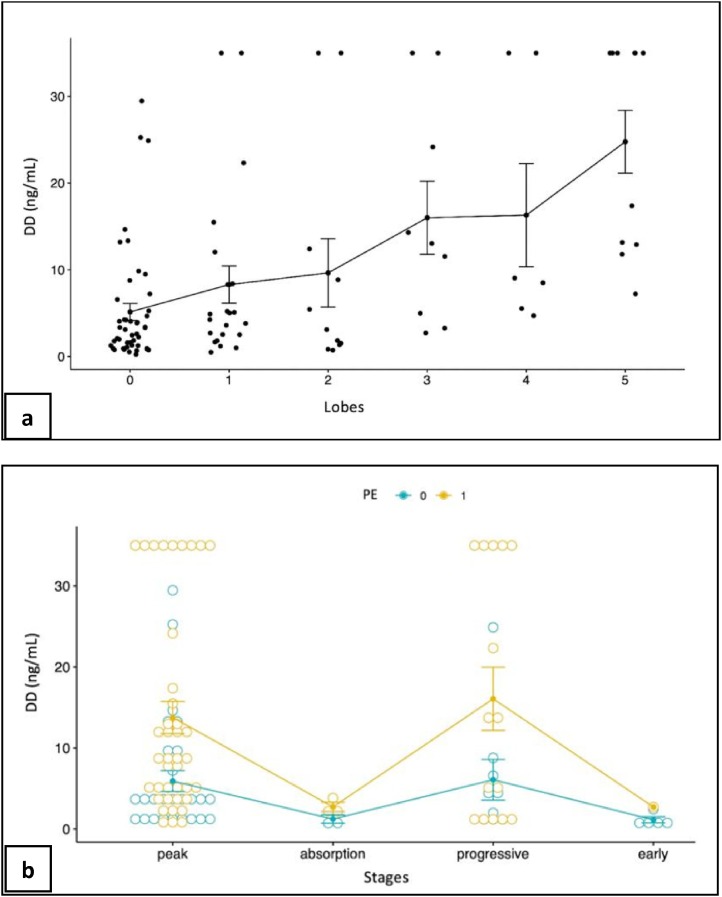
According to the Scheirer-Ray-Hare, DD blood levels showed a significant correlation both with PE occurrence (p < 0.001) and distribution (segmental/subsegmental pulmonary arteries (p < 0.001) and trunk/main pulmonary arteries (p-value 0.003)). DD levels significantly increased in cases of main pulmonary arteries involvement (4.06–18.7 u g/mL in median, p = 0.003) and with increasing number of lobes involved (especially for the left lower lobe) (Fig. 2 ).
Fig. 2.
a: d-Dimer levels distribution (ng/mL) for different number of involved lobes. The vertical bars represent the interval: mean ± standard deviation. b: D-Dimer levels distribution (ng/mL) for different stages and PE occurrence. The vertical bars represent the interval: mean ± standard deviation. PE 0: no PE occurrence; PE 1: PE occurrence.
The most common parenchymal stages were the peak (74, 64.9 %) and the progressive (26, 22.8 %) (Table 3 ). The majority of PE (60, 92.3 %) occurred during the peak (44, 67.7 %) and the progressive (16, 24.6 %) stages (Fig. 3, Fig. 4 ). The interobserver agreement for assigning parenchymal stages was excellent (Kappa 0.966). The Scheirer-Ray-Hare test also revealed a statistically significant relation between the pulmonary stage and DD levels, albeit with non-significant interaction effect with the presence of PE (Fig. 2).
Table 3.
Parenchymal stages and pulmonary embolism.
| Total (n = 114) | PE (n = 65) | Non-PE (n = 49) | p-value (p < 0.001) | |
|---|---|---|---|---|
| Stages | 0.229 | |||
| Early | 6 (5.3 %) | 1 (1.5 %) | 5 (10.2 %) | |
| Progressive | 26 (22.8 %) | 16 (24.6 %) | 10 (20.4 %) | |
| Peak | 74 (64.9 %) | 44 (67.7 %) | 30 (61.2 %) | |
| Absorption | 8 (7%) | 4 (6.2 %) | 4 (8.2 %) |
Data presented as n (%). Differences with two-sided p-values above 0.001 not deemed as statistically strongly significant.
Fig. 3.
CT images from a 37 years old COVID-19 female patient with history of rheumatoid arthritis: peripheral lower lobe subocclusive embolic defects (arrows, a and c) and lower lobes extensive consolidations mixed to GGO, with pleural effusions (peak stage). D-dimer levels were 5.09 ug/mL.
Fig. 4.
Axial (a and b) and paracoronal (c and d) CT images in a 49 years old male with no comorbidities, admitted to CT from a non-ICU ward. Multiple bilateral embolic defects involving the segmental and subsegmental pulmonary arteries (branches arrows, a and c). This patient showed also a concomitant progressive stage of lung disease (bilateral extensive GGO and crazy paving). D-dimer levels were > 35 ug/mL.
4. Discussion
Our data show a higher PE frequency in patients with severe COVID-19 pneumonia if compared to data available in literature [8].
This is one of the few studies available in which PE on imaging and its clinical significance in COVID-19 has been described [9,10]. Chen and colleagues performed 25 CTA in 1008 patients diagnosing PE in 10 (mainly small branches). In our population we confirmed PE in 65/114 suspected cases. In about 83 % of cases, thromboembolism involved only the smaller segmental/subsegmental arteries, less frequently also the pulmonary trunk/main arteries (16.9 %). This discrepancy remains unclear, but it may be partially explained by the small number of CTA performed by Chen and colleagues; therefore, some PE may have been missed. Also, there could be a difference for venous thromboembolism (VTE) incidence between European and Asian populations [11]. Moreover, Authors do not explain if patients were under anticoagulant treatment, as heparin usually improves the hypercoagulable state of sepsis and reduces disseminated intravascular coagulation (DIC) incidence [12]. DIC has been described common in deaths with COVID-19 pneumonia and abnormal coagulation results (particularly DD) are suggested to guide therapy and prognosis [13]. Ninety-one of our patients (79.9 %) were hospitalized at the time of CTA. Despite the anticoagulant therapy since their hospitalization, they demonstrated a higher PE incidence (54, 83.1 %); no significant differences were found with patients without anticoagulant therapy (p-value 0.353). Moreover, the findings of a normal platelets count in PE patients may collocate the PE occurrence in a pre-DIC period [12].
Klok and colleagues [14] evaluated the PE incidence, DVT, ischemic stroke, myocardial infarction and systemic arterial embolism in 184 critically ill COVID-19 patients. Diagnostic tests were only applied if thrombotic complications were clinically suspected. They reported thrombotic complications (especially PE) in 31 % of ICU COVID-19 patients despite systematic thrombosis prophylaxis, but no DIC.
Cui and colleagues [15] enrolled 81 ICU patients with severe COVID-19 pneumonia and retrospectively evaluated the lower limbs sonographies performed, reporting VTE in 20 cases (25 %); 8 died. Authors argued that patients with and without VTE differed in terms of DD levels, proposing a DD cut-off value of 1.5 ug/mL to improve accuracy in predicting VTE. In a French cohort of 106 pulmonary CTA, a DD threshold of 2660 μg/L detected all PE [10]. Among COVID-19 patients, DD has been related to a more severe disease [16], poor prognosis [15,17], and mortality [13]. Guan and colleagues [16] reported significantly higher DD levels in deceased people as compared to survivors. Conversely other Authors [18] advise against DD levels as a screening tool. The role of DD in COVID-19 patients still remains unclear [19].
Our study outlined abnormal DD values in all patients, but we also found a significant correlation between DD and PE occurrence (p-value < 0.001). Moreover, DD correlated with PE extension in segmental/subsegmental arteries (p-value < 0.001) and the eventual involvement of the pulmonary trunk/main branches (p-value 0.003). Therefore, DD should be considered predictive of PE occurrence and extent. As DD levels in severe COVID-19 patients are almost constantly above the upper limit of the usual range (0.5 ug/mL), a different cut-off value in this peculiar group of patients could be a remarkable suggestion.
As confirmed by international guidelines [20], in COVID-19 fibrinogen serum levels assessment is useful. Tang and colleagues observed fibrinogen to be significantly lower in non-survivors [13]. Median fibrinogen level was higher than normal in some of our cases (534 mg/dL, range 396–750), without significant differences between PE and non-PE groups (p-value 0.163). Other studies confirm the requirement for further consideration of fibrinogen levels in COVID-19 [21,22].
No studies still investigated a correlation between COVID-19 parenchymal involvement and PE occurrence. Our results demonstrated a statistically significant correlation between pulmonary stage and DD levels, despite a non-significant interaction effect. The most relevant number of PE occurred during the progressive (16/65, 24.6 %) or peak stages (44/65, 67.7 %). These results may be partially biased by the characteristics of our population, clinically severe at the time of the CTA with a corresponding critical parenchymal stage (26 progressive, 74 peak). Considering this prevalence of PE during the critical stages of parenchymal disease may help in those scenarios where only unenhanced CT examinations are available. Moreover, the high degree of interobserver agreement among readers with different experiences supports a good CT reproducibility.
Advanced age [17], male gender and comorbidities are suggested risks factors for severe disease and death from COVID-19 [16]. Comorbidities in our population were frequent (at least one in 88 patients), especially arterial hypertension (35.9 %), diabetes mellitus (14.9 %) and overweight/obesity (11.4 %), in line with other results [23]. However, there were no differences between comorbidities and PE/non-PE groups.
In our cohort PE did not appear to affect both short-term (7 patients from PE group and 5 from non-PE group dead at the first follow-up, p-value 0.032) and medium-term mortality (11 patients from PE group and 6 from non-PE group dead at the last follow-up, p-value 0.179), in contrast with other studies [19].
Moreover, a strict correlation between PE and anatomopathological data may be useful in order to match radiological imaging and histopathological feature; one main point of discussion is if imaging might miss PE occurring in the smallest caliber pulmonary branches below CT spatial resolution. According to this rationale, we here report the figurative correlation between imaging and anatomopathological picture in two of our deceased patients (Fig. 5, Fig. 6 ).
Fig. 5.
Axial (a) and paracoronal (f) CT images in a COVID-19 43 years old female with no comorbidities, showing a peak stage of lung disease (extensive consolidations complicated by right pneumothorax; orange stars, a and f). CTA (d and e) revealed bilateral embolic defects (orange arrows, e). Patient died 3 days later. (b) Large pulmonary artery branch sub-total luminal thrombosis; perivascular lung parenchyma shows diffuse vascular congestion, multiple thrombosed septal capillaries and focal alveolar edema (Hematossilin and Eosin, 400X). c) Complete thrombosis of a medium size pulmonary artery branch in emphysematous lung (Hematossilin and Eosin, 400X).
Fig. 6.
Axial CT images (a and d) in a 71 years old male with D-dimer levels of 1.99 ug/mL. CTA revealed extensive lower lobes consolidation (peak stage), bronchiectasis and distal airspaces distension (orange arrows, a and d). CTA did not show any embolic defect, later demonstrated in small caliber branches during the anatomopathological analysis. b) Severely emphysematous lung parenchyma showing few septal capillaries with dilated and thrombosed lumen (Hematossilin and Eosin, 400X). c) Details of massive emphysema and focal septal capillaries thrombosis (Hematossilin and Eosin, 400X).
The first limitation of our retrospective study is the short follow-up period, potentially missing PE in patients who were negative for PE at the time of CTA examination but still hospitalized at last follow-up. Another limitation is the different anticoagulant therapy administered to the patients: not having correlate PE occurrence with anticoagulant dosages, it was not possible to evaluate whether PE was less frequent in patients under higher anticoagulant therapy doses.
5. Conclusions
PE is a very frequent occurrence in COVID-19 severe pneumonia, representing one of the major concerns in critically ill patients. DD demonstrated to have the role of predicting both PE occurrence and extent. On the other hand, an ongoing anticoagulant therapy at standard doses should not rule out the possibility of PE occurrence. Therefore, imaging plays a crucial role in the assessment of suspected PE. This, along with pulmonary findings, can aid in guiding clinical decisions and patient’s management.
Author contributions
V.C., B. P. A. and S. S. substantially contributed to the design of the work; M. E., F. P. N., D. C. M., S.A. and G.A. contributed to the acquisition and interpretation of data for the work; V. G. substantially contributed to the analysis of data for the work. V.C. drafted the work; B. P. A., S. S., M. E., F. P. N., D. C. M., S. A., G.A. and V. G. revised the work critically for important intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Role of the sponsors
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
The ethical approval was obtained from the local committee of Papa Giovanni XXIII Hospital, Bergamo (protocol number COVIDPGXXIIIRAD001).
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
All participants gave written or oral (in case of physically isolated patients due to COVID-19 infection) informed consent.
Methodology
-
•
Retrospective
-
•
Revision of the Literature
-
•
Performed at one Institution
Guarantor
The scientific guarantor of this publication is Professor Sandro Sironi.
CRediT authorship contribution statement
C. Valle: Conceptualization, Writing - original draft, Investigation, Methodology, Project administration, Visualization. P.A. Bonaffini: Conceptualization, Writing - review & editing, Methodology, Validation, Project administration. M. Dal Corso: Data curation, Investigation. E. Mercanzin: Data curation, Investigation. P.N. Franco: Data curation, Investigation, Writing - review & editing, Visualization, Resources, Project administration. A. Sonzogni: Data curation, Resources. G. Vacca: Formal analysis, Software. A. Gianatti: Data curation. S. Sironi: Conceptualization, Validation, Supervision.
Declaration of Competing Interest
All authors declare no competing interests.
References
- 1.Inui S., Fujikawa A., Jitsu M., et al. Chest CT findings in cases from the cruise ship “diamond princess” with coronavirus disease 2019 (COVID-19) Radiol Cardiothorac Imaging. 2020 doi: 10.1148/ryct.2020204002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin G.D., Ryerson C.J., Haramati L.B., et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Chest. 2020;158(1):106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh Y.J., Hong H., Ohana M., et al. Pulmonary embolism and Deep Vein Thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2020 doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mossa-Basha M., Medverd J., Linnau K.F., et al. Policies and guidelines for COVID-19 preparedness: experiences from the University of Washington. Radiology. 2020;296(2):E26–E31. doi: 10.1148/radiol.2019201326. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . 2020. Clinical Management of Severe Acute Respiratory Infection When COVID-19 Is Suspected.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 24 Apr 2020) [Google Scholar]
- 6.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Wang X., Zhang S., et al. Characteristics of acute pulmonary embolism in patients with COVID-19 associated pneumonia from the city of Wuhan. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Léonard-Lorant I., Delabranche X., Séverac F., et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-Dimer levels. Radiology. 2020;296(3):E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakai N.A., McClure L.A. Racial differences in venous thromboembolism. J. Thromb. Haemost. 2011;9(10):1877–1882. doi: 10.1111/j.1538-7836.2011.04443.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu X.L., Wang X.Z., Liu X.X., et al. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: a prospective clinical study. Exp. Ther. Med. 2014;3:604–608. doi: 10.3892/etm.2013.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;91:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casey K., Iteen A., Nicolini R., Auten J. COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. Am. J. Emerg. Med. 2020;38(7) doi: 10.1016/j.ajem.2020.04.011. 1544.e1-1544.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br. J. Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranucci M., Ballotta A., Di Dedda U., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. 2020;18(7):1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J., Tong Z., Guan X., Du B., Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]