Abstract
A decreased lung diffusing capacity for carbon monoxide (DLCO) has been reported in a variable proportion of subjects over the first 3 months of recovery from severe coronavirus disease 2019 (COVID‐19). In this study, we investigated whether measurement of lung diffusing capacity for nitric oxide (DLNO) offers additional insights on the presence and mechanisms of gas transport abnormalities. In 94 subjects, recovering from mild‐to‐severe COVID‐19 pneumonia, we measured DLNO and DLCO between 10 and 266 days after each patient was tested negative for severe acute respiratory syndrome coronavirus 2. In 38 subjects, a chest computed tomography (CT) was available for semiquantitative analysis at six axial levels and automatic quantitative analysis of entire lungs. DLNO was abnormal in 57% of subjects, independent of time of lung function testing and severity of COVID‐19, whereas standard DLCO was reduced in only 20% and mostly within the first 3 months. These differences were not associated with changes of simultaneous DLNO/DLCO ratio, while DLCO/VA and DLNO/VA were within normal range or slightly decreased. DLCO but not DLNO positively correlated with recovery time and DLCO was within the normal range in about 90% of cases after 3 months, while DLNO was reduced in more than half of subjects. Both DLNO and DLCO inversely correlated with persisting CT ground glass opacities and mean lung attenuation, but these were more frequently associated with DLNO than DLCO decrease. These data show that an impairment of DLNO exceeding standard DLCO may be present during the recovery from COVID‐19, possibly due to loss of alveolar units with alveolar membrane damage, but relatively preserved capillary volume. Alterations of gas transport may be present even in subjects who had mild COVID‐19 pneumonia and no or minimal persisting CT abnormalities.
Trial registry
ClinicalTrials.gov PRS: No.: NCT04610554 Unique Protocol ID: SARS‐CoV‐2_DLNO 2020.
Keywords: alveolar membrane diffusive conductance, carbon monoxide, COVID‐19, ground glass opacities, lung diffusing capacity, nitric oxide
An impairment of lung diffusing capacity for nitric oxide exceeding standard DLCO may be present during the recovery from COVID‐19, possibly due to loss of alveolar units with alveolar membrane damage

1. INTRODUCTION
Infection with the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been the cause, in a variable number of subjects, of a disease named severe coronavirus disease 2019 (COVID‐19) showing clinical manifestations ranging from mild upper airway symptoms to interstitial pneumonia with or without acute hypoxemic respiratory failure (Guan et al., 2020). Among the distinctive features of COVID‐19, in comparison with influenza virus pneumonia, are an increase of serum D‐dimer and, at autopsy, the presence of alveolar damage with widespread thrombotic microangiopathy (Ackermann et al., 2020). SARS‐CoV‐2 targets preferentially type II alveolar cells (Mason et al., 2020), which are the precursors for type I cells; thus, it can be hypothesized that COVID‐19 survivors might develop gas exchange abnormalities because of aberrant alveolar wound healing, or loss of pulmonary vascular bed, or both.
Three preliminary studies found a mild decrement of lung diffusing capacity for carbon monoxide (DLCO) in about half of subjects 1 month after symptom onset (Frija‐Masson et al., 2020; Mo et al., 2020) or hospital discharge (Huang et al., 2020). Two studies found DLCO be reduced in 21% (Sonnweber et al., 2020) and 24% (Lerum et al., 2020) of subjects about 3 months after hospital admission, and one study in 34% of subjects 3 months after recovery from the acute phase of disease (van den Borst et al., 2020). Two of these studies (Lerum et al., 2020; Mo et al., 2020) also reported values of DLCO‐to‐alveolar volume (DLCO/VA) ratio, that is, KCO, to be slightly decreased (Mo et al., 2020) or within the normal range (Lerum et al., 2020) in the majority of subjects, which would suggest an alveolar damage associated with diffuse microvascular destruction (Hughes & Pride, 2012). However, the interpretation of the above findings is complicated by differences in the cutoffs for defining DLCO abnormality, coexisting comorbidities, time of lung function studies, and severity of disease in the acute phase. Moreover, the major limit to lung CO uptake is its slow binding with intracapillary hemoglobin (Hb), which makes DLCO unable to distinguish between reductions of alveolar membrane diffusive conductance (DM) and pulmonary capillary blood volume (VC) (Borland & Hughes, 2020; Guénard et al., 1987). By contrast, nitric oxide (NO) has a much greater affinity and faster reaction rate with Hb than CO (Gibson & Roughton, 1957), which make the lung diffusing capacity for NO (DLNO) more sensitive to changes in DM than VC (Borland & Hughes, 2020; Guénard et al., 1987). Indeed, recent studies on interstitial lung diseases (Barisione et al., 2016, 2019) have shown that DLNO reflects fibrotic changes more accurately than standard DLCO.
Thus, considering the complex pathophysiology of COVID‐19 (Ackermann et al., 2020; Mason et al., 2020), we undertook the present study to investigate whether measurements of DLNO and DLCO can provide different information on gas exchange abnormalities persisting after COVID‐19 that may be related to radiological findings, severity of pneumonia, and time of recovery.
2. MATERIALS AND METHODS
2.1. Study subjects
This study included 94 Caucasian subjects who attended our pulmonary function laboratory as outpatients for follow‐up after in‐hospital treatment for COVID‐19 pneumonia, confirmed by ground glass opacities (GGO) or band‐like consolidations on chest roentgenogram or computed tomography (CT) and positive nasopharyngeal swabs for SARS‐CoV‐2. Pulmonary function tests were obtained between 10 and 266 days after hospital discharge, which occurred only after each patient had been tested negative for SARS‐CoV‐2. To be included in the study, subjects were required not to have history of comorbidities potentially affecting lung diffusing capacity, that is, bronchial asthma, chronic obstructive pulmonary disease, pulmonary interstitial fibrosis or vasculitis, systemic collagen disease, congestive heart failure, liver or renal diseases, and morbid obesity. They were classified in three groups based on the presence or severity of acute hypoxemic respiratory failure and the respiratory support received during hospitalization (Table 1). Acute hypoxemic respiratory failure was diagnosed whenever the measured oxygen partial pressure (PaO2) in an arterial blood sample drawn from the radial artery during room air breathing was below the age‐adjusted lower limit of normal (Cerveri et al., 1995). The first group included 34 subjects who had no arterial hypoxemia, a second group included 34 subjects who had mild‐to‐moderate arterial hypoxemia treated by O2‐supplementation with (n = 31) or without (n = 3) helmet continuous positive airway pressure, and a third group included 26 subjects who had severe arterial hypoxemia treated by O2‐supplementation and invasive mechanical ventilation via tracheal intubation (n = 23) or tracheostomy (n = 3). During hospitalization, they had received antibiotics (n = 63), oral hydroxychloroquine (n = 49), corticosteroids (n = 43), enoxaparin (n = 37), tocilizumab or anakinra,
TABLE 1.
Subjects’ anthropometric characteristics and lung function data (n = 125)
| Controls | COVID‐19 Severity | p value | |||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| Male/Female | 22/9 | 21/13 | 21/13 | 23/3 | 0.20 |
| Age (years) | 57 ± 12 | 62 ± 14 | 61 ± 10 | 60 ± 11 | 0.38 |
| Stature (cm) | 171 ± 11 | 167 ± 9 | 169 ± 10 | 171 ± 8 | 0.29 |
| BMI (kg·m−2) | 25 ± 3 | 27 ± 4 | 29 ± 4* | 28 ± 4 | 0.003 |
| Smokers (current‐former/never) | 16/15 | 22/12 | 18/16 | 12/14 | 0.41 |
| FVC (L) | 4.68 ± 1.28 | 4.00 ± 0.88* | 3.90 ± 1.04* | 4.00 ± 0.87 | 0.011 |
| (% predicted) | 112 ± 14 | 108 ± 14 | 102 ± 16* | 97 ± 15* , † | <0.001 |
| (z‐score) | 0.79 ± 0.93 | 0.53 ± 0.89 | 0.11 ± 1.11* | −0.25 ± 0.98* , † | <0.001 |
| FEV1 (L) | 3.61 ± 0.99 | 3.04 ± 0.71* | 3.08 ± 0.77* | 3.10 ± 0.66 | 0.015 |
| (% predicted) | 110 ± 13 | 105 ± 14 | 104 ± 18 | 96 ± 15* | 0.004 |
| (z‐score) | 0.71 ± 0.90 | 0.37 ± 0.96 | 0.25 ± 1.17 | −0.25 ± 0.99* | 0.003 |
| TLC (L) | 6.68 ± 1.43 | 5.86 ± 1.01* | 5.60 ± 1.24* | 5.68 ± 1.28* | 0.003 |
| (% predicted) | 106 ± 12 | 100 ± 13 | 94 ± 15* | 87 ± 14* , † | <0.001 |
| (z‐score) | 0.51 ± 1.08 | −0.06 ± 1.10 | −0.61 ± 1.42* | −1.16 ± 1.33* , † | <0.001 |
| DLCO (mL·min−1·mmHg−1) | 30.3 ± 8.80 | 23.2 ± 6.71* | 21.4 ± 6.65* | 22.4 ± 5.60* | <0.001 |
| (% predicted) | 118 ± 19 | 100 ± 22* | 89 ± 20*, † | 87 ± 19* , † | <0.001 |
| (z‐score) | 0.99 ± 1.04 | −0.06 ± 1.29* | −0.80 ± 1.41*, † | −0.91 ± 1.30* , † | <0.001 |
| DLCO/VA (mL·min−1·mmHg−1·L−1) | 4.52 ± 0.69 | 4.04 ± 0.77 | 3.90 ± 0.76* | 4.04 ± 0.74* | 0.005 |
| (% predicted) | 103 ± 15 | 94 ± 17 | 90 ± 19* | 92 ± 16* | 0.007 |
| (z‐score) | 0.22 ± 0.93 | −0.41 ± 1.08 | −0.68 ± 1.26* | −0.59 ± 1.04* | 0.007 |
| DLNO (mL·min−1·mmHg−1) | 124.8 ± 37.1 | 96.9 ± 29.7* | 89.5 ± 28.5* | 91.7 ± 23.0* | <0.001 |
| (% predicted) | 90 ± 10 | 78 ± 17* | 69 ± 15* | 65 ± 13* , † | <0.001 |
| (z‐score) | −0.69 ± 0.71 | −1.44 ± 1.10* | −1.98 ± 1.05* | −2.47 ± 1.02* , † | <0.001 |
| DLNO/VA (mL·min−1·mmHg−1·L−1) | 19.2 ± 3.02 | 17.8 ± 3.40 | 17.3 ± 3.14 | 17.3 ± 3.06 | 0.06 |
| (% predicted) | 88 ± 9 | 86 ± 14 | 82 ± 13 | 82 ± 13 | 0.10 |
| (z‐score) | −0.90 ± 0.70 | −1.08 ± 1.02 | −1.38 ± 0.96 | −1.40 ± 0.99 | 0.10 |
| DLNO /DLCO | 4.22 ± 0.43 | 4.19 ± 0.59 | 4.34 ± 0.66 | 4.35 ± 0.43 | 0.37 |
Data are absolute numbers or mean ± SD; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; DLCO, standard single‐breath lung diffusing capacity for carbon monoxide; DLNO, single‐breath lung diffusing capacity for nitric oxide; VA, alveolar volume; DLNO/DLCO, ratio of simultaneous DLNO and DLCO measurements.
Significantly different from controls.
Significantly different from mild group.
(n = 24), and various antiviral drugs (n = 18). As a control group, we selected 31 healthy subjects, matched for anthropometric characteristics and smoking habit, among health professionals and their relatives studied before the onset of COVID‐19 pandemic.
2.2. Lung function measurements
Spirometry (Graham et al., 2019) and lung volumes (Wanger et al., 2005) were determined with subjects sitting in a whole‐body plethysmograph (V62 J, SensorMedics‐Viasys, CareFusion; Höchberg, Germany) and breathing quietly with a nose clip in place. Forced vital capacity (FVC), forced expiratory volume in one second (FEV1), their ratio (FEV1/FVC), and total lung capacity (TLC) were measured and compared with predicted values (Quanjer et al., 1993, 2012).
Standard DLCO was measured (MasterScreen PFT System, Jaeger‐Viasys, CareFusion, Höchberg, Germany) by single‐breath technique with a measured breath‐hold time of 11 ± 0.4 s. Maneuvers with inspired volume ≥85% of vital capacity, 8–12 s breath‐hold time, and sample collection ≤4 s were retained for analysis (Graham et al., 2017). Results were compared with the predicted values from Stanojevic et al. (2017) after adjustment for effective Hb measured in available arterial or venous blood samples ( (Cotes et al., 1972).
At least 5–10 min after standard DLCO, single‐breath DLNO and DLCO were simultaneously measured with an actual breath‐hold time of 5 ± 0.3 s as detailed elsewhere (Barisione et al., 2016, 2019), and the DLNO/DLCO ratio calculated. Predicted values for DLNO and DLNO/VA were from Zavorsky et al. (2017).
Personnel wearing equipment against exposure to SARS‐CoV‐2 did all testing and instrument cleaning disinfection procedures.
2.3. Chest CT
In 38 subjects, a thin‐section CT scan obtained between 0 and 207 days after hospital discharge and 34 days (median 8 days; interquartile range 25–75% [IQR25‐75%] 0–19) before or after pulmonary function measurements was available. Scans of the entire chest were obtained in a supine position, during breath‐holding at full inspiration, by a multi‐detector row‐spiral scanner (SOMATOM Emotion 6, Siemens AG Medical, Forchheim, Germany). Images were acquired by 110 kVp tube voltage at 1.25‐mm slice thickness and reconstructed at 1‐mm increments using smooth (B41 s) and sharp (B70 s) convolution kernels. CT scans acquired at an absolute lung volume ≥80% of plethysmographic TLC were retained for semiquantitative calculation of voxel percentages with GGO at six axial levels (Barisione et al., 2016, 2019) and automatic quantitative 3D analysis of mean lung attenuation (MLA) and its coefficient of variation (MLA CV %) for the entire lung (ITK‐Snap 3.8.0, Philadelphia, PA, US) (Yushkevich et al., 2006).
2.4. Statistical analysis
For each lung function measure, we calculated the percentage of predicted and z‐score values. As lower limits of normality for DLNO and standard DLCO, we considered both 5th (LLN5, z‐score −1.645) and 2.5th (LLN2.5, z‐score −1.96) percentiles of the reference population. Categorical variables were compared by z‐test with Yates correction, while Fisher's exact test was used to compare their distributions. Continuous variables were tested by one‐way pairwise ANOVA with Holm‐Sidak post hoc test for multiple comparisons. Associations between variables were tested for significance by the coefficient of
determination (R2). The difference between two dependent correlations with one variable in common was calculated by an asymptotic two‐tailed z‐test, with values >1.96 considered significant (Steiger 1980). Data are presented as mean ±SD or median with IQR25‐75% whenever appropriate. In all analyses, the acceptable type I error was set at p < 0.05.
3. RESULTS
Collectively, all standard lung function measures and DLNO were significantly lower in the three COVID‐19 groups than in the control group, whereas DLNO/VA and DLNO/DLCO ratios did not differ significantly.
There was a significant correlation between DLNO and standard DLCO z‐scores (R2: 0.59; p < 0.0001) (Figure 1a). However, considering individual data, 35 subjects (37%) had DLNO but not DLCO below the LLN5, and 30 of them also below the LLN2.5, 19 subjects (20%) had both DLNO and DLCO below the LLN5 and 16 of them also below the LLN2.5, 40 subjects (43%) had both DLNO and DLCO above the LLN5 and 47 of them also above the LLN2.5, and only one subject had DLCO but not DLNO below the LLN2.5. There were no significant differences in the distribution of subjects with reduced DLNO, DLCO, or both in relation to the presence or severity of acute hypoxemic respiratory failure and type of respiratory support received during hospitalization. The DLNO/DLCO ratio was in the majority of COVID‐19 subjects within 1.96 SD of the values observed in the control group (Figure 1b).
FIGURE 1.

Panel a: Relationship between z‐scores of standard lung diffusing capacity for carbon monoxide (DLCO) and lung diffusing capacity for nitric oxide (DLNO). Horizontal and vertical lines correspond to the 5th (dashed) and 2.5th (dotted) percentiles of reference values, that is, −1.645 and −1.96 z‐scores, respectively. The numbers within brackets indicate the subjects falling into each quadrant (Q1‐Q4) bounded within 5th or 2.5th percentiles. Symbols indicate subjects recovering from mild (white), moderate (gray), and severe (black) COVID‐19 pneumonia. Panel b: Correlation between simultaneous measures of DLNO and DLCO. Upper and lower oblique dashed lines indicate the 95% confidence interval for DLNO/DLCO ratio in healthy controls
There was a weak albeit significant positive correlation between standard DLCO (R2 = 0.06; p = 0.014) but not DLNO (R2 = 0.02; p = 0.15) and the time elapsed between negative test for SARS‐CoV‐2 and lung function studies (Figure 2a,b). Notably, of the 58 subjects studied after 3 months, 30 had DLNO below the LLN5 and 25 also below the LLN2.5, while only six had DLCO below the LLN5 and LLN2.5 (p < 0.001).
FIGURE 2.
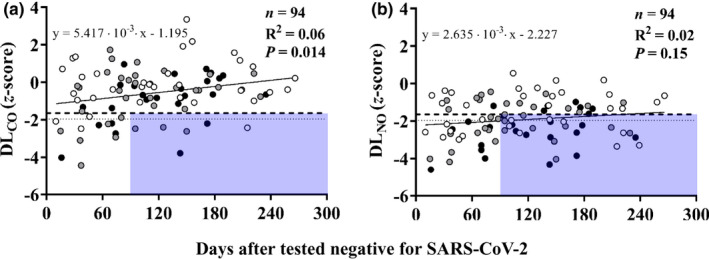
Relationships between standard DLCO (panel a) or DLNO (panel b) and time elapsed from negative testing for SARS‐CoV‐2 to lung function studies. Symbols indicate subjects who recovered from mild (white), moderate (gray), and severe (black) COVID‐19 pneumonia. Horizontal lines correspond to the 5th (dashed) and 2.5th (dotted) percentiles of reference values, that is, −1.645 and −1.96 z‐scores, respectively. The shaded areas include the subjects with abnormal standard DLCO or DLNO values after the first 3 months of recovery
The CT scans obtained within 34 days from lung function studies showed GGO above 5% of total lung volume be present in 21 (55%) of the 38 subjects examined (Figure 3a,b). Both DLNO and standard DLCO z‐scores were inversely related to the extent of GGO with correlation coefficients insignificantly different (p = 0.61) between each other but y‐intercepts significantly (p < 0.0001) lower for DLNO than standard DLCO. Therefore, reduced DLNO was associated with GGO more frequently than DLCO. Similar correlations were observed between DLNO or standard DLCO with MLA or MLA CV% (Figure 3c‐f). Figure 4 shows an example of wide discrepancy between DLNO and standard DLCO in a subject with moderate CT abnormality. Quantitative analysis of the entire lung and qualitative analysis at six axial levels did not reveal areas of reticular opacities, honeycombing, or hypoattenuation (<−950 HU) in any subject.
FIGURE 3.
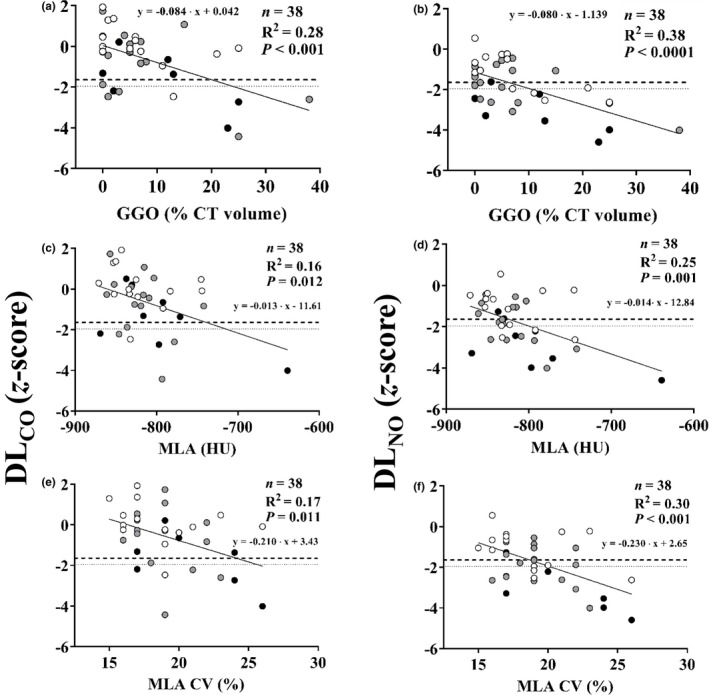
Correlations between standard DLCO (panels a, c, and e), or DLNO (panels b, d, and f) and ground glass opacities (GGO), as percentage of total CT volume, mean lung attenuation (MLA) in Hounsfield units (HU), and its coefficient of variation (MLA CV%). Symbols indicate subjects who recovered from mild (white), moderate (gray), and severe (black) COVID‐19 pneumonia. Horizontal lines correspond to the 5th (dashed) and 2.5th (dotted) percentiles of reference values, that is, −1.645 and −1.96 z‐scores, respectively
FIGURE 4.

Axial CT scan acquired at the bifurcation of main bronchi (carina) in supine position in a representative subject who had severe COVID‐19 pneumonia treated by invasive mechanical ventilation. Note the discrepancy between DLNO and standard DLCO in the presence of moderate GGO extent. Abbreviations as in Table 1
4. DISCUSSION
The main findings of the present study are that 1) abnormal DLNO was present in more than half of the subjects over 8 months of recovery from mild‐to‐severe COVID‐19 pneumonia, whereas standard DLCO was abnormal in only 20%, 2) standard DLCO but not DLNO was positively correlated with recovery time, and 3) both standard DLCO and DLNO were inversely correlated with persisting CT abnormalities, but DLNO was more frequently associated with their presence.
4.1. Comments on methodology
In this study, we measured DLCO by standard technique and in combination with DLNO, which required breath‐hold times of 11 ± 0.4 s and 5 ± 0.3 s, respectively. Such a difference seems to have a negligible effect on final values of DLCO both in healthy subjects and restrictive disorders, that is, idiopathic pulmonary fibrosis (Barisione et al., 2016) and systemic sclerosis‐associated interstitial lung disease (Barisione et al., 2019). Also in the present investigation, absolute values of DLCO measured by the two methods were strongly correlated (R2 = 0.85; p < 0.0001) (Figure 5a) without systematic differences (Figure 5b). Therefore, we used standard DLCO values for comparison with DLNO and the results of previous studies.
FIGURE 5.

Panel a: Correlation between absolute values of DLCO measured by standard method with breath‐hold time of 11 ± 0.4 s (DLCO,11±0.4 s) or by simultaneous DLNO‐DLCO method with breath‐hold time of 5 ± 0.3 s (DLCO,5±0.3 s). Asterisks (*) indicate healthy controls while circles indicate subjects who recovered from mild (white), moderate (gray), and severe (black) COVID‐19 pneumonia. Panel b: Bland‐Altman plot of difference vs. mean DLCO measured by the two methods. Shaded area is the standard deviation of differences, and horizontal dashed lines indicate the 95% confidence interval
Although the 5th percentile (z‐score −1.645) is generally assumed as the lower limit of normal for standard lung function measurements including DLCO (Quanjer et al., 1993), the 2.5th percentile (z‐score −1.96) has been suggested for DLNO with the currently available predictive equations (Munkholm et al., 2018; Zavorsky et al., 2017). Therefore, we have used both LLN5 and LLN2.5 to reduce false negative or false positive biases. As reference values for DLNO and DLNO/VA, we used the set of equations that provided the lower SD of z‐scores from our local data set of healthy subjects, that is, 0.71 and 0.70, respectively.
The alveolar concentration of endogenous NO increases in several inflammatory interstitial lung diseases (Cameli et al., 2020), which could theoretically bias DLNO measures. However, the mean NO concentration in the gas mixtures inhaled in the present study was 63.7 ± 10 ppm, resulting in alveolar concentrations ranging from 5.4 to 21.9 ppm, thus >1,000 times the threshold considered as a marker of pulmonary alveolitis. Hence, it is reasonable to assume that any effect of endogenous NO backpressure on DLNO measurements was negligible. Furthermore, 40 ppm of NO in the inspired gas could decrease hypoxic pulmonary vasoconstriction (Glenny & Robertson, 2011), but this effect was observed with<60 mmHg (Asadi et al., 2015), thus well below the 102 ± 4 mmHg of this study.
The present study has two major limitations. First, lung function tests were obtained in a sitting posture and CT in supine position. The latter might have increased VC (Cotton et al., 1990), thus possibly affecting differently the relationships of DLNO and standard DLCO with CT density distribution data. Second, the study was cross‐sectional, which may limit the clinical relevance of results but does not seem to invalidate their pathophysiological meaning and interpretation.
4.2. Comments on results
To our knowledge, this is the first study using DLNO and DLCO to investigate the pathophysiology of alveolar‐to‐capillary gas exchange in patients recovering from COVID‐19. Clinically, COVID‐19 pneumonia is associated in a variable number of subjects with acute hypoxemic respiratory failure ranging from mild‐to‐severe, whereas other subjects have no apparent gas exchange abnormalities (Guan et al., 2020). At autopsy of patients who died from severe COVID‐19, diffuse alveolar damage, capillary endothelialitis, and fibrinous microthrombi with angiogenesis within the interalveolar septa has been observed (Ackermann et al., 2020). A question is whether these abnormalities occurring in the acute phase of the disease might leave late pathophysiological sequelae over the recovering phase and these depend on the presence or severity of acute hypoxemic respiratory failure. A mild reduction of standard DLCO has been reported in about half of survivors as early as 30 days after acute infection (Frija‐Masson et al., 2020; Mo et al., 2020) or hospital discharge (Huang et al., 2020). In the present study, we found a much lower prevalence of decreased standard DLCO, that is, 20% and 18% with LLN5 and LLN2.5, respectively, over 8 months after negative SARS‐CoV‐2 testing. There are three main reasons that may have contributed to this discrepancy. First, we used lower limits of normal based on z‐scores instead of 80% of predicted (Huang et al., 2020; Mo et al., 2020), which tends to overestimate the presence of abnormality due to age‐, sex‐, and size biases (Miller & Brusasco, 2016). Indeed, our results are in keeping with the decrease of DLCO found in 24% of subjects in one study using z‐scores (Lerum et al., 2020). Second, the proportion of subjects with reduced DLCO tended to decrease with the time elapsed from the negative testing for SARS‐CoV‐2 as suggested by Sonnweber et al. (2020). Instead, we found that more than half of subjects had DLNO below the LLN5 and 49% below the LLN2.5, and this proportion remained near constant over 8 months. Third, almost all previous studies included several patients with comorbidities potentially affecting the final value of DLCO independent of COVID‐19 severity (van den Borst et al., 2020; Frija‐Masson et al., 2020; Huang et al., 2020; Mo et al., 2020; Sonnweber et al., 2020). Collectively, our results support the hypothesis that a more severe and prolonged abnormality of DLNO may be present after COVID‐19 pneumonia, reflecting a prevailing decrement of DM.
Several physiological mechanisms can explain a disproportionate reduction of DLNO and DLCO. Since the alveolar‐to‐capillary transfer of CO is mostly limited by its slow reaction rate with Hb (Carlsen & Comroe, 1958), DLCO is relatively less sensitive to changes in DM than VC. By contrast, NO has a much greater affinity and fast reaction rate with Hb, which make DLNO more sensitive to DM than VC (Borland & Hughes, 2020). Thus, the findings of the present study suggest that a decreased DM is more frequent and persistent than the reduction of VC in the recovery phase after COVID‐19 pneumonia. One reason for decreased DM could be simply a loss of lung volume, but this would have caused an increase of DLNO/VA, which was instead slightly below the LLN5 or LLN2.5 in about one third of subjects. Moreover, TLC was significantly lower than in controls and in subjects with moderate‐to‐severe than mild pneumonia, while there were no differences in the distribution of DLNO and DLCO abnormalities. DLNO/DLCO ratio was in most cases within the normal range suggesting that alveolar damage rather than loss of lung volume was the major determinant of diffusion limitation (Hughes & van der Lee, 2013). A possible mechanism for the differences in DLNO and DLCO over recovery time could be that SARS‐CoV‐2, by targeting type II and eventually type I pneumocytes (Mossel et al., 2008), may cause a persistent damage of alveolar membrane while vasculopathy with capillary microthrombi is possibly reversing more rapidly after the acute phase of the disease. However, while VC reflects pulmonary blood volume only, DM reflects alveolar membrane thickness and surface but also vessel surface (Kang & Sapoval, 2016). The latter may be reduced as a consequence of capillary remodeling or obliteration with blood volume being redistributed to unaffected lung regions (Oppenheimer et al., 2006; Pande et al., 1975), or uneven red cell distribution within the alveolar capillaries (Hsia et al., 1997). Another reason for decreased DM without VC changes could be the presence of interstitial edema (Zavorsky et al., 2014), which would be consistent with the closer associations of DLNO than standard DLCO with CT abnormalities.
In the present study, GGO was the only qualitative CT abnormality persisting after COVID‐19 and correlated with decrement of DLNO and standard DLCO. In interstitial pulmonary fibrosis (Barisione et al., 2016) or interstitial lung disease associated with systemic sclerosis (Barisione et al., 2019), we found DLNO be correlated with CT fibrotic abnormalities but not GGO. This may suggest that interstitial edema by itself may not be sufficient to alter substantially the alveolar‐to‐capillary gas transport, owing to the high solubility of both NO and CO (Wilhelm et al., 1977). Moreover, we observed reduced DLNO even in the absence or minimal‐to‐moderate GGO, which suggests that mechanisms other than alveolar membrane thickening may contribute to diffusion abnormality after COVID‐19.
5. CONCLUSIONS
In subjects recovering from COVID‐19 pneumonia, DLNO is impaired more frequently and more persistently than standard DLCO, suggesting an impairment of DM due to alveolar‐capillary damage and loss of alveolar units with VC relatively preserved. DLNO was more frequently abnormal than standard DLCO even in subjects with minimal or absent CT abnormalities, suggesting persistent alveolar damage in these subjects. Further long‐term studies are necessary to investigate whether these medium‐term changes may evolve into chronic morphological and functional abnormalities.
CONFLICT OF INTEREST
G.B. and V.B have no financial/nonfinancial interests to disclose.
AUTHOR CONTRIBUTIONS
G.B. conceived and designed the research and performed the experiments; G.B. and V.B. analyzed the data; G.B. and V.B. interpreted results of experiments; G.B. prepared figures; G.B. and V.B. drafted the manuscript; G.B. and V.B. edited and revised the manuscript; and G.B. and V.B. approved the final version of the manuscript.
ETHICAL APPROVAL
The study was approved by the Regional Ethics Committee (CER Liguria Registry No.: 412/2020 ‐ DB id 10794) and each subject gave written informed consent to use his/her anonymized personal data.
Contributor Information
Giovanni Barisione, Email: giovanni.barisione@hsanmartino.it.
Vito Brusasco, Email: vito.brusasco@unige.it.
REFERENCES
- Ackermann, M. , Verleden, S. E. , Kuehnel, M. , Haverich, A. , Welte, T. , Laenger, F. , Vanstapel, A. , Werlein, C. , Stark, H. , Tzankov, A. , Li, W. W. , Li, V. W. , Mentzer, S. J. , & Jonigk, D. (2020). Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. New England Journal of Medicine, 383, 120–128. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi, A. K. , Sá, R. C. , Kim, N. H. , Theilmann, R. J. , Hopkins, S. R. , Buxton, R. B. , & Prisk, G. K. (2015). Inhaled nitric oxide alters the distribution of blood flow in the healthy human lung, suggesting active hypoxic pulmonary vasoconstriction in normoxia. Journal of Applied Physiology, 118, 331–343. 10.1152/japplphysiol.01354.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisione, G. , Brusasco, C. , Garlaschi, A. , Baroffio, M. , & Brusasco, V. (2016). Lung diffusing capacity for nitric oxide as a marker of fibrotic changes in idiopathic interstitial pneumonias. Journal of Applied Physiology, 120, 1029–1038. 10.1152/japplphysiol.00964.2015 [DOI] [PubMed] [Google Scholar]
- Barisione, G. , Garlaschi, A. , Occhipinti, M. , Baroffio, M. , Pistolesi, M. , & Brusasco, V. (2019). Value of lung diffusing capacity for nitric oxide in systemic sclerosis. Physiological Reports, 7, e14149 10.1152/japplphysiol.00964.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland, C. D. R. , & Hughes, J. M. B. (2020). Lung diffusing capacities (DL) for nitric oxide (NO) and carbon monoxide (CO): The evolving story. Comprehensive Physiology, 10, 73–97. [DOI] [PubMed] [Google Scholar]
- Cameli, P. , Bargagli, E. , Bergantini, L. , d’Alessandro, M. , Pieroni, M. , Fontana, G. A. , Sestini, P. , & Refini, R. M. (2020). Extended exhaled nitric oxide analysis in interstitial lung diseases: A systematic review. International Journal of Molecular Sciences, 21(17), 6187 10.3390/ijms21176187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen, E. , & Comroe, J. H. Jr (1958). The rate of uptake of carbon monoxide and of nitric oxide by normal human erythrocytes and experimentally produced spherocytes. Journal of General Physiology, 42, 83–107. 10.1085/jgp.42.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveri, I. , Zoia, M. C. , Fanfulla, F. , Spagnolatti, L. , Berrayah, L. , Grassi, M. , & Tinelli, C. (1995). Reference values of arterial oxygen tension in the middle‐aged and elderly. American Journal of Respiratory and Critical Care Medicine, 152, 934–941. 10.1164/ajrccm.152.3.7663806 [DOI] [PubMed] [Google Scholar]
- Cotes, J. E. , Dabbs, J. M. , Elwood, P. C. , Hall, A. M. , McDonald, A. , & Saunders, M. J. (1972). Iron‐deficiency anaemia: Its effect on transfer factor for the lung (diffusing capacity) and ventilation and cardiac frequency during sub‐maximal exercise. Clinical Science, 42, 325–335. 10.1042/cs0420325 [DOI] [PubMed] [Google Scholar]
- Cotton, D. J. , Graham, B. L. , & Mink, J. T. (1990). Pulmonary diffusing capacity in adult cystic fibrosis: reduced positional changes are partially reversed by hyperoxia. Clinical and Investigative Medicine, 13, 82–91. [PubMed] [Google Scholar]
- Frija‐Masson, J. , Debray, M.‐P. , Gilbert, M. , Lescure, F.‐X. , Travert, F. , Borie, R. , Khalil, A. , Crestani, B. , d'Ortho, M.‐P. , & Bancal, C. (2020). Functional characteristics of patients with SARS‐CoV‐2 pneumonia at 30 days post‐infection. European Respiratory Journal, 56, 2001754 10.1183/13993003.01754-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, Q. H. , & Roughton, F. J. W. (1957). The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. Journal of Physiology, 136, 507–524. 10.1113/jphysiol.1957.sp005777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny, R. W. , & Robertson, H. T. (2011). Spatial distribution of ventilation and perfusion: mechanisms and regulation. Comprehensive Physiology, 1, 375–395. [DOI] [PubMed] [Google Scholar]
- Graham, B. L. , Brusasco, V. , Burgos, F. , Cooper, B. G. , Jensen, R. , Kendrick, A. , MacIntyre, N. R. , Thompson, B. R. , & Wanger, J. (2017). 2017 ERS/ATS standards for single‐breath carbon monoxide uptake in the lung. European Respiratory Journal, 49, 1600016 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- Graham, B. L. , Steenbruggen, I. , Miller, M. R. , Barjaktarevic, I. Z. , Cooper, B. G. , Hall, G. L. , Hallstrand, T. S. , Kaminsky, D. A. , McCarthy, K. , McCormack, M. C. , Oropez, C. E. , Rosenfeld, M. , Stanojevic, S. , Swanney, M. P. , & Thompson, B. R. (2019). Standardization of spirometry 2019 update: An Official American Thoracic Society and European Respiratory Society Technical Statement. American Journal of Respiratory and Critical Care Medicine, 200, e70–e88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐J. , Ni, Z.‐Y. , Hu, Y. U. , Liang, W.‐H. , Ou, C.‐Q. , He, J.‐X. , Liu, L. , Shan, H. , Lei, C.‐L. , Hui, D. S. C. , Du, B. , Li, L.‐J. , Zeng, G. , Yuen, K.‐Y. , Chen, R.‐C. , Tang, C.‐L. , Wang, T. , Chen, P.‐Y. , Xiang, J. , … Zhong, N.‐S. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guénard, H. , Varène, N. , & Vaida, P. (1987). Determination of lung capillary blood volume and membrane diffusing capacity by measurement of NO and CO transfer. Respiration Physiology, 70, 113–120. [DOI] [PubMed] [Google Scholar]
- Hsia, C. C. , Chuong, C. J. , & Johnson, R. L. Jr (1997). Red cell distortion and conceptual basis of diffusing capacity estimates: Finite element analysis. Journal of Applied Physiology, 83, 1397–1404. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Tan, C. , Wu, J. , Chen, M. , Wang, Z. , Luo, L. , Zhou, X. , Liu, X. , Huang, X. , Yuan, S. , Chen, C. , Gao, F. , Huang, J. , Shan, H. , & Liu, J. (2020). Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respiratory Research, 21, 163 10.1186/s12931-020-01429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, J. M. B. , & Pride, N. B. (2012). Examination of the carbon monoxide diffusing capacity (DLCO) in relation to Its KCO and VA components. American Journal of Respiratory and Critical Care Medicine, 186, 132–139. [DOI] [PubMed] [Google Scholar]
- Hughes, J. M. , & van der Lee, I. (2013). The TL, NO/TL, CO ratio in pulmonary function test interpretation. European Respiratory Journal, 41, 453–461. [DOI] [PubMed] [Google Scholar]
- Kang, M. Y. , & Sapoval, B. (2016). Time‐based understanding of DLCO and DLNO. Respiratory Physiology and Neurobiology, 225, 48–59. 10.1016/j.resp.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Lerum, T. V. , Aaløkken, T. M. , Brønstad, E. , Aarli, B. , Ikdahl, E. , Lund, K. M. A. , Durheim, M. T. , Rodriguez, J. R. , Meltzer, C. , Tonby, K. , Stavem, K. , Skjønsberg, O. H. , Ashraf, H. , & Einvik, G. (2020). Dyspnoea, lung function and CT findings three months after hospital admission for COVID‐19. European Respiratory Journal, 2003448, 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, R. J. (2020). Pathogenesis of COVID‐19 from a cell biology perspective. European Respiratory Journal, 55, 2000607 10.1183/13993003.00607-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. R. , & Brusasco, V. (2016). Risk of COPD in smokers with low transfer factor. European Respiratory Journal, 47, 1885–1886. 10.1183/13993003.02218-2015 [DOI] [PubMed] [Google Scholar]
- Mo, X. , Jian, W. , Su, Z. , Chen, M. U. , Peng, H. , Peng, P. , Lei, C. , Chen, R. , Zhong, N. , & Li, S. (2020). Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. European Respiratory Journal, 55, 2001217 10.1183/13993003.01217-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel, E. C. , Wang, J. , Jeffers, S. , Edeen, K. E. , Wang, S. , Cosgrove, G. P. , Funk, C. J. , Manzer, R. , Miura, T. A. , Pearson, L. D. , Holmes, K. V. , & Mason, R. J. (2008). SARS‐CoV replicates in primary human alveolar type II cell cultures but not in type I‐like cells. Virology, 372, 127–135. 10.1016/j.virol.2007.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkholm, M. , Marott, J. L. , Bjerre‐Kristensen, L. , Madsen, F. , Pedersen, O. F. , Lange, P. , Nordestgaard, B. G. , Mortensen, J. (2018). Reference equations for pulmonary diffusing capacity of carbon monoxide and nitric oxide in adult Caucasians. European Respiratory Journal, 52, 1500677. [DOI] [PubMed] [Google Scholar]
- Oppenheimer, B. W. , Berger, K. I. , Hadjiangelis, N. P. , Norman, R. G. , Rapoport, D. M. , & Goldring, R. M. (2006). Membrane diffusion in diseases of the pulmonary vasculature. Respiratory Medicine, 100, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Pande, J. N. , Gupta, S. P. , & Guleria, J. S. (1975). Clinical significance of the measurement of membrane diffusing capacity and pulmonary capillary blood volume. Respiration, 32, 317–324. [DOI] [PubMed] [Google Scholar]
- Quanjer, P. H. , Stanojevic, S. , Cole, T. J. , Baur, X. , Hall, G. L. , Culver, B. H. , Enright, P. L. , Hankinson, J. L. , Ip, M. S. M. , Zheng, J. , & Stocks, J. ; ERS Global Lung Function Initiative . (2012). Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. European Respiratory Journal, 40, 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanjer, P. H. , Tammeling, G. J. , Cotes, J. E. , Pedersen, O. F. , Peslin, R. , & Yernault, J. C. (1993). Lung volumes and forced ventilatory flows. Report Working Party, Standardization of Lung Function Tests, European Community for Steel and Coal and European Respiratory Society. European Respiratory Journal, 6(Suppl 16):5–40. [PubMed] [Google Scholar]
- Sonnweber, T. , Sahanic, S. , Pizzini, A. , Luger, A. , Schwabl, C. , Sonnweber, B. , Kurz, K. , Koppelstätter, S. , Haschka, D. , Petzer, V. , Boehm, A. , Aichner, M. , Tymoszuk, P. , Lener, D. , Theurl, M. , Lorsbach‐Köhler, A. , Tancevski, A. , Schapfl, A. , Schaber, M. , … Tancevski, I. (2020). Cardiopulmonary recovery after COVID‐19 ‐ an observational prospective multi‐center trial. European Respiratory Journal, 2003481, 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojevic, S. , Graham, B. L. , Cooper, B. G. , Thompson, B. R. , Carter, K. W. , Francis, R. W. , Hall, G. L. ; Global Lung Function Initiative TLCO working group; Global Lung Function Initiative (GLI) TLCO (2017). Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. European Respiratory Journal, 50, 1700010. [DOI] [PubMed] [Google Scholar]
- Steiger J. H. (1980). Testing Pattern Hypotheses On Correlation Matrices: Alternative Statistics And Some Empirical Results. Multivariate Behavioral Research, 15, 335–352. 10.1207/s15327906mbr1503_7. [DOI] [PubMed] [Google Scholar]
- van den Borst, B. , Peters, J. B. , Brink, M. , Schoon, Y. , Bleeker‐Rovers, C. P. , Schers, H. , van Hees, H. W. H. , van Helvoort, H. , van den Boogaard, M. , van der Hoeven, H. , Reijers, M. H. , Prokop, M. , Vercoulen, J. , & van den Heuvel, M. (2020). Comprehensive health assessment three months after recovery from acute COVID‐19. Clinical Infectious Diseases, ciaa1750, 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger, J. , Clausen, J. L. , Coates, A. , Pedersen, O. F. , Brusasco, V. , Burgos, F. , Casaburi, R. , Crapo, R. , Enright, P. , van der Grinten, C. P. M. , Gustafsson, P. , Hankinson, J. , Jensen, R. , Johnson, D. , Macintyre, N. , McKay, R. , Miller, M. R. , Navajas, D. , Pellegrino, R. , & Viegi, G. (2005). Standardisation of the measurement of lung volumes. European Respiratory Journal, 26, 511–522. [DOI] [PubMed] [Google Scholar]
- Wilhelm, E. , Battino, R. , & Wilcock, R. J. (1977). Low pressure solubility of gases in liquid water. Chemical Reviews. 77:219–262. [Google Scholar]
- Yushkevich, P. A. , Piven, J. , Hazlett, H. C. , Smith, R. G. , Ho, S. , Gee, J. C. , Gerig, G. (2006). User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage, 31, 1116–1128. [DOI] [PubMed] [Google Scholar]
- Zavorsky, G. S. , Hsia, C. C. W. , Hughes, J. M. B. , Borland, C. D. R. , Guénard, H. , van der Lee, I. , Steenbruggen, I. , Naeije, R. , Cao, J. , & Dinh‐Xuan, A. T. (2017). Standardisation and application of the single‐breath determination of nitric oxide uptake in the lung. European Respiratory Journal, 49(2), 1600962– 10.1183/13993003.00962-2016 [DOI] [PubMed] [Google Scholar]
- Zavorsky, G. S. , Milne, E. N. C. , Lavorini, F. , Rienzi, J. P. , Cutrufello, P. T. , Kumar, S. S. , & Pistolesi, M. (2014). Small changes in lung function in runners with marathon‐induced interstitial lung edema. Physiological Reports, 2(6), e12056. [DOI] [PMC free article] [PubMed] [Google Scholar]


