Abstract
Background
Endometrial cancer (EC) is the fourth most common neoplasm and the eighth leading cause of cancer death in females worldwide. PTPN18 is a member of the protein tyrosine phosphatases (PTP) family, which is associated with the occurrence and progression of various human cancers. PTPN18 was up-regulated in endometrial cancer tissues and high level of PTPN18 promoted proliferation and metastasis of EC cells.
Methods
The expression of PTPN18, GPX4 and xCT in endometrial cancer tissues and KLE cells was detected by immunohistochemistry and Western blot, respectively. Lentiviral transfection were used to silence PTPN18 level in KLE cells. The Ros level in KLE cells was examined by ELISA assay.
Results
In the present study, we found that silencing of PTPN18 induced ferroptosis in KLE endometrial cancer cells. PTPN18 knockdown increased intracellular ROS level and down-regulated GPX4 and xCT expression. Besides, silencing of PTPN18 also induced the expression of p-p38.
Conclusion
We concluded that silencing of PTPN18 might induce ferroptosis by targeting the p-p38/GPX4/xCT axis. The results provide critical insight into the application of PTPN18 knockdown in EC intervention.
Keywords: PTPN18, endometrial cancer, ferroptosis, p-p38, GPX4/xCT
Introduction
Endometrial carcinoma (EC) is one of the most common gynecological malignancy cancer types, which affects the health of many women worldwide.1 As the eighth leading cause of cancer-related death in women, the incidence and mortality rates of EC are increasing rapidly.2 The prognosis is unfavorable for EC patients with metastasis or recurrence, who are at a higher risk of mortality significantly and always live a life with a low quality.3 The median overall survival time of patients with EC resistance to chemotherapy and hormone therapy is usually less than 16 weeks.3 Accepted risk factors for EC include prolonged exposure to endogenous or exogenous estrogens, age at menopause, age at menarche, obesity, history of infertility, polycystic ovarian syndrome, diabetes, and prior pelvic radiation therapy.4 It remains vague how genetic regulatory networks modulate the initiation of EC. It is still hard to predict the prognosis and seek convenient and effective biomarkers for EC patients though great improvements have been made in classifying EC patients by estrogen receptor status.5
Ferroptosis is a recently discovered unique form of programmed cell death driven by mitochondrial lipid peroxidation and dysregulation of cellular iron metabolism.6 Unlike necroptosis or apoptosis, ferroptosis is independent of receptor-interacting protein 1 (RIPK1) kinase activity and caspase activity.7 With the in-depth study, multiple lines have indicated that ferroptosis is related to various types of diseases, especially carcinoma.8–10 Emerged reports reveal that ferroptosis facilitates the selective eradication of cancer cells.6,10 However, investigations regarding the function of ferroptosis in EC progression are still rare.
PTPN18, also known as BDP1, located in Chromosome 2q21.1, belongs to the PTP family. PTPN18 can regulate the expression of HER2, which belongs to the epidermal growth factor receptor family of receptor tyrosine kinases.11 A considerable amount of literatures indicated that PTPN18 is involved in the progression of various cancers. PTPN18 is correlated with the pathological grade in digestive tract cancers12 and independent prognostic factors in hepatocellular carcinoma.13 The expression of PTPN18 increased in breast tumor, illustrating that PTPN18 might be a critical mediator in oncogenesis.14 We previously demonstrated that PTPN18 is up-regulated in endometrial cancer tissues, and promoted the proliferation and metastasis of endometrial cancer cells.15 However, there few studies to investigate the relationship between PTPN18 and ferroptosis.
In this study, we want to investigate the underlying role PTPN18 played in ferroptosis of EC to search suitable biomarkers for the screening and treatment of EC.
Methods
Cell Culture and Main Regents
KLE endometrial cancer cells were obtained from American Type Culture Collection (ATCC) and cultured in DEME/F12 medium containing 10% fetal bovine serum (FBS), 1% penicillin (100 U/mL), and streptomycin (100 U/mL). The cells were maintained at 37°C in a humidified incubator with 5% CO2 and 95% air.
DMEM/F12 medium, FBS, and trypsin were obtained from Gibco. Penicillin and streptomycin double antibodies were purchased from Genview. Cell Proliferation and Activity Assay Kit (CCK-8) was obtained from Shanghai Best-Bio. The primary antibodies PTPN18, GPX4, xCT and GAPDH were purchased from ABclonal. The primary antibody p-p38 was provided by Cell Signaling Technology and p38 was obtained from Proteitech. Reactive Oxygen Species Assay Kit (No. S0033) was purchased from Beyotime Biotechnology.
Cell Viability Assay
The transfected KLE cells (2 × 103 cells/well) were seeded in 96-well culture plates. Forty-eight hours after inoculation, the cell proliferation for 5 consecutive days was examined via CCK-8 assay. The cells were incubated in medium with 20 µL/well CCK-8 solutions for 1–2 h at 37°C. Absorbance was measured at 450 nm by a Thermo Fisher spectrometer.
Detection of Intracellular ROS
ROS level in each group KLE cell was analyzed by Flow cytometry using DCFH-DA as a fluorescence probe staining. The cells in each group were collected by centrifugation. The cells were then washed twice by cold PBS, resuspended by 500 μL binding buffer to adjust the concentration to 106/mL. One hundred microliter cell suspension was transferred into a 5 mL flow tube, 5 μL ROS test reagent was added into the flow tube and mix, incubate at 37°C in the dark for 15 minutes. Flow cytometry was performed on a BECKMAN CogtoFLEX flow cytometer and the data were analyzed with FlowJo software.
RNA Interference
To silence PTPN18 expression, KLE cells were transfected lentiviral vectors harboring PTPN18 siRNA (5ʹ - GTGGCTGAATGAGGACATCAT - 3ʹ). KLE cells were seeded in 6-well plates (2 × 105) and were transfected with 20 μM PTPN18 siRNA using Lipofectamine 3000 (Invitrogen) for 4 h. Then, the medium was replaced with fresh DEME/F12 medium containing 10% FBS. After another 24 h culture, cells were harvested to extract protein for the next analysis.
Western Blot
Total protein was obtained from KLE cells using RIPA buffer containing 1% protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were measured by BCA assay (Beyotime). The protein samples of each group were separated by SDS-PAGE and blotted onto PVDF membranes (Millipore) following incubating at 4°C overnight with primary antibodies against PTNT18, xCT, GPX4, p38, p-p38 and GAPDH. HRP-conjugated goat anti-rabbit/mouse IgG (Boster) was used as a secondary antibody. Immunoreactive protein bands were visualized by a ClinxChemiScope 6000 scanning system. The gray-scale values were quantified by Image-J software.
ELISA Assay
GPX4 levels in KLE cells supernatant were determined by an ELISA assay kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Immunohistochemistry (IHC)
All the EC tissue samples were collected from Hainan General Hospital. The EC tissue samples were fixed, embedded, and cut into 5 sections as previously described with slight modification.16 The tissue sections were incubated for 30 minutes at 60°C and then deparaffinized with xylene (2 × 10 min), rehydrated with graded ethanol solutions (2 × 100%, 80%, 70%; 4 × 5 min), then blocked with 3% H2O2 in PBS for 10 minutes to delete endogenous peroxidases. Next, the sections were incubated at 95°C in 10 mM citric acid (pH 6.0) for 10 min to retrieve the antigen followed by a 10-min wash with PBS. Unspecific binding sites were blocked with 5% normal calf serum (NCS) in PBS for 30 min at RT. The following incubation with primary antibodies against PTNT18, xCT, GPX4 diluted in 5% NCS/PBS was carried out overnight at 4°C in a humidified chamber. Next, sections were washed 3 times with PBS (3 × 10 min) before adding a biotinylated secondary antibody (1:500 dilution in 5% NCS/PBS). After a 2-h incubation period at RT, the excess antibody was removed by 3 washes with PBS (3 × 10 min). The 3, 3ʹ-diaminobenzidine (DAB) substrate (0.01% H2O2 and 0.05% DAB in H2O) was applied for visualization of immunoreactivity. To obtain optimal labeling intensity, staining development was followed under visual control and reaction was stopped by incubation in distilled water for 5 min. For nuclear counterstaining, sections were incubated in the hematoxylin solution for 1 min and rinsed in running tap water for 10 min. Subsequently, sections were dehydrated with ascending ethanol solutions (70%, 80%, 2 × 100%; 4 × 5 min), and finally cleared in xylene. Control sections were treated using the same incubation conditions, except that slides were incubated with 5% NCS/PBS instead of the primary antibody. Samples were subsequently coated with Entellan mounting medium and examined using a Leica microscope (Leica. DM2000 LED).
Statistical Analysis
GraphPad Prism 5 was used to conduct statistical analysis. All p values were obtained from three independent experiments and expressed as mean ± SD. p <0.05 was regarded as statistically significant by one-way ANOVA or Pearson’s χ2 test using SPASS 18.0.
Results
Patients’ Characteristics
The present study included 80 female patient endometrial tissues, including 20 cases of simple endometrial hyperplasia (SEH), 20 cases of atypical endometrial hyperplasia (AEH), 20 cases of endometrioid adenocarcinomas grade Ⅰ (EC), and 20 cases of healthy control tissues (CON), which all collected from Hainan General Hospital between Feb 2018 and Feb 2020. Data gathered included patient age at diagnosis, body mass index, number of pregnancies and abortions.
Table 1 presents selected general features of the studied population. There were no apparent differences in age and mean body mass index (BMI) between each group of patients. At the same time, there were statistically significant differences in the number of pregnancies and abortions between each group of patients. Pregnancy times of CON and SHE patients are significantly higher than that of AEH and EC group, while abortion times in the CON group is remarkably lower than the other three groups.
Table 1.
General Features of Patients Included in the Study
| Variables | CON | SHE | AEH | EC |
|---|---|---|---|---|
| Cases | 20 | 20 | 20 | 20 |
| Age (y) | 36.4 ± 10.24 | 38.64 ± 8.24 | 37.94 ± 8.50 | 40.56 ± 10.50 |
| BMI (kg/m2) | 24.19 ± 3.54 | 26.40 ± 3.86 | 28.50 ± 3.80 | 24.53 ± 3.76 |
| Pregnancy (times) | 2.13 ± 1.25 | 3.00 ± 1.00 | 1.60 ± 1.45 | 1.50 ± 1.25 |
| Abortion (times) | 0.67 ± 0.54 | 2.54 ± 2.21 | 1.68 ± 1.32 | 2.44 ± 2.66 |
The Expression of PTPN18, GPX4 and xCT in Endometrial Cancer (EC)
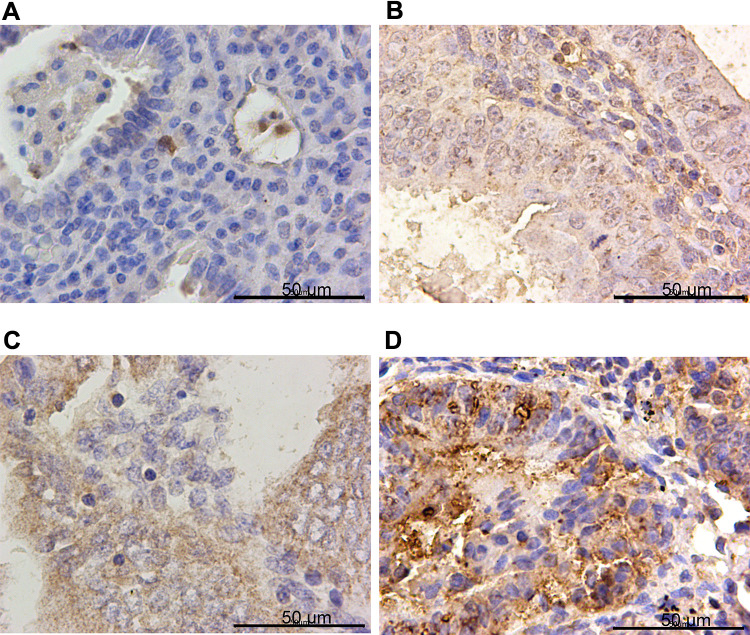
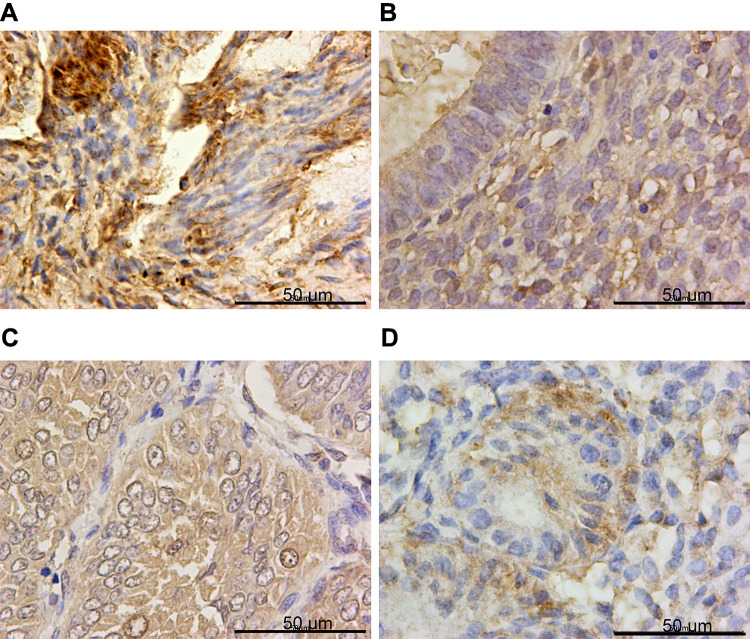
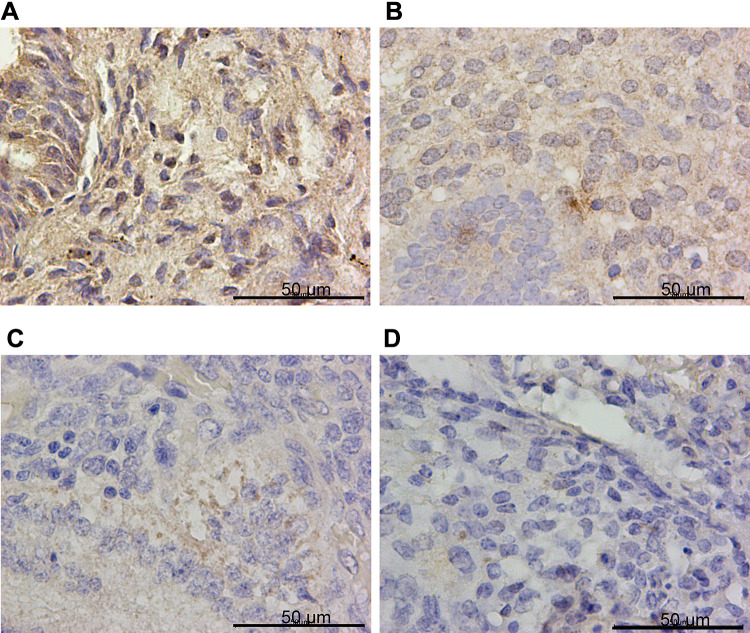
IHC confirmed the difference of PTPN18, GPX4 and xCT expression in CON, SHE, AEH and EC tissues. PTPN18 expression in EC tissue was significantly higher than that in normal tissue (Figure 1A–D), while the level of Glutathione peroxidase 4 (GPX4) and xCT transporters lower in EC tissue compared with that in normal tissue (Figure 2A–D and Figure 3A–D). GPX4 and xCT are marker genes of ferroptosis. Therefore, we predicted that PTPN18 might induce ferroptosis in EC. Detailed information is listed in Table 2.
Figure 1.
PTPN18 was overexpressed in endometrial cancer specimens. Representative images of PTPN18 immunohistochemistry staining in (A) control tissues (CON), (B) simple endometrial hyperplasia (SEH), (C) atypical endometrial hyperplasia (AEH) and (D) endometrial cancer tissues (EC).
Figure 2.
GPX4 was down-regulated in endometrial cancer specimens. Representative images of GPX4 immunohistochemistry staining in (A) CON, (B) SEH, (C) AEH and (D) EC specimens.
Figure 3.
xCT was down-regulated in endometrial cancer specimens. Representative images of xCT immunohistochemistry staining in (A) CON, (B) SEH, (C) AEH and (D) EC specimens.
Table 2.
The Expression of PTPN18, GPX4 and xCT in Tissues
| Variables | CON | SHE | AHE | EC |
|---|---|---|---|---|
| PTPN18 positive (n/%) | 7 (35%) | 17 (85%) | 15 (75%) | 20 (100%) |
| GPX4 positive (n/%) | 19 (95%) | 16 (80%) | 14 (70%) | 6 (30%) |
| xCT positive (n/%) | 19 (95%) | 15 (75%) | 14 (70%) | 7 (35%) |
Silencing of PTPN18 Induced Ferroptosis via GPX4
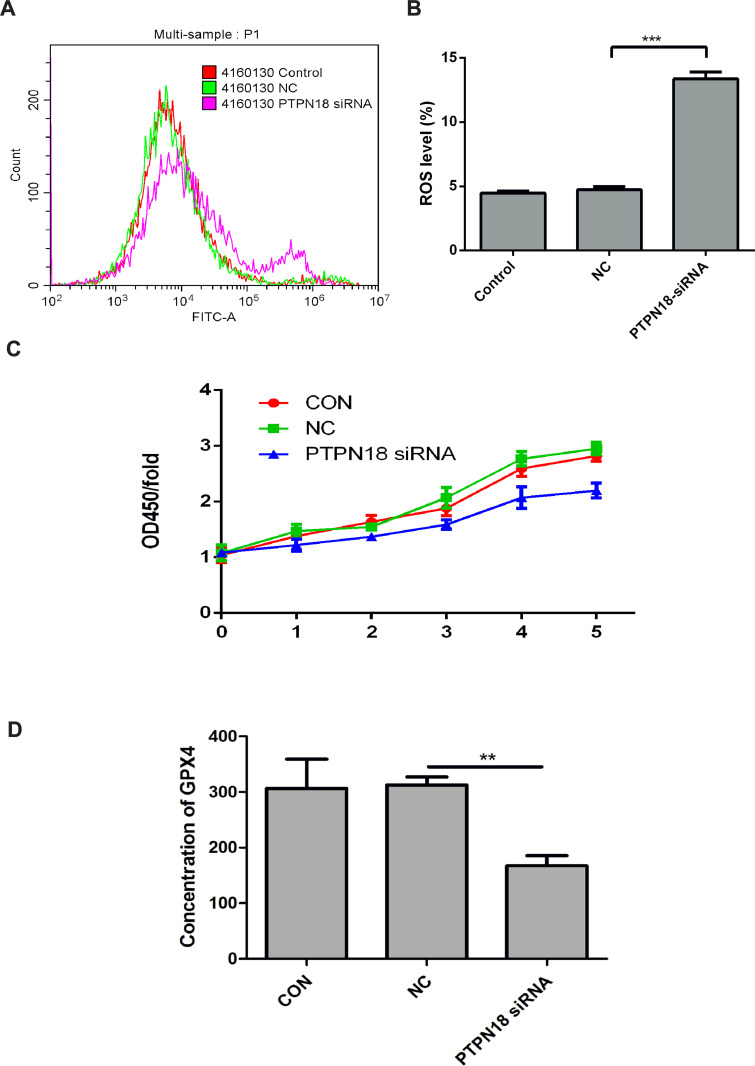
ROS plays a vital role in lipid peroxidation, thus increased intracellular ROS level might also cause ferroptosis.17 In this study, we revealed that PTPN18 knockdown stimulated ROS production significantly (Figure 4A and B). Besides, PTPN18 silencing also suppressed proliferation, induced apoptosis of KLE cells (Figure 4C). In addition, GPX4 levels in KLE cells supernatant decreased notably (Figure 4D). The above results revealed that silencing of PTPN18 induced ferroptosis by targeting GPX4.
Figure 4.
PTPN18 silencing induced ferroptosis via GPX4. (A, B) PTPN18 knockdown induced the intracellular ROS expression detected by Flow cytometry. (C) PTPN18 knockdown blocked the proliferation of KLE endometrial cancer cells measured by CCK-8. (D) PTPN18 knockdown suppressed the expression of GPX4 detected by ELISA. Results are expressed as means ± SD (**p < 0.01, *** p < 0.001 vs CON).
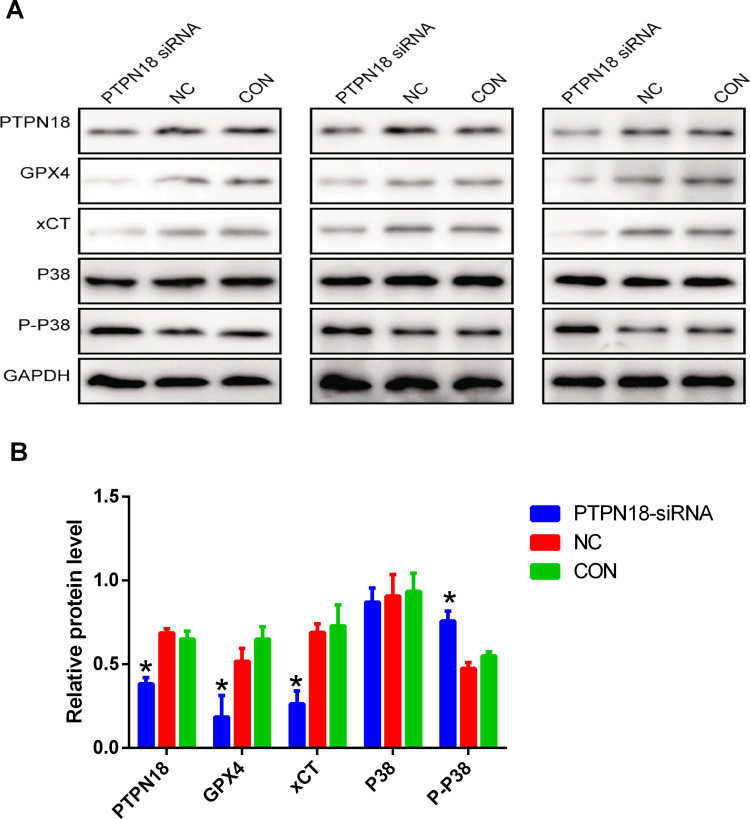
PTPN18 Silencing Blocked GPX4 Expression by Up-Regulation of p-P38
In the current study, we want to explore whether p-p38 has been involved in PTPN18 mediated ferroptosis. Silencing of PTPN18 blocked the expression of GPX4 and xCT revealed by Western blot (Figure 5A and B). Besides, the expression of p-p38 increased significantly after PTPN18 silencing (Figure 5A and B). Thus, we concluded that silencing of PTPN18 induced ferroptosis via up-regulation of p-p38 in KLE endometrial cancer cells. Taken together, these results suggested silencing of PTPN18 might induce ferroptosis by targeting the p-p38/GPX4/xCT axis.
Figure 5.
PTPN18 silencing blocked GPX4 expression by up-regulation of p-p38. (A) Protein expression of PTPN18, GPX4, xCT, p38 and p-p38 in KLE cells after PTPN18 silencing examined by Western blot. (B) The expression levels of proteins in (A) were quantified by densitometry and normalized to the expression of GAPDH. Densitometry data are shown as mean ± SD. *p < 0.05 versus CON.
Discussion
Endometrial cancer (EC), as the most common gynecologic cancer, ranks the fourth most common neoplasm and the eighth cancer-related death in females.1 In recent years, PTPN18, a member of the protein tyrosine phosphatases (PTP) family predicted to be tumor suppressors or oncogenes, has been confirmed to participate in the occurrence and progression of many cancers.14 The present study uncovered that PTPN18 expression increased in EC tissues and could induce proliferation of EC cells. Our current study employed KLE endometrial cancer cells and EC specimens to test the expression and role of PTPN18 in the progression of EC, and identified a novel mechanism that PTPN18 silencing induced ferroptosis via p-p38-mediated GPX4 and xCT down-regulation. The above result might provide critical insight into the application of PTPN18 knockdown in EC intervention.
Ferroptosis is a newly discovered form of cell death, which is characterized by the iron-dependent accumulation of lipid reactive oxygen species (ROS).6 Emerged reports reveal that cancer treatment options based on the induction of ferroptosis effectively eliminate the number of cancer cells. Sorafenib, which induces the induction of ferroptosis, has been approved by the FDA to treat hepatocellular carcinoma (HCC).18 Actinidia chinensis Planch (ACP), which achieves anti-tumor effects via stimulating ferroptosis, is an approved anti-tumor drug to treat gastric cancer for clinical use.19 Besides, treatment based on induction of ferroptosis has also been used in the treatment of ovarian,20 pancreatic,21 colorectal,22 breast carcinoma.23
Increased ROS plays a vital role in lipid peroxidation, which is required by ferroptosis. Factors that affect intracellular ROS level are involved in ferroptosis.24 In this study, knockdown of PTPN18 induced the expression of intracellular ROS. Glutathione peroxidase 4 (GPX4) is one of the critical regulators of ferroptosis, which can convert lipid hydroperoxides to lipid alcohol to protect cells from death.25 xCT, the cystine/glutamate antiporter solute carrier family 7 member 11 (SLC7A11), prevents the accumulation of lipid peroxidation products, thereby protecting cells from ferroptosis.26 The inhibition of GPX4 and xCT have been confirmed to cause ferroptosis.25,26 We discovered in the present study that PTPN18 negatively regulated the expressions of GPX4 and xCT in KLE cells and EC specimens. The above results demonstrated that PTPN18 regulated the proliferation of EC might by targeting ferroptosis.
The P38 mitogen-activated protein kinase (MAPK) pathway activation results in cell apoptosis in EC.27 Emerging evidence suggests that the activation of p38 MAPK is mediated by ferroptosis.28,29 To explore the role p-p38 played in PTPN18 mediated ferroptosis, we measured the expression of p-p38 after silencing the expression of PTPN18. We showed in this study that the expression of p-p38 increased in PTPN18-silenced KLE cells, while the expression of p38 had no obvious change, indicating silencing of PTPN18 might induce ferroptosis by targeting p-p38/GPX4/xCT axis. However, further investigations regarding the targeting regulation relationship between p-p38 and GPX4/xCT are still required.
Conclusions
Taken together, the outcomes of the present study suggest that PTPN18 affected the proliferation of EC cells and took part in the regulation of EC cell ferroptosis by targeting the p-p38/GPX4/xCT axis. The findings of this study offer direction for a new avenue of targets and strategies for EC treatment.
Acknowledgments
This work was supported by the Hainan Key Program of Research and Development (ZDYF2019123) and Major Science and Technology Program of Hainan Province (ZDKJ2017007).
Ethical Approval
Our study complied with the Declaration of Helsinki. The studies involving human participants were reviewed and approved by Hainan General Hospital Medical Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Makker A, Goel MM. Tumor progression, metastasis, and modulators of epithelial-mesenchymal transition in endometrioid endometrial carcinoma: an update. Endocr Relat Cancer. 2016;23(2):R85–R111. doi: 10.1530/ERC-15-0218 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr Relat Cancer. 2009;16(2):363–380. doi: 10.1677/ERC-08-0266 [DOI] [PubMed] [Google Scholar]
- 4.Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007;17(5):964–978. doi: 10.1111/j.1525-1438.2007.00897.x [DOI] [PubMed] [Google Scholar]
- 5.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. doi: 10.1016/S0140-6736(15)00130-0 [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Ping J, Wen Y, Wu Y. The mechanism of ferroptosis and applications in tumor treatment. Front Pharmacol. 2020;11:1061. doi: 10.3389/fphar.2020.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu D, Yang Z, Xia Q, et al. ACADSB regulates ferroptosis and affects the migration, invasion and proliferation of colorectal cancer cells. Cell Biol Int. 2020;44(11):2334–2343. doi: 10.1002/cbin.11443 [DOI] [PubMed] [Google Scholar]
- 8.Guan Z, Chen J, Li X, Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53 mediated-SLC7A11 down-regulation. Biosci Rep. 2020;40(8). doi: 10.1042/BSR20201807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasan M, Reddy SM, Das NK. Ferritinophagy is not required for colon cancer cell growth. Cell Biol Int. 2020;44(11):2307–2314. doi: 10.1002/cbin.11439 [DOI] [PubMed] [Google Scholar]
- 10.Yao X, Yang B, Wang S, et al. A novel multifunctional FePt/BP nanoplatform for synergistic photothermal/photodynamic/chemodynamic cancer therapies and photothermally-enhanced immunotherapy. J Mater Chem B. 2020;8(35):8010–8021. doi: 10.1039/D0TB00411A [DOI] [PubMed] [Google Scholar]
- 11.Held MA, Greenfest-Allen E, Su S, Stoeckert CJ, Stokes MP, Wojchowski DM. Phospho-PTM proteomic discovery of novel EPO- modulated kinases and phosphatases, including PTPN18 as a positive regulator of EPOR/JAK2 signaling. Cell Signal. 2020;69:109554. doi: 10.1016/j.cellsig.2020.109554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Zhao X, Yuan Y, Jing JJ. The expression patterns and the diagnostic/prognostic roles of PTPN family members in digestive tract cancers. Cancer Cell Int. 2020;20(1):238. doi: 10.1186/s12935-020-01315-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhangyuan G, Yin Y, Zhang W, et al. Prognostic value of phosphotyrosine phosphatases in hepatocellular carcinoma. Cell Physiol Biochem. 2018;46(6):2335–2346. doi: 10.1159/000489625 [DOI] [PubMed] [Google Scholar]
- 14.Lucci MA, Orlandi R, Triulzi T, Tagliabue E, Balsari A, Villa-Moruzzi E. Expression profile of tyrosine phosphatases in HER2 breast cancer cells and tumors. Cell Oncol. 2010;32(5–6):361–372. doi: 10.3233/CLO-2010-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai J, Huang S, Yi Y, Bao S. Downregulation of PTPN 18 can inhibit proliferation and metastasis and promote apoptosis of endometrial cancer. Clin Exp Pharmacol Physiol. 2019;46(8):734–742. doi: 10.1111/1440-1681.13098 [DOI] [PubMed] [Google Scholar]
- 16.Ozawa H. Principles and basics of immunohistochemistry. Nippon Yakurigaku Zasshi. 2019;154(4):156–164. doi: 10.1254/fpj.154.156 [DOI] [PubMed] [Google Scholar]
- 17.Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Z, Deng G, Li Y, et al. Actinidia chinensis planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed Pharmacother. 2020;126:110092. doi: 10.1016/j.biopha.2020.110092 [DOI] [PubMed] [Google Scholar]
- 20.Zhou HH, Chen X, Cai LY, et al. Erastin reverses ABCB1-mediated docetaxel resistance in ovarian cancer. Front Oncol. 2019;9:1398. doi: 10.3389/fonc.2019.01398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2(5):517–532. doi: 10.18632/oncoscience.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen MS, Wang SF, Hsu CY, et al. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget. 2017;8(70):114588–114602. doi: 10.18632/oncotarget.23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skouta R, Dixon SJ, Wang J, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136(12):4551–4556. doi: 10.1021/ja411006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forcina GC, Dixon SJ. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19(18):e1800311. doi: 10.1002/pmic.201800311 [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa M, Takahashi H, Rajabi H, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7(11):11756–11769. doi: 10.18632/oncotarget.7598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donohoe F, Wilkinson M, Baxter E, Brennan DJ. Mitogen-activated protein kinase (MAPK) and obesity-related cancer. Int J Mol Sci. 2020;21(4):1241. doi: 10.3390/ijms21041241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Q, Zeng C, Luo C, Wu Y. Ferrostatin-1 mitigates cognitive impairment of epileptic rats by inhibiting P38 MAPK activation. Epilepsy Behav. 2020;103:106670. doi: 10.1016/j.yebeh.2019.106670 [DOI] [PubMed] [Google Scholar]
- 29.Hattori K, Ishikawa H, Sakauchi C, Takayanagi S, Naguro I, Ichijo H. Cold stress-induced ferroptosis involves the ASK 1-p38 pathway. EMBO Rep. 2017;18(11):2067–2078. doi: 10.15252/embr.201744228 [DOI] [PMC free article] [PubMed] [Google Scholar]