Abstract
Purpose
To predict patient survival in early-stage hepatocellular carcinoma (HCC) following hepatic resection. We evaluated the prognostic potential of the aspartate aminotransferase to platelet ratio index (APRI) in order to use it to model a nomogram.
Patients and Methods
We randomized 901 early-stage HCC patients treated with hepatic resection at our center into training and validation cohorts that were followed from January 2009 to December 2012. X-tile software was used to establish the APRI cut-off threshold in the training cohort. The validation cohort was subsequently assessed to determine threshold value accuracy. Data generated from the multivariate analysis in the training cohort were used to design a prognostic nomogram. Decision curve analyses (DCA), concordance index values (C-index) and calibration curves were used to determine the performance of the nomogram.
Results
X-tile software revealed that the optimal APRI cut-off threshold in the training cohort that distinguished between patients with different prognoses was 0.9. We, therefore, validated its prognostic value. Multivariate analyses showed that poor overall survival was associated with APRI above 0.9, blood loss of more than 400 mL, liver cirrhosis, multiple tumors, tumor size greater than 5 cm, microvascular invasion and satellite lesions. When the independent risk factors were integrated into the prognostic nomogram, it performed well with accurate predictions. Indeed, the performance was better than comparative prognosticators (P<0.05 for all) with 0.752 as the C-index (95% CI: 0.706–0.798). These results were verified by the validation cohort.
Conclusion
APRI was a noninvasive and accurate predictive indicator for patients with early-stage HCC. Following hepatic resection to treat early-stage HCC, individualized patient survival predictions can be aided by the nomogram based on APRI.
Keywords: hepatocellular carcinoma, hepatic resection, survival, nomogram, aspartate aminotransferase to platelet ratio index, APRI
Introduction
Among causes of global cancer-related death, hepatocellular carcinoma (HCC) is the third highest in rank and also the sixth most prevalent tumor malignancy.1 The main risk factors of HCC include hepatitis virus infection, aflatoxin B1 exposure and excessive alcohol consumption.1 Meanwhile, in developed countries, non-alcoholic fatty liver disease has become a leading HCC etiology.2 Liver cancer is endemic in many countries, but seriously so in China as it accounts for half of the patients globally.3 Liver transplants and therapeutic surgical tumor removal are preferred treatments for early-stage HCC patients.1 However, only 25% of HCC patients in China are treated by hepatic surgery.4 The high recurrence rates depress the long-term prognosis of HCC, despite some instances of curative treatment being reported.5
Inflammation has been regarded as a typical symptom of cancer.6 A number of studies suggest that HCC tumor development and spread are closely associated with inflammation occurring in the host.7 Prognostic factors based on hematological indicators of systemic inflammation including aspartate aminotransferase-to-alanine aminotransferase (AST/ALT) ratio, neutrophil-to-lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) are currently used to predict cancer survival.7–9 Serological markers of immune status or liver function are potential HCC prognostic factors in addition to those based on inflammation. Unlike the advanced stage, early-stage HCC is more dependent on reserve liver function as liver cirrhosis is closely associated with HCC mortality.10 Thus, a noninvasive and accurate biomarker reflecting liver function and inflammation is urgently required as an alternative to liver biopsy. Aspartate aminotransferase (AST) to platelet ratio index (APRI) was reported as a rapid and reliable tool for evaluating the liver function and cirrhosis severity, even to the point of replacing liver biopsy.11 Other inflammatory markers with predictive value for HCC prognosis including PLR, NLR and AST/ALT had no such advantage. Recently, HCC prognosis was also demonstrated to be associated with APRI.12 However, APRI has not been definitively validated following hepatic resection in early-stage HCC patients. Moreover, a model based on APRI to accurately predict early-stage HCC prognosis has not been developed so far.
HCC prognosis has been predicted using various staging systems. The Hong Kong Liver Cancer (HKLC) staging system developed in 2014 is based on a large number of HBV-related HCC patients. It is designed to be more sensitive at identifying patients requiring more aggressive treatment than the Barcelona Clinic Liver Cancer (BCLC) staging system.13 Despite several studies reporting that the HKLC system more effectively predicted survival than the BCLC system,14,15 it lacks external validation in different cohorts. Thus, the currently most validated and reliable prediction systems are still BCLC1 and AJCC eighth edition.16 However, both systems lack definite prognostic thresholds.
Accurate and reliable prognostic nomograms have been developed and validated in several cancer types.17,18 Nomograms can quantify patient scores based on risk factors to predict survival outcomes and can help clinicians to make clinical decisions. Nomograms can be used preoperatively to determine whether surgical resection or other treatments should be applied. For instance, transarterial chemoembolization (TACE) combined with radiofrequency ablation (RFA) has been reported to be as good as surgery with less post-treatment morbidity.19 Postoperative nomograms can be used to help surgeons select patient subgroups for further treatment, such as TACE or immunological therapy. Thus, nomogram is considered to be a new prognostic criterion compared with other staging systems. We, therefore, set out to establish a predictive APRI threshold in order to construct an APRI-based nomogram to provide an individualized prognosis for early-stage HCC patients treated with hepatic resection.
Patients and Methods
Patients
The Barcelona Clinic Liver Cancer guidelines were used to diagnose early-stage HCC patients at the Eastern Hepatobiliary Surgery Hospital (EHBH, Shanghai, China) who underwent liver resection between January 2009 and December 2012. After attaining informed consent and ethical approval from the EHBH Clinical Research Ethics Committee, we retrospectively analyzed patient data in accordance with the tenets of the Helsinki Declaration (1964).
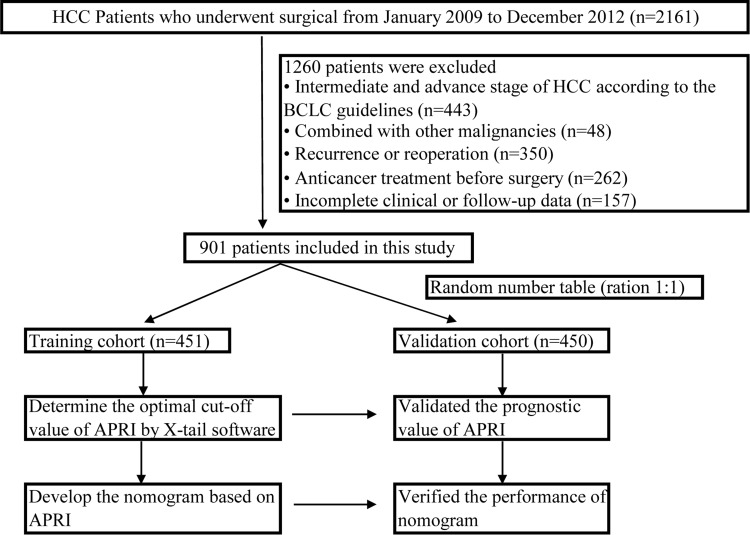
For inclusion into the study, patients were required to have the following: (1) histopathologically confirmed HCC diagnosis; (2) BCLC staging system classification equivalent to stage 0 and A1 (the definition of early-stage HCC); (3) Child-Pugh class A or B; (4) R0 resection with negative margin; (5) no pre-surgery history of any anticancer treatment. Additionally, patients were screened based on the following exclusion criteria: (1) combined morbidity with other malignancies; (2) lymph node metastasis or extrahepatic metastasis confirmed by imaging examination or postoperative pathology; (3) inferior vena cava, portal, hepatic vein or similar macrovascular invasion; (4) patients with recurrence and reoperation; (5) clinical follow-up data that was missing or inadequate. The study design and the recruited patient flowchart are shown in Figure 1.
Figure 1.
The included patient flowchart and study design.
Data Collection and Definitions
Clinic characteristics of each patient including gender, age, platelets (PLT), liver function data, hepatitis virus data and tumor biomarkers were collected before surgery. Tumor status, pathological reports, Edmondson-Steiner grade, TNM stage and BCLC grade were determined when surgery was complete. The 8th edition of the American Joint Committee on Cancer (AJCC) was used to classify TNM stage.16 The differentiation grade of tumors was established using the Edmondson-Steiner classification.20 The threshold level of HBV-DNA, microvascular invasion (MVI) and satellite lesions were considered as we previously described.21 To mathematically derive APRI, we used the following formula: [AST (IU/L) ÷ highest normal level] ÷ PLT (×109/L).22
Surgical Treatment
All patients underwent routine assessment within 7 days before surgery, including preoperative elaborate blood tests, coagulation function test, biochemical indicators and other examinations. Chest radiographs, abdominal b-ultrasound, spirometry, blood gas analysis and liver imaging examinations were also performed routinely. Magnetic resonance imaging (MRI), contrast-enhanced computed tomography (CT), or three-dimensional CT images were imaging techniques used to capture images of the liver in order to assess tumor status and determine the extent of surgical resection. Furthermore, imaging was used to establish whether the future liver remnant was sufficient in size. Surgery was only conducted once contraindications had been excluded.
Liver resection was performed by the right subcostal incision and extended to the left subcostal and xiphoid process if necessary. The abdominal cavity, primary focus and other organs were carefully probed by the surgeon. Anatomic resection was preferred for tumors hemi hepatic or located in liver lobes or segments. The hepatic parenchyma was separated by a clamp-crushing method and the related hepatic pedicles and veins were ligated carefully. Pringle’s maneuver was routinely carried out to occlude the hepatic portal vein in order to manage and restrict intraoperative bleeding, with clamping and unclamped cycles of 15 and 5 minutes respectively. For large, deep or paravascular tumors, preoperative three-dimensional CT images were used to determine tumor boundaries and resection ranges. Large tumor size can make complete liver mobilization and extraparenchymal inflow control difficult. In such cases, extraparenchymal control of the main hepatic veins or the inferior vena cava is preferred. This method can substantially reduce both operative bleeding and the risk of rupture.23 Microscopic absence of remaining tumors including around the margin was defined as R0 resection.24
Follow-Up
During the first year, patients who underwent hepatectomy were followed up by telephone or outpatient visits every 3 months then once every 6 months subsequently. Routine follow-up examination included abdominal b-ultrasound, liver function and level of serum a-fetoprotein (AFP). CT with or without MRI of the liver was conducted once every 6 months. Tumor recurrence was considered when new lesions were detected on two imaging examinations or AFP continued to rise. The interval between the surgical operation date to the final date of follow-up or death was used to measure overall survival (OS), while the duration from the date of surgery to the initial diagnosed recurrence was used to determine recurrence-free survival (RFS).21 March 2016 was the last date of follow-up for all patients.
Statistical Analysis
In this study, data were presented in categorical variables, stated as percentages, while Fisher’s exact or the Chi-squared were used to test for differences between variables. Multivariate and univariate analyses through the Cox proportional hazards model were employed to determine independent prognostic factors for survival. The rms package of R software (version 3.5.1) was applied to the training cohort to construct a nomogram based on the independent predictors. The nomogram’s performance was tested using calibration curves and the concordance index (C-index) which were derived and presented for this purpose. The comparison of C-indices between the nomogram and other predictors was performed using the R Hmisc package, specifically, the rcorrp.cens function.18 In the validation cohort, calibration evaluation and validation of the nomogram were conducted with a bootstrap of 1000 samples.25 From the training cohort, version 3.6.1 of X-tile software from Yale University School of Medicine (Connecticut, United States)26 calculated the optimum APRI threshold level. The validation cohort then verified its precision. The R package survminer was used to measure variable survival distribution, while the rmda package was employed for a net benefit-dependent decision curve analysis (DCA).27 The 23rd version of SPSS (IBM, New York, United States) was used alongside R software to carry out statistical tests and two-tailed probability values of P<0.05 were considered statistically significant.
Results
Patients Clinicopathologic Characteristics
This study observed early-stage HCC patients amounting to 901 in total. Validation and training cohorts were established using the random number table method to achieve a 1:1 distribution for each cohort. Table 1 outlines the patient clinicopathologic traits. The proportion of male patients was 84.0%, while among all patients, ages ranged from 18 to 83 years with 52.2±10.7 years being the average. The most common etiology was hepatitis B (88.6%). Patients with HBV-DNA level exceeding 2000 IU/mL were 46.2% of the total, while 56.6% of patients developed liver cirrhosis. Additionally, almost all patients (97.6%) had a Child-Pugh score of A. With respect to the tumor status, 876 (97.2%) cases presented with a single nodule and 342 (38.0%) patients had tumor size greater than 5 cm. Regarding pathological reports, 265 (29.4%) patients were MVI positive and 253 (28.1%) cases had satellite lesions. Edmondson-Steiner grade III+IV was identified in 714 (79.2%) patients. As for tumor staging, 90.0% and 61.2% of patients were classified as BCLC stage A and AJCC eighth stage IB, respectively. Apart from age, the validation and training cohorts were otherwise similar in most respects. All included patients underwent open surgery, with an average operation time of 127.9±40.4 minutes and an average hospital stay of 17.3±8.2 days. Post-hepatectomy liver failure was observed in 6 (0.6%) patients, and postoperative 30-day and 90-day mortality were observed in 6 (0.6%) and 21 (2.3%) patients, respectively. Perioperative complications mainly included pleural effusion in 81 cases (9.0%), pneumonia in 9 cases (1.0%), bile leakage in 52 cases (5.8%), postoperative hemorrhage in 11 cases (1.2%), incision infection in 20 cases (2.2%), and gastric retention in 8 cases (0.9%).
Table 1.
Clinicopathologic Characteristics of Patients with Early Stage HCC in the Training and Validation Cohorts
| Total Patients | Training Cohort | Validation Cohort | ||
|---|---|---|---|---|
| Variables | n=901 | n=451 | n=450 | P value |
| Sex | ||||
| Male | 757 (84.0%) | 383 (84.9%) | 374 (83.1%) | 0.458 |
| Female | 144 (16.0%) | 68 (15.1%) | 76 (16.9%) | |
| HBsAg | ||||
| Negative | 103 (11.4%) | 46 (10.2%) | 57 (12.7%) | 0.245 |
| Positive | 798 (88.6%) | 405 (89.8%) | 393 (87.3%) | |
| Age, y | ||||
| ≤60 | 693 (76.9%) | 328 (72.7%) | 365 (81.1%) | 0.003 |
| >60 | 208 (23.1%) | 123 (27.3%) | 85 (18.9%) | |
| PLT, x10^9/L | ||||
| ≤100 | 158 (17.5%) | 81 (18.0%) | 77 (17.1%) | 0.738 |
| >100 | 743 (82.5%) | 370 (82.0%) | 373 (82.9%) | |
| TB, μmol/L | ||||
| <34.2 | 891 (98.9%) | 445 (98.7%%) | 446 (99.1%%) | 0.753 |
| ≥34.2 | 10 (1.1%) | 6 (1.3%%) | 4 (0.9%%) | |
| ALB, g/L | ||||
| ≥40 | 646 (71.7%) | 322 (71.4%) | 324 (72.0%) | 0.841 |
| <40 | 255 (28.3%) | 129 (28.6%) | 126 (28.0%) | |
| ALT, IU/L | ||||
| ≤50 | 676 (75.0%) | 345 (76.5%) | 331 (73.6%) | 0.308 |
| >50 | 225 (25.0%) | 106 (23.5%) | 119 (26.4%) | |
| AST, IU/L | ||||
| ≤40 | 592 (65.7%) | 295 (65.4%) | 297 (66.0%) | 0.852 |
| >40 | 309 (34.3%) | 156 (34.6%) | 153 (34.0%) | |
| GGT, IU/L | ||||
| ≤60 | 472 (52.4%) | 234 (51.9%) | 238 (52.9%) | 0.763 |
| >60 | 429 (47.6%) | 217 (48.1%) | 212 (47.1%) | |
| LDH, IU/L | ||||
| ≤225 | 725 (80.5%) | 361 (80.0%) | 364 (80.9%) | 0.749 |
| >225 | 176 (19.5%) | 90 (20.0%) | 86 (19.1%) | |
| ALP, U/L | ||||
| ≤130 | 792 (87.9%) | 393 (87.1%) | 399 (88.7%) | 0.482 |
| >130 | 109 (12.1%) | 58 (12.9%) | 51 (11.3%) | |
| HBV-DNA load, IU/mL | ||||
| <2000 | 485 (53.8%) | 245 (54.3%) | 240 (53.3%) | 0.766 |
| ≥2000 | 416 (46.2%) | 206 (45.7%) | 210 (46.7%) | |
| AFP, μg/L | ||||
| ≤400 | 594 (65.9%) | 299 (66.3%) | 295 (65.6%) | 0.814 |
| >400 | 307 (34.1%) | 152 (33.7%) | 155 (34.4%) | |
| CEA, μg/L | ||||
| ≤10 | 892 (99.0%) | 447 (99.1%) | 445 (98.9%) | 0.997 |
| >10 | 9 (1.0%) | 4 (0.9%) | 5 (1.1%) | |
| CA19-9, μg/L | ||||
| ≤39 | 738 (81.9%) | 376 (83.4%) | 362 (80.4%) | 0.254 |
| >39 | 163 (18.1%) | 75 (16.6%) | 88 (19.6%) | |
| APRI | ||||
| ≤0.9 | 686 (76.1%) | 344 (76.3%) | 342 (76.0%) | 0.923 |
| >0.9 | 215 (23.9%) | 107 (23.7%) | 108 (24.0%) | |
| Blood loss, mL | ||||
| <400 | 691 (76.7%) | 341 (75.6%) | 350 (77.8%) | 0.442 |
| ≥400 | 210 (23.3%) | 110 (24.4%) | 100 (22.2%) | |
| Tumor number | ||||
| Single | 876 (97.2%) | 438 (97.1%) | 438 (97.3%) | 0.844 |
| Multiple | 25 (2.8%) | 13 (2.9%) | 12 (2.7%) | |
| Tumor size, cm | ||||
| ≤5 | 559 (62.0%) | 273 (60.5%) | 286 (63.6%) | 0.350 |
| >5 | 342 (38.0%) | 178 (39.5%) | 164 (36.4%) | |
| MVI | ||||
| No | 636 (70.6%) | 314 (69.6%) | 322 (71.6%) | 0.524 |
| Yes | 265 (29.4%) | 137 (30.4%) | 128 (28.4%) | |
| Satellite lesions | ||||
| No | 648 (71.9%) | 327 (72.5%) | 321 (71.3%) | 0.695 |
| Yes | 253 (28.1%) | 124 (27.5%) | 129 (28.7%) | |
| Liver cirrhosis | ||||
| No | 391 (43.4%) | 194 (43.0%) | 197 (43.8%) | 0.817 |
| Yes | 510 (56.6%) | 257 (57.0%) | 253 (56.2%) | |
| Edmondson-Steiner grade | ||||
| I+II | 187 (20.8%) | 95 (21.1%) | 92 (20.4%) | 0.819 |
| III+IV | 714 (79.2%) | 356 (78.9%) | 358 (79.6%) | |
| Child-Pugh (A/B) | ||||
| A | 879 (97.6%) | 439 (97.3%) | 440 (97.8%) | 0.670 |
| B | 22 (2.4%) | 12 (2.7%) | 10 (2.2%) | |
| TNM stage (AJCC eighth) | ||||
| IA | 90 (10.0%) | 49 (10.9%) | 41 (9.1%) | 0.445 |
| IB | 551 (61.2%) | 267 (59.2%) | 284 (63.1%) | |
| II | 260 (28.9%) | 135 (29.9%) | 125 (27.8%) | |
| BCLC stage | ||||
| 0 | 90 (10.0%) | 49 (10.9%) | 41 (9.1%) | 0.380 |
| A | 811 (90.0%) | 402 (89.1%) | 409 (90.9%) |
Notes: P value: training cohort versus validation cohort. P<0.05 was defined as statistical significance.
Abbreviations: HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; PLT, platelets; TB, total bilirubin; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; HBV-DNA, hepatitis B virus deoxyribonucleic acid; AFP, a-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; APRI, aspartate aminotransferase-to-platelet ratio index; MVI, microvascular invasion; TNM, TNM Classification of Malignant Tumors; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer.
Patients Characteristics Stratified by APRI and Prognostic Value of APRI
The validation and training cohort median APRI values were 0.54 (range, 0.12–2.79) and 0.58 (range, 0.08–2.71), respectively. X-tile software facilitated a clear distinction between poor and favorable prognostic outcomes. It revealed an APRI threshold of 0.9 in the training cohort (Supplemental Figure S1). The threshold value was used to classify patients into low and high (≤0.9, >0.9, respectively) APRL categories (Supplemental Table S1). High ARPI patients in both cohorts had a poorer liver function. For instance, a higher proportion of them had elevated total bilirubin, alanine transaminase, aspartate aminotransferase, γ-glutamyl transferase, lactate dehydrogenase and alkaline phosphatase, while levels of serum albumin and platelets were lower (all P <0.05). They also had higher rates of liver cirrhosis and comprised a greater proportion with elevated serum CA199 and HBV-DNA levels, compared with low APRI patients (P<0.05). However, there was no significant difference between high and low APRI groups in tumor status.
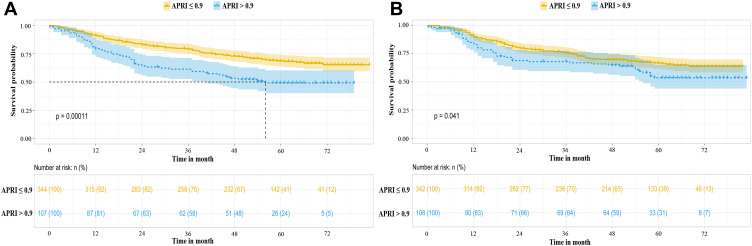
As shown in Figure 2A, the high ARPI group had significantly lower one-, three- and five-year OS rates than the low ARPI group (79.4%, 59.3% and 49.2%, respectively, versus 91.0%, 79.1% and 68.2%, respectively, P<0.01). The validation cohort confirmed the prognostic accuracy of APRI. The validation results (Figure 2B) showed that the high ARPI group also had significantly lower one-, three- and five-year OS rates than the low ARPI group (83.1%, 66.9% and 53.6%, respectively, versus 89.8%, 75.8% and 66.2%, respectively, P=0.041).
Figure 2.
HCC patient Kaplan–Meier curves stratified by APRI in the training cohort (A) and validation cohort (B).
Abbreviations: HCC, hepatocellular carcinoma; APRI, aspartate aminotransferase-to-platelet ratio index.
Survival Analysis
All patients were followed up for 3–86 months with a median duration of 64 months. The training cohort’s two-, three- and four-year cumulative RFS rates were 68.6%, 61.6% and 52.7%; while OS rates were 78.6%, 74.9% and 67.8%, respectively. The validation cohort’s cumulative two-, three- and four-year RFS rates were 63.7%, 58.2% and 52.3%, while OS rates were 77.3%, 73.9% and 68.3%, respectively. The respective validation and training cohort median RFS durations were 54 and 56 months.
Independent Risk Factors of OS and RFS in the Training Cohort
Table 2 summarizes the multivariate and univariate analyses of the training cohort which showed that when APRI was above 0.9 (RFS: HR=1.535, 95% CI:1.093–2.156, P=0.013; OS: HR=1.667, 95% CI:1.096–2.536, P=0.017), blood loss was more than 400 mL (RFS: HR=1.419, 95% CI:1.044–1.928, P=0.025; OS: HR=1.671, 95% CI:1.178–2.370, P=0.004), multiple tumors (RFS: HR=2.389, 95% CI:1.219–4.681, P=0.011; OS: HR=3.957, 95% CI:1.904–8.222, P<0.001), tumors larger than five centimeters (RFS: HR=1.754, 95% CI:1.270–2.422, P=0.001; OS: HR=2.637, 95% CI:1.756–3.961, P<0.001), MVI positivity (RFS: HR=1.571, 95% CI:1.169–2.112, P=0.003; OS: HR=1.905, 95% CI:1.342–2.705, P<0.001), satellite lesions (RFS: HR=1.370, 95% CI:1.019–1.840, P=0.037; OS: HR=1.645, 95% CI:1.162–2.329, P=0.005) and liver cirrhosis (RFS: HR=1.485, 95% CI:1.091–2.021, P=0.012; OS: HR=1.553, 95% CI:1.072–2.249, P=0.020) correlated with poorer RFS and OS. In addition, male gender (HR=1.583, 95% CI:1.024–2.449, P=0.039) and ALB less than 40 g/L (HR=1.445, 95% CI:1.085–1.925, P=0.012) also correlated with diminished RFS.
Table 2.
Univariate and Multivariate Analysis of the Training Cohort for OS and RFS
| OS | RFS | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P value | HR | 95% CI | P value |
| Univariate | ||||||
| Sex (Female/Male) | 1.404 | 0.869–2.268 | 0.165 | 1.663 | 1.098–2.519 | 0.016 |
| HBsAg (Negative/Positive) | 0.872 | 0.534–1.424 | 0.584 | 1.269 | 0.802–2.008 | 0.309 |
| Age (≤60/>60, y) | 1.295 | 0.926–1.811 | 0.131 | 1.168 | 0.880–1.550 | 0.281 |
| PLT (≤100/>100, x10^9/L) | 0.877 | 0.592–1.299 | 0.514 | 0.832 | 0.602–1.150 | 0.265 |
| TB (≥34.2/<34.2, μmol/L) | 1.915 | 0.611–6.009 | 0.265 | 1.262 | 0.404–3.943 | 0.689 |
| ALB (<40/≥40, g/L) | 1.302 | 0.934–1.814 | 0.120 | 1.674 | 1.278–2.191 | <0.001 |
| ALT (>50/≤50, IU/L) | 1.320 | 0.932–1.869 | 0.118 | 1.495 | 1.125–1.987 | 0.006 |
| AST (>40/≤40, IU/L) | 1.883 | 1.376–2.577 | <0.001 | 1.675 | 1.288–2.178 | <0.001 |
| GGT (>60/≤60, IU/L) | 1.910 | 1.389–2.627 | <0.001 | 1.668 | 1.286–2.163 | <0.001 |
| LDH (>225/≤225, IU/L) | 2.495 | 1.783–3.492 | <0.001 | 1.930 | 1.437–2.591 | <0.001 |
| ALP (>130/≤130, U/L) | 2.273 | 1.540–3.353 | <0.001 | 1.696 | 1.177–2.445 | 0.005 |
| HBV-DNA (≤2000/>2000, IU/mL) | 1.688 | 1.232–2.312 | 0.001 | 1.495 | 1.154–1.936 | 0.002 |
| AFP (>400/≤400, μg/L) | 1.899 | 1.387–2.598 | <0.001 | 1.367 | 1.046–1.787 | 0.022 |
| CEA (≤10/>10, μg/L) | 0.827 | 0.116–5.907 | 0.850 | 1.057 | 0.263–4.253 | 0.938 |
| CA199 (>39/≤39, μg/L) | 1.238 | 0.836–1.834 | 0.286 | 1.307 | 0.943–1.810 | 0.107 |
| APRI (≤0.9/>0.9) | 1.910 | 1.369–2.663 | <0.001 | 1.821 | 1.374–2.413 | <0.001 |
| Blood loss (≥400/<400, mL) | 2.388 | 1.731–3.294 | <0.001 | 1.733 | 1.310–2.292 | <0.001 |
| Tumor number (Single/Multiple) | 2.903 | 1.479–5.697 | 0.002 | 2.616 | 1.383–4.946 | 0.003 |
| Tumor size (>5/≤5, cm) | 3.151 | 2.288–4.340 | <0.001 | 1.934 | 1.493–2.505 | <0.001 |
| MVI (No/Yes) | 3.021 | 2.209–4.132 | <0.001 | 1.949 | 1.491–2.549 | <0.001 |
| Satellite lesions (No/Yes) | 2.159 | 1.571–2.967 | <0.001 | 1.650 | 1.255–2.171 | <0.001 |
| Livers cirrhosis (No/Yes) | 1.410 | 1.019–1.950 | 0.038 | 1.545 | 1.180–2.024 | 0.002 |
| Edmondson-Steiner grade (I+II/III+IV) | 1.900 | 1.211–2.982 | 0.005 | 1.294 | 0.932–1.798 | 0.124 |
| Child-Pugh (A/B) | 0.708 | 0.226–2.221 | 0.554 | 0.632 | 0.235–1.700 | 0.364 |
| Multivariate | ||||||
| APRI (≤0.9/>0.9) | 1.667 | 1.096–2.536 | 0.017 | 1.535 | 1.093–2.156 | 0.013 |
| Blood loss (≥400/<400, mL) | 1.671 | 1.178–2.370 | 0.004 | 1.419 | 1.044–1.928 | 0.025 |
| Tumor number (Single/Multiple) | 3.957 | 1.904–8.222 | <0.001 | 2.389 | 1.219–4.681 | 0.011 |
| Tumor size (>5/≤5, cm) | 2.637 | 1.756–3.961 | <0.001 | 1.754 | 1.270–2.422 | 0.001 |
| MVI (No/Yes) | 1.905 | 1.342–2.705 | <0.001 | 1.571 | 1.169–2.112 | 0.003 |
| Satellite lesions (No/Yes) | 1.645 | 1.162–2.329 | 0.005 | 1.370 | 1.019–1.840 | 0.037 |
| Livers cirrhosis (No/Yes) | 1.553 | 1.072–2.249 | 0.020 | 1.485 | 1.091–2.021 | 0.012 |
| Sex (Female/Male) | 1.583 | 1.024–2.449 | 0.039 | |||
| ALB (<40/≥40, g/L) | 1.445 | 1.085–1.925 | 0.012 | |||
Note: P<0.05 was defined as statistical significance.
Abbreviations: OS, overall survival; RFS, recurrence-free survival; HBsAg, hepatitis B surface antigen; PLT, platelets; TB, total bilirubin; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; HBV-DNA, hepatitis B virus deoxyribonucleic acid; AFP, a-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; APRI, aspartate aminotransferase-to-platelet ratio index; MVI, microvascular invasion.
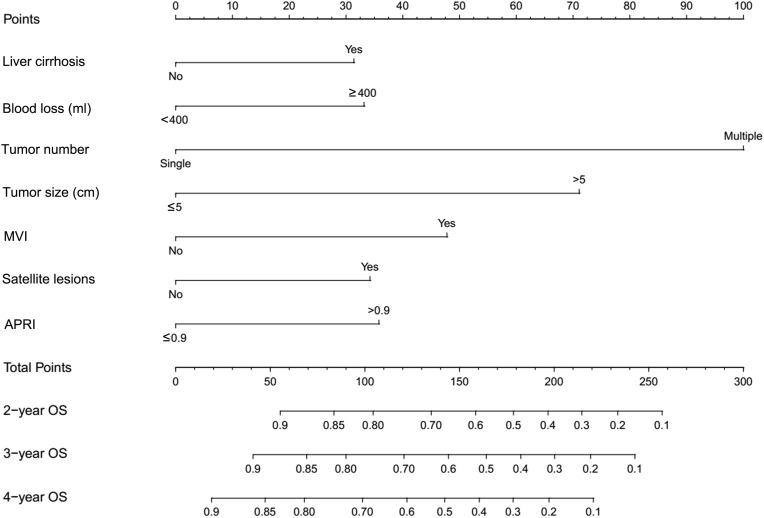
Construction and Validation of the OS Nomogram
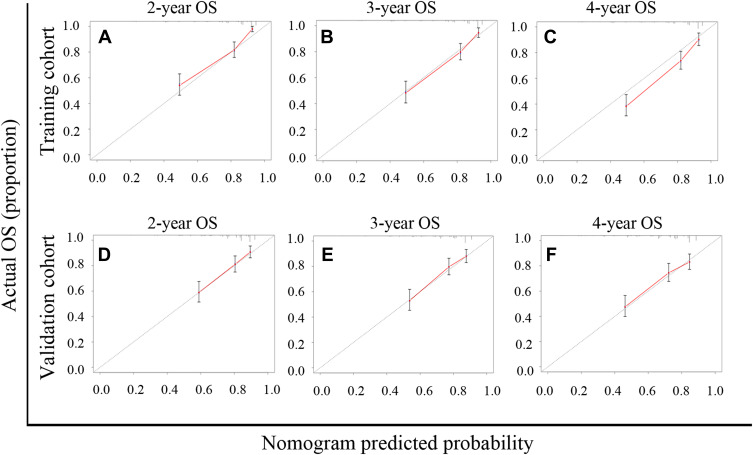
The prognostic nomogram was constructed from all seven separate OS risk factors in the training group (Figure 3). Each individual risk factor had a specific score, and the individualized grade of each included patient was defined by the sum of points from the seven predictors. The projections from the total points on the scale (range, 0–300) indicated the probability of survival at 2-, 3-, and 4-year. From the training group, the nomogram’s C-index for predicting survival was 0.752 (95% CI: 0.706–0.798), while enhanced alignment between two-, three- and four-year predictions and manifested outcomes was shown by the calibration curves (Figure 4A–C). In parallel, the OS nomogram’s C-index from the validation group was 0.701 (95% CI: 0.654–0.748), while the idealized 45° line was harmonious with two-, three- and four-year OS calibration curves (Figure 4D–F).
Figure 3.
Survival predicting nomogram based on APRI for early-stage HCC patients.
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; MVI, microvascular invasion.
Figure 4.
APRI-based nomogram calibration curves for predicting overall survival (OS) at two-, three-and four-year. (A–C) Two-, three-and four-year OS in the training cohort. (D–F) Two-, three-and four-year OS in the validation cohort.
Abbreviation: APRI, aspartate aminotransferase-to-platelet ratio index.
Performance Comparison of the APRI-Based Nomogram and Other Predictors
The APRI-based nomogram we established was compared with other predictors including seven independent risk factors, AJCC eighth and BCLC staging systems to determine the optimal prediction model (Table 3). When compared to C-indices of APRI (0.568), blood loss (0.593), tumor number (0.520), tumor size (0.651), MVI (0.632), satellite lesions (0.593), liver cirrhosis (0.538), AJCC eighth (0.664) and BCLC stage (0.550), the training cohort nomogram C-index (0.752) was significantly higher (all P<0.001). Concurrently, comparing C-indices of APRI (0.537), blood loss (0.552), tumor number (0.500), tumor size (0.620), MVI (0.592), satellite lesions (0.608), liver cirrhosis (0.516), AJCC eighth (0.609) and BCLC stage (0.533) to the nomogram’s C-index (0.701) in the validation cohort revealed that it was also significantly higher (all P<0.001). A nomogram based on APRI was hence demonstrated to be the most optimal prediction model for OS in patients with early-stage HCC following liver resection.
Table 3.
The Nomogram C-Index for OS and Other Predictors
| Training Cohort | Validation Cohort | |||||
|---|---|---|---|---|---|---|
| Predictors | C-Index | 95% CI | P value | C-Index | 95% CI | P value |
| Nomogram | 0.752 | 0.706–0.798 | 0.701 | 0.654–0.748 | ||
| APRI | 0.568 | 0.535–0.601 | <0.001 | 0.537 | 0.503–0.571 | <0.001 |
| Blood loss | 0.593 | 0.560–0.626 | <0.001 | 0.552 | 0.519–0.585 | <0.001 |
| Tumor number | 0.520 | 0.508–0.532 | <0.001 | 0.500 | 0.486–0.513 | <0.001 |
| Tumor size | 0.651 | 0.613–0.689 | <0.001 | 0.620 | 0.582–0.658 | <0.001 |
| MVI | 0.632 | 0.597–0.668 | <0.001 | 0.592 | 0.557–0.627 | <0.001 |
| Satellite lesions | 0.593 | 0.558–0.628 | <0.001 | 0.608 | 0.574–0.643 | <0.001 |
| Livers cirrhosis | 0.538 | 0.498–0.578 | <0.001 | 0.516 | 0.475–0.556 | <0.001 |
| TNM stage | 0.664 | 0.624–0.704 | <0.001 | 0.609 | 0.570–0.648 | <0.001 |
| BCLC stage | 0.550 | 0.524–0.577 | <0.001 | 0.533 | 0.508–0.557 | <0.001 |
Notes: P value: nomogram versus other predictors. P<0.05 was defined as statistical significance.
Abbreviations: C-index, concordance index; OS, overall survival; CI, confidence interval; APRI, aspartate aminotransferase-to-platelet ratio index; MVI, microvascular invasion; BCLC, Barcelona Clinic Liver Cancer; TNM, TNM Classification of Malignant Tumors.
Assessment of the Discriminative Ability of the APRI-Based Nomogram
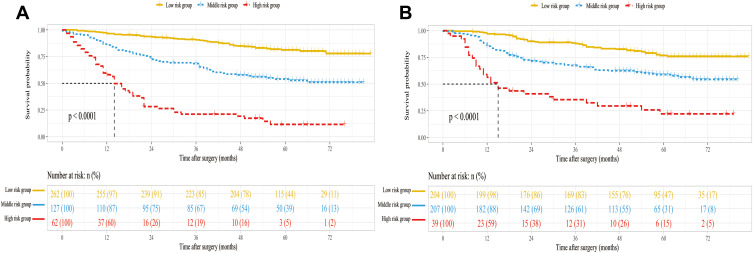
In the APRI-based nomogram, each risk factor corresponds to a specific score. The total points for each patient were derived from the nomogram. In the training and validation cohorts, these total points ranged from 0 to 253. Using X-tile software, all patients were split into low-, middle- and high-risk groups by cut-off scores of 101 and 179 for the training cohort, and 69 and 171 for the validation cohort. As shown in Figure 5, the Kaplan–Meier curves of OS indicated clear and distinct prognosis rates in each risk group for training (Figure 5A) and validation (Figure 5B) cohorts. This means that the APRI-based nomogram can accurately stratify patients into different risk groups for early-stage HCC.
Figure 5.
Kaplan–Meier curves of overall survival (OS) for risk groups in the training cohort (A) and validation cohort (B).
Decision Curve Analysis of the APRI-Based Nomogram
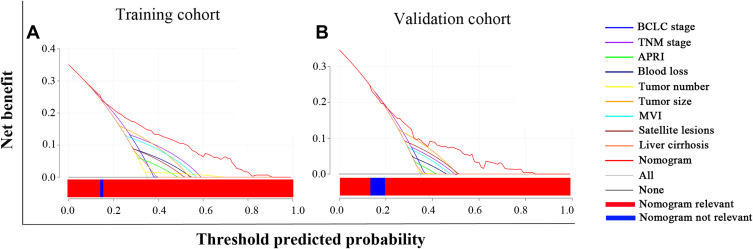
DCA is a new technique for assessing clinical predictive models by examining the range of threshold probabilities and overall therapeutic advantage.27 The greatest overall advantage attained through predicting survival over a broad range of threshold probabilities in the DCA was shown by the nomogram based on APRI which performed better than other predictors in the training (Figure 6A) and validation (Figure 6B) cohorts. Moreover, it showed that the APRI-based nomogram was more suitable for clinical application in early-stage HCC.
Figure 6.
Decision curve analysis (DCA) of the APRI-based nomogram and other predictors for overall survival (OS) in the training cohort (A) and validation cohort (B). The x-axis and the y-axis represent threshold predicted probability and net benefit, respectively. Solid black line: absence of patients experiencing the event. Solid gray line: all patients will die. Each predictor had a line with a corresponding color. The blue bar within the red horizontal line indicated that the nomogram was not the optimal model in this section. Generally, the APRI-based nomogram showed more net benefit with a wider range of threshold probabilities than other predictors.
Abbreviation: APRI, aspartate aminotransferase-to-platelet ratio index.
Discussion
We retrospectively assessed a total of 901 early-stage HCC patients following hepatic resection in this study. Several major results were identified and validated. Firstly, 0.9 as the cut-off value of APRI in the training cohort enabled a clear distinction of poor outcomes and favorable prognoses. Importantly, the criterion was validated in the validation cohort. Secondly, APRI was a poor, separate determinant of survival and recurrence for post-surgery early-stage HCC patients. Thirdly, a prognostic nomogram based on APRI for the prediction of individualized patient survival of early-stage HCC was constructed and also validated. Our prognostic nomogram showed more accurate prediction and optimal distinctive capability. Validation and training cohort C-indices of 0.701 and 0.752, respectively, were obtained when compared with other predictors. Moreover, DCA also demonstrated that the prognostic nomogram was more suitable for clinical application in early-stage HCC with a superior net benefit to other predictors.
The current gold standard for histological liver analysis in chronic hepatitis patients is a liver biopsy. Nevertheless, its clinical application is restricted due to its invasiveness, risk of painful tissue damage, injury to the bile duct, hemorrhage and its expense.11 Thus, several noninvasive tools have been developed to surrogate liver biopsy and applied on patients with hepatitis to measure the extent of liver fibrosis. Recent studies suggested that APRI was a simple, effective and noninvasive method in ascertaining liver cirrhosis and fibrosis stages for chronic hepatitis B or C patients.28,29 Moreover, APRI has been reported to be a separate determinant in HCC patients following curative resection, radiofrequency ablation and transarterial chemoembolization.30,31 Consistent with the above results, our study also demonstrated that elevated APRI measurements correlated with dismal prognoses. We used X-tile software, relying on the principles of the time-dependent technique to establish the APRI threshold offering optimal performance. Moreover, a validation cohort was developed to assess the stability of APRI. The results showed that an APRI threshold of 0.9 was the best predictor of prognosis for patients with early-stage HCC treated by curative resection. Importantly, APRI stability was verified in the validation cohort. The APRI threshold we present here is slightly different from that in previous reports. Using receiver operating characteristic curves for post-surgical hepatitis B-related hepatocellular carcinoma patients, Hung et al12 and Shen et al32 developed thresholds of 0.47 and 0.62, respectively. Divergent cut-off value analysis methods and different etiology of HCC may be responsible for the differences with this study. Additionally, tumor status, sample size and criteria for patient inclusion were also important reasons for the differences. Hung et al12 study enrolled 76 patients with small (<5 cm) solitary HBV-related HCC, the median tumor size was 2.5 cm, which was significantly smaller than the tumor size in our study (median tumor size of 4.2 cm). Shen et al32 included 332 HCC patients with a mean tumor size of 8.76 cm, while 25.6% patients with macrovascular invasion and 20.8% patients with portal vein tumor thrombi. However, such patients were not included in our study. Although the cut-off values of APRI were different, the views were consistent that a low APRI predicts better overall survival.
The exact mechanism operating between APRI and poor prognosis of HCC patients after hepatectomy is still unclear. It may be related to the following reasons. Firstly, hepatocellular inflammation caused by hepatitis virus infection and alcohol consumption is a leading cause of HCC pathogenesis.22 AST, which exists in the mitochondria of hepatocytes, is a reliable and sensitive biological indicator of inflammation in the liver. AST from the liver can be released into blood serum following disease of the liver that injures hepatic mitochondria, which indicates that liver function has been seriously damaged. Moreover, hepatocyte injury is closely associated with liver carcinogenesis.33 Secondly, circulating platelets have a dual role in the liver. Platelets are closely related to liver regeneration, which can promote hepatocellular regeneration and reflect the degree of liver function.34 In contrast, it has been reported that platelets can interact with cancer cells and promote tumor growth.35 Thus, an APRI encompassing AST and platelets could assess the reserve liver function of a patient and predict an individualized prognosis after surgery. As the study results have indicated, HCC patients with APRI >0.9 had a worse prognosis and recurrence. Therefore, an elevated APRI indicates severe impairment of liver function and poor tumor prognosis.
A nomogram, constructed using independent risk factors, is an intuitive statistical model that can maximize prediction accuracy and estimate individualized prognosis. In several cancer types, nomograms with more accurate prediction and performance than other models for evaluating prognosis have been developed and validated.18,36 In the present study, of all the predictors incorporated into the prognostic nomogram, that is: liver cirrhosis, blood loss of more than 400 mL, multiple tumor, tumor size greater than 5 cm, MVI and satellite lesions have been demonstrated to be associated with the prognosis of HCC.37–39 The APRI was shown to have a superior survival predictive ability than AST and PLT from multivariate analysis. Moreover, there is no literature reporting previous use of an APRI-based nomogram for predicting early-stage HCC patient prognosis. Thus, the APRI-based nomogram consisting of tumor status, blood loss and liver function was constructed to offer personalized survival for early-stage HCC patients after radical resection. The APRI-based nomogram displayed more accurate survival prediction and superior performance than other predictors according to the C-indexes, calibration plots and DCA. The validation group verified the nomogram’s stability.
Among all staging systems for HCC, BCLC and AJCC eighth TNM stage are the most widely used tools. In this study, both above-mentioned systems demonstrated the ability to classify HCC patients into different risk categories (Supplemental Figure S2A and B). However, our APRI-based nomogram compared with AJCC eighth TNM and BCLC staging systems showed more accuracy in stratify patients into different risk groups using X-tile software.26 X-tile software which is based on principles of the time-dependent technique can divide a population into low-, middle-, and high-risk groups and display an associated Kaplan–Meier curve with statistical significance. This can provide more patient information and help surgeons to make clinical decisions. Furthermore, the C-index, calibration plots and DCA provided overwhelming support for the APRI-based nomogram as the early-stage HCC optimal survival predictive model. Using this nomogram, surgeons can develop favorable postoperative decision-making and individualized surveillance strategies.
The utility of data produced by this study has several drawbacks. Firstly, the nomogram was retrospectively derived using a single center’s data with selective bias and further validation of the results through other prospective studies is needed. Secondly, most patients included in this study had a background of HBV infection (88.6%), thus, a multicenter trial with a large cohort stratified by different etiologies is needed to validate our results. Thirdly, our nomogram was not compared with HKLC system in prediction ability to identify which model was better, it should be further verified in the follow-up study. Fourthly, the nomogram was only suitable for the prediction of survival in early-stage HCC patients and may ineligible for intermediate-stage and advanced HCC.
Conclusions
In conclusion, APRI was a noninvasive and accurate prognostic marker for early-stage HCC patients. APRI elevations were linked to dismal HCC prognosis. In addition, we produced and validated an APRI-based nomogram for early-stage HCC patients after hepatic resection. The results showed that the APRI-based nomogram was associated with more accurate prediction and better performance compared with other predictors in prognosticating two-, three- and four-year patient HCC survival, which plays an important role for surgeons when guiding decision-making and individualized surveillance strategies in patients.
Acknowledgments
Thank all the staff authors for their contributions to this study.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (81672721, 81772529, 81970453) and the Innovation Program of Shanghai Municipal Education Commission (12ZZ077).
Abbreviations
APRI, aspartate aminotransferase to platelets; HCC, hepatocellular carcinoma; DCA, decision curve analyses; C-index, concordance index values; AST/ALT, aspartate aminotransferase-to-alanine aminotransferase; NLR, neutrophil-to-lymphocyte; PLR, platelet to lymphocyte; BCLC, Barcelona Clinic Liver Cancer; PLT, platelets; AJCC, American Joint Committee on Cancer; MVI, microvascular invasion; MRI, magnetic resonance imaging; CT, contrast-enhanced computed tomography; AFP, a-fetoprotein; OS, overall survival; RFS, recurrence-free survival.
Data Sharing Statement
Research data are not shared, owing to the privacy or ethical restrictions.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 2.Kanwal F, Kramer JR, Duan Z, Yu X, El-Serag HB. Trends in the burden of non-alcoholic fatty liver disease in a US cohort of veterans. Clin Gastroenterol Hepatol. 2015;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58(4):724–729. doi: 10.1016/j.jhep.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi: 10.1038/457036b [DOI] [PubMed] [Google Scholar]
- 7.Shelat VG. Role of inflammatory indices in management of hepatocellular carcinoma-neutrophil to lymphocyte ratio. Ann Transl Med. 2020;8(15):912. doi: 10.21037/atm-2020-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabir T, Ye M, Mohd Noor NA, Woon W, Junnarkar SP, Shelat VG. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes after curative resection for hepatocellular carcinoma. Int J Hepatol. 2019;2019:4239463. doi: 10.1155/2019/4239463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Wang MC, Tian T, et al. A high preoperative platelet-lymphocyte ratio is a negative predictor of survival after liver resection for hepatitis b virus-related hepatocellular carcinoma: a retrospective study. Front Oncol. 2020;10:576205. doi: 10.3389/fonc.2020.576205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CY, Lin JT, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147(1):143–151.e5. doi: 10.1053/j.gastro.2014.03.048 [DOI] [PubMed] [Google Scholar]
- 11.Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018 [DOI] [PubMed] [Google Scholar]
- 12.Hung HH, Su CW, Lai CR, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4(4):691–699. doi: 10.1007/s12072-010-9213-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–1700.e3. doi: 10.1053/j.gastro.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 14.Selby LK, Tay RX, Woon WW, et al. Validity of the Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer staging systems for hepatocellular carcinoma in Singapore. J Hepatobiliary Pancreat Sci. 2017;24(3):143–152. doi: 10.1002/jhbp.423 [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Sinn DH, Gwak GY, et al. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol. 2016;22(2):250–258. doi: 10.3350/cmh.2016.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin MB, Gress DM. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. [Google Scholar]
- 17.Bochner BH, Kattan MW, Vora KC, International Bladder Cancer Nomogram Consortium. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol. 2006;24(24):3967–3972. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi: 10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 19.Gui CH, Baey S, D’Cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma – a meta-analysis. Eur J Surg Oncol. 2020;46(5):763–771. doi: 10.1016/j.ejso.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Rui JA, Ye DX, Wang SB, Chen SG, Qu Q. Edmondson-Steiner grading increases the predictive efficiency of TNM staging for long-term survival of patients with hepatocellular carcinoma after curative resection. World J Surg. 2008;32(8):1748–1756. doi: 10.1007/s00268-008-9615-8 [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Liu FC, Li L, Zhou WP, Jiang BG, Pan ZY. Nomograms to predict the long-time prognosis in patients with alpha-fetoprotein negative hepatocellular carcinoma following radical resection. Cancer Med. 2020;9(8):2791–2802. doi: 10.1002/cam4.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu GQ, Wang K, Wang B, et al. Aspartate aminotransferase-to-platelet ratio index predicts prognosis of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization. Cancer Manag Res. 2018;11:63–79. doi: 10.2147/CMAR.S186150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelat VG, Cipriani F, Basseres T, et al. Pure laparoscopic liver resection for large malignant tumors: does size matter. Ann Surg Oncol. 2015;22(4):1288–1293. doi: 10.1245/s10434-014-4107-6 [DOI] [PubMed] [Google Scholar]
- 24.Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol Res Pract. 1994;190(2):115–123. doi: 10.1016/S0344-0338(11)80700-4 [DOI] [PubMed] [Google Scholar]
- 25.Ni JY, Fang ZT, Sun HL, et al. A nomogram to predict survival of patients with intermediate-stage hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Eur Radiol. 2020;30(4):2377–2390. doi: 10.1007/s00330-019-06438-8 [DOI] [PubMed] [Google Scholar]
- 26.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 27.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. doi: 10.1177/0272989X06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boursier J, Brochard C, Bertrais S, et al. Combination of blood tests for significant fibrosis and cirrhosis improves the assessment of liver-prognosis in chronic hepatitis C. Aliment Pharmacol Ther. 2014;40(2):178–188. doi: 10.1111/apt.12813 [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Jiang Y, Gong G. Evaluation of seven noninvasive models in staging liver fibrosis in patients with chronic hepatitis B virus infection. Eur J Gastroenterol Hepatol. 2013;25(4):428–434. doi: 10.1097/MEG.0b013e32835cb5dd [DOI] [PubMed] [Google Scholar]
- 30.Tang T, Qiu J-L, Li G-W, et al. Aspartate aminotransferase-to-platelet ratio predicts response to transarterial chemoembolisation and prognosis in hepatocellular carcinoma patients. Clin Radiol. 2018;73(3):259–265. [DOI] [PubMed] [Google Scholar]
- 31.Peng W, Li C, Wen TF, et al. Postoperative aspartate aminotransferase to platelet ratio index change predicts prognosis for hepatocellular carcinoma. Medicine (Baltimore). 2016;95(30):e4160. doi: 10.1097/MD.0000000000004160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen SL, Fu S, Chen B, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis b-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21(12):3802–3809. doi: 10.1245/s10434-014-3771-x [DOI] [PubMed] [Google Scholar]
- 33.Takashi K, Hidenori T, Seiki K, et al. Predictive value of tumor markers for hepatocarcinogenesis in patients with hepatitis C virus. J Gastroenterol. 2011;46(4). [DOI] [PubMed] [Google Scholar]
- 34.Liao R, Li DW, Du CY, Li M. Combined preoperative ALBI and FIB-4 is associated with recurrence of hepatocellular carcinoma after curative hepatectomy. J Gastrointest Surg. 2018;22(10):1679–1687. doi: 10.1007/s11605-018-3810-1 [DOI] [PubMed] [Google Scholar]
- 35.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desseroit MC, Visvikis D, Tixier F, et al. Erratum to: development of a nomogram combining clinical staging with 18F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur J Nucl Med Mol Imaging. 2016;43(10):1933. doi: 10.1007/s00259-016-3450-1 [DOI] [PubMed] [Google Scholar]
- 37.Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long-term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14(10):2817–2823. doi: 10.1245/s10434-007-9518-1 [DOI] [PubMed] [Google Scholar]
- 38.Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14(1):38. doi: 10.1186/1471-2407-14-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]