Abstract
Background
Rivastigmine is used to treat cognitive impairment in Alzheimer’s disease (AD); however, the efficacy of Rivastigmine in patients with AD and concomitant small vessel cerebrovascular disease (svCVD) remains unclear. We investigated the effectiveness of Rivastigmine Patch in patients with AD and svCVD.
Methods
In this open-label study, 100 patients with AD and MRI confirmed svCVD received 9.5mg/24 hours Rivastigmine transdermal treatment for 24 weeks. The primary outcome was global cognition indexed using the ADAS-Cog. Secondary outcomes included clinical-rated impression of change (indexed using (ADCS‐CGIC), activities of daily living (indexed using ADCS-ADL) and side effects.
Results
Overall, performance on the ADAS-Cog after 24 weeks deteriorated by 1.78 (SD = 5.29) points. Fifty-two percent of the sample demonstrated improvement or remained stable, while 48% demonstrated worsening of ADAS-Cog scores. Of the 52%, significant improvement (2 or more-point decline) on the ADAS-Cog was observed in 25% of the sample, with a mean change of −5.08 (SD = 3.11). A decline on the ADAS-Cog was observed in 48% of the sample, with a mean change of 6 (SD = 2.98) points. Cognitive outcome did not interact with severity of svCVD. ADCS-ADL scores remained stable from baseline to week 24 and ADCS‐CGIC reports indicated that 81% of the patients remained stable after treatment. Side effects were reported by 16% of the patients, with contact dermatitis being the most common.
Conclusion
Our findings suggest that Rivastigmine may have a role in the management of patients having AD and concomitant mild-severe svCVD, with minimal side effects.
Keywords: rivastigmine, Alzheimer’s disease, small vessel cerebrovascular disease, treatment
Introduction
Alzheimer’s disease (AD) and cerebrovascular disease are leading causes of dementia, with cerebrovascular disease, specifically small vessel cerebrovascular disease (svCVD) co-existing in one-third of AD patients worldwide.1 In Asia, the comorbidity of AD and svCVD is believed to be even higher at 50%.2 Cholinesterase inhibitors such as rivastigmine is an approved treatment for the symptomatic management of cognitive deficits in AD. This inhibitor reduces the activity of the esterase enzyme and results in improved activity of the acetylcholine neurotransmitter, which has been clearly established to reduce cognitive decline in patients with AD.3–5 There is evidence that levels of acetylcholine are also reduced in patients with cerebrovascular disease,6 and emerging evidence suggests that Rivastigmine may have stronger treatment effects in patients with comorbid AD and svCVD7 and less side effects8 compared to AD patients without svCVD. Collectively these studies suggest that patients with AD having concomitant svCVD may experience greater clinical benefits with Rivastigmine treatment than AD patients without svCVD; however, direct evidence for the role of rivastigmine in patients with AD and concomitant svCVD is limited.
The presence of comorbid svCVD is most reliably detected on MRI FLAIR images in the form of white matter hyperintensities (WMH). WMH have been associated with a twofold increased risk of dementia, a threefold increased risk of stroke and a threefold increased risk of death.9 To date, MRI confirmed svCVD patients have yet to be assessed for their response to Rivastigmine. Due to the high prevalence of svCVD among Asian patients with AD, there is an urgent need to study the effectiveness of Rivastigmine patch in subjects with svCVD. In an open-label trial, we sought to identify the effectiveness of Rivastigmine Patch in patients with AD having MRI confirmed svCVD. We hypothesized that over a 24-week period, patients with AD having concomitant svCVD treated with Rivastigmine patch 9.5mg/24 hours will experience improvements in cognition and daily function.
Methods
Participants
One hundred consecutive patients diagnosed with AD and MRI confirmed svCVD were recruited from a memory clinic at National Neuroscience Institute, Singapore. Mild to moderate probable AD was diagnosed by cognitive neurologists using the NIA-AA Criteria10 and a Clinical Dementia Rating (CDR)11 scale of 1 or 2. The diagnosis of svCVD was based on the presence of moderate to severe white matter disease, indexed using the Fazekas scale score12 of 2–3.
Inclusion criteria were as follows: 1) age between 50 and 85 years; 1) MRI brain (with T2 or FLAIR sequences) performed within a 12-month period from time of recruitment; and 3) a Mini-Mental State Examination (MMSE)13 score of 12–28. Exclusion criteria were as follows: 1) severe neurological, psychiatric or systemic disease which in the opinion of the clinician could interfere with trial assessments; 2) use of any investigational drugs, antipsychotic or dopaminergic agents, NMDA receptor antagonist, cholinesterase inhibitors or anti-cholinergic agents during the last 4 weeks prior to recruitment; and 3) known skin allergy or previous allergic reaction to transdermal treatment.
Study Design
In this open-label study, each patient received transdermal Rivastigmine treatment for 24 weeks. For the first 4 weeks of the study, subjects received Rivastigmine patch 4.6mg/24 hours. Thereafter, subjects received Rivastigmine patch 9.5mg/24 hours. Dose adjustments (interruptions or down-titrations for up to 2 weeks at a time, for a maximum of 2 times) were permitted to address perceived safety or tolerability issues. The Rivastigmine patch was applied by the patient or their caregivers to clean, dry, hairless skin and worn for 24 hours, during which normal activities including bathing were allowed. Cognitive assessments were conducted at baseline, week 16 and week 24.
This study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki and local clinical research guidelines. The study received approval from the SingHealth Centralized Review Board and was registered with the Health Science Authority Singapore, CTA9900218 (registered 28/11/2014). Prior to data collection, written informed consent was received from all patients.
Outcomes
The primary outcome was change in global cognition, measured using the ADAS-Cog.14 The ADAS-Cog measures 11 cognitive functions: spoken language ability, comprehension of spoken language, recall of test instructions, word-finding difficulty, following commands, naming objects, construction drawing, ideational praxis, orientation, word recall and word recognition. The total score ranges from 0 to 70, with a higher score indicating greater impairment.
A secondary outcome included clinician’s impression of global change, rated using the Alzheimer’s Disease Cooperative Study – Clinical Global Impression of Change scale (ADCS‐CGIC).15 The ADCS‐CGIC reports change from baseline using a seven‐point scale, where 1 represents marked improvement and 7 represents marked worsening. A second secondary outcome was change in daily functioning, measured using the Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS-ADL) scale.16 The ADCS-ADL is a 19‐item scale assessing basic and complex abilities in people with dementia. Items include activities such as eating, bathing, operating taps, and switching off lights. Scores range from 0 (severe impairment) to 54 (no impairment). A third outcome was safety of treatment, whereby adverse events were recorded using a structured interview.
Statistical Analysis
Data Preparation
An intent-to-treat (ITT) study design was employed, where all patients who had a baseline cognitive assessment and received at least one dose of transdermal Rivastigmine were included in the study. Missing data for the ITT group was imputed using last observation carried forward (LOCF). A t-test was used to determine individual differences between participants included in the ITT-LOCF group and observed cases (OC).
Change in Cognition
Change on the ADAS-Cog from baseline to week 16 and to week 24 was assessed using repeated measure analysis controlling for age, gender and education.
Patients were split into three groups based on their pattern of cognitive change from baseline to week 24; 1) improvers, rated as a 2- or more point decline on the ADAS-Cog; 2) stable, rated as a change score of 2 to −2 on the ADAS-Cog; and 3) decliners, rated as a 2 or more increase on the ADAS-Cog. The frequency of patients in each group and mean change score was calculated for each group. A second measure of cognitive change was the clinician-rated ADCS-CGIC reported at week 24. Scores were reported as frequencies.
Interaction Between Treatment Response and Severity of svCVD
To determine whether severity of svCVD affected treatment response, a repeated measure interaction model was conducted using baseline and week 24 ADAS-Cog scores and Fazekas score as the interaction factor. Interaction models were analysed separately for each treatment responder group (improvers, patients who remained stable and decliners).
Differences Between Patients Who Improved, Remained Stable and Declined in Cognition
Differences in age, education, Fazekas, baseline cognition and baseline daily functioning between patients who improved, remained stable and those that declined was assessed using analysis of variance with post hoc Tukey’s test. A Chi-square test was used to determine differences in gender.
Change in Activities of Daily Living
Change on the ADL from baseline to week 16–week 24 was assessed using repeated measure analysis, controlling for age, gender and education.
Adverse Events
Adverse events were qualitatively reported using structured interviews at week 8, week 16 and week 24. Vitals, including systolic and diastolic blood pressure, heart rate and body weight, were measured at baseline, week 16 and week 24. Differences in vitals between baseline and week 16, week 16 and week 24, and baseline and week 24 were assessed using a paired t-test. We imputed missing data (baseline: blood pressure [70%], heart rate [70%]; week 16: blood pressure [18%)], [18%], weight [13%] and week 24: blood pressure [18%], heart rate [13%] and weight [13%]) with the individuals average across all three time points.
Availability of Data
Data are available upon reasonable request.
Results
Participants
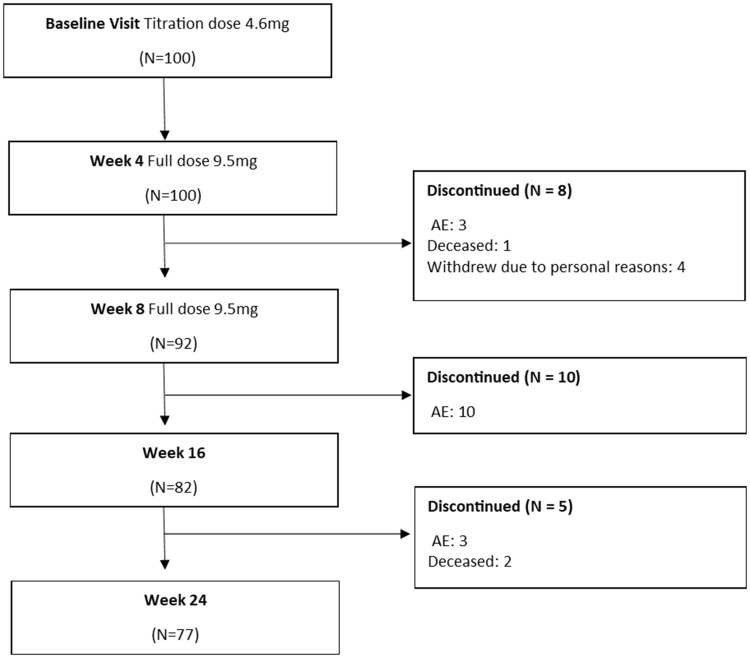
From the 100 patients recruited, there were 69 OC that had full data at all three time points. Following the ITT model, LOCF was conducted for 15 patients (15%) at week 16, and for 14 patients (14%) at week 24. Reasons for withdrawal mainly included adverse events after treatment (Figure 1). No differences in cognitive or functional scores were observed between OC and LOCF groups at baseline (Table 1).
Figure 1.
Consort diagram.
Table 1.
Participant Characteristics
| OC (N = 69) | ITT-LOCF (N = 100) | P value | ||||
|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Mean | Std. Deviation | T-Statistic (DF) | ||
| Age | 74.38 | 6.98 | – | – | – | – |
| Males (%) | 48 (48%) | – | – | – | – | – |
| Body weight (kg) | 59 | 11.48 | ||||
| Blood pressure – Systolic | 137.45 | 19.09 | ||||
| Blood pressure – diastolic | 71.03 | 12.13 | ||||
| Heart rate (beats per min) | 67.52 | 10.25 | ||||
| Years of education | 7.58 | 4.64 | – | – | – | – |
| Fazekas Score | 2.23 | 0.42 | – | – | – | – |
| MMSE | ||||||
| Baseline (OC N=99) | 21.45 | 4.48 | 21.52 | 4.45 | 0.11 (197) | 0.91 |
| Week 16 (OC N=81) | 20.12 | 4.63 | 20.51 | 4.74 | 0.55 (179) | 0.57 |
| Week 24 (OC N=87) | 20.58 | 5.21 | 20.83 | 5.04 | 0.33 (185) | 0.73 |
| ADAS Cog | ||||||
| Baseline (OC N=95) | 48.93 | 8.72 | 48.93 | 8.12 | 0.00 (193) | 1.00 |
| Week 16 (OC N=75) | 50.42 | 9.06 | 50.21 | 9.21 | −0.15 (173) | 0.88 |
| Week 24 (OC N=81) | 51.25 | 9.47 | 50.72 | 9.59 | −.35 (179) | 0.72 |
| ADL | ||||||
| Baseline (OC = 100) | 61.19 | 10.26 | 61.19 | 10.26 | – | – |
| Week 16 (N= 81) | 60.39 | 11.42 | 60.26 | 11.93 | 0.07 (179) | 0.94 |
| Week 24 (N= 85) | 61.02 | 10.96 | 60.43 | 11.58 | 0.35 (183) | 0.73 |
Abbreviations: OC, observed cases; ITT-LOCF, intent to treat-last observation carried forward sample; MMSE, Mini-Mental State Examination; ADAS-Cog, Alzheimer’s disease Assessment Scale-Cognitive subscale; ADL, activities of daily living.
Change in Global Cognition
Overall, the mean change on the ADAS-COG from baseline to week 16 was 1.27 (SD = 5.63). The mean change on the ADAS-Cog from baseline to week 24 was 1.78 (SD = 5.29). Repeated measure analysis indicated that there were no significant differences on the ADAS-Cog from baseline, to week 16 to week 24.
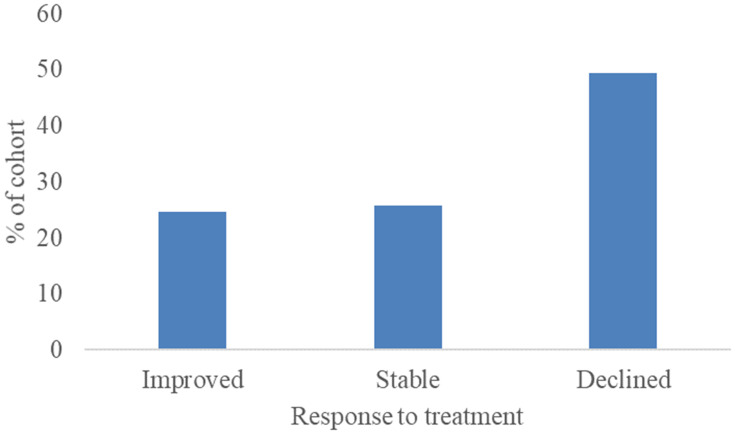
Figure 2 shows that 25% of the sample experienced a significant improvement in cognition after 24 weeks of treatment (2 or more-point decline on the ADAS-Cog). For these treatment responders, the mean change on the ADAS-Cog was −5.08 (SD = 3.11). Performance on the ADAS-Cog remained stable for 27% of the sample, with mean change 0.38 (SD =0 0.71). A decline in performance on the ADAS-Cog was observed in 49% of the sample, with a mean ADAS-Cog change of 6 (SD = 2.98).
Figure 2.
The proportion of patients that either responded to treatment and improved in cognitive performance, remained stable or that declined in cognitive performance after 24 weeks.
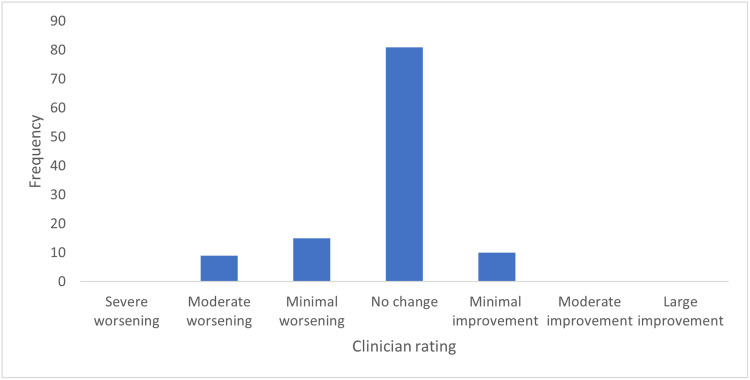
On the clinician-rated ADCS-CGIC, the mean impression of change in global function was “no change”, reported for 81% of the patients (Figure 3). Minimal improvement was rated for 5% of the patients, while worsening in global function was rated for 14% of the patients.
Figure 3.
Frequency of responses on the clinician-rated Clinical Global Impression of Change scale at week 24.
Interaction Between Treatment Response and Severity of svCVD
Overall, there was no interaction effect between Fazekas score and treatment response on the ADAS-Cog. When looking at the three treatment groups individually, interactions remained insignificant.
Differences Between Patients Who Improved, Remained Stable and Declined in Cognition
Comparing differences in individual characteristics between patients who improved, remained stable and declined in cognition over the 24-week period, there were no differences in age, Fazekas score or cognition, as indexed using the MMSE and ADAS-Cog, or baseline daily functioning, indexed using the ADL (Table 2). Patients that remained stable had significantly more years of education compared to patients that improved or declined.
Table 2.
Differences in Demographics Between Patients Who Improved, Remained Stable or Who Declined in Cognitive Performance
| Mean (SD) | |||
|---|---|---|---|
| Improved | Stable | Declined | |
| (N = 25) | (N = 27) | (N = 48) | |
| Age | 75.61 (6.27) | 75.04 (6.44) | 73.26 (4.09) |
| Gender (male) | 15 (60%) | 15 (55%) | 18 (37%) |
| Education | 6.52 (3.84) | 10.14 (5.37) | 6.68 (4.09) |
| Fazekas score | 2.32 (0.47) | 2.25 (0.44) | 2.16 (0.37) |
| Baseline MMSE | 19.84 (4.42) | 22.53 (4.41) | 21.72 (4.36) |
| Baseline ADAS | 48.75 (9.53) | 51.68 (8.67) | 47.60 (8.15) |
| Baseline ADL | 60.36 (10.35) | 58.40 (12.55) | 63.18 (8.42) |
Daily Functioning
Repeated measure analysis indicated that there were no significant differences on the ADL from baseline, to week 16 to week 24, while controlling for age, gender and education.
Safety
Treatment was not associated with any serious adverse events as reported by clinical interview and analysis of vitals. The structured interview indicated that mild adverse events were observed at the 8-week time point by 3% of the patients (Figure 1). At the 16-week time point, 10% of the patients experienced mild adverse events and at the 24-week time point, 3% of the patients experienced adverse events. The most reported adverse event was rash, limited to the site of application of the transdermal patch (Table 3). Analysis of vitals indicated that systolic blood pressure significantly decreased from baseline to week 16 (t(93) = 2.01, p = 0.04) (Table 4). There were no differences from week 16 to week 24, and from baseline to week 24. A significant decrease in body weight was observed from baseline to week 24 (t(99) = −2.24, p = 0.02). No differences in body weight were observed between baseline and week 16, and week 16 to week 24. No changes in diastolic blood pressure and heart rate were observed across the study duration. Three patients deceased during the study period (Figure 1). None of the deaths were considered to be treatment-related, with causes typical of an elderly AD population.
Table 3.
Side Effects Reported by Clinical Interview
| Week 8 (N= 3) | |
|---|---|
| Pruritus | |
| Palpitation | |
| Nausea, giddiness | |
| Week 16 (N=10) | |
| Peripheral vestibulopathy | |
| Neutropenia, rash | |
| Rash, dyspepsia | |
| Nausea, giddiness | |
| Headache, behavioural change (aggression/low mood) | |
| Rash contact dermatitis (N=4) | |
| Week 24 (N=3) | |
| Rash (N=2) | |
| Worsening behavioral symptoms | |
Table 4.
Vitals Measured at Baseline, Week 16 and Week 24
| Vitals | Timeframe | Mean | SD |
|---|---|---|---|
| Systolic blood pressure (mmHg) | Baseline | 142.28 | 29.24 |
| Week 16 | 137.76 | 19.72 | |
| Week 24 | 138.28 | 20.28 | |
| Diastolic blood pressure (mmHg) | Baseline | 70.74 | 10.54 |
| Week 16 | 69.87 | 10.90 | |
| Week 24 | 70.31 | 11.74 | |
| Heart rate (beats per min) | Baseline | 72.43 | 10.68 |
| Week 16 | 72.20 | 12.48 | |
| Week 24 | 67.98 | 20.85 | |
| Weight (kg) | Baseline | 58.76 | 11.48 |
| Week 16 | 58.46 | 12.05 | |
| Week 24 | 58.31 | 12.01 |
Discussion
This open-label study investigated the efficacy of transdermal Rivastigmine patch in patients with AD having concomitant MRI confirmed mild to severe svCVD over a 24-week period. Significant improvements in cognition after treatment was observed in a quarter of our sample, with an average 5-point improvement on the ADAS-Cog measure of global cognition. A quarter of the sample remained stable in their cognitive functions, while almost half experienced a decline in cognition, with an average 6-point decline. These findings were irrespective of svCVD severity. Clinician-rated impressions of global change suggested that majority (81%) of patients appeared to remain stable in their global function after 24 weeks of treatment. Similarly, a measure of activities of daily living remained stable from baseline to week 24, suggesting Rivastigmine may maintain daily living functions. Side effects were reported by 16% of the patients, with rash being the most common. Our findings suggest that transdermal Rivastigmine may be useful in delaying cognitive decline in almost half the population of patients with AD having concomitant svCVD, with minimal side effects.
Our findings are consistent with previous double-blind, placebo-controlled studies7 demonstrating that Rivastigmine slows down cognitive decline in patients with AD and concomitant svCVD in a significant portion of the population. In one study, Kumar et al7 found that in a cohort of patients with AD and svCVD, indexed using the clinician-rated Modified Hachinski Ischemic Scale (MHIS), 32% of the sample experienced a >4-point improvement on the ADAS-Cog. Similarly, we observed a quarter of our sample experienced a >2-point improvement on the ADAS-Cog. Our cohort was comparable to Kumar et al7 cohort with regards to age and education, however our cohort had greater svCVD burden and baseline cognitive impairment, suggesting that the benefits of transdermal patch extend to AD patients with MRI confirmed mild-severe svCVD. Kumar et al further reported that on average, patients experienced a 1.9-point improvement on the ADAS-Cog test while the placebo group experienced a 4.2-decline in performance. In the current study, we observed that treatment responders experienced a 5-point increase in performance on the ADAS-Cog. Collectively, these findings suggest that over a quarter of the population with AD and concomitant svCVD may benefit from Rivastigmine treatment, and the magnitude of improvement may be clinically relevant.
In AD without svCVD, the benefits of Rivastigmine is often small and some have argued that it may have little clinical importance.17,18 Previous clinical studies in patients with AD and no svCVD reported that transdermal patch has on average a 0.5 point improvement on the ADS-Cog3 or up to a 2 point decline in performance.4,19 In a meta-analysis with 13 placebo-controlled clinical trials in patients with AD,17 the mean difference between the treatment group and the placebo group on the ADAS-Cog was 1.79 points. In comparison, Kumar et al7 reported that the treatment difference between the treatment group and placebo group for patients with AD having concomitant svCVD was 6.15 points, while the treatment difference for AD without svCVD was 4.03 points. These findings suggest that a more responsive treatment population to Rivastigmine patch may be patients with AD having concomitant svCVD.
One possible mechanism for the favorable effect of rivastigmine on patients with svCVD may be its capacity to modify cardiovascular risk factors such as blood pressure. A recent study in Parkinson’s disease20 demonstrated that 24 weeks of rivastigmine capsule was more effective in improving cognition compared to placebo in the hypotensive group than the non-hypotensive group. This suggests that rivastigmine may have a larger function on cognitive function by attenuating hypotension, rather than its enhancing effect on brain cholinergic tone. Thus, patients with subcortical dementias such as those with svCVD or Parkinson’s disease may experience not only the acetylcholine inhibition activity of Rivastigmine but also a powerful butyryl-cholinesterase inhibition effect.6 In addition to the cognitive benefits, we observed that daily living functions remained stable over the treatment period. This finding is consistent with AD studies, whereby compared to the treatment group, placebo groups exhibited large declines in daily functioning.3 In the present study, we further observed minimal adverse events. The percentage of patients affected by adverse effects in the current study (16%) is consistent with the safety profile of Rivastigmine reported in pure AD patients.21 Thus, we propose Rivastigmine patch is safe in patients with AD having concomitant mild to severe svCVD.
One strength of our study was that we used MRI biomarkers to confirm svCVD. All patients had moderate to severe WMH; thus, our findings are specific to this population. Another strength is that we used a naturalistic open-label study design which allowed us to test efficacy and safety in a real-world setting. One limitation of our study was the lack of a control group. A control group of pure AD or pure vascular disease would have informed us whether rivastigmine is superior in treating mixed dementia with svCVD, compared to pure dementia as previously suggested.7,22 Future research may benefit from collecting AD biomarkers and MRI to discriminate between pure AD and AD with svCVD. We note we did not report cardiovascular risk factors of patients, which may have played a role in cognitive impairment in addition to the presence of WMH.23 Previous research has shown that rivastigmine is sensitive to the severity of cardiovascular risk factors7 and future research would benefit from determining whether they act as moderators to treatment effects in patients with AD and concomitant svCVD”. We note that 29% of the data required imputation; however, we do not suspect any bias in the data as there were no group differences between patients that had imputed data vs patients with complete data.
Conclusion
We demonstrate that transdermal Rivastigmine patch is effective in targeting cognitive impairment in patients with AD with concomitant mild to severe svCVD, with minimal side effects. Transdermal Rivastigmine may demonstrate a huge benefit in cognition in a quarter of patients with AD and concomitant svCVD, with clinically relevant effects on cognitive performance. Clinical significance of these findings are important given patients with AD with concomitant svCVD are highly comorbid and that vascular injury is a large risk for the progression of AD pathology.24,25
Trial Registration
Health Science Authority, CTA9900218. Registered 28/11/2014.
Acknowledgments
We would like to thank National Neuroscience Institute, Singapore, for sponsoring this study.
Data Sharing Statement
Data will be made available upon reasonable request to corresponding author.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Novartis (Singapore) Pte Ltd partially funded the study and the cost of the investigational product. Novartis did not have any role in the concept of the study, data analyses or writing of the manuscript. This was an investigator-initiated study wherein the study design, data collection, data analyses as well as writing of this manuscript was carried out by the study team at NNI with no involvement from Novartis. The principal investigator and study team members did not receive any honorarium or personal fees from Novartis for carrying out this study. However, Dr Nagaendran Kandiah received honorarium from Novartis for lectures not related to any aspect of this study. Dr Nagaendran Kandiah reports grants from Novartis Pharmaceuticals, during the conduct of the study; and received honoraria for speaker engagements and personal fees from Novartis Pharmaceuticals, and honoraria and grant support from Eisai Pharmaceuticals and Schwabe Pharmaceuticals, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
- 1.Esiri MM, Joachim C, Sloan C, et al. Cerebral subcortical small vessel disease in subjects with pathologically confirmed alzheimer disease: a clinicopathologic study in the Oxford Project to Investigate Memory and Ageing (OPTIMA). Alzheimer Dis Assoc Disord. 2014;28:30–35. doi: 10.1097/WAD.0b013e31829b72f1 [DOI] [PubMed] [Google Scholar]
- 2.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu‐Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x [DOI] [PubMed] [Google Scholar]
- 3.Winblad B, Grossberg G, Frölich L, et al. IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for alzheimer disease. Neurology. 2007;69(Issue 4, Supplement 1):S14–S22. doi: 10.1212/01.wnl.0000281847.17519.e0 [DOI] [PubMed] [Google Scholar]
- 4.Feldman HH, Lane R. Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1056–1063. doi: 10.1136/jnnp.2006.099424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J, Froelich L, Black SE, et al. Randomized, double-blind, parallel-group, 48-week study for efficacy and safety of a higher-dose rivastigmine patch (15 vs. 10 cm2) in alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33:341–353. doi: 10.1159/000340056 [DOI] [PubMed] [Google Scholar]
- 6.Kandiah N, Pai M-C, Senanarong V, et al. Rivastigmine: the advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and parkinson’s disease dementia. Clin Interv Aging. 2017;12:697. doi: 10.2147/CIA.S129145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar V, Anand R, Messina J, Hartman R, Veach J. An efficacy and safety analysis of exelon® in alzheimer’s disease patients with concurrent vascular risk factors. Eur J Neurol. 2000;7:159–169. doi: 10.1046/j.1468-1331.2000.00046.x [DOI] [PubMed] [Google Scholar]
- 8.Erkinjuntti T, Skoog I, Lane R, Andrews C. Rivastigmine in patients with Alzheimer’s disease and concurrent hypertension. Int J Clin Pract. 2002;56:791–796. [PubMed] [Google Scholar]
- 9.Debette S, Markus H. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 12.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683. doi: 10.1212/WNL.43.9.1683 [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 14.Rosen W, Mohs R, Davis K. Alzheimer’s Disease Assessment Scale—Cognitive and Non-Cognitive sections (ADAS-Cog, ADAS Non-Cog). J Psychiatry. 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 15.Schneider L, Olin J, Doody R, Morris JRB, Schmitt F. Validity and reliability of the ADCSU clinical global impression of change (ADCS-CGIC). Alzheimer Dis Assoc Disord. 1997;11:522–532. doi: 10.1097/00002093-199700112-00004 [DOI] [PubMed] [Google Scholar]
- 16.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in alzheimerʼs disease. Alzheimer Dis Assoc Disord. 1997;11:33–39. doi: 10.1097/00002093-199700112-00005 [DOI] [PubMed] [Google Scholar]
- 17.Birks JS, Evans JG. Rivastigmine for alzheimer’s disease. Cochrane Database Syst Rev. 2015;4. [DOI] [PubMed] [Google Scholar]
- 18.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Imai Y, Shigeta M, et al. A 24-week, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety and tolerability of the rivastigmine patch in Japanese patients with alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2011;1:163–179. doi: 10.1159/000328929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espay AJ, Marsili L, Mahajan A, et al. Rivastigmine in parkinson’s disease dementia with orthostatic hypotension. Ann Neurol. 2021;89(1):91–98. doi: 10.1002/ana.25923 [DOI] [PubMed] [Google Scholar]
- 21.Moretti R, Torre P, Antonello RM, Cazzato G. Rivastigmine in subcortical vascular dementia: a comparison trial on efficacy and tolerability for 12 months follow‐up. Eur J Neurol. 2001;8:361–362. doi: 10.1046/j.1468-1331.2001.00224.x [DOI] [PubMed] [Google Scholar]
- 22.Noufi P, Khoury R, Jeyakumar S, Grossberg GT. Use of cholinesterase inhibitors in non-alzheimer’s dementias. Drugs Aging. 2019;1–13. [DOI] [PubMed] [Google Scholar]
- 23.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertens. 2013;62(5):810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Zhou K, Wang R, et al. Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J Biol Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200 [DOI] [PubMed] [Google Scholar]
- 25.Hachinski V, Munoz DG. Cerebrovascular pathology in alzheimer’s disease: cause, effect or epiphenomenon? Ann N Y Acad Sci. 1997;826:1–6. doi: 10.1111/j.1749-6632.1997.tb48456.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.