Abstract
Objective:
Midlife women experience elevated risk for cardiovascular disease and often receive advice to increase physical activity to mitigate this risk. Use of accelerometers to measure ambulatory physical activity requires selection of appropriate thresholds for estimating moderate-to-vigorous physical activity (MVPA), and choice of cut points may lead to meaningfully different conclusions about midlife women’s physical activity (PA) engagement. This is particularly important given the recent elimination of 10-minute bout requirements for MVPA. This two-phase study examined differences between four cut point methods among midlife women with cardiovascular disease (CVD) risk. We used findings from Study 1 (exploratory) to generate hypotheses for Study 2 (confirmatory).
Methods:
Across studies, participants (N = 65) were midlife women with an additional CVD risk factor (eg, hypertension). Participants wore waistband accelerometers for seven days. Daily totals were calculated for minutes in light and MVPA using four common quantification methods (Freedson, Matthews, Swartz, and Troiano).
Results:
Multilevel models showed meaningful differences between methods (P < 0.0001). For total (non-bouted) minutes of MVPA, Freedson and Troiano methods showed that participants barely met MVPA recommendations (30 min per day), whereas Matthews and Swartz methods showed that participants greatly exceeded this goal. As differences between methods were smaller using MVPA bouts of 10 minutes or more (though remained significant), the observed variation was due in part to small bursts of MVPA dispersed throughout the day.
Conclusions:
Findings demonstrate the need for careful consideration of PA quantification among midlife women with CVD risk, and for further investigation to determine the most appropriate quantification method.
Keywords: Accelerometer, Cardiovascular risk, Midlife, Physical activity, Women’s health
Midlife women (aged 40–60) experience elevated risk for cardiovascular disease (CVD) due to contributions from advancing age,1 the onset of menopause,2 weight gain,3 and related health conditions such as hypertension and type 2 diabetes.4,5 Regular physical activity (PA) protects against these risks. However, men engage in greater activity than women at all life stages,6 and this disparity becomes further pronounced during midlife,7 which exacerbates CVD risk for midlife women. These women could experience meaningful cardiovascular benefits from increasing PA—particularly aerobic activities associated with moderate-to-vigorous physical activity (MVPA)— and often receive this advice from health care providers.8 Consequently, accurate assessment and estimation of midlife women’s current PA patterns is critical for maximizing the rigor of research in this area, and will provide useful contributions to CVD risk reduction in this population.
A common method for assessing ambulatory PA is accelerometry; this method often uses an ActiGraph device worn at the waist as close as possible to the iliac crest (Actigraph Corp., Pensacola, FL). These devices show high reliability and validity for assessing PA and account for individual differences in posture and gait.9–11 To interpret PA data from accelerometers, however, researchers have proposed different count-per-minute thresholds, or cut points, to capture PA at various intensities. Use of different cut point methods have led to substantial heterogeneity in estimates of PA, particularly activity that reaches moderate or vigorous intensity (ie, MVPA). For example, comparisons of different cut point methods generate estimates ranging from 4 to 231 minutes of MVPA per day, and the proportions of these samples that met MVPA recommendations ranged from 8% to 100%.12,13 As such, the selection of cut point method can have a meaningful effect on the conclusions drawn from a study assessing PA via accelerometer, including the proportion of a population that does not meet recommended PA goals and who may therefore benefit from intervention.14,15
To date, little research has examined different methods for quantifying PA among midlife women with elevated CVD risk, in order to understand true PA estimates. As is the case in populations such as pregnant women,16 patterns of PA engagement and intensity among midlife women with elevated CVD risk may not align with those of healthy groups. In addition, recent PA guidelines for health removed the recommendation that MVPA occur in bouts of at least 10 minutes,15 and it is not clear what effect this change will have on estimates of MVPA in midlife women. In particular, understanding whether accelerometer estimation methods generate clinically versus statistically meaningful differences is more challenging without the threshold of a 10-minute bout. Together, these factors suggest that additional information is needed to accurately measure PA among midlife women with elevated CVD risk.
Common methods used to estimate activity intensity among groups of overweight and midlife adults include those proposed by Freedson et al,17 Matthews et al,18 Swartz et al,19 and Troiano et al.6,20–27 Each of these methods has shown associations with health outcomes (eg, obesity, blood pressure, LDL levels) in previous work,28,29 though they differ in their count-per-minute thresholds for moderate and vigorous-intensity activity. Moderate activity count thresholds that range from 574 to 2,020, and vigorous thresholds that range from 4,945 to 5,999 (see Table 1), based on data from a range of populations and subsets of activities.30 Thus, achieving the minimum activity count to earn minutes of MVPA will differ by the estimation method applied to accelerometer data. In this respect, methods by Freedson et al17 and Troiano et al6 are more conservative than the others, and thus classify more minutes at light intensity. Methods by Matthews et al18 and Swartz et al19 are less conservative, in contrast, and classify more minutes at moderate intensity.
TABLE 1.
Cut points indicated by each of the four methods considered: Freedson et al,17 Matthews et al,18 Swartz et al,19 and Troiano et al6
| Freedson |
Matthews |
Swartz |
Troiano |
|||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | Minimum | Maximum | Minimum | Maximum | |
| Sedentary activity | 0 | 99 | 0 | 99 | 0 | 99 | 0 | 99 |
| Light activity | 100 | 1,951 | 100 | 759 | 100 | 573 | 100 | 2,019 |
| Moderate activity | 1,952 | 5,724 | 760 | 5,724 | 574 | 4,944 | 2,020 | 5,998 |
| Vigorous activity | 5,725 | 9,498 | 5,725 | and above | 4,945 | 9,316 | 5,999 | and above |
Aims of the present research
An initial step toward identifying an optimal method for estimating PA among midlife women with CVD risk is to directly compare common estimation methods using data collected from this population.12,13,22,31,32 In the present research, we took a two-phase approach to this task. We first conducted exploratory multilevel and descriptive analyses comparing four common estimation methods, in a small sample of midlife women who were recruited for an observational study. Based on the differences in cut points between methods, we expected to observe a statistically significant effect of cut point method for total (non-bouted) minutes of light and moderate PA per day, as well as MVPA per day. We did not expect to observe these differences using MVPA bouts of 10 minutes or more. Using findings from this study to generate specific hypotheses, we then conducted confirmatory analyses in a larger sample of midlife women who were recruited for a behavioral weight loss clinical trial. Participants in each study wore a waistband accelerometer for seven days. In line with American College of Sports Medicine guidelines,14 we use a threshold of 10 or more minutes of MVPA as a benchmark for clinically significant differences.
STUDY 1: MATERIALS AND METHODS
Participants and procedures
All procedures were approved by the relevant Institutional Review Board. Participants were recruited from a small city via print and web advertisement to participate in an observational study. Eligibility criteria were women aged 40 to 60 (inclusive), fluent in English, BMI between 27 and 50 kg/m2, and reporting a diagnosis of one or more additional CVD risk factors (eg, hypertension). Women who met these criteria attended an interview at an academic research center, where study staff explained all procedures and engaged participants in an informed consent process. Study staff also recorded each participant’s height and weight, which were used to calibrate the accelerometer to their unique movement patterns, and explained proper wear and care of the accelerometer. Participants wore the accelerometer for the following seven days. They returned to the research center at the end of this period to return the accelerometer and receive compensation of a $25 gift card. The sample of 13 women (MAge = 48, MBMI = 32.3 kg/m2) was predominantly white (77%) and had at least some college experience (including bachelor’s degrees or higher; 85%). The largest subsets identified as married (48%) and reported previous diagnoses of hyperlipidemia (62%) and (pre)hypertension (54%; see Table 2 for additional demographic information).
TABLE 2.
Demographic information
| Study 1 (N = 13) | Study 2 (N = 52) | |
|---|---|---|
| M (SD) | M (SD) | |
| Age, years | 48 (5.09) | 53 (4.47) |
| BMI, kg/m2 | 32.3 (7.16) | 35.6 (4.36) |
| Frequency (%) | Frequency (%) | |
| Racial/Ethnic identification | ||
| Caucasian | 10 (77%) | 22 (44.2%) |
| Hispanic/Latina | 2 (16%) | 0 (0%) |
| Asian | 1 (7%) | 0 (0%) |
| Black/African American | 0 (0%) | 25 (48.1%) |
| More than one race | 0 (0%) | 4 (7.7%) |
| Education | ||
| Graduate or professional degree | 0 (0%) | 21 (42.3%) |
| Bachelor’s degree or higher | 11 (85%) | 16 (30.8%) |
| Associate’s, tech degree or partial | 0 (0%) | 11 (21.2%) |
| High school degree/GED | 2 (15%) | 3 (5.8%) |
| Marital status | ||
| Married | 6 (48%) | 29 (55.8%) |
| Separated | 4 (32%) | 0 (0%) |
| Divorced | 3 (20%) | 12 (23.1%) |
| Widowed | 0 (0%) | 1 (1.9%) |
| Never married | 0 (0%) | 8 (17.3%) |
| Reported medical conditions | ||
| Hypertension | 7 (54%) | 51 (99%) |
| Type 2 diabetes | 5 (38%) | 7 (14%) |
| Hyperlipidemia | 8 (62%) | 0 (0%) |
| Metabolic syndrome | 2 (15%) | 0 (0%) |
| Smoker | 1 (8%) | 0 (0%) |
| Menopause status | ||
| Premenopause (regular cycles) | 2 (15%) | 11 (18%) |
| Perimenopause (irregular cycles) | 4 (31%) | 14 (22%) |
| Postmenopause (last cycle > 12 mo ago) | 7 (54%) | 37 (60%) |
Participants in Study 2 were significantly older than those in Study 1, and Study 2 had higher proportions of women who identified as Black/African American and as having hypertension (P < 0.02). GED, General Education Diploma.
Measures
Height and weight were measured by study staff using a standard stadiometer and balance beam scale, respectively. Participants were asked to wear an Actigraph GT3X+ accelerometer around their waists for seven consecutive days. Days with 10 or more hours of wear time were included in the present analyses.33 Total minutes of time spent in activities at each level of intensity (ie, light, moderate, vigorous, MVPA) were calculated using the different cut points proposed by Freedson et al,17 Matthews et al,18 Swartz et al,19 and Troiano et al,6 via the ActiLife software platform.
Data analysis
A total of 348 observations were included in exploratory analyses. Differences between cut point methods for estimating time spent at each intensity level were tested using three-level multilevel models, which are optimal for nested, repeated assessments (ie, cut point method nested within day of assessment nested within participant). Separate models were run for total (non-bouted) number of minutes at each intensity level and for time in bouts of MVPA. All models employed a restricted maximum likelihood method to address missing data (ie, 16 of 364 possible observations missing due to nonwear). Alphas were set to P < 0.05. We examined the within-person effect of cut point method, then estimated daily means by cut point method and described the substantive differences among methods. We conducted posthoc pairwise comparisons between methods. Results are presented in model estimates (B, SE) with corresponding F- and P-values. Finally, we calculated the percent of days on which participants met MVPA recommendations of 30 minutes or more, and the percent of the overall sample that engaged in 30 or more minutes of MVPA on five or more of the seven days of observation (total non-bouted and in bouts of 10 minutes or more14).
STUDY 1: RESULTS AND DISCUSSION
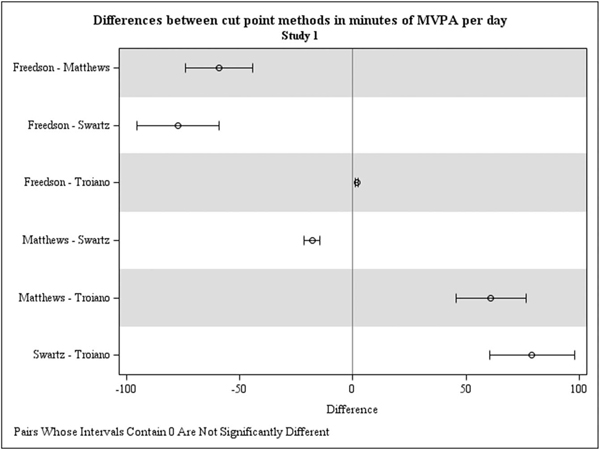
Average number of total (non-bouted and bouted) minutes per day spent at each level of PA intensity are displayed in Table 3 (upper panel), by cut point method. The effect of cut point method was significant for total (non-bouted) minutes of light (F[4,35] = 41.94), moderate (F[4,35] = 37.38), and MVPA per day (F[4,35] = 34.69; Ps < 0.0001), indicating that cut point methods generated divergent activity estimates. Differences between cut point methods for number of vigorous minutes per day were not statistically significant but showed a potentially noteworthy trend (F[4,35] = 2.56, P = 0.06). As shown in Table 3 and Fig. 1, the largest differences were between methods by Swartz et al19 and Troiano et al6 (P < 0.05), with average differences of 79 minutes per day for light activity and MVPA. Contrasts between methods by Freedson and Matthews, Freedson and Swartz, and Matthews and Troiano also produced differences of 60 or more minutes of light, moderate, and MVPA per day (P < 0.001).
TABLE 3.
Mean estimates for total minutes spent at each physical activity intensity based on the four cut point methods considered
| Study 1 | Freedson B (SE) | Matthews B (SE) | Swartz B (SE) | Troiano B (SE) |
|---|---|---|---|---|
| Light activity | 169.44 (15.65) | 110.34 (9.15) | 92.26 (7.54) | 171.21 (15.89) |
| Moderate activity | 31.91 (5.86) | 91.11 (12.61) | 107.88 (13.87) | 30.43 (5.75) |
| Vigorous activity | 1.66 (1.21) | 1.73 (1.26) | 2.93 (1.76) | 1.45 (1.08) |
| MVPA | 33.69 (6.75) | 92.86 (13.12) | 110.95 (14.72) | 31.92 (6.44) |
| MVPA in bouts | 2.49 (2.49) | 9.34 (2.49) | 9.34 (2.49) | 2.49 (2.49) |
| Study 2 | Freedson B (SE) | Matthews B (SE) | Swartz B (SE) | Troiano B (SE) |
| Light activity | 179.59 (5.18) | 120.64 (3.54) | 102.05 (2.95) | 181.38 (5.22) |
| Moderate activity | 37.21 (2.60) | 96.08 (3.91) | 112.70 (2.63) | 35.82 (2.63) |
| Vigorous activity | 1.23 (0.42) | 1.34 (0.44) | 3.19 (0.10) | 0.95 (0.29) |
| MVPA | 38.63 (2.77) | 97.51 (4.04) | 116.10 (4.39) | 36.85 (2.73) |
| MVPA in bouts | 10.88 (2.28) | 16.89 (2.75) | 20.36 (2.90) | 10.56 (2.25) |
Study 1 included 348 total days of observation for n = 13, Study 2 included 1,340 total days of observation for n = 52.
MVPA, moderate-to-vigorous physical activity.
FIG. 1.
Estimated differences in minutes of moderate-vigorous physical activity (MVPA) based on direct (pairwise) contrasts between methods, for Study 1. MVPA (total) = total (non-bouted) minutes of moderate-to-vigorous physical activity. MVPA, moderate-vigorous physical activity.
Total (non-bouted) minutes of MVPA
Methods by Freedson et al17 and Troiano et al6 showed that, based on total (non-bouted) minutes of MVPA, participants met MVPA recommendations on 40% and 38% of individual days, respectively (see Table 4). Across the week of data collection, these methods both showed that 33% of participants achieved the recommended 30 minutes of MVPA per day on five or more days. In comparison, methods by Matthews et al18 and Swartz et al19 showed that participants met MVPA recommendations on 89% and 92% of individual days. Both methods indicated that 92% of participants achieved the recommended 30 minutes of MVPA per day on five or more days.
TABLE 4.
Proportion of days meeting recommended 30 minutes of MVPA and recommended 30 or more minutes of MVPA on five or more days per week
| Met daily MVPA recommendation (n, %) | Met weekly MVPA recommendation (n, %) | |
|---|---|---|
| Study 1—total minutes (non-bouted) | ||
| Freedson | 35 (40%) | 4 (33%) |
| Matthews | 77 (89%) | 11 (92%) |
| Swartz | 80 (92%) | 11 (92%) |
| Troiano | 33 (38%) | 4 (33%) |
| Study 1—bouts of 10 or more minutes | ||
| Freedson | 3 (3%) | 0 (0%) |
| Matthews | 12 (13%) | 1 (8%) |
| Swartz | 12 (13%) | 1 (8%) |
| Troiano | 3 (3%) | 0 (0%) |
| Study 2—total minutes (non-bouted) | ||
| Freedson | 180 (54%) | 21 (40%) |
| Matthews | 331 (99%) | 48 (92%) |
| Swartz | 332 (99%) | 48 (92%) |
| Troiano | 168 (50%) | 17 (32%) |
| Study 2—bouts of 10 or more minutes | ||
| Freedson | 51 (15%) | 2 (4%) |
| Matthews | 71 (21%) | 5 (10%) |
| Swartz | 88 (26%) | 7 (13%) |
| Troiano | 48 (14%) | 2 (4%) |
Study 1 included 348 total days of observation for n = 13, Study 2 included 1,340 total days of observation for n = 52.
MVPA, moderate-to-vigorous physical activity.
Minutes in MVPA bouts
The effect of cut point method also was significant for minutes per day spent in MVPA bouts of 10 minutes or more (F[3,26] = 16.37; P < 0.0001), though differences between methods were smaller (see Table 3 and Fig. 1). Methods by Matthews et al18 and Swartz et al19 generated the same estimates, of 9.34 minutes per day (SE=2.49), which was significantly higher than estimates by Freedson et al17 (B = 2.49, SE = 2.49) and Troiano et al6 (B = 2.35, SE = 2.49, pairwise contrast P<0.0001). Differences between Matthews et al18 and Swartz et al,19 and between Freedson et al17 and Troiano et al,6 were not significant (P > 0.90). Using methods by Freedson et al17 and Troiano et al,6 participants in the present study met MVPA recommendations of 30 minutes or more on 13% of individual days, though no participants met this recommendation on five or more (of seven) days (see Table 3). Participants met MVPA recommendations on 3% of individual days using methods by both Matthews et al18 and Swartz et al,19 and 8% of participants reached the goal on five or more (of seven) days.
Summary
The size of differences in PA based on cut point method (and thus, their potential real-world significance) may depend on whether minutes are considered in 10-minute bouts or not. In this study, pairwise differences were as large as 79 total (non-bouted) minutes per day, though comparisons between time in 10-minute bouts of MVPA were much smaller (eg, 7 min per day). Specifically, methods by Freedson et al17 and Troiano et al6 suggest that midlife women with elevated CVD risk fail to meet weekly MVPA recommendations for adults (for both non-bouted and bouted minutes), whereas methods by Matthews et al18 and Swartz et al19 suggest that they may achieve this goal if total (non-bouted) minutes are emphasized. These findings were used to generate hypotheses for confirmatory analyses in a larger sample in Study 2, comprising a subset of participants enrolled in a behavioral weight loss trial.
STUDY 2: MATERIALS AND METHODS
Participants and procedures
All procedures were approved by the Institutional Review Board at the supporting institution. Participants included in analyses were recruited from a large urban area for a clinical trial of behavioral weight loss treatment (NCT02363010).34 Eligible individuals had a BMI of 27 to 45 kg/m2, were fluent in English, were able to engage in PA (ie, walk at least two blocks without stopping), and had no medical contraindications to weight loss treatment (eg, uncontrolled psychiatric illness). Participants attended a baseline visit where they engaged in an informed consent process. Study staff then measured each participant’s height and weight, explained how to use the ActiGraph accelerometer, and led participants through a variety of other research tasks.34 Participants wore the accelerometer for the seven days following their clinic visit and mailed it back to study staff using a prepaid envelope. Participants received $25 for their participation in this assessment. Data included for the present analyses were collected at baseline, before any treatment was delivered.
Individuals included in this substudy were women between 40 and 60 years old who met the criteria above and reported at least one of the following CVD risk factors: high blood pressure, type 2 diabetes, or were currently a smoker. The resulting sample of 52 women (MAge = 53 years, MBMI = 35.64) was 48.1% Black/African American. The sample was highly educated, with the largest subset (42.3%) achieving a graduate or professional degree. The majority of participants were married (55.8%) and reported a diagnosis of high blood pressure (99%; see Table 2 for additional demographics).
Measures
Participants reported their demographic and health information using the Weight and Lifestyle Inventory.35 Height and weight were measured by study staff using the Tanita model WB-3000 physician’s digital scale. Participants were instructed to wear an ActiGraph GT3X+ accelerometer at their waist near the iliac crest for seven consecutive days following their assessment.34 Validity criteria and PA estimation processes were consistent with Study 1.
Data analysis
A total of 1,340 observations were included in confirmatory analyses; 116 of the possible 1,456 observations were missing due to nonwear. The statistical approach was repeated from Study 1 to Study 2, with the addition of the planned comparisons described next. Based on findings from Study 1, we hypothesized that the within-person difference between the four methods would be statistically significant for total minutes of light and moderate activity, as well as for MVPA (total [non-bouted] minutes and 10-min bouts; P < 0.05). We expected to observe the largest pairwise differences between methods by Swartz and Troiano, and to observe substantial differences between methods by Freedson and Matthews, Freedson and Swartz, and Matthews and Troiano. We predicted that fewer individual days and fewer participants (overall) would meet MVPA recommendations, using methods by Freedson et al17 and Troiano et al6 versus methods by Matthews et al18 and Swartz et al.19 Finally, we expected to observe smaller differences in these pairwise comparisons and proportions meeting MVPA recommendations when MVPA was calculated in bouts of 10 minutes or more (vs total [non-bouted] minutes).
STUDY 2: RESULTS AND DISCUSSION
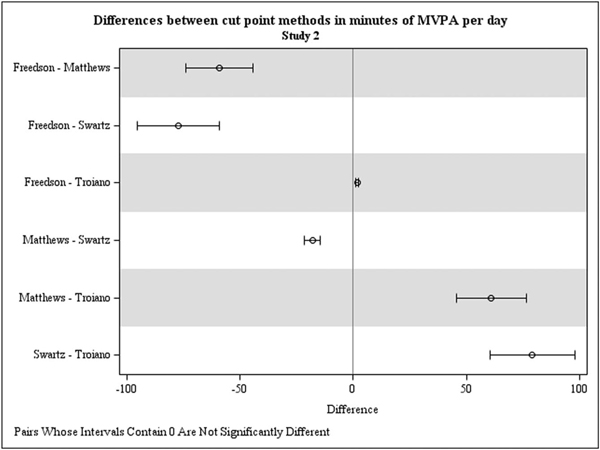
Average number of minutes per day at each PA intensity by cut point method are shown in Table 3 (lower panel). As expected, the effect of cut point method was significant for total non-bouted minutes of light (F[4,152] = 435.63), moderate (F[4,152] = 214.49), and MVPA (F[4,152] = 250.54, P < 0.0001), showing distinctions between estimates. The effect of cut point method also was significant for vigorous-intensity activity (F[4,152] = 5.30, P = 0.0005). Consistent with findings from Study 1, the largest pairwise differences were between Swartz et al19 and Troiano et al6 (see Table 3 and Fig. 2). For example, relative to the method proposed by Troiano et al,6 the method proposed by Swartz et al19 estimated an average of 79.25 additional minutes of MVPA per day (SE = 2.94). The average difference between methods by Freedson et al17 and Swartz et al19 was similar, with Swartz estimating 77.46 additional minutes of MVPA per day (SE = 2.88). The method proposed by Matthews et al18 also generated estimates of nearly 60 more minutes of MVPA per day than methods by Freedson et al17 and Troiano et al6 (see Table 3 and Fig. 2). The effect of cut point method also was significant for time in 10-minute bouts of MVPA (F[4,152] = 17.76, P < 0.0001), and all pairwise comparisons were statistically significant. The absolute values of these differences were smaller, however; these ranged from 0.35 to 9.8 minutes, with the largest difference between methods by Swartz et al19 and Troiano et al6 (B = 9.81, SE = 1.30, F[4,152] = 7.57, P < 0.0001; see Table 3 and Fig. 2).
FIG. 2.
Estimated differences in minutes of moderate-vigorous physical activity (MVPA) based on direct (pairwise) contrasts between methods, for Study 1. MVPA (total) = total (non-bouted) minutes of moderate-to-vigorous physical activity. MVPA, moderate-vigorous physical activity.
With respect to MVPA recommendations on individual days, when total (non-bouted) minutes were considered, methods by Freedson et al17 and Troiano et al6 estimated that participants reached 30 minutes of MVPA on 54% and 50% of days, respectively (see Table 4). Across the week of data collection, methods by Freedson et al17 and Troiano et al6 showed that 40% and 33% of participants, respectively, achieved the recommended 30 minutes of MVPA per day on five or more days. Using methods by both Matthews et al18 and Swartz et al,19 participants achieved 30 minutes of MVPA on 99% of individual days; the subset that achieved the recommended 30 minutes of MVPA per day on five or more days was 92% for both methods.
When bouts of 10 minutes or more were considered, however, all methods indicated that recommendations were achieved on less than 30% of days. Methods by Freedson et al17 and Troiano et al6 estimated that participants engaged in 30 or more minutes of MVPA on 15% and 14% of individual days, respectively; methods by Matthews et al18 and Swartz et al19 showed that participants met the recommendation on 21% and 26% of days, respectively. At the participant level, methods by Freedson et al17 and Troiano et al6 both indicated that 4% of the sample achieved 30 minutes per day on five (of seven) days. These estimates were 10% and 13% using methods by Matthews et al18 and Swartz et al,19 respectively.
Summary
Overall, findings from Study 2 confirmed those of Study 1 and demonstrate statistically and potentially clinically meaningful differences between cut point methods—particularly when estimating total (non-bouted) minutes of MVPA. Further, methods by Freedson et al17 and Troiano et al6 indicate that midlife women with elevated CVD risk do not meet weekly MVPA recommendations for adults based on their total (non-bouted) minutes, whereas methods by Matthews et al18 and Swartz et al19 suggest that they may reach or even exceed this goal. As expected, however, these differences were much smaller when considering MVPA in 10-minute bouts, and none of the methods tested indicated that these women met weekly MVPA recommendations.
DISCUSSION
Different methods for estimating PA from accelerometers generate divergent conclusions about PA engagement in various populations, leading some authors to suggest that knowing the true proportion of a population that meets MVPA recommendations is impossible.12 Midlife women with elevated CVD risk are of particular interest in prevention research, and often receive advice from providers to increase PA to prevent CVD morbidity and mortality.36 However, as research shows heterogeneity in the cut point methods used to estimate PA in this population,20,23,37 it is not clear exactly how much PA these women do in a given week (without or before intervention). To our knowledge, the present study is the first to directly compare common accelerometer cut point methods among midlife women with CVD risk factors. It is also one of the few that employed a two-study approach to generate and then confirm specific hypotheses related to accelerometer cut point methods. This investigation is particularly timely, given that the United States Department of Health and Human Services recently removed its recommendation that MVPA be considered in bouts of 10 minutes or more.15 This change offered the opportunity to examine comparisons between cut point methods using both total (non-bouted) minutes of MVPA and time in bouts of 10 minutes or more.
This two-study approach produced consistent findings regarding differences between cut point methods. Given their higher thresholds for moderate activity, methods proposed by Freedson et al17 and Troiano et al6 categorize significantly more minutes as light activity than those proposed by Matthews et al18 and Swartz et al.19 This difference led to significantly higher estimates of moderate activity and MVPA using methods by Matthews et al18 and Swartz et al,19 particularly when emphasizing total (non-bouted) minutes. In Study 2, methods by Matthews et al18 and Swartz et al19 also were significantly higher for vigorous activity, which may have been due to power afforded by a larger sample size (vs Study 1). The size of these differences ranged from 3 to 79 minutes of MVPA, which is wider than the observed range of differences in related populations: postmenopausal women (eg, 2 to 45 min),38 postmenopausal women with cancer (eg, 42 to 75 min),39 and women age 70 and older (eg, 13 to 44 min).40 The use of different combinations of cut point methods in each of these studies precludes strong conclusions about the overall size of observed differences for women nearing or beyond menopause. However, comparisons of cut point methods consistently show statistically and clinically significant differences in PA estimates for women in these groups.
Such discrepancies could have important implications for PA research and promotion efforts among midlife women. For example, when considering only total minutes at MVPA intensity, as opposed to 10-minute bouts as in previous guidelines, all four methods estimated that women in both of the present studies achieved the recommended 30 or more minutes of MVPA per day, on average. Further, methods by Matthews et al18 and Swartz et al19 showed that women achieved more than 90 minutes of MVPA per day, on average, and that the vast majority met the goal of 30 minutes five days per week. These estimates stand in contrast to much lower previous descriptions of MVPA among midlife women,7,23 though they further support the differences previously observed between total (non-bouted) minutes and 10-minute bouts of MVPA in this population.41
As such, midlife women’s MVPA may be more sporadic than previously thought. Capturing their activity using total (non-bouted) minutes would provide important empirical information about the timing and fluctuation of MVPA throughout the day; in the future, such information could be useful for informing just-in-time PA interventions (eg, as delivered via smartphone app in response to a user’s PA engagement).42 Although MVPA estimates from all four methods were much lower when 10-minute bouts were evaluated, which is consistent with previous work,43 differences between methods remained significant, ranging from 2.50 to 10 minutes of MVPA.
Together, the present findings demonstrate the need for increased attention to cut point methods in future PA work with midlife women who have elevated risk for CVD. The choice of cut point method may lead to dramatically different conclusions about midlife women’s baseline level of PA, and thus, the targeting and evaluation of particular subgroups in future PA promotion programs. Importantly, some methods may underestimate these women’s PA – particularly methods by Freedson et al,17 which were calibrated for a young and healthy sample. Methods by Matthews et al18 and Swartz et al19 are used less widely, both generally and among midlife women. Greater emphasis on these methods may provide useful information about midlife women’s PA, particularly as it occurs throughout the day (vs in 10-min bouts). However, we cannot determine from the current findings whether these provide overestimates of midlife women’s PA. Future research to compare all four cut point methods to energy output assessments such as doubly labeled water44 would provide needed clarification regarding the appropriate PA estimation method(s) for midlife women with elevated CVD risk.
Strengths, limitations, and future directions
This study benefitted from an emphasis on an at-risk subgroup (ie, midlife women with CVD risk markers), recruitment of an ethnically diverse sample (particularly in Study 2), and emphasis on within-person analyzes (which maximizes power to detect effects). Its two-study design also represents a strength, as specific hypotheses generated by findings from Study 1 could be tested in Study 2. As these studies had different aims (ie, short-term observation vs long-term weight loss treatment), participants from the overall population were likely to enroll for a range of reasons. Thus, the observed consistency of findings between the two studies increases the generalizability of our conclusions. However, the overall sample size (N = 65) was small, participants were highly educated, and accelerometer assessment occurred over a single week that may not have captured their typical PA behavior. We also did not have information about participants’ CVD risk factors other than those assessed. And although we selected the four cut point methods used herein for their common use in samples of midlife adults, other methods were not included in the present analyses.41 Consequently, there is need to replicate these findings in larger, more representative samples using longer data collection periods, and it may be useful to compare additional cut point methods in future research. This work would inform strong recommendations regarding best practices for using specific accelerometer cut point methods among midlife women with CVD risk.
CONCLUSIONS
These limitations notwithstanding, the present findings highlight the need for careful consideration of cut point methods applied to accelerometer data from midlife women with elevated CVD risk. The selection of cut point method, as well as emphasis on total (non-bouted) minutes versus time in 10-minute bouts of MVPA, could meaningfully affect conclusions about this group’s overall activity levels. These conclusions, in turn, could meaningfully influence both clinical recommendations for these women and inferences regarding the effects of PA promotion efforts tailored specifically for them. Specifically, methods by Matthews et al18 and Swartz et al19 may provide the most useful estimates of midlife women’s PA throughout the day (vs. in bouts of ≥10 min), and use of these methods could inform adjustments to CVD risk reduction approaches for this group. Additional work in this area would further inform assessment and intervention recommendations for promoting PA among midlife women with CVD risk.
Acknowledgments:
The authors thank Emily Vendetta, Laura Travers, Darlene Jules, Autumn Bowman, and Sabrina DiBisceglie for their assistance with data collection for this manuscript.
Funding/support: This work was supported by the National Institutes of Health under NHLBI K23136657 (PI: D. Arigo), NIDDK R01DK100345 (PI: M. Butryn), and NHLBI R01HL119245 (PI: D.S. Downs) and internal funding awarded to the first author.
Disclaimers: These findings were presented as a poster at the 2019 annual meeting of the Society of Behavioral Medicine in Washington, D.C. Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
Footnotes
Financial disclosure/conflicts of interest: None reported.
Video Summary: http://links.lww.com/MENO/A545.
REFERENCES
- 1.Lakatta EG. Age-associated cardiovascular changes in health: Impact on cardiovascular disease in older persons. Heart Fail Rev 2002;7: 29–49. [DOI] [PubMed] [Google Scholar]
- 2.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009;54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovejoy JC. Weight gain in women at midlife: the influence of menopause. Obes Manag 2009;5:52–56. [Google Scholar]
- 4.Braun LT, Wilbur J, Buchholz SW, et al. Cardiovascular risk in midlife African American women participating in a lifestyle physical activity program. J Cardiovasc Nurs 2016;31:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins P, Webb CM, De Villiers TJ, et al. Cardiovascular risk assessment in women—an update. Climacteric 2016;19:329–336. [DOI] [PubMed] [Google Scholar]
- 6.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40: 181–188. [DOI] [PubMed] [Google Scholar]
- 7.Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc 2000;32:1601–1609. [DOI] [PubMed] [Google Scholar]
- 8.Woodward MJ, Lu CW, Levandowski R, et al. The exercise prescription for enhancing overall health of midlife and older women. Maturitas 2015;82:65–71. [DOI] [PubMed] [Google Scholar]
- 9.Lugade V, Fortune E, Morrow M, et al. Validity of using tri-axial accelerometers to measure human movement—Part I: posture and movement detection. Med Eng Phys 2014;36:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aadland E, Ylvis\aaker E. Reliability of the Actigraph GT3X+ accelerometer in adults under free-living conditions. PLoS One 2015;10: e0134606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly LA, McMillan DG, Anderson A, et al. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys 2013;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migueles JH, Cadenas-Sanchez C, Tudor-Locke C, et al. Comparability of published cut-points for the assessment of physical activity: implications for data harmonization. Scand J Med Sci Sports 2019;29:566–574. [DOI] [PubMed] [Google Scholar]
- 13.Gába A, Dygr?n J, Mitáš J, et al. Effect of accelerometer cut-off points on the recommended level of physical activity for obesity prevention in children. PLoS One 2016;11:e0164282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medicine, AC of S. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 15.U.S. Department of Health, Human Services. Physical Activity Guidelines for Americans, 2nd ed Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- 16.Downs DS, Chasan-Taber L, Evenson KR, et al. Physical activity and pregnancy: past and present evidence and future recommendations. Res Q Exerc Sport 2012;83:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–781. [DOI] [PubMed] [Google Scholar]
- 18.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 2008;167:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swartz AM, Strath SJ, Bassett DR, et al. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc 2000;32:450–456. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel KP, Sidney S, Jacobs DR, et al. Ten-year changes in accelerometer-based physical activity and sedentary time during midlife. Am J Epidemiol 2018;187:2145–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Rey E, Maldonado-Martín S, Palacios-Samper N, et al. Objectively measured absolute and relative physical activity intensity levels in postmenopausal women. Eur J Sport Sci 2018;19:539–548. [DOI] [PubMed] [Google Scholar]
- 22.Kerr J, Marinac CR, Ellis K, et al. Comparison of accelerometry methods for estimating physical activity. Med Sci Sports Exerc 2017;49:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishida M, Elavsky S. An intensive longitudinal examination of daily physical activity and sleep in midlife women. Sleep Health 2016;2:42–48. [DOI] [PubMed] [Google Scholar]
- 24.Maddison R, Jiang Y, Foley L, et al. The association between the activity profile and cardiovascular risk. J Sci Med Sport 2016;19:605–610. [DOI] [PubMed] [Google Scholar]
- 25.Orsini N, Bellocco R, Bottai M, et al. Profile of physical activity behaviors among Swedish women aged 56–75 years. Scand J Med Sci Sports 2008;18:95–101. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez A, Norman GJ, Sallis JF, et al. Patterns and correlates of multiple risk behaviors in overweight women. Prev Med 2008;46:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sternfeld B, Bhat AK, Wang H, et al. Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc 2005;37:1195–1202. [DOI] [PubMed] [Google Scholar]
- 28.Loprinzi PD, Lee H, Cardinal BJ, et al. The relationship of Actigraph accelerometer cut-points for estimating physical activity with selected health outcomes: results from NHANES 2003–06. Res Q Exerc Sport 2012;83:422–430. [DOI] [PubMed] [Google Scholar]
- 29.Stone MR, Rowlands AV, Eston RG. Relationships between accelerometer-assessed physical activity and health in children: impact of the activity-intensity classification method. J Sports Sci Med 2009;8:136. [PMC free article] [PubMed] [Google Scholar]
- 30.Watson KB, Carlson SA, Carroll DD, et al. Comparison of accelerometer cut points to estimate physical activity in US adults. J Sports Sci 2014;32:660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince SA, Reed JL, Mark AE, et al. A comparison of accelerometer cut-points among individuals with coronary artery disease. PLoS One 2015;10:e0137759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Remoortel H, Camillo CA, Langer D, et al. Moderate intense physical activity depends on selected Metabolic Equivalent of Task (MET) cut-off and type of data analysis. PLoS One 2013;8:e84365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tudor-Locke C, Camhi SM, Troiano RP. Peer reviewed: a catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Prev Chronic Dis 2012;9:110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butryn ML, Martinelli MK, Remmert JE, et al. Executive functioning as a predictor of weight loss and physical activity outcomes. Ann Behav Med 2019;53:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadden TA, Foster GD. Weight and lifestyle inventory (WALI). Obesity 2006;14:99–118. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Townsend MK, Okereke OI, et al. Physical activity at midlife in relation to successful survival in women at age 70 years or older. Obstet Gynecol Surv 2010;65:377–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butryn ML, Arigo D, Raggio GA, et al. Enhancing physical activity promotion in midlife women with technology-based self-monitoring and social connectivity: a pilot study. J Health Psychol 2016;21:1548–1555. [DOI] [PubMed] [Google Scholar]
- 38.Diniz TA, Rossi FE, da Costa Rosa CS, et al. Moderate-to-vigorous physical activity among postmenopausal women: discrepancies in accelerometry-based cut-points. J Aging Phys Act 2017;25:20–26. [DOI] [PubMed] [Google Scholar]
- 39.Nelson SH, Natarajan L, Patterson RE, et al. Physical activity change in an RCT: comparison of measurement methods. Am J Health Behav 2019;43:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thralls KJ, Godbole S, Manini TM, et al. A comparison of accelerometry analysis methods for physical activity in older adult women and associations with health outcomes over time. J Sports Sci 2019;37:2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laakkonen EK, Kulmala J, Aukee P, et al. Female reproductive factors are associated with objectively measured physical activity in middle-aged women. PLoS One 2017;12:e0172054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-Time Adaptive Interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med 2018;52:446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banda JA, Hutto B, Feeney A, et al. Comparing physical activity measures in a diverse group of midlife and older adults. Med Sci Sports Exerc 2010;42:2251–2257. [DOI] [PubMed] [Google Scholar]
- 44.Chomistek AK, Yuan C, Matthews CE, et al. Physical activity assessment with the ActiGraph GT3X and doubly labeled water. Med Sci Sports Exerc 2017;49:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]