Summary:
Objective:
We aimed to evaluate the performance of a survey that quantifies intensity of household tuberculosis (TB) exposure among children.
Methods:
Children ages 0-14 in Lima, Peru with >=1 signs and/or symptoms of TB and a history of contact with an adult TB patient were included. The 10-question survey was administered to caregivers and addressed sleep proximity, frequency of exposure and infectiousness of contact. Infection status was determined using tuberculin skin testing (TST). The scale was evaluated for association with TST positivity using mixed effects regression analyses.
Results:
The exposure score was significantly associated with TST positivity (age-adjusted odds ratio [aOR]: 1.14, 95% Confidence Interval [CI] 1.02- 1.28; Table 2). We observed a stronger association with TST positivity in children five years old and under; (aOR: 1.23; 95% CI: 1.07-1.41) and no association in children 6-14 years old; aOR: 0.99; 95% CI: 0.82-1.20).
Conclusion:
This survey was easy-to-use and modestly successful in predicting TST positivity in children five years old and under. It may be a useful resource to clinicians in diagnosing TB in children, and for national TB programs aiming to scale up preventive therapy initiatives.
Keywords: latent tuberculosis infection, pediatric tuberculosis, screening, preventive therapy, contact tracing, household exposure
Introduction:
Treatment of latent tuberculosis infection (LTBI) to prevent progression to TB disease (i.e., TB preventive therapy, (TPT)) remains an important, yet severely under-implemented part of tuberculosis (TB) control and disease prevention. The World Health Organization (WHO) recommends that children under five years of age who are close contacts of TB patients receive TPT after TB disease is ruled out.1,2,3 Until recently, although TPT also offers protection from TB in children six to 14 years of age, the recommendations did not provide guidance on its use in this group. Updated guidelines now suggest TPT for children and adolescent household contacts of pulmonary TB patients.3 However, despite the global recommendations, only 23% of eligible children were prescribed TPT in 2017.2,3
Though not mandated by WHO, in some settings, a child must have a test of infection prior to initiation of TPT.4,5,6 Clinicians rely on infection status as a key consideration when making decisions related to clinical diagnosis. This is especially important information in the absence of microbiological confirmation, which is obtained in only 30-40% of children with TB disease.7,8,9
Though no gold standard exists for tests of infection, tuberculin skin testing (TST) is widely used to determine infection status. TST has limited utility given the well-known challenges it presents. Stock-outs and global tuberculin shortages, required return visits for patients, and suboptimal test performance, including reduced specificity in previously BCG-vaccinated children and limited sensitivity in immunocompromised or malnourished children are well documented shortcomings.10,11,12,13 Interferon Gamma Release Assay (IGRA) tests improve specificity in BCG-vaccinated children and only require one clinic visit, however the tests have limited sensitivity in the immunocompromised and require expensive supplies and highly trained lab technicians.11,14 These issues prevent widespread implementation in low and middle-income settings. Prior studies suggest that characterizing the intensity of exposure to TB may serve as a proxy for TB infection status.15,16,17,18
Work in South Africa suggests that a short, easy-to-use survey evaluating intensity of household-based exposure to TB predicts infection status in children 3 months to <15 years old.18 Results from subsequent studies of the survey in high burden settings have varied. In India, the tool’s sensitivity approximated that of TST and IGRA results among a study population of both adult and child household contacts.19 However, in Tanzania it did not predict infection status among children under five.20 The present study aims to examine the performance of the survey in an urban, low-HIV prevalence setting in Latin America.
Methods:
Study population:
From May 2015 through February 2018, we recruited children ages 0-14 years from 49 urban public sector health centers in Lima, Peru to participate in a pediatric tuberculosis diagnostics study [1U19AI109755, Centers for Excellence in Translation Research]. Eligible children had a history of contact with an adult with pulmonary TB within the two years preceding enrollment and met at least one of the following criteria for inclusion in pediatric TB diagnostic studies, as defined by an expert panel21: persistent, unremitting, and unexplained cough for > 2 weeks, unexplained weight loss, unexplained fever for >1 week, or unexplained fatigue or lethargy. Eligible children were identified in one of three possible ways: 1) through contact investigations completed by health center staff after an adult in the home presented to a clinic for diagnosis 2) via the child’s presentation to a health center or, 3) through study staff’s review of health center adult TB registries. In the latter scenario, the study team identified adults diagnosed with TB within the prior two years and conducted a subsequent household contact investigation.
Ethics committees at the Peru National Institutes of Health and Harvard Medical School granted study approval. Trained study personnel obtained written consent from guardians and written assent from children eight years of age and older.
Data collection:
Study workers asked guardians to respond to ten yes/no questions18 regarding the intensity of the child’s TB exposure from index cases (Figure 1). We diagnosed latent TB infection status based on TST results. TSTs were prospectively applied to participants according to Peru National Tuberculosis Program guidelines5, and read within 48-72 hours of application by study staff. If the test had been completed within the six months prior to enrollment, TST results were collected from the child’s health record. Skin reactions with an induration of ≥10mm were considered positive.22 All data were collected using standardized electronic data collection forms.
Figure 1: Original tuberculosis exposure scale developed by Mandalakas and colleagues1.

1 Mandalakas A M, Kirchner H L, Lombard C, et al. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int J Tuberc Lung Dis 2012; 16(8)1033-1039
2This question was excluded from the scale due to the low prevalence of extra pulmonary TB in this population.
Analysis:
We evaluated each survey for missing answers and excluded children with ≥1 missing survey responses or who lacked a TST result. A missing answer included responses of ‘refused’ and ‘don’t know’. Although the original scale contained ten items, we removed one question: “Does the index case have reported pulmonary TB” because there were no cases of extra pulmonary TB among index cases in this cohort. For each child, a survey score was calculated as a sum of yes answers.
We conducted mixed effects regression analyses with a logit link to assess the association between exposure score and TST positivity. To adjust variance for household clustering (i.e., multiple children could be enrolled from the same household), we included a random intercept for each household. Because TB infection correlates positively with older age, we adjusted for age as a continuous linear variable. Additionally, to examine whether the association between exposure score and TST positivity varied across older and younger children, we stratified analyses by age group (0-5 and 6-14 year olds).
Some of the children in our study were diagnosed with TB. To examine whether any association between exposure score and TST positivity was driven by children with a TB diagnosis, we conducted a sensitivity analysis in which we excluded all children with clinically diagnosed TB or who lacked data on TB diagnosis.
Finally, we evaluated scale sensitivity and specificity and plotted each against exposure score distribution to understand trade-offs in sensitivity and specificity across different scale thresholds.
Results:
Among 628 children eligible for inclusion, 608 (97%) completed the survey, of which 51 (8%) were excluded: 30 (5%) for missing TST results and 21 (3%) for missing one or more survey responses. The median age among the 557 children included in the analysis was 5.1 years [IQR 2.4 - 8.7]. Table 1 shows that 41% of the cohort was TST positive; among those, 54% were children ages five and under and 46% were six to 14 years old. Most children had a contact with TB who had a current cough (86%), who was in daily contact with the child (91%), and/or who lived in the same household (91%) as the child. On a scale from 0-9, median survey score among all children included was five [IQR 4-7].
Table 1.
Characteristics of children with symptoms of tuberculosis and tuberculin skin test positivity (n=557)
| TST-negative n=326 |
TST-positive n=231 |
Total n=557 |
|
|---|---|---|---|
| Characteristics | |||
| Age, years, median [IQR] | 4.5 [2.1-7.9] | 5.7 [3.4-9.2] | 5.1 [2.4-8.7] |
| Age groups n (%) | |||
| 0 – 5 years | 208 (64) | 125 (54) | 333 (60) |
| 6 - 14 years | 118 (36) | 106 (46) | 224 (40) |
| Female n (%) | 147 (45) | 113 (49) | 260 (47) |
| BMI, median [IQR] | 16.9 [15.7-18.8] | 16.8 [15.7-18.6] | 16.9 [15.7-18.8] |
| BMI for age z-score, mean ± SD | 0.68 (1.24) | 0.61 (1.15) | 0.65 (1.21) |
| Severe malnutrition*, n(%) | 1 (.3) | 0 (0) | 1 (.001) |
| HIV seropositivity n(%) | 2 (1) | 0 | 2 (.4) |
| Children exposed to index cases with the following characteristics n(%): | |||
| Sleeps in the same bed as child | 97 (30) | 71 (31) | 168 (30) |
| Currently has cough | 280 (86) | 199(83) | 479 (86) |
| In daily contact with child | 294 (90) | 212 (92) | 506 (91) |
| Child’s primary caregiver | 126 (39) | 103 (45) | 229 (41) |
| Lives in same household as child | 294 (90) | 211 (91) | 505 (91) |
| Child’s mother | 87 (27) | 81 (35) | 168 (30) |
| More than one contact in child’s household | 46 (14) | 57 (25) | 103 (18) |
| Has reported pulmonary TB? | 326 (100) | 231 (100) | 557 (100) |
| Sleeps in same room as child | 159 (49) | 122 (53) | 281 (50) |
| Has smear-positive pulmonary TB | 274 (84) | 205 (89) | 479 (86) |
| Median survey score [IQR] | 5 [4-6] | 5 [4-7] | 5 [4-7] |
Defined as < −3 standard deviations from the median value, per WHO Growth Indicators16
Scale performance
Overall, we found that each one-point increase in exposure scale score was associated with a 14% higher odds of having a positive TST result (age-adjusted odds ratio [aOR]: 1.14, 95% Confidence Interval [CI] 1.02- 1.28; Table 2). In children five years old and under, each additional one-point increase in scale score was associated with a 20% higher odds of TST positivity; (aOR: 1.23; 95% CI: 1.07-1.41). We found no association between scale score and infection in children six to 14 years old; aOR: 0.99; 95% CI: 0.82-1.20). Results were similar in sensitivity analyses in which we excluded children who were clinically diagnosed with TB (n=112) or whose diagnosis status was unattainable (n=16; Table 2).
Table 2:
Association between tuberculin skin test positivity and household exposure score, stratified by age
| 0-14 years | 0-5 years | 6-14 years | ||||
|---|---|---|---|---|---|---|
| n= 557 | n= 224 | n= 333 | ||||
| OR (95% CI) | aOR** (95% CI) | OR (95% CI) | aOR** (95% CI) | OR (95% CI) | aOR** (95% CI) | |
| Score, all children | 1.10 (0.98-1.22) | 1.14 (1.02-1.28) | 1.21 (1.06-1.38) | 1.23 (1.07-1.41) | 0.99 (0.82-1.21) | 0.99 (0.82-1.20) |
| Score, excluding 128 children*** | 1.08 (0.96-1.23) | 1.14 (0.99-1.30) | 1.22 (1.04-1.43) | 1.27 (1.07-1.51) | 0.96 (0.77-1.20) | 0.96 (0.77-1.20) |
Odds ratios [ORs] represent the increase in odds of TST positivity for each one-point increase in exposure scale score.
Adjusted for age as a continuous variable
Children who had tuberculosis disease (n=112) or who lacked a TB diagnosis (n=16) were excluded
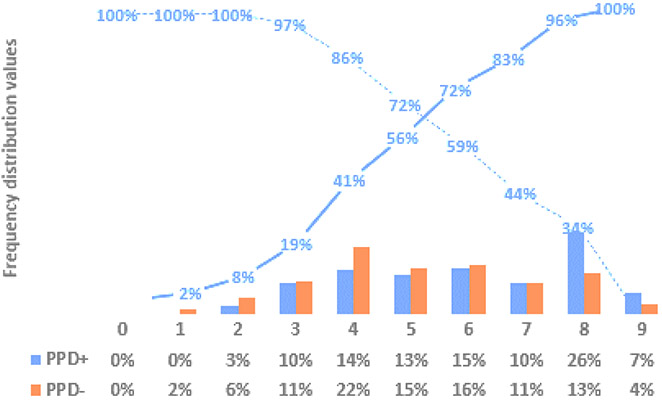
Figure 1 presents sensitivity and specificity of the exposure score in identifying children five and under with TST positivity. A score of three or higher was associated with a sensitivity of 97% and a specificity of 19%. A household exposure score of five resulted in sensitivity and specificity at 72% and 56%, respectively.
Discussion:
Our findings suggest that the survey developed by Mandalakas and colleagues18 predicts TST positivity in children five years old and under in an urban, low-HIV prevalence Latin American setting. Notably, the association between household exposure score and TST status was weaker among children in this age group in Peru as compared to the South African cohort (aOR 1.23 vs 1.74). One possible explanation for this difference is that in Lima, it is common for extended families to live in multi-unit constructions where sleeping quarters are separate from common areas that are shared for meals and leisure activities. Therefore, a yes response to the question “Does the index case live in the same household as the child?” may overestimate the exposure intensity for some living arrangements common to Lima. In future studies, culturally adapting and tailoring the questions to the setting where the scale is implemented may address this issue. In contrast to our findings, researchers in Tanzania found that this scale did not predict TST positivity in young children under the age of five and authors speculate that community-based exposure may play an important role in latent TB infection in this setting. 20 An emerging body of literature suggests that a majority of exposure to TB in high burden settings occurs outside the home.23,24,25 Increased exposure outside the home may be a key factor that affects this scale’s performance across settings and highlights the need for contact tracing outside of the home.
As was the case in South Africa, this scale did not predict TST positivity in children over the age of five in Peru. Differential performance of the scale across ages may be due an increased likelihood of community-based TB exposure among older children, who spend more of their time outside the home than younger children. In Peru, the probability of acquiring TB from a community setting has been shown to be higher than from within the household,26 though this may not be true for individuals with a known close contact, as was the case in our study. Another possible explanation for differential performance across age groups is that TST does not distinguish between recent and remote infection, and older children may have been infected when they were younger. Therefore, exposure intensity at the time of the survey may not reflect exposure intensity at the time of infection. This could result in lower exposure scores, despite infection. A third reason is that older children may have less intense contact with the adults in their household as compared to toddlers or infants. For example, one survey question asks whether the index case is the mother. The intensity of contact between a mother and an infant and a mother and an older child may be different, and the survey may not adequately capture these nuances in exposure patterns across the age spectrum.
We found that using a score threshold of three or higher predicted TST positivity with 97% sensitivity and specificity of 19% whereas a score of five resulted in a sensitivity of 72% and specificity of 56%. These thresholds are based on a total score of nine possible points after removal of the question referring to pulmonary TB, whereas the original scale from South Africa and those implemented in other settings were based on a score of ten total points. Determining appropriate score thresholds may vary by setting and intended use; however, TPT is safe and so when used to inform prescription, priority should be placed on optimizing sensitivity, if and when health systems resources allow.
The enrollment criteria for the larger cohort study limited participation to only children who had symptoms of tuberculosis. This could have resulted in an overestimate in scale performance given that some of the indicators for infection may also overlap with clinical criteria for evaluating disease status. In order to determine whether predictors of TB disease were driving the association, we excluded children with a diagnosis of TB. We found similar associations for the scale as compared to the analysis inclusive of all children, suggesting this was an unlikely source of bias in our study. A second limitation of this study relates to the singular use of TST as the indicator of infection status and the lack of sensitivity and specificity of this test, especially in children who are immunocompromised or malnourished. Because malnutrition27 was uncommon in this sample (severe malnutrition (<1%)) and Peru has a low prevalence of HIV, we expect that false negative TST results may be rare relative to other cohorts. On the other hand, vaccination with BCG is common in Peru, and therefore, false-positives may have occurred, especially in young children. This misclassification likely would have underestimated scale performance in this group.
Conclusion:
This survey was easy-to-use and modestly successful in predicting TST positivity in children five years old and younger. Given the frequent global shortages of tuberculin that thwart access to TST testing, limited implementation and uptake of TPT globally, and clinical importance of known infection status, the scale may be a useful resource to clinicians who are tasked with diagnosing and treating TB disease in young children. Lastly, contact tracing outside of the home, and the development of methods to assess community-based TB exposure will be critical for TB prevention in older children.
Figure 2: Household exposure score stratified by tuberculin skin test result and test performance among children five and under. Sensitivity (dotted line) and specificity (solid line).
Note: Total scores are based on a possible total of nine points, whereas the original scale was scored out of ten points.
Acknowledgements:
National Institutes of Health (NIH/NIAID grant 5U19AI109755)
References:
- 1.WHO: Global Tuberculosis Report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.WHO: Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second Edition. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 3.WHO: Latent TB Infection: Updated and consolidated guidelines for programmatic management. Geneva: World Health Organization; 2018 [PubMed] [Google Scholar]
- 4.Jagger A, Reiter-Karam S,Hamadab Y & Getahunb H. National policies on the management of latent tuberculosis infection: review of 98 countries. Bull World Health Organ 2018;96:173–184F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministerio de Salud (MINSA). Norma tecnica de salud para la atencion integral de las personas afectadas por tuberculosis. Lima: MINSA; 2013. http://www.tuberculosis.minsa.gob.pe/portaldpctb/recursos/20180308083418.pdf [Google Scholar]
- 6.National Tuberculosis Control Program. Manual of Procedures of the National Tuberculosis Control Program. Fifth Edition. Manila, Philippines; 2014. http://www.doh.gov.ph/sites/default/fi les/ntp_2001.html and at itis.doh.gov.ph/mop_2014.pdf [Google Scholar]
- 7.Starke JR. Tuberculosis in the pediatric population of Houston, Texas. Pediatrics. 1989. July; 84(1): 28–35. [PubMed] [Google Scholar]
- 8.Iriso R, Mudido PM, Karamagi C, Whalen C. The diagnosis of childhood tuberculosis in an HIV-endemic setting and the use of induced sputum. Int J Tuberc Lung Dis. 2005; 9:716–26. [PubMed] [Google Scholar]
- 9.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005. January 8-14;365(9454):130–4. [DOI] [PubMed] [Google Scholar]
- 10.Trajman A, Steffen RE, Menzies D. Interferon-Gamma Release Assays versus Tuberculin Skin Testing for the Diagnosis of Latent Tuberculosis Infection: An Overview of the Evidence. Pulm Med. 2013;2013: 601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebruegge M, Bogyi M, Soriano-Arandes A, et al. Shortage of purified protein derivative for tuberculosis testing. The Lancet. 2014; 384(9959); 2026. [DOI] [PubMed] [Google Scholar]
- 12.Marais BJ, Gie RP, Schaaf S, et al. Childhood pulmonary tuberculosis, old wisdom and new challenges. Am J Respir Crit Care Med. 2006; 173: 1078–1090. [DOI] [PubMed] [Google Scholar]
- 13.Seddon JA, Paton J, Nademi Z, et al. The impact of BCG vaccination on tuberculin skin test responses in children is age dependent: evidence to be considered when screening children for tuberculosis infection Thorax 2016;71: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandalakas A, Detjen A, Hesseling A. Can we accurately diagnose tuberculosis infection in children? Pediatr Infect Dis 2011; 30: 817–818. [DOI] [PubMed] [Google Scholar]
- 15.Adetifa I M, Ota M O, Jeffries D J, et al. Commercial interferon gamma release assays compared to the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection in childhood contacts in the Gambia. Pediatr Infect Dis J 2010; 29: 439–443. [DOI] [PubMed] [Google Scholar]
- 16.Hill PC, Brookes RH, Adetifa IM, et al. Comparison of enzyme-linked immunospot assay and tuberculin skin test in healthy children exposed to Mycobacterium tuberculosis. Pediatrics. 2006;117:1542–1548 [DOI] [PubMed] [Google Scholar]
- 17.Nakaoka H, Lawson L, Squire SB, et al. Risk for tuberculosis among children. Emerg Infect Dis 2006; 12: 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandalakas AM, Kirchner HL, Lombard C, et al. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int J Tuberc Lung Dis 2012; 16(8)1033–1039. [DOI] [PubMed] [Google Scholar]
- 19.Chandrasekaran P, Mave V, Thiruvengadam K, et al. (2018) Tuberculin skin test and QuantiFERON-Gold In Tube assay for diagnosis of latent TB infection among household contacts of pulmonary TB patients in high TB burden setting. PLoS ONE 13(8): e0199360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said K, Hella J, Ruzegae M et al. Immunologic-Based Diagnosis of Latent Tuberculosis among Children Less Than 5 Years of Age Exposed and Unexposed to Tuberculosis in Tanzania: Implications for Tuberculosis Infection Screening. Pediatr. Infect. Dis. J 2018 [DOI] [PubMed] [Google Scholar]
- 21.Cuevas L E, Browning R, Bossuyt P, et al. Evaluation of Tuberculosis Diagnostics in Children: 2. Methodological Issues for Conducting and Reporting Research Evaluations of Tuberculosis Diagnostics for Intrathoracic Tuberculosis in Children. Consensus From an Expert Panel. J. Infect. Dis 2012;205:S209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention: Tuberculosis Fact Sheets. (May 11, 2016). Retrieved from: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.htm [Google Scholar]
- 23.Auld S, Shah N, Cohen T, et al. Where is tuberculosis transmission happening? Insights from the literature, new tools to study transmission and implications for the elimination of tuberculosis. APSR Respirology (2018) 23, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson B, Morrow CD, Kohls D, et al. Mapping sites of high TB transmission risk: integrating the shared air and social behaviour of TB cases and adolescents in a South African township. Sci. Total Environ 2017; 583: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowdy DW, Azman AS, Kendall EA, et al. Transforming the fight against tuberculosis: targeting catalysts of transmission. Clin. Infect. Dis 2014; 59: 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks-Pollock E, Becerra MC, Goldstein E, et al. Epidemiologic inference from the distribution of tuberculosis cases in households in Lima, Peru. J. Infect. Dis 2011; 203: 1582–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blossner Monika, de Onis Mercedes. Malnutrition: quantifying the health impact at national and local levels. Geneva, World Health Organization, 2005. (WHO Environmental Burden of Disease Series, No. 12) https://www.who.int/quantifying_ehimpacts/publications/MalnutritionEBD12.pdf [Google Scholar]