Abstract
See also the editorial by Little in this issue.
Summary
In patients suspected of having coronavirus disease 2019 (COVID-19) who have two consecutive negative nasopharyngeal swab tests, high-probability CT infection measured with Society of Thoracic Radiology/Radiological Society of North America and COVID-19 Reporting and Data System scores were associated with bronchoalveolar lavage findings.
Introduction
The diagnosis of severe acute respiratory coronavirus 2 (SARS-CoV-2) infection is based on real-time reverse transcriptase polymerase reaction (RT-PCR) virus positivity on nasopharyngeal swab (1). The Society of Thoracic Radiology/Radiological Society of North America (STR/RSNA) and Prokop et al (2,3) introduced two distinct COVID-19 CT probability scores, here labeled STR/RSNA and COVID-19 Reporting and Data System (CO-RADS). Studies frequently use a single RT-PCR with nasopharyngeal swab as the standard of reference compared with CT (4,5). To our knowledge, no prior study used the more sensitive bronchoalveolar lavage (BAL) as a reference standard for comparison to CT for the diagnosis of COVID-19 infection.
We evaluated the association of STR/RSNA and CO-RADS CT probability scores with BAL results in patients with two negative nasopharyngeal swab RT-PCR tests.
Materials and Methods
We conducted a retrospective single-center study approved by the institutional committee on human research (protocol number, CE 97/20). Patients included in the current study are part of a previously published study (6). We included all the consecutive patients from March 16 to May 19, 2020, who underwent a chest CT scan after two consecutive negative nasopharyngeal swabs and followed by bronchoscopy with BAL with RT-PCR testing for SARS-CoV-2 RNA. RT-PCR testing was performed with nasopharyngeal swabs (Xpert [Cepheid] or eNAT [Copan Diagnostics]); the viral RNA was extracted, amplified, and detected (GeneFinder; Osang Healthcare). For each patient demographic, clinical and laboratory data were recorded. Information obtained from the bronchoscopy were noted, as well as the main chest CT features (Fig). This research letter differs from the previous article (6) for methodologic evaluation of CT scans: In particular, each patient’s CT scan was evaluated in consensus by two expert radiologists (Z.F., A.P.) and for each case, the likelihood of COVID-19 pneumonia was reported based on STR/RSNA and CO-RADS standards (2,3). The two readers acquired their COVID-19 experience by participating in a previous study (4) where they reviewed in consensus 773 CT images in patients suspected of having SARS-CoV-2 infection.
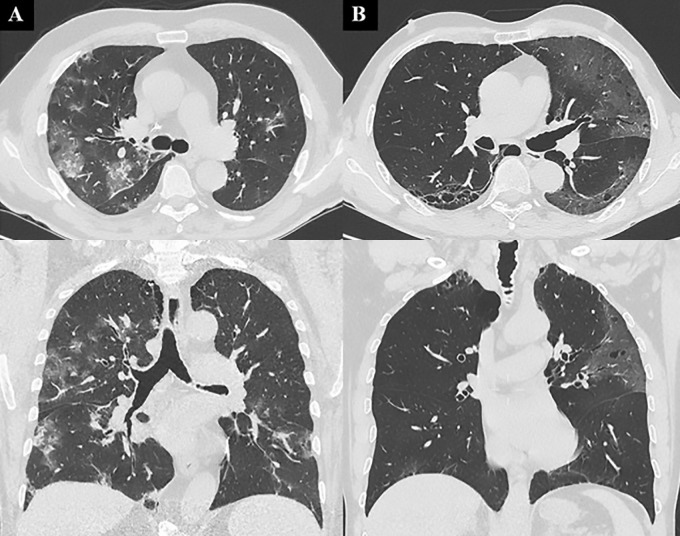
Images show chest CT features of patients with true-positive and false-positive results. A, In a 60-year-old man with bilateral diffuse areas of consolidation associated with ground-glass opacity (GGO), chest CT was classified as coronavirus disease 2019 (COVID-19) Reporting and Data System (CO-RADS) category 5 and as typical with Society of Thoracic Radiology/Radiological Society of North America (STR/RSNA) probability score, with subsequent confirmed diagnosis of COVID-19 based on fluid obtained at bronchoalveolar lavage (BAL). B, In a 54-year-old man with bilateral peripheral patchy areas of GGO who was hospitalized but not treated for COVID-19, an alternative diagnosis of viral pneumonia due to metapneumovirus was determined after BAL. Chest CT was classified as CO-RADS category 4 and as indeterminate with STR/RSNA probability score.
Results
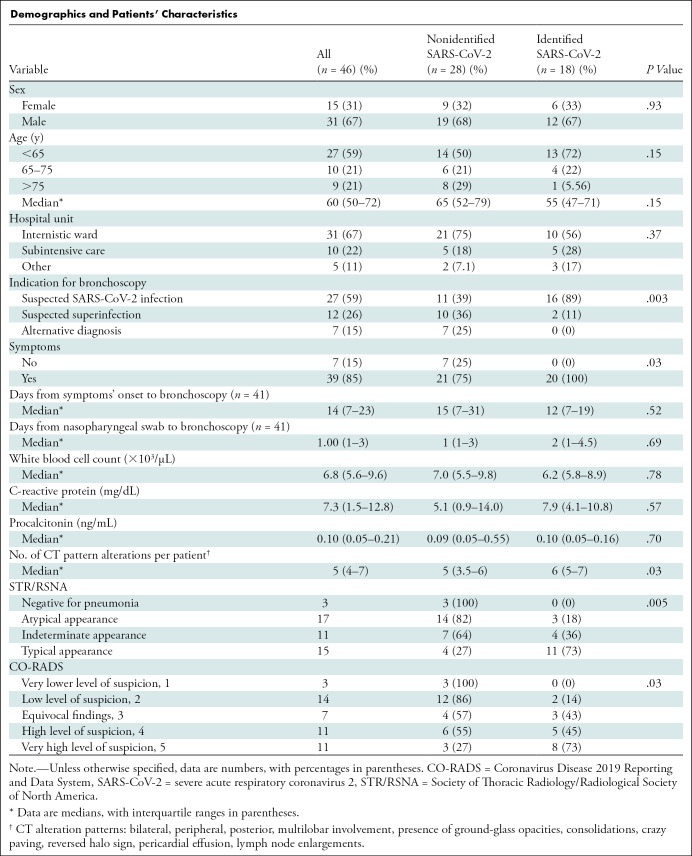
We included 46 patients; their characteristics, BAL results, and CT findings are described in the Table. SARS-CoV-2 was isolated in 18 patients (39%). By using STR/RSNA guidelines, 15 of 46 (32%) patients were classified as having typical COVID-19 patterns, 11 of 46 (24%) as having indeterminate patterns, and 17 of 46 (37%) as having atypical patterns, while three of 46 (6.5%) CT scans were negative for pneumonia. By using CO-RADS guidelines, very high and high levels of suspicion of COVID-19 pneumonia were present in 11 of 46 (24%) and 11 of 46 (24%), respectively. Seventeen of 46 other patients (37%) were classified as low or very low levels of suspicion. We found a positive association between the two scoring system (P < .001). The STR/RSNA and CO-RADS classification systems were positively associated with the presence of SARS-CoV-2 on BAL (P = .006 and P = .03, respectively).
Demographics and Patients’ Characteristics
The STR/RSNA typical pattern had a sensitivity of 61% (95% CI: 36, 83), a specificity of 86% (95% CI: 67, 96), and a positive predictive value of 73% (95% CI: 51, 88). Atypical and negative patterns in our small population showed a negative predictive value of 82% (95% CI: 59, 96) and 100%, respectively.
For CO-RADS score, we consider a score greater than or equal to 4 as positive for COVID-19 and a score less than or equal to 3 as negative: The sensitivity, specificity, positive predictive value, and negative predictive value were 72% (95% CI: 46, 90), 68% (95% CI: 48, 84), 59% (95% CI: 44, 73), and 79% (95% CI: 63, 89).
BAL was able to identify the presence of SARS-CoV-2 RNA in 10 of 11 patients with both a typical STR/RSNA pattern and a white blood cell count less than 11 000 cells/µL (P = .005) and in seven of eight patients with both a CO-RADS 5 classification and a normal white blood cell count (P = .03).
The areas under the receiver operating characteristic curve were similar for STR/RSNA and CO-RADS (0.79 [95% CI: 0.66, 0.92]) and 0.78 [95% CI: 0.64, 0.91]); P = .67 for the comparison).
Discussion
The majority of studies evaluating the accuracy of CT in the diagnosis of coronavirus disease 2019 (COVID-19) used a single nasopharyngeal reverse transcriptase polymerase reaction (RT-PCR) as reference standard and a minority used multiple RT-PCR tests (4,5). Our results seem to confirm the hypothesis that the diagnosis of COVID-19 should not rely exclusively on the RT-PCR testing. In our cohort, the integration of clinical, radiologic, and bronchoalveolar lavage results led us to a definitive diagnosis in 78% of patients (36 of 46). Society of Thoracic Radiology/Radiological Society of North America (STR/RSNA) and COVID-19 Reporting and Data System (CO-RADS) diagnostic accuracies were evaluated: Sensitivity, specificity, and positive predictive value were slightly lower than those described by previous studies (4,5). We believe that this difference is due to a smaller population and the requirement for two consecutive negative nasopharyngeal swabs with the presence of continued symptoms.
We found the association of a typical STR/RSNA pattern or a CO-RADS 5 and a normal white blood cell count extremely specific in identifying uncomplicated SARS-CoV-2 infection. This association may help the clinician distinguishing between COVID-19 and an interstitial pneumonia with bacterial etiology, and it could be used as the additional step as a proxy for BAL.
In conclusion, the integration of bronchoalveolar lavage in diagnostic flowchart in patients with two consecutive negative reverse transcriptase polymerase reaction nasopharyngeal swabs could lead a diagnosis in almost 80% of patients; typical and/or high-suspicion CT pattern, as well as white blood cell count, could orientate decision to perform other invasive testing. Further research will be needed to validate our findings.
P.S. and P.E.B. contributed equally to this work.
Disclosures of Conflicts of Interest: F.P. disclosed no relevant relationships. A.C. disclosed no relevant relationships. Z.F. disclosed no relevant relationships. A.P. disclosed no relevant relationships. F.G. disclosed no relevant relationships. C.A. disclosed no relevant relationships. M.B. disclosed no relevant relationships. P.P.S. disclosed no relevant relationships. P.S. disclosed no relevant relationships. P.E.B. disclosed no relevant relationships.
Abbreviations:
- BAL
- bronchoalveolar lavage
- CO-RADS
- COVID-19 Reporting and Data System
- COVID-19
- coronavirus disease 2019
- RT-PCR
- reverse transcriptase polymerase reaction
- SARS-CoV-2
- severe acute respiratory coronavirus 2
- STR/RSNA
- Society of Thoracic Radiology/Radiological Society of North America
References
- 1.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology 2020;296(2):E97–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging 2020;35(4):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falaschi Z, Danna PSC, Arioli R, et al. Chest CT accuracy in diagnosing COVID-19 during the peak of the Italian epidemic: A retrospective correlation with RT-PCR testing and analysis of discordant cases. Eur J Radiol 2020;130:109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccarese F, Coppola F, Spinelli D, et al. Diagnostic Accuracy of North America Expert Consensus Statement on Reporting CT Findings in Patients with Suspected COVID-19 Infection: An Italian Single Center Experience. Radiol Cardiothorac Imaging 2020;2(4):e200312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrucco F, Albera C, Bellocchia M, et al. SARS-CoV-2 Detection on Bronchoalveolar Lavage: An Italian Multicenter experience. Respiration 2020. 10.1159/000511964. Published online October 19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]