Abstract
Anterior segment optical coherence tomography (AS-OCT) imaging is a non-contact imaging technique that produces high-resolution images and quantitative measurements of the anterior segment and its anatomical structures. There has been rapid development of OCT technology over the past two decades, with the transition from time-domain to Fourier-domain OCT devices. By integrating these advancements in OCT technology, AS-OCT devices have evolved into versatile clinical and research tools for studies of the anterior segment and ocular surface. The primary purpose of this article is to review OCT technology and AS-OCT devices as well as applications of AS-OCT for clinical practice and scientific research. We first describe the different types of OCT technology, how they have been adapted for AS-OCT imaging, and differences between various AS-OCT devices. We then review the applications of AS-OCT for characterizing the anatomical structures of the anterior segment and aqueous outflow pathways, including the anterior chamber angle, trabecular meshwork, and Schlemm’s canal. We also describe glaucoma-related applications of AS-OCT imaging, which include evaluating patients for static and dynamic biometric risk factors of primary angle closure disease (PACD) and assessing the efficacy of glaucoma interventions, such as laser peripheral iridotomy (LPI) and glaucoma surgery. Finally, we review other clinical applications of AS-OCT imaging for detection and management of diseases of the ocular surface, cornea and lens.
Introduction
Optical coherence tomography (OCT) is a non-invasive, non-contact imaging technique that produces in vivo images of anatomical structures throughout the body. OCT imaging has been used extensively in the eye for micrometer resolution imaging of various structures, including the cornea, lens, iris, retina and optic nerve. OCT allows for cross-sectional or 3-dimensional imaging of biological tissues. Image resolution in the 1 to 15 um range can be achieved with depth of penetration from 2 to 7 mm, which is generally limited by signal attenuation. OCT has finer resolution but less depth penetration than ultrasound and lower resolution but more depth penetration than confocal microscopy. Given that most ocular structures are millimeters deep, OCT has proven to be an ideal tool for ocular imaging.1
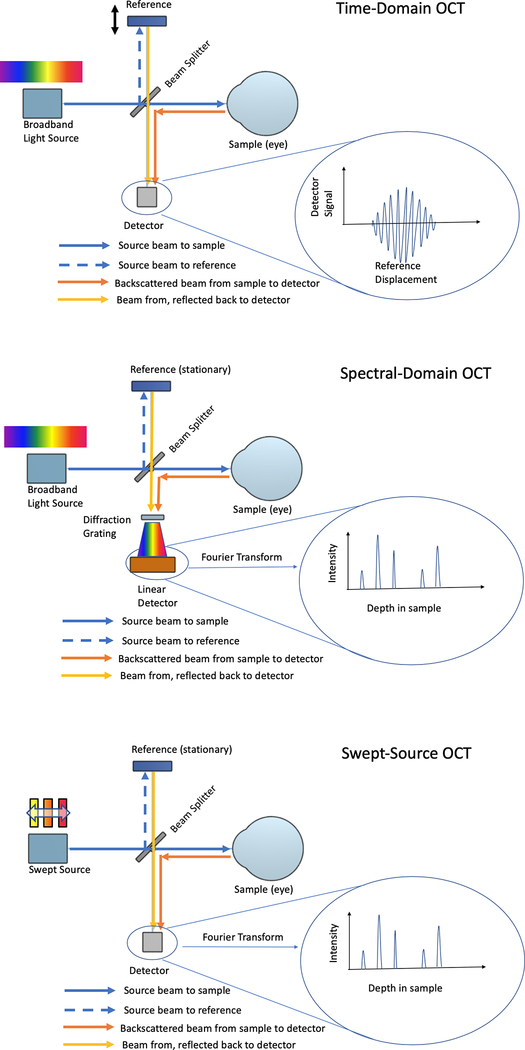
OCT is based on the principle of low-coherence interferometry and relies on the backscattering of light as it travels through various tissue structures. Infrared light is emitted by a light source and reflected by the material or tissue that it travels through. Back-reflected light is detected by a sensor, which then compares this with light from a reference beam. In practice, there are three commercially available OCT technologies that have been adapted for anterior segment imaging: time-domain OCT and Fourier-domain OCT, which is further sub-categorized into spectral-domain OCT and swept source OCT (Figure 1).
Figure 1:
Schematic diagram of time domain OCT and Fourier-domain OCT (spectral-domain and swept source) technologies.
Time-domain OCT consists of a low coherence light source, split into a reference beam and a sample beam with a beam splitter. The reference arm of the beam is reflected off a reference sample, which can be scanned for depth. The sample beam travels to the tissue (eye) for imaging. The two arms are then combined and brought to a point detector. When the reference length and reflection from the sample are similar in length (within the coherence length), the intensity of the combined beam is high, leading to a measurable signal. Different depths can be measured by scanning the reference sample. In order to construct a 2- or 3-dimensional image, the beams must move to different lateral positions. As moving the beams takes time, other techniques were developed to reduce scan times.
Fourier-domain OCT has a similar layout to time-domain. However, the reference mirror remains stationary and interference between the reference and sample arms are detected as a spectrum. With spectral-domain OCT, the reference and sample arms are combined and the spectral components are split apart by a diffraction grating, which is captured by a CCD line camera. This technique captures the depth scan in a single CCD image. Swept-source OCT is different form of Fourier-domain technology. In this scenario, the wavelength of the light source is swept and captured by a point detector. Scanning the wavelength of the source is similar to the scanning the reference mirror. However, as swept-source provides optical scanning without moving parts, acquisition rates of up to several MHz are possible.
The resolution of OCT is dependent on the source central wavelength of light, λo, as well as the spectral width of the source, Δλ. It can be approximated as:
Therefore, a source with a shorter central wavelength, or a larger spectral width will have higher resolution.
The transverse resolution, Δx, is similar to a microscope, and is related to the focus of the OCT itself. It is described as:
where d is the spot size on the objective lens and f is the focal length of the objective lens. Therefore, to increase theoretical transverse resolution, it is possible to increase the spot size at the objective lens, decrease the focal length (i.e. increase numerical aperture), or decrease the wavelength of the light source. As the wavelength of the light source is increased, axial resolution decreases. It is important to note that as numerical aperture increases, depth of focus decreases.2
Anterior Segment OCT Imaging
Anterior segment optical coherence tomography (AS-OCT) is a form of OCT technology that has been adapted for clinical care and scientific research of ocular surface and anterior segment diseases.2–4 Anterior segment imaging has a different set of requirements than posterior segment imaging. First, there is a desire to image the full anterior segment, which requires a larger width and depth of scan than is typically used in the posterior segment. As the anterior segment is more accessible optically than the posterior segment, longer wavelengths of light, which are normally attenuated by the vitreous, can be used. In addition, transverse resolution can be increased through adjustment of numerical aperture (as described above), while in the posterior segment, a higher level of optical aberrations limits the realized transverse resolution. Given these requirements, longer wavelength sources are often preferred for anterior segment imaging as they provide increased depth of penetration.2
Early anterior segment OCT (AS-OCT) devices, such as the Zeiss Visante (Carl Ziess Meditec, Dublin, CA) and Heidelberg SL-OCT (Heidelberg Engineering, Heidelberg, Germany), used time-domain OCT technology and 1310 nm superluminescent diodes, allowing for penetration depths of up to 7 mm. However, these devices were slow with long image acquisition times (2000 and 200 scans per second, respectively).
More recently, Fourier-domain OCT devices with wavelengths ranging between 820 to 880 nm have been adapted for anterior segment imaging. These include the Zeiss Cirrus (Carl Ziess Meditec, Dublin, CA), Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany), Optovue iVue (Optovue, Fremont, CA), and Nidek RS 3000 (Nidek, Fremont, CA), which have scan depths of 2 to 3 mm and scan speeds from 26k to 110k scans per second. These devices, which are nearly ubiquitous in modern ophthalmology practices, are particularly adept at high-resolution imaging of individual sections of the anterior chamber angle. However, these devices require specialized lens adapters to image across the entire anterior chamber in a single scan as they are primarily designed for posterior segment imaging.
Two swept-source OCT systems are commercially available for anterior segment use. The CASIA SS-1000 (Tomey Corporation, Nagoya, Japan) uses a 1310 nm light source, which is capable of 16×16 mm wide imaging and 6 mm deep scanning at a rate of 30k scans per second. Axial resolution is 10 um, with 30 um transverse resolution in tissue. The Triton (Topcon Medical Systems, Oakland, NJ) was developed for posterior segment use but features an anterior segment adaptor. It uses a 1050 nm light source with a 100 kHz scan rate and 3 mm depth of scan (Table 1).
Table 1.
Comparison of AS-OCT Devices
| Zeiss Visante | Heidelberg SL-OCT | Zeiss Cirrus | Heidelberg Spectralis | Optovue iVue | Nidek RS 3000 | CASIA ss-1000 | Topcon Triton | |
|---|---|---|---|---|---|---|---|---|
| Technology | Time Domain | Spectral-Domain | Spectral-Domain Swept Source | |||||
| Light Source | Superluminescent Diodc | Swept Source | ||||||
| Wavelength (nm) | 1310 | 1310 | 840 | 870 | 840 | 880 | 1310 | 1050 |
| Sean rate (kHz) | 2 | 0.2 | 27 | 40 | 26 | 53 | 30 | 100 |
| Axial Resolution (um) | 18 | <25 | 5 | 7 | 5 | 7 | <10um | 8 |
| Sean Depth (mm) | 6 | 7 | 5.8 | 1.8 | 2.3 | 2.1 | 6 | 3 |
| Sean Width (mm) | 16 | 15 | 3 | 9 | 13 | 9 | 16 | 12 |
Intra- and inter-device AS-OCT measurement repeatability and reproducibility are important to consider in clinical and research studies. In general, intra-device measurements of AS-OCT parameters based on the location of the scleral spur are less reproducible than measurements based on the location of Schwalbe’s line.5 Also, intra-device measurements from time-domain devices are also less reproducible than Fourier-domain devices, although all AS-OCT devices demonstrate good to excellent reproducibility.6–10 Inter-device reproducibly of measurements varies depending on the devices being compared. There was poor agreement between the Visante and SL-OCT.7 In contrast, inter-device reproducibility of measurements between the Vistante and CASIA SS-1000 were good for most parameters, except anterior chamber depth and scleral spur angle.11 The CASIA2 (Tomey Corporation, Nagoya, Japan) and Spectralis also demonstrated good inter-device reproducibility, especially among small measurement values.12 Although Fourier-domain devices tend to have good inter-device measurement reproducibility, differences still exist between measurements. Therefore, AS-OCT measurements from different devices should not be considered interchangeable, especially since these devices also differ in their OCT technology, modeling parameters, de-warping algorithms, and assumptions about refractive indices.
Anterior Chamber Angle and Aqueous Humor Outflow Anatomy
Intraocular pressure (IOP), an important risk factor for the development of glaucomatous optic neuropathy, is determined by a balance of aqueous humor production and outflow. Aqueous humor is produced by the ciliary body and secreted into the posterior chamber, where it moves through the pupil into the anterior chamber and exits the eye via the trabecular or uveoscleral outflow pathway (Figure 2).
Figure 2.
Cross-sectional illustration of the conventional aqueous outflow pathway and its structures. Blue arrows indicate normal flow of aqueous.
The trabecular pathway is pressure-sensitive and predominates under physiological conditions, accounting for ~90% of aqueous outflow. In this pathway, aqueous humor filters through the trabecular meshwork, a multi-layered porous structure, into Schlemm’s canal. Schlemm’s canal connects to aqueous veins via 25 – 35 collector channels, from where the aqueous drains into episcleral veins, then into anterior ciliary and superior ophthalmic veins, and ultimately into the cavernous sinus.
Anterior segment optical coherence topography (AS-OCT) imaging provides information about pre-trabecular outflow pathways and can describe the configuration of the anterior chamber angle, either qualitatively or through various quantitative AS-OCT parameters (Figure 3).2,13–17 Quantitative AS-OCT studies of the anterior chamber angle reveal significant anatomical variations along the 360-span of an angle.18 In general, angles are more narrow superiorly and inferiorly and most open temporally and nasally. These anatomical variations may affect the contribution of various trabecular segments to aqueous outflow, especially in angle closure eyes. These findings, coupled with recent advances in imaging technology, have prompted interest in characterizing the anterior chamber angle using a multi-image approach in lieu of a single cross-sectional image acquired along the temporal-nasal meridian.
Figure 3.
Image taken with the Tomey CASIA SS-1000 AS-OCT device. The cornea (C), iris (I), lens (L), scleral spur (SS, yellow arrows) and anterior chamber angle (A) are marked for reference.
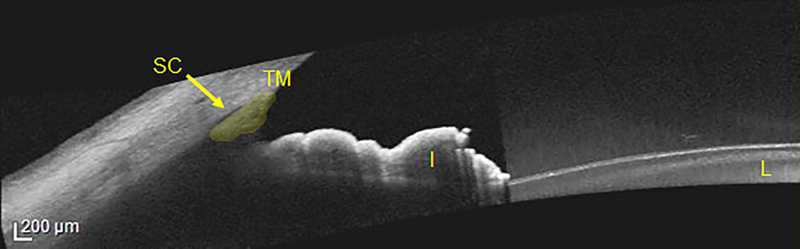
AS-OCT imaging has also been applied to the study of post-trabecular outflow pathways. Schlemm’s canal and collector channels are more easily visible on shorter wavelength spectral-domain OCT devices, such as the Cirrus and Spectralis (Figure 4).19 These devices permits in vivo, 360-degree visualization of first-order aqueous humor outflow pathways. For example, AS-OCT imaging of Schlemm’s canal has been used to confirm that pilocarpine increases its lumen size in eyes with and without glaucoma.20
Figure 4.
Image taken with the Heidelberg Spectralis with anterior segment module. The iris (I), lens (L), trabecular meshwork (TM, highlighted in yellow) and Schlemm’s canal (SC, yellow arrow) are marked for reference.
Clinical Methods for Assessing the Anterior Chamber Angle
Angle assessment has wide clinical applications in glaucoma, ranging from screening for angle closure, to the evaluation of structural causes of angle closure, and assessment of procedure efficacy. As such, there is increased research focused on improving the utility of AS-OCT for the diagnosis, management and monitoring of angle closure disease.
The current reference method for visualizing the structures of the anterior chamber angle is gonioscopy.21 Techniques can be categorized as either direct or indirect, but all gonioscopy methods are contact-based and subjective. In addition, numerous factors at the time of exam such as room light intensity, slit lamp light beam dimensions and pressure applied to the goniolens can all alter the appearance of the angle. Consequently, there is poor reproducibility, both among different examiners and among different sessions with the same examiner.22,23
Ultrasound biomicroscopy (UBM) has been employed to assess anterior segment anatomy in several types of glaucoma. This test uses sound waves that are shorter in wavelength than those used in conventional ocular ultrasonography, leading to reduced penetration through the sclera but increased resolution. UBM is able to provide detailed images of the anterior segment, the posterior chamber and the ciliary body.14,16
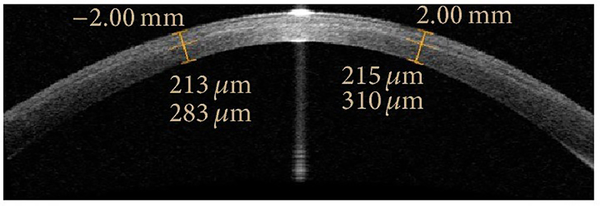
AS-OCT imaging and gonioscopy both provide qualitative assessments of the anterior chamber angle, although descriptive definitions of angle closure differ between the two methods. Angle closure in AS-OCT images is defined as any degree of contact between the anterior iris surface and angle wall anterior to the scleral spur, which lies at the junction of the trabecular meshwork and ciliary body and is defined as the inward protrusion of the sclera where a change in curvature of the corneoscleral junction is observed. This is in contrast to gonioscopy, where angle closure is defined as inability to visualize the pigmented trabecular meshwork. There is limited agreement in angle closure detected by the two methods based on the disparate definitions.24,25 However, there is a strong relationship between gonioscopic angle closure and quantitative AS-OCT measurements of angle width (Figure 5), such as angle opening distance (AOD), angle recess area (ARA), and trabecular-iris space area (TISA). One study suggests that angle opening distance at 750 um anterior to the scleral spur (AOD750) is the most useful angle measurement for identifying gonioscopic angle closure in AS-OCT images.26
Figure 5.
Anterior segment parameters measurable by AS-OCT. AOD; angle opening distance. ARA; angle recess area. TISA; trabecular iris angle. TISA; trabecular iris space area. SSAngle; scleral spur angle. ACD; anterior chamber depth. LV; lens vault. CAD; corneal arcuate distance. ACW; anterior chamber width. PD; pupillary diameter. ACA; anterior chamber area. 500 and 750 denote distance from scleral spur in um.
Very few studies have compared the results of angle measurements between UBM and AS-OCT.2 Dada et al. analyzed 63 eyes using the two methods and concluded that they correlate well.27 AS-OCT offers better resolution and is thus able to provide sharper images of the scleral spur.26 However, the use of infrared light in AS-OCT limits penetration of iris pigment epithelium, resulting in poor visualization of the ciliary body and more posterior structures. Overall, there was a tendency towards smaller measurements of angle and slightly higher measurements of the anterior chamber depth in AS-OCT compared to UBM.27
AS-OCT Imaging and Primary Angle Closure Disease
Angle closure refers to the mechanical blockage of the trabecular meshwork in the anterior chamber drainage angle by the peripheral iris. Primary angle closure disease (PACD) is typically divided based on the following gonioscopic classification system:28
Primary angle closure suspect (PACS), in which the eye is at increased risk of angle closure. PACS is defined as having one or fewer quadrants of the angle open to pigmented trabecular meshwork, without evidence of trabecular meshwork dysfunction or glaucomatous optic neuropathy.
Primary angle closure (PAC), in which exam findings confirm the suspicion of angle closure. PAC is defined as PACS with peripheral anterior synechiae, excessive pigment deposition on the trabecular meshwork, or elevated intraocular pressure.
Primary angle closure glaucoma (PACG), in which the eye has sustained optic nerve damage. PACG is defined as PAC with glaucomatous optic neuropathy
The worldwide prevalence of PACG is estimated to be 16 million.29 While a number of risk factors (race, sex, family history, ocular biometrics, medications) predispose eyes to angle closure, there is currently no reliable method to determine whether at-risk eyes will develop PACG. The clinical standard for assessing angle structures, gonioscopy, exhibits limited efficacy in identifying which patients are at a higher risk for elevated IOP – a prominent feature of PACD – even when performed by experienced clinicians.2,16,30 Recent studies have explored the use of AS-OCT as a quantitative assessment method to detect patients at risk for PACG. These studies have identified ocular biometrics quantifiable by AS-OCT as predictors of PACD. These factors can be classified as either static risk factors, which can be measured on a single AS-OCT image; or dynamic risk factors, which are evoked by structural changes associated with pupillary dilation or constriction.
Static Risk Factors
The strongest and most consistently reported static risk factor is lens vault, defined as the perpendicular distance that separates the anterior pole of the lens from an imaginary horizontal line joining the two scleral spurs.31–34 Larger values of lens vault are suggestive of increased crowding of the anterior chamber by a thick lens. In a study examining angle closure disease in Chinese subjects, Nongpuir et al. noted a significant correlation between angle closure and lens vault. 31 Specifically, after dividing eyes into quartiles based on lens vault, those in the highest quartile were at a 48 times higher risk of angle closure than those in the lowest quartile. This association was further shown to be independent of other angle closure risk factors such as age and gender, as well as other ocular biometrics, such as anterior chamber depth, lens thickness and relative lens position. These findings were corroborated by another study in Japanese subjects, which reported an odds ratio of 24.2 for angle closure when comparing the lowest and highest quartiles of lens vault.32
A number of iris-related AS-OCT parameters have been described as anatomical risk factors for PACD, though studies disagree on whether these associations are statistically significant.33–35 Iris thickness (defined as the largest perpendicular distance along the iris connecting the anterior and posterior iris borders) was found to have an odds ratio of 2.2 to 2.7 for angle closure when compared with normal eyes. Iris curvature (defined as the perpendicular distance between the iris pigment epithelium and an imaginary line connecting the most peripheral and most central points of iris pigment epithelium, at the point of greatest convexity) and iris area (calculated as the cross-sectional area of the full length of the iris) were found to have odds ratios of 0.4 to 2.5 and 1.1 to 2.7, respectively.
A study by Wu et al. further examined the usefulness of AS-OCT biometrics as predictors for PACD.36 Smaller anterior chamber area (defined as the cross-sectional area bounded by endothelium, anterior surface of iris, and anterior surface of lens within the pupil) and smaller anterior chamber volume (calculated by rotating the anterior chamber area 360 degrees around a vertical axis drawn through the midpoint of the anterior chamber area) were found to have odds ratios of 53.2 and 40.2, respectively. This translates into 89.9% sensitivity and 85.5% specificity if anterior chamber area measured by AS-OCT is used as a screening parameter.
Dynamic Risk Factors
Primary dynamic risk factors for PACD revolve around structural changes of the iris with dilation. Studies have shown that iris behavior in the dark versus light differ significantly between PACD and normal eyes. Early studies using AS-OCT showed less decrease in iris area and iris volume with dilation in PACD patients when compared with normal subjects.37–40 Some studies even reported a paradoxical increase in iris volume with dilation in PACD patients, though this has since been shown to be an artifact of the formula used to calculate iris volume, caused by an increase of centroid-to-centroid distance (CCD, the distance between the centers of the nasal and temporal iris masses).37,38,40
More recently, Zhang et al. found that post-dilation, larger, more peripherally distributed irises increase the risk of angle closure.33 They specifically report that PACD subjects showed less loss of iris area per mm of pupillary distance (PD) increase after physiologic dilation (0.18mm/0.13mm respectively) when compared with normal subjects (0.24 mm). Logistic regression analysis confirmed that less iris area loss per mm PD increase was a risk factor for an occludable angle (defined as non-visibility of posterior trabecular meshwork for at least 180 degrees). Furthermore, the change in CCD per mm PD increase was significantly greater in PACD subjects compared with normal subjects. CCD per PD increase was proposed in this study as a measure of iris area redistribution peripherally; the more peripheral the iris area, the higher the ratio.
Together, these studies on static and dynamic biometric risk factors support an expanded role for AS-OCT in the clinical management of angle closure patients. Future directions include automated methods to analyze AS-OCT images and a more detailed delineation of the relationship between angle width and IOP, a key risk factor for PACG.30,41
AS-OCT Imaging and Glaucoma Surgery
Glaucoma surgery encompasses laser, incisional and minimally invasive procedures. These are usually employed in conjunction with medical therapy to control IOP in patients with progressive glaucomatous damage. A role for AS-OCT has been proposed in guiding glaucoma surgeries, assessing the safety of procedures and evaluating treatment outcomes.
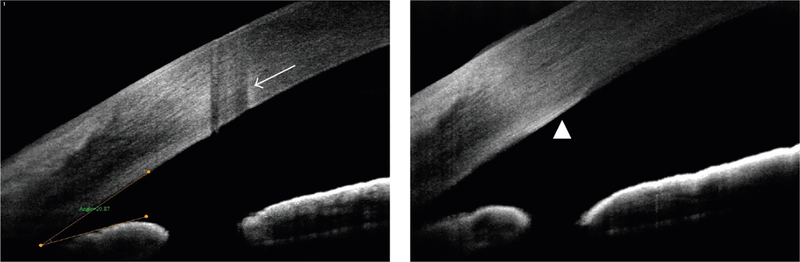
Laser surgery in primary angle closure most frequently consists of laser peripheral iridotomy to widen the angle. This procedure utilizes a Nd:YAG laser to create an opening in the iris as an alternate route of aqueous outflow from the posterior chamber to the anterior chamber. In assessing the effects of this procedure, Lee et al. reported that AOD500, TISA500, and ARA500 changed significantly after laser iridotomy, but anterior chamber angle remained unchanged in some narrow-angle eyes despite iridotomy. 42 Stromal damage and thickened Descemet’s membrane are procedure-related complications that have been reported to be observable on AS-OCT following laser iridotomy (Figure 6).16
Figure 6.
AS-OCT visualization of complications of laser peripheral iridotomy. Left: stromal damage. Right: thickened Descemet’s membrane. Reprinted with permission from Lim.16
The gold standard glaucoma surgery is trabeculectomy, which creates a surgical corneoscleral opening under a partial-thickness scleral flap. This opening serves as a new pathway for aqueous outflow from the anterior chamber to the sub-tenon and subconjunctival spaces, leading to the formation of a bleb. AS-OCT provides detailed visualizations of the trabeculectomy bleband several studies have reported an association of bleb morphology with level of IOP control.43–47 Features of bleb morphology associated with successful IOP lowering include a multi-layered appearance45 and low reflectivity of the bleb wall, presence of episcleral fluid, as well as lower internal reflectivity of the fluid-filled cavity.46,47 With 3-dimensional anterior-segment OCT techniques, it is even possible to precisely identify the filtration opening on the scleral flap margin.44 In the office, AS-OCT could be used to determine which blebs are suitable for needling, to evaluate bleb changes after laser suture lysis and to aid in the planning of bleb revision surgeries.48–50 Furthermore, Hong et al. have shown that AS-OCT can quantify changes in angle structures post procedure, specifically reporting that Schlemm’s canal expands with trabeculectomy.51
Progressive enlargement of the crystalline lens contributes to pupillary block, the primary mechanism contributing to PACD. Cataract extraction in these cases can widen the angle and lower IOP. Thus, cataract surgery may be considered as the primary procedure to relieve angle closure and elevated IOP in patients with PACG.52–54 AS-OCT studies have shown that dilations of Schlemm’s canal are observable after phacoemulsification surgery and correlated with decreases in IOP.55
A number of minimally invasive glaucoma surgeries (MIGS) have been introduced to the management of glaucoma patients over the past decade. MIGS devices have been developed to restore, enhance, or provide an alternative to the eye’s natural aqueous outflow pathways by shunting aqueous from the anterior chamber directly into the Schlemm’s canal, suprachoroidal space or sub-tenon/subconjunctival space. These procedures typically have a better safety profile than traditional glaucoma surgeries, such as trabeculectomy and glaucoma drainage devices, but also tend to be less effective in lowering IOP. AS-OCT has been shown to aid in the assessment of MIGS surgical placements and effects. Saheb et al. have reported that AS-OCT provides adequate visualization of the angle, the supraciliary space and aqueous fluid drainage post implantation of CyPass into the suprachoroidal space.56 Lenzhoffer et al. used AS-OCT to study bleb appearance after ab interno implantation of the XEN gel stent or ab externo trabeculectomy. They noted that while there are differences in morphology between XEN gel stent and trabeculectomy blebs, there remains a correlation between bleb morphology on AS-OCT and IOP or long term treatment success; specifically, the presence of small diffuse cysts are associated with better IOP control while cystic encapsulation portends higher surgical failure rates.57
AS-OCT for Corneal Disorders and Surgery
Keratoconus, diagnosis and treatment
Keratoconus is a bilateral, progressive, non-inflammatory progressive thinning of the cornea, characterized by collagen structure change and decreased corneal rigidity. It typically involves the central two-thirds of the cornea and is a relatively common disorder, with local prevalence of 54 per 100,000, but variable among different parts of the world.58,59 The corneal epithelial cells and basement membrane are disrupted, creating irregularities in epithelial thickness. Stromal thinning occurs, along with endothelial pleomorphism and polymegathism. Although the clinical diagnosis of moderate to advanced keratoconus is typically straightforward, diagnosis can be challenging early in the disease’s course. Current diagnosis of keratoconus relies on Placido-disk-based corneal topography to reveal characteristic signs of the disease. Measurements can be used with predictive indices, such as keratoconus prediction index and KISA% index which relies on measurements of central corneal steepening, inferior-superior dioptric asymmetry, skew percentage, and astigmatism.60 Challenges using topography include difficulty with topography if poor tear film is present, contact lens warpage, or other corneal distortions such as scars and lid artifacts.
AS-OCT has been used to create pachymetry maps to diagnose keratoconus based on asymmetry of the corneal thinning. This technique showed a statistically similar area under the receiver operating characteristic curve (AUC) when compared with standard KISA% index.61 In a similar manner, OCT has also been used to measure epithelial disruption and irregularity in keratoconus with diagnosis based on pattern standard deviation from individual subjects and a normal reference population.62
AS-OCT has also been used to measure the efficacy of collagen cross-linking when treating keratoconus. On biomicroscopy, a demarcation line can be seen, indicating a transition zone between treated and untreated cornea. Deeper demarcation lines correspond with a deeper depth of treatment. AS-OCT has been used to measure the depth of the demarcation line, thereby allowing the clinician to quantitatively assess efficacy of treatment.63,64
Intracorneal rings have been approved for use to reduce irregular astigmatism and myopia associated with keratoconus. However, depth is critical, as shallow depth has been associated with stromal thinning, epithelial breakdown, and ring extrusion. AS-OCT has been used to assess the positioning of intracorneal rings intra- and post-operatively.66,67
Ocular Surface Neoplasia and Surface Lesions
Ocular surface squamous neoplasia (OSSN) is a spectrum of disease with abnormal growth of dysplastic squamous epithelial cells on the surface of the eye. It can vary from conjunctival intraepithelial neoplasia, squamous cell carcinoma, and mucoepidermoid carcinoma. In addition, corneal epithelial dysmaturation, dysplasia and intraepithelial neoplasia fall under the category of OSSN. The gold standard for diagnosis is biopsy and histology, but OCT has been shown as a potential non-invasive technique to assist with diagnosis.
AS-OCT is effective complement to biopsy in diagnosis of OSSN. Ultra-high resolution OCT devices (<5 um resolution) can differentiate OSSN from other conjunctival lesions. Characteristically, OSSN has a thickened and hyper-reflective epithelial layer, and an abrupt transition from normal to abnormal epithelium.68 OSSN has been differentiated from nevi, primary acquired melanosis (PAM), amelanotic melanoma, conjunctival lymphoma, conjunctival amyloidosis, histiocytosis, and pterygium based on physical characteristics and reflective properties assessed by AS-OCT.69–71 However, limitations in AS-OCT include difficulty differentiating invasive from non-invasive carcinoma.69
Ultra-high resolution OCT has been used to ensure adequate treatment and surveillance of medically managed OSSN as it can detect lesions after what appears to be clinical resolution. An average latency between clinical resolution and resolution as seen by OCT was found to be 16 weeks, indicating the potential use for monitoring progression during treatment and surveillance after treatment.68
Corneal Dystrophies
AS-OCT has been applied to assisting clinicians with diagnosis of corneal dystrophies. By locating the depth, character, and extent of lesions, both diagnosis and therapy can be guided by imaging. Vajzovic et al. used a custom 2 um high-resolution AS-OCT to characterize and differentiate corneal dystrophy of Bowman’s layer, macular dystrophy, granular dystrophy, anterior basement dystrophy, Salzmann’s degeneration, Meesmann’s dystrophy, and corneal verticillate.73 Other authors have been able to differentiate Lattice corneal dystrophy as well as epithelial basement membrane dystrophy. 74,75 A summary of select corneal dystrophies and detailed findings on OCT can be found by Siebelmann et al. 75
AS-OCT in Microbial Keratitis
AS-OCT can be used in addition to slit-lamp biomicroscopy for diagnosis and monitoring treatment of microbial keratitis. Advantages include serial standardized exams, quantitative measurements, and qualitative information regarding specific etiologies.76 Konstantopoulos et al. used AS-OCT to monitor corneal thickness and infiltrate during treatment. They demonstrated that within 3 days, corneal thickness as well as infiltrate thickness decreased and the rate of change depended on length of treatment. Soliman et. al used AS-OCT to define morphologic patterns of bacterial and fungal keratitis. Yamazaki et al. were able to evaluate radial keratoneuritis in early stage Acanthamoeba Keratitis, as well as monitor resolution with treatment.77.
AS-OCT imaging for LASIK
AS-OCT has been used to evaluate biometrics of the cornea, such as the anterior and posterior cornea surface before and after LASIK. This is important for IOL planning, post-LASIK enhancement and monitoring for proper healing and complications. AS-OCT has been shown to have higher reliability than Scheimpflug imaging for post-LASIK corneal measurements.78 It has been used to evaluate flap dimensions and flap related complications, such as epithelial ingrowth, microstriae, macrostriae, and interface debris. 79 In addition, subtle clinical findings, such as minimally displaced LASIK flap, may be better visualized and diagnosed with AS-OCT.80
AS-OCT Imaging and Cataract Surgery
AS-OCT has been used to improve the accuracy of intraocular lens (IOL) power calculations for cataract surgery. Many of the current measurements (automated keratometry, Placido-ring topography) of corneal power measure the anterior corneal surface and assume the posterior corneal curvature based on a fixed-ratio relationship. After refractive surgery, alterations to the eye increase the risk of error in standard IOL calculations. First, there is an incorrect estimation of the effective lens position. Second, there is incorrect corneal refractive power estimate due to incorrect anterior corneal refractive power measurement and changes to the ratio of anterior to posterior radius of curvature of the cornea. Standard techniques to correct for refractive surgery include knowledge of pre-refractive surgery K values (not always recorded), or the use of rigid plano contact lenses (difficult with corneal opacities). However, direct measurement of both anterior and posterior cornea radii of curvature can be performed with AS-OCT with or without corneal opacities and can be used to improve IOL calculations.
A combination of biometry and AS-OCT has been used to create an IOL calculation which was shown to be comparable to standard IOL formulae.81 In addition, an OCT based IOL calculator for eyes with LASIK and PRK was shown to have smaller or similar prediction error to current techniques, leading to its recent adoption into the ASCRS IOL calculator.82 AS-OCT imaging of the lens has also been used to measure cataract density by evaluating the intensity of OCT signal in the lens nucleus, which is correlated with grades based on the Lens Opacification Classification System III.83 In addition, AS-OCT imaging has been used to identify eyes with posterior polar cataracts at risk for posterior capsule rupture.84
OCT technology is integrated into many modern femto-second laser systems and operating microscopes, which facilitates its use for intraoperative guidance during cataract surgery. For example, intraoperative OCT has been used with cataract surgery, helping surgeons visualize wound morphology and closure, lens hydrodissection, depth of trenching during phacoemulsification, and final position of the IOL.85
Conclusion
AS-OCT is a versatile non-invasive in vivo imaging technique that has gained popularity among clinicians and researchers. AS-OCT imaging facilitates qualitative and quantitative studies of the ocular surface, cornea, lens and anterior chamber angle. It has applications in the characterization of anatomical structures, diagnosis and staging of disease, and assessment of treatment efficacy. However, its adoption in routine clinical care significantly lags behind OCT studies of the posterior segment. Therefore, further research is needed that demonstrates and validates the benefit of novel OCT-based methods compared to current clinical standards of care in the management of anterior segment diseases.
Figure 7:
AS-OCT of a demarcation line after corneal collagen crosslinking. Reprinted with permission from Ozgurhan et al.65
Figure 8.
AS-OCT of corneal intraepithelial neoplasia showing a line (arrow) between normal and neoplastic epithelium. Reprinted with permission from Ong et al.72
Figure 9.
AS-OCT of a patient with granular dystrophy, demonstrating the depth of the corneal lesions. Reprinted with permission from Han et al.14
REFERENCES
- 1.Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical Coherence Tomography: An Emerging Technology for Biomedical Imaging and Optical Biopsy. Neoplasia. 2000;2(1–2):9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang M, Baskaran M, Werkmeister RM, et al. Anterior segment optical coherence tomography. Prog Retin Eye Res. 2018;66:132–156. [DOI] [PubMed] [Google Scholar]
- 3.Wang SB, Cornish EE, Grigg JR, McCluskey PJ. Anterior segment optical coherence tomography and its clinical applications: a review. Clin Exp Optom. January 2019. [DOI] [PubMed] [Google Scholar]
- 4.Jiao H, Hill LJ, Downie LE, Chinnery HR. Anterior segment optical coherence tomography: its application in clinical practice and experimental models of disease. Clin Exp Optom. October 2018. [DOI] [PubMed] [Google Scholar]
- 5.Cheung CY, Zheng C, Ho CL, et al. Novel anterior-chamber angle measurements by high-definition optical coherence tomography using the Schwalbe line as the landmark. Br J Ophthalmol. 2011;95(7):955–959. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Leung CKS, Cheung CYL, et al. Repeatability and reproducibility of anterior chamber angle measurement with anterior segment optical coherence tomography. Br J Ophthalmol. 2007;91(11):1490–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung CKS, Li H, Weinreb RN, et al. Anterior chamber angle measurement with anterior segment optical coherence tomography: A comparison between slit lamp OCT and visante OCT. Investig Ophthalmol Vis Sci. 2008;49(8):3469–3474. [DOI] [PubMed] [Google Scholar]
- 8.Pan X, Marion K, Maram J, et al. Reproducibility of Anterior Segment Angle Metrics Measurements Derived From Cirrus Spectral Domain Optical Coherence Tomography. J Glaucoma. 2015;24(5):e47–e51. [DOI] [PubMed] [Google Scholar]
- 9.Qin B, Francis BA, Li Y, et al. Anterior chamber angle measurements using Schwalbe’s line with high-resolution fourier-domain optical coherence tomography. J Glaucoma. 2013;22(9):684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akil H, Dastiridou A, Marion K, Francis B, Chopra V. Repeatability, reproducibility, agreement characteristics of 2 SD-OCT devices for anterior chamber angle measurements. Can J Ophthalmol. 2017;52(2):166–170. [DOI] [PubMed] [Google Scholar]
- 11.Aptel F, Chiquet C, Gimbert A, et al. Anterior Segment Biometry Using Spectral-Domain Optical Coherence Tomography. J Refract Surg. 2014;30(5):354–360. [DOI] [PubMed] [Google Scholar]
- 12.Xu BY, Mai DD, Penteado RC, Saunders L, Weinreb RN. Reproducibility and Agreement of Anterior Segment Parameter Measurements Obtained Using the CASIA2 and Spectralis OCT2 Optical Coherence Tomography Devices. J Glaucoma. 2017;26(11):974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers JE, Seif MW. Global perspective of legal abortion - Trends analysis and accessibility. Best Pract Res Clin Obstet Gynaecol. 2010;24(4):457–466. [DOI] [PubMed] [Google Scholar]
- 14.Han SB, Liu YC, Noriega KM, Mehta JS. Applications of Anterior Segment Optical Coherence Tomography in Cornea and Ocular Surface Diseases. J Ophthalmol. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Jhanji V, Dorairaj S, Liu A, Lam DSC, Leung CK. Anterior segment optical coherence tomography and its clinical applications in glaucoma. J Curr Glaucoma Pract. 2012;6(2):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SH. Clinical applications of anterior segment optical coherence tomography. J Ophthalmol. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos JLB, Li Y, Huang D. Clinical and research applications of anterior segment optical coherence tomography - A review. Clin Exp Ophthalmol. 2009;37(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu BY, Israelsen P, Pan BX, Wang D, Jiang X, Varma R. Benefit of measuring anterior segment structures using an increased number of optical coherence tomography images: The Chinese American Eye Study. Investig Ophthalmol Vis Sci. 2016;57(14):6313–6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang AS, Belghith A, Dastiridou A, Chopra V, Zangwill LM, Weinreb RN. Automated circumferential construction of first-order aqueous humor outflow pathways using spectral-domain optical coherence tomography. J Biomed Opt. 2017;22(6):66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skaat A, Rosman MS, Chien JL, et al. Effect of pilocarpine hydrochloride on the schlemm canal in healthy eyes and eyes with open-angle glaucoma. In: JAMA Ophthalmology. Vol 134. 2016:976–981. [DOI] [PubMed] [Google Scholar]
- 21.Porporato N, Baskaran M, Aung T. Role of anterior segment optical coherence tomography in angle-closure disease: a review. Clin Exp Ophthalmol. 2018;46(2):147–157. [DOI] [PubMed] [Google Scholar]
- 22.Campbell P, Redmond T, Agarwal R, Marshall LR, Evans BJW. Repeatability and comparison of clinical techniques for anterior chamber angle assessment. Ophthalmic Physiol Opt. 2015;35(2):170–178. [DOI] [PubMed] [Google Scholar]
- 23.Rigi M, Bell NP, Lee DA, et al. Agreement between Gonioscopic Examination and Swept Source Fourier Domain Anterior Segment Optical Coherence Tomography Imaging. J Ophthalmol. 2016;2016:1727039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu BY, Pardeshi AA, Burkemper B, et al. Quantitative Evaluation of Gonioscopic and EyeCam Assessments of Angle Dimensions Using Anterior Segment Optical Coherence Tomography. Transl Vis Sci Technol. 2018;7(6):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolan WP, See JL, Chew PTK, et al. Detection of Primary Angle Closure Using Anterior Segment Optical Coherence Tomography in Asian Eyes. Ophthalmology. 2007;114(1):33–39. [DOI] [PubMed] [Google Scholar]
- 26.Narayanaswamy A, Sakata LM, He MG, et al. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: An anterior segment OCT study. Arch Ophthalmol. 2010;128(10):1321–1327. [DOI] [PubMed] [Google Scholar]
- 27.Dada T, Sihota R, Gadia R, Aggarwal A, Mandal S, Gupta V. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cataract Refract Surg. 2007;33(5):837–840. [DOI] [PubMed] [Google Scholar]
- 28.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. [DOI] [PubMed] [Google Scholar]
- 30.Xu BY, Burkemper B, Lewinger JP, et al. Correlation Between Intraocular Pressure and Angle Configuration Measured by Optical Coherence Tomography: The Chinese American Eye Study. Ophthalmol Glaucoma. 2018;1:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in chinese subjects with angle closure. Ophthalmology. 2011;118(3):474–479. [DOI] [PubMed] [Google Scholar]
- 32.Ozaki M, Nongpiur ME, Aung T, He M, Mizoguchi T. Increased lens vault as a risk factor for angle closure: Confirmation in a Japanese population. Graefe’s Arch Clin Exp Ophthalmol. 2012;250(12):1863–1868. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Li SZ, Li L, He MG, Thomas R, Wang NL. Dynamic iris changes as a risk factor in primary angle closure disease. Investig Ophthalmol Vis Sci. 2016;57(1):218–226. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Sakata LM, Friedman DS, et al. Quantitative Iris Parameters and Association with Narrow Angles. Ophthalmology. 2010;117(1):11–17. [DOI] [PubMed] [Google Scholar]
- 35.Wang BS, Narayanaswamy A, Amerasinghe N, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011;95(1):46–50. [DOI] [PubMed] [Google Scholar]
- 36.Wu RY, Nongpiur ME, He MG, et al. Association of narrow angles with anterior chamber area and volume measured with anterior-segment optical coherence tomography. Arch Ophthalmol. 2011;129(5):569–574. [DOI] [PubMed] [Google Scholar]
- 37.Aptel F, Chiquet C, Beccat S, Denis P. Biometric evaluation of anterior chamber changes after physiologic pupil dilation using Pentacam and anterior segment optical coherence tomography. Investig Ophthalmol Vis Sci. 2012;53(7):4005–4010. [DOI] [PubMed] [Google Scholar]
- 38.Aptel F, Denis P. Optical Coherence Tomography Quantitative Analysis of Iris Volume Changes after Pharmacologic Mydriasis. Ophthalmology. 2010;117(1):3–10. [DOI] [PubMed] [Google Scholar]
- 39.Quigley HA, Silver DM, Friedman DS, et al. Iris cross-sectional area decreases with pupil dilation and its dynamic behavior is a risk factor in angle closure. J Glaucoma. 2009;18(3):173–179. [DOI] [PubMed] [Google Scholar]
- 40.Seager FE, Jefferys JL, Quigley HA. Comparison of dynamic changes in anterior ocular structures examined with anterior segment optical coherence tomography in a cohort of various origins. Investig Ophthalmol Vis Sci. 2014;55(3):1672–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong RS, Sakata LM, Narayanaswamy AK, et al. Relationship between intraocular pressure and angle configuration: An anterior segment OCT study. Investig Ophthalmol Vis Sci. 2013;54(3):1650–1655. [DOI] [PubMed] [Google Scholar]
- 42.Lee KS, Sung KR, Kang SY, Cho JW, Kim DY, Kook MS. Residual anterior chamber angle closure in narrow-angle eyes following laser peripheral iridotomy: Anterior segment optical coherence tomography quantitative study. Jpn J Ophthalmol. 2011;55(3):213–219. [DOI] [PubMed] [Google Scholar]
- 43.Savini G, Zanini M, Barboni P. Filtering blebs imaging by optical coherence tomography. Clin Exp Ophthalmol. 2005;33(5):483–489. [DOI] [PubMed] [Google Scholar]
- 44.Inoue T, Matsumura R, Kuroda U, Nakashima KI, Kawaji T, Tanihara H. Precise identification of filtration openings on the scleral flap by three-dimensional anterior segment optical coherence tomography. Investig Ophthalmol Vis Sci. 2012;53(13):8288–8294. [DOI] [PubMed] [Google Scholar]
- 45.Nakano N, Hangai M, Nakanishi H, et al. Early trabeculectomy bleb walls on anterior-segment optical coherence tomography. Graefe’s Arch Clin Exp Ophthalmol. 2010;248(8):1173–1182. [DOI] [PubMed] [Google Scholar]
- 46.Pfenninger L, Schneider F, Funk J. Internal reflectivity of filtering blebs versus intraocular pressure in patients with recent trabeculectomy. Investig Ophthalmol Vis Sci. 2011;52(5):2450–2455. [DOI] [PubMed] [Google Scholar]
- 47.Tominaga A, Miki A, Yamazaki Y, Matsushita K, Otori Y. The assessment of the filtering bleb function with anterior segment optical coherence tomography. J Glaucoma. 2010;19(8):551–555. [DOI] [PubMed] [Google Scholar]
- 48.Guthoff R, Guthoff T, Hensler D, Grehn F, Klink T. Bleb needling in encapsulated filtering blebs: Evaluation by optical coherence tomography. Ophthalmologica. 2010;224(4):204–208. [DOI] [PubMed] [Google Scholar]
- 49.Sng CCA, Singh M, Chew PTK, et al. Quantitative assessment of changes in trabeculectomy blebs after laser suture lysis using anterior segment coherence tomography. J Glaucoma. 2012;21(5):313–317. [DOI] [PubMed] [Google Scholar]
- 50.Kim WK, Seong GJ, Lee CS, Kim YG, Kim CY. Anterior segment optical coherence tomography imaging and histopathologic findings of an overhanging filtering bleb. Eye. 2008;22(12):1520–1522. [DOI] [PubMed] [Google Scholar]
- 51.Hong J, Yang Y, Wei A, et al. Schlemm’s canal expands after trabeculectomy in patients with primary angle-closure glaucoma. Investig Ophthalmol Vis Sci. 2014;55(9):5637–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harasymowycz PJ, Papamatheakis DG, Ahmed I, et al. Phacoemulsification and goniosynechialysis in the management of unresponsive primary angle closure. J Glaucoma. 2005;14(3):186–189. [DOI] [PubMed] [Google Scholar]
- 53.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: The ocular hypertension treatment study. Ophthalmology. 2012;119(9):1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang ZM, Niu Q, Nie Y, Zhang J. Reduction of intraocular pressure and improvement of vision after cataract surgeries in angle closure glaucoma with concomitant cataract patients. Int J Clin Exp Med. 2015;8(9):16557–16563. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Z, Zhu X, He W, Jiang C, Lu Y. Schlemm’s canal expansion after uncomplicated phacoemulsification surgery: An optical coherence tomography study. Investig Ophthalmol Vis Sci. 2016;57(15):6507–6512. [DOI] [PubMed] [Google Scholar]
- 56.Saheb H, Ianchulev T, Ahmed IK. Optical coherence tomography of the suprachoroid after CyPass Micro-Stent implantation for the treatment of open-angle glaucoma. Br J Ophthalmol. 2014;98(1):19–23. [DOI] [PubMed] [Google Scholar]
- 57.Lenzhofer M, Strohmaier C, Hohensinn M, et al. Longitudinal bleb morphology in anterior segment OCT after minimally invasive transscleral ab interno Glaucoma Gel Microstent implantation. Acta Ophthalmologica. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–273. [DOI] [PubMed] [Google Scholar]
- 59.Gokhale N Epidemiology of keratoconus. Indian J Ophthalmol. 2013;61(8):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Espandar L, Meyer J. Keratoconus: Overview and update on treatment YR - 2010/1/1. Middle East Afr J Ophthalmol. 2010;(1 UL-http://www.meajo.org/article.asp?issn=0974-9233;year=2010;volume=17;issue=1;spage=15;epage=20;aulast=Espandar;t=5):15 OP-20 VO-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Meisler DM, Tang M, et al. Keratoconus Diagnosis with Optical Coherence Tomography Pachymetry Mapping. Ophthalmology. 2008;115(12):2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119(12):2425–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kymionis GD, Tsoulnaras KI, Grentzelos MA, et al. Corneal stroma demarcation line after standard and high-intensity collagen crosslinking determined with anterior segment optical coherence tomography. J Cataract Refract Surg. 2014;40(5):736–740. [DOI] [PubMed] [Google Scholar]
- 64.Doors M, Tahzib NG, Eggink FA, Berendschot TTJM, Webers CAB, Nuijts RMMA. Use of Anterior Segment Optical Coherence Tomography to Study Corneal Changes After Collagen Cross-linking. Am J Ophthalmol. 2009;148(6). [DOI] [PubMed] [Google Scholar]
- 65.Ozgurhan EB, Sezgin Akcay BI, Yildirim Y, Karatas G, Kurt T, Demirok A. Evaluation of corneal stromal demarcation line after two different protocols of accelerated corneal collagen cross-linking procedures using anterior segment optical coherence tomography and confocal microscopy. J Ophthalmol. 2014;2014:981893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai MM, Tang M, Andrade EMM, et al. Optical coherence tomography to assess intrastromal corneal ring segment depth in keratoconic eyes. J Cataract Refract Surg. 2006;32(11):1860–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehlers JP, Dupps WJ, Kaiser PK, et al. The prospective intraoperative and perioperative ophthalmic imaging with optical CoherEncE TomogRaphy (PIONEER) study: 2-year results. Am J Ophthalmol. 2014;158(5):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kolobova EV, Dvorianskii SA, Tsirkin VI. Otsenka beta-adrenoreaktivnosti éritrotsitov u rozhaiushchikh zhenshchin. Fiziol Cheloveka. 1998;24(3):134–142. [PubMed] [Google Scholar]
- 69.Esser B, Noack R, Schmidt G. Verfahrensentwicklung an Sm-Ofen Und Mikroprozessorsteuerung. Neue Hutte. 1987;32(6):219–223. [Google Scholar]
- 70.Shousha MA, Karp CL, Canto AP, et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology. 2013;120(5):883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nanji AA, Sayyad FE, Galor A, Dubovy S, Karp CL. High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology. Ocul Surf. 2015;13(3):226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ong SS, Vora GK, Gupta PK. Anterior Segment Imaging in Ocular Surface Squamous Neoplasia. J Ophthalmol. 2016;2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vajzovic LM, Karp CL, Haft P, et al. Ultra high-resolution anterior segment optical coherence tomography in the evaluation of anterior corneal dystrophies and degenerations. Ophthalmology. 2011;118(7):1291–1296. [DOI] [PubMed] [Google Scholar]
- 74.Nowinska AK, Teper SJ, Janiszewska DA, et al. Comparative Study of Anterior Eye Segment Measurements with Spectral Swept-Source and Time-Domain Optical Coherence Tomography in Eyes with Corneal Dystrophies. Biomed Res Int. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siebelmann S, Scholz P, Sonnenschein S, et al. Anterior segment optical coherence tomography for the diagnosis of corneal dystrophies according to the IC3D classification. Surv Ophthalmol. 2018;63(3):365–380. [DOI] [PubMed] [Google Scholar]
- 76.Konstantopoulos A, Kuo J, Anderson D, Hossain P. Assessment of the Use of Anterior Segment Optical Coherence Tomography in Microbial Keratitis. Am J Ophthalmol. 2008;146(4):534–542.e2. [DOI] [PubMed] [Google Scholar]
- 77.Yamazaki N, Kobayashi A, Yokogawa H, et al. In vivo imaging of radial keratoneuritis in patients with acanthamoeba keratitis by anterior-segment optical coherence tomography. Ophthalmology. 2014;121(11):2153–2158. [DOI] [PubMed] [Google Scholar]
- 78.Chan TCY, Biswas S, Yu M, Jhanji V. Longitudinal evaluation of cornea with swept-source optical coherence tomography and Scheimpflug imaging before and after lasik. Med (United States). 2015;94(30):e1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdelazeem K, Sharaf M, Saleh MGA, Fathalla AM, Soliman W. Relevance of Swept-Source Anterior Segment Optical Coherence Tomography for Corneal Imaging in Patients With Flap-Related Complications After LASIK. Cornea. 2019;38(1):93–97. [DOI] [PubMed] [Google Scholar]
- 80.Rosas Salaroli CH, Li Y, Huang D. High-resolution optical coherence tomography visualization of LASIK flap displacement. J Cataract Refract Surg. 2009;35(9):1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang M, Li Y, Huang D. An intraocular lens power calculation formula based on optical coherence tomography: a pilot study. … Refract Surg (Thorofare, NJ 1995). 2010;26:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L, Tang M, Huang D, Weikert MP, Koch DD. Comparison of newer intraocular lens power calculation methods for eyes after corneal refractive surgery. Ophthalmology. 2015;122(12):2443–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong AL, Leung CKS, Weinreb RN, et al. Quantitative assessment of lens opacities with anterior segment optical coherence tomography. Br J Ophthalmol. 2009;93(1):61–65. [DOI] [PubMed] [Google Scholar]
- 84.Chan TCY, Li EYM, Yau JCY. Application of anterior segment optical coherence tomography to identify eyes with posterior polar cataract at high risk for posterior capsule rupture. J Cataract Refract Surg. 2014;40(12):2076–2081. [DOI] [PubMed] [Google Scholar]
- 85.Das S, Kummelil MK, Kharbanda V, et al. Microscope Integrated Intraoperative Spectral Domain Optical Coherence Tomography for Cataract Surgery: Uses and Applications. Curr Eye Res. 2016;41(5):643–652. [DOI] [PubMed] [Google Scholar]