Abstract
Background and Aim:
Reconstruction of interdental papillae (IDP) is among the most difficult periodontal therapy. Papillary recession is multifactorial, and several surgical, nonsurgical, and minimally invasive techniques have been suggested. The purpose of this study was to evaluate the clinical application of injectable hyaluronic acid (HA) gel for the reconstruction of IDP in Nordland and Tarnow's Class I and II papillary recession cases.
Materials and Methods:
In the present in vivo clinical trial, 7 patients (2 males, 5 females) with 25 defects were selected. A volume of 0.2 ml HA gel was injected at the respective areas and massaged for 2–3 min. Photographs were obtained, and the assessment of the data was performed clinically (CP-GM, interproximal width [IPW]) and by Image analysis software (black triangle height [BTH], black triangle width [BTW]). Comparison of mean values was performed using the analysis of variance, followed by Post hoc Bonferroni test. Value of P ≤ 0.05 was considered statistically significant.
Results:
Application of HA gel for the reconstruction of IDP was successful in 6 months. CP-GM, BTH, IPW, and BTW showed a statistically significant difference from baseline to 3 and 6 months interval (P = 0.01). Post hoc Bonferroni test for CP-GM, BTH, BTW, and IPW revealed a statistically significant difference from baseline to 3 months (P ≤ 0.05) and 6 months (P ≤ 0.05) and a nonsignificant difference at 3–6 months (P ≥ 0.05).
Conclusion:
Injectable HA gel is a promising minimally invasive therapy for enhancing papillary esthetics.
Keywords: Hyaluronic acid filler, image analysis method, interdental papilla, minimally invasive technique, reconstruction of papilla, regeneration
INTRODUCTION
The existence of interdental papilla (IDP) and healthy gingiva harmonized with natural dentition plays a critical role to protect the periodontal structure and maintain periodontal health. The presence of papillae in the maxillary anterior region is the key of esthetic; therefore, an open gingival embrasure creates a diverse array of complications such as food impaction, increase plaque accumulation, lack of self-cleansing action and consequently leads toward periodontal disease. It also helps to maintain the resonance of the voice, and loss of it creates problems in phonetics as well. All these clubbed together can lead to a lack of self-esteem in public, if left untreated. Loss of IDP is multifactorial, and treatment needs a holistic approach concerning the correction of causative factors. Regeneration of IDP, lost from either disease or previous periodontal therapy, is among the most difficult periodontal regenerative treatment, due to its peculiarity of blood supply.[1,2] Over the decades, many surgical and nonsurgical techniques have been proposed.[3] In surgical techniques, till date, autogenous sub epithelial, connective tissue graft remains gold standard and provides predictability, long term stability, and superior esthetics besides its disadvantages of the surgically exposed donor site.[4] Hence, we had focused on minimally invasive periodontal therapy as a safer and convenient therapeutic approach. Becker et al., in 2010, first used injectable hyaluronic acid (HA) filler to treat papillary recession and showed a promising result.[5] The aim of this present study was to evaluate the efficacy of injectable HA filler for the reconstruction of IDP as well as to evaluate its handling property.
MATERIALS AND METHODS
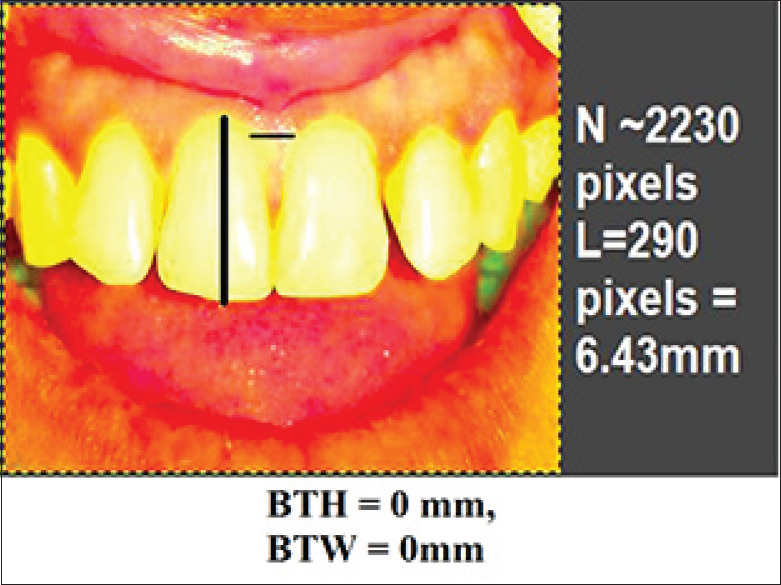
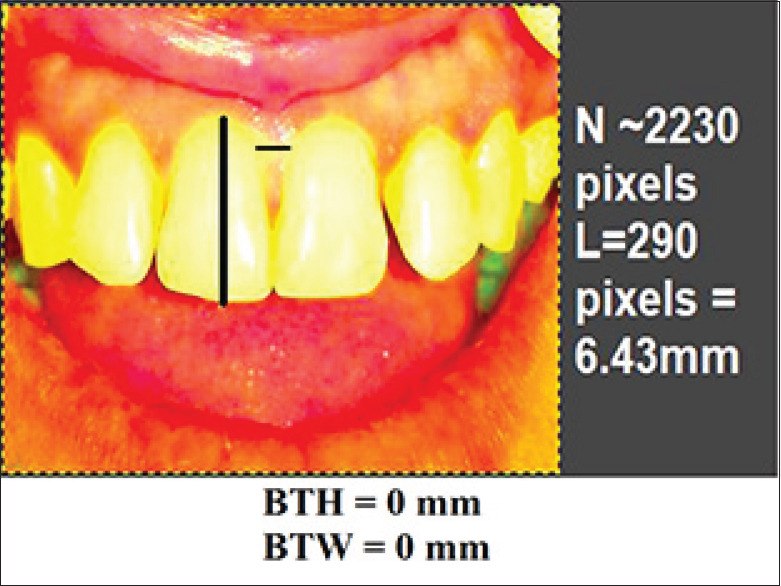
In this in vivo clinical trial, treatment of Nordland's Class I and II papillary recession in maxillary anterior dentition were performed with 2% injectable HA filler.[6] Total 7 patients (2 males, 5 females) with 25 defects were selected from the outpatient department with a mean age 30.96 years. All the patients were in good health, no radiographic evidence of interdental bone loss, pocket depth ≤4 mm, good plaque control and tooth mobility score 0. Patient with systemic disease, blood disorders, pregnant or lactating mothers and tobacco users were excluded. Informed consent was signed those fulfilled the inclusion criteria and agreed to participate voluntarily after a thorough explanation of the nature, risk, and benefit of the clinical investigations and associated procedure. The University Ethical Committee approved the consent form and experimental protocol (IEC No.IEC/MDCRC/2017–18/001). A thorough plaque control program was initiated 3 weeks before procedure, including oral hygiene instruction, patient education, and motivation. For clinical measurements, a prefabricated standardized acrylic stent and the University of North Carolina-15 probe was used to measure the papillary recession height, i.e., distance from contact point of teeth to gingival margin (CP-GM) and interproximal width (IPW) i.e., the width of recession at the gingival margin (IPW) at the nearest millimeters [Figures 1-3]. Photographic analysis was performed by using the Image analysis software-GNU Image Manipulation Program (GIMP), a free raster graphics editor as described by Becker et al.[5] The contrast was increased by using GIMP level tool in such a way that the dark area on the photograph corresponding to the deficient papillae became completely black, while the rest of the image turned white. A software program counted the number of black pixels in a raster image, and pixels were converted to a physical length. By running this program with the increased contrast version of the photograph as input, the number of pixels in the papillae deficient area was determined. The physical size corresponding to a pixel is different on different photographs. To compare the absence of papillae from different photographs on the same patient, pixels were converted to a physical length. This was done in GIMP by measuring the size in pixels of the reference object in the image (using GIMP's measure tool). The reference distances used were the width of a central incisor from mesial to distal and distance between marks on the ruler's image. This was repeated for each patient, measuring the teeth adjacent to the treated site. This was done in GIMP by measuring the size in pixels of the reference object in the image. This program predefined the height as the difference between the maximum and minimum denoted as y coordinates of the pixels within a designated area, while the width was denoted as x coordinates. The reference distances used were the width of a central incisor from mesial to distal. The portion of the black triangle that was measured and demarcated on the image and black triangle height (BTH) and black triangle width (BTW) were automatically converted into millimeters[Figure 4].
Figure 1.
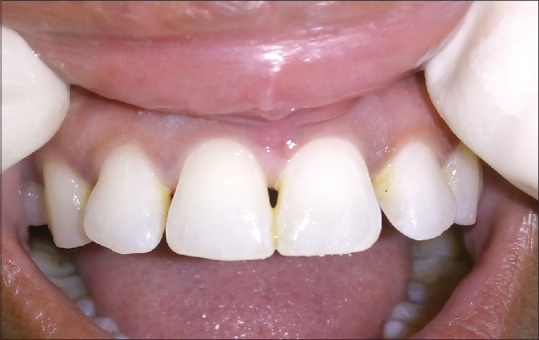
Preoperative view of papillary recession between 11 and 21
Figure 3.
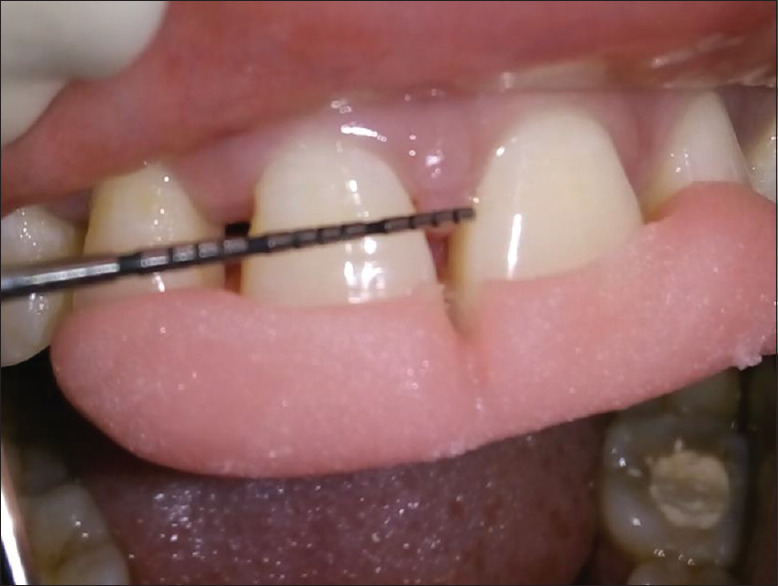
Preoperative measurement of interproximal width (interproximal width)
Figure 4.
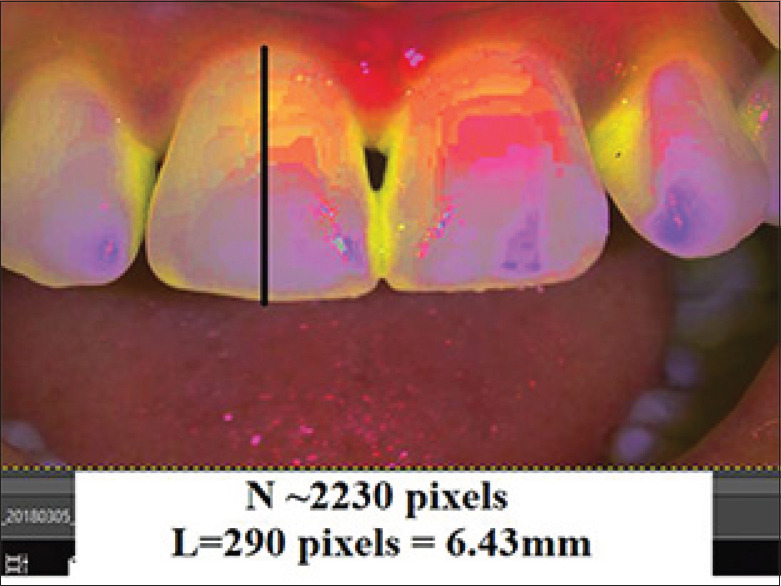
Preoperative photographic measurement of black triangle height and Black triangle width
Figure 2.
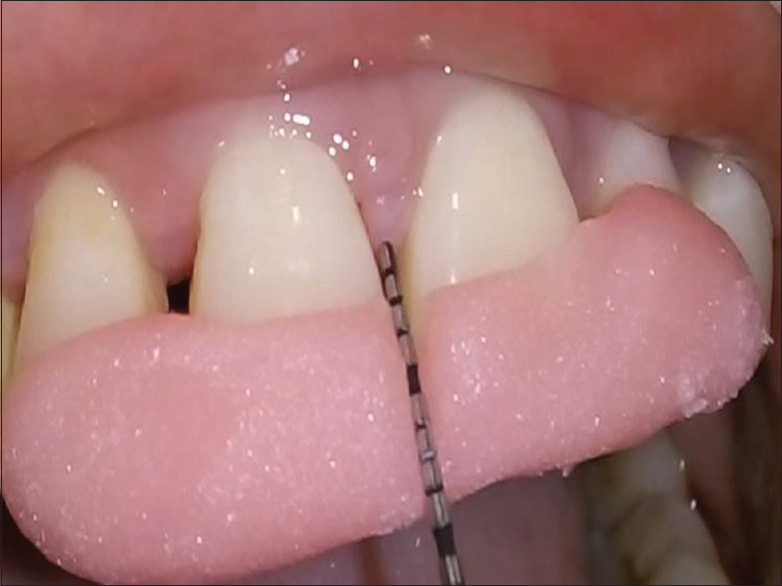
Preoperative measurement of papillary recession height (CP-GM)
A commercially available pure cross-linked mild type, 20 mg/ml concentrated, 400 μm (particle size), was obtained from GENOSS® (MONALISA, Genoss Co. Ltd., Gyeonggi R&DB Center IF, Suwon-si Yeongtong-gu, Gyeonggi-do, Korea) which was available in 1.0 ml syringe for clinical use. 0.2 ml of HA filler (20 mg/ml concentration) was injected at the respective sites 2–3 mm apical to the coronal tip of papillae with a 23G × 25 mm intraoral injection needle at a 45° angle [Figure 5]. The area was gently massaged for 2–3 min to ensure the uniform distribution of filler [Figure 6]. Postoperative instructions to follow a soft diet and not to attempt to incise with their anterior teeth were emphasized. A periodontal maintenance program weekly for the first 4 weeks and then monthly until the end of the study period was performed. Clinical measurements were recorded at the 3rd [Figures 7-9] and 6th month for evaluation [Figures 10-12].
Figure 5.
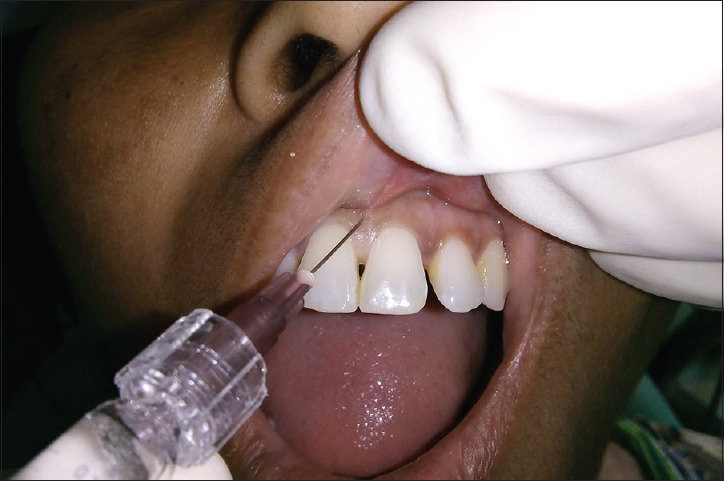
Injection of hyaluronic acid filler was given
Figure 6.

Massage the area with adequate pressure
Figure 7.
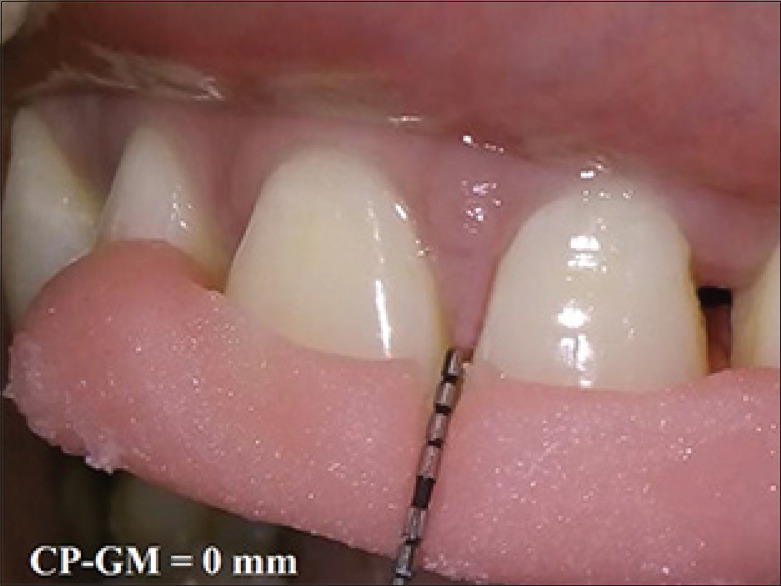
Postoperative measurement of CP-GM at 3 month
Figure 9.
Postoperative measurement of black triangle height and black triangle width at 3 month
Figure 10.
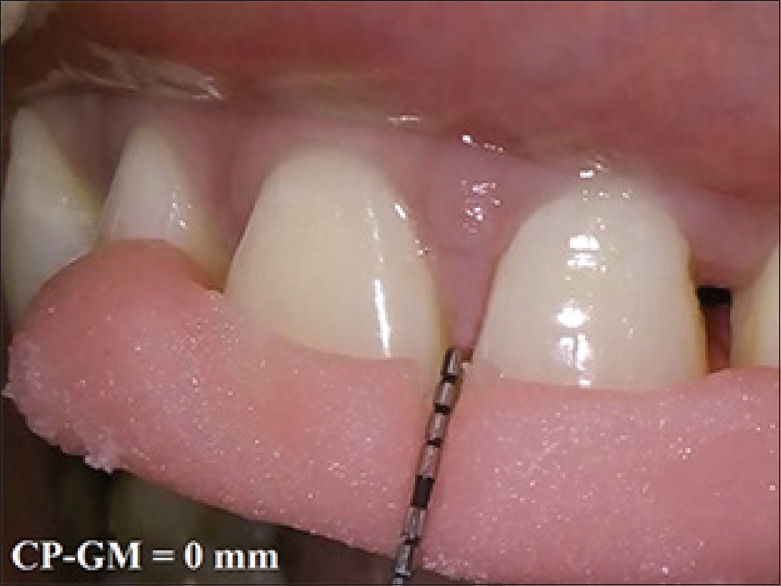
Postoperative measurement of CP-GM at 6 month
Figure 12.
Postoperative measurement of black triangle height and black triangle width at 6 month
Figure 8.
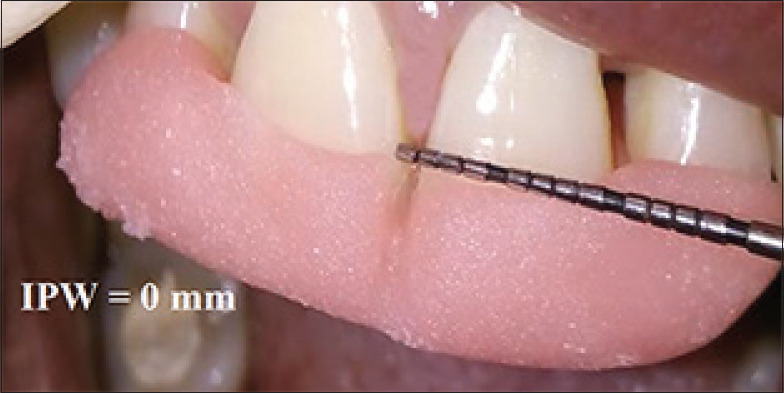
Postoperative measurement of interproximal width at 3 month
Figure 11.
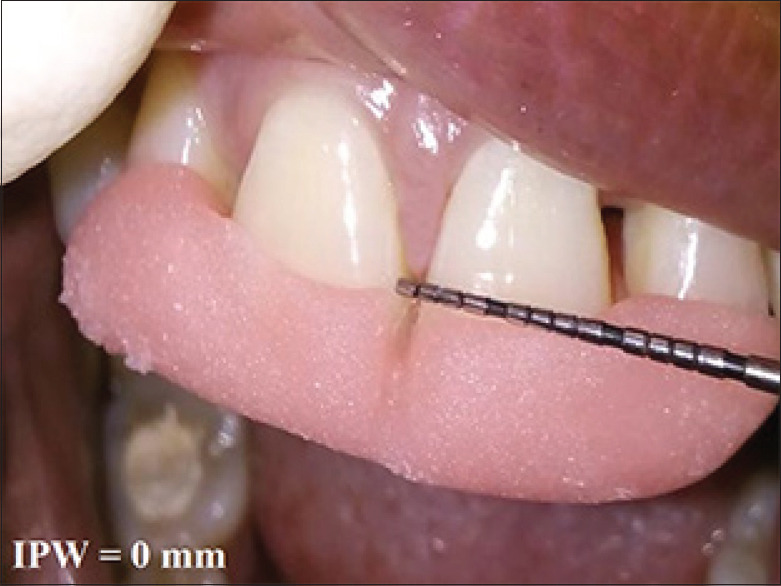
Postoperative measurement of interproximal width at 6 month
Statistical data analysis
The data obtained were fed into Microsoft Excel 2000 package, and statistical analysis was carried out using SPSS software version 20.0 for windows (IBM, Developed by Norman H. Nie, Dale H. Bent, C. Hadlai Hull, Chicago, USA). The mean and standard deviation values of all the parameters were calculated at baseline, 3, and 6 months postoperatively. Probability value, i.e., P ≤ 0.05 was considered statistically significant, P ≤ 0.01 was considered highly significant. Comparison of mean values was performed using analysis of variance followed by Post hoc Bonferroni test.
RESULTS
The result of this study showed improvement in the regeneration of recession defects at 3 and 6 months [Figures 13 and 14]. CP-GM showed a statistically significant difference from baseline to 3 and 6 months interval (P = 0.01) [Table 1]. BTH correlated with the finding of CP-GM and showed a statistically significant difference from baseline to 3 and 6 months results (P = 0.01) [Table 1]. IPW also showed a statistically significant difference from baseline to 3 and 6 months (P = 0.01) [Table 1]. Similarly, BTW was also found statistically significant at baseline to 3 and 6 months interval (P = 0.01) [Table 1]. Post hoc Bonferroni test for CP-GM, BTH, BTW and IPW revealed a statistically significant difference from baseline to 3 months (P ≤ 0.05) and 6 months (P ≤ 0.05) and a nonsignificant difference at 3–6 months (P ≥ 0.05) [Table 2]. At 3 months, clinical measurements showed 52% sites (13 out of 25 sites) had complete papillary coverage, and 48% sites (12 out of 25 sites) had varying degrees of partial papillary coverage. Image analysis method showed 48% sites (12 out of 25 sites) had achieved complete papillary coverage, and 52% sites (13 out of 25 sites) had achieved partial coverage. 6-month clinical measurement showed, 48% sites (12 out of 25 sites) had complete papillary coverage and 52% sites (13 out of 25 sites) had partial papillary coverage. Image analysis method showed 48% sites (12 out of 25 sites) remained as complete papillary fill and 52% sites (13 out of 25 sites) had a varying degree of partial coverage at 6 months. This variation of the result between clinical measurement and measurement through image analysis could be justified by the fact that, photographic image analysis provides more precise evaluation and even minute changes can be evaluated through it which cannot be detected by clinical visualization with periodontal probe.
Figure 13.
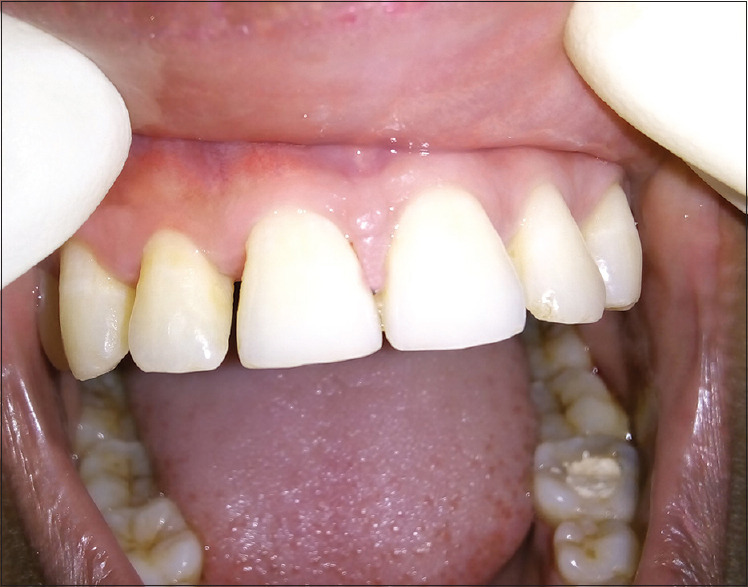
Postoperative view of papillary regeneration between 11 and 21 at 3 months
Figure 14.
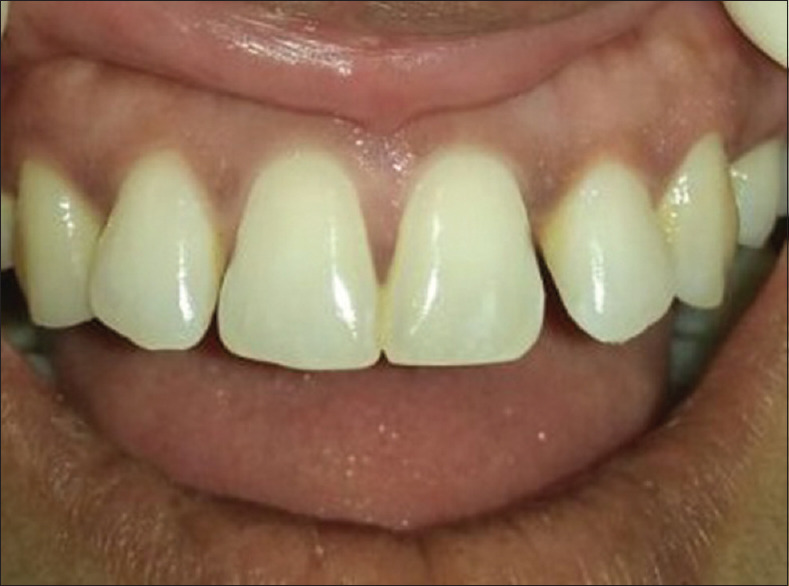
Six months postoperative view of showing persistent result of papillary regeneration between 11 and 21
Table 1.
Mean, standard deviation, F and P values of all the parameters, measured at baseline, 3 months, and 6 months intervals
| Parameters | Time interval | Mean | SD | F | P |
|---|---|---|---|---|---|
| CP-GM | Baseline | 1.7200 | 1.17331 | 10.355 | 0.01* |
| 3 months | 0.5600 | 0.86987 | |||
| 6 months | 0.6400 | 0.95219 | |||
| BTH | Baseline | 1.6952 | 1.25837 | 9.003 | 0.01* |
| 3 months | 0.5876 | 0.90469 | |||
| 6 months | 0.6352 | 0.92979 | |||
| IPW | Baseline | 1.2800 | 0.54160 | 19.159 | 0.01* |
| 3 months | 0.4000 | 0.57735 | |||
| 6 months | 0.4400 | 0.58310 | |||
| BTW | Baseline | 1.2408 | 0.69809 | 14.558 | 0.01* |
| 3 months | 0.3860 | 0.58798 | |||
| 6 months | 0.4292 | 0.60129 |
*Statistically significant value, P≤0.05: Statistically significant, P<0.01: Statistically highly significant, P≥0.05: Statistically nonsignificant. P – Probability value; F – F statistics value; SD – Standard deviation; CP-GM – Contact point to gingival margin distances; IPW – Interproximal width; BTH – Black triangle height; BTW – Black triangle width
Table 2.
Post-hoc Bonferroni test for comparison of contact point to gingival margin distances, black triangle height, interproximal width, black triangle width
| Parameters | Time intervals | Mean difference | Sig |
|---|---|---|---|
| CP-GM | Baseline | ||
| 3 months | 1.16000 | 0.01* | |
| 6 months | 1.08000 | 0.01* | |
| 3 months | |||
| 6 months | −0.08000 | 0.957 | |
| BTH | Baseline | ||
| 3 months | 1.10760 | 0.01* | |
| 6 months | 1.06000 | 0.02* | |
| 3 months | |||
| 6 months | −0.04760 | 0.986 | |
| IPW | Baseline | ||
| 3 months | 0.88000 | 0.00* | |
| 6 months | 0.84000 | 0.00* | |
| 3 months | |||
| 6 months | −0.04000 | 0.966 | |
| BTW | Baseline | ||
| 3 months | 0.85480 | 0.01* | |
| 6 months | 0.81160 | 0.01* | |
| 3 months | |||
| 6 months | −0.04320 | 0.968 |
*Statistically significant values, P≤0.05: Statistically significant, P<0.01: Highly statistically significant, P≥0.05: Statistically nonsignificant. CP-GM – Contact point to gingival margin distances; IPW – Interproximal width; BTH – Black triangle height; BTW – Black triangle width; Sig – Significance value; P – Probability value
DISCUSSION
HA is a mucopolysaccharide naturally present in human body and commercially available as biotechnologically prepared gel. Unique properties of HA showed promising result in various esthetic corrections. When injected into papillary gingiva, HA integrates well into the adjacent area, microcirculatory perfusion is enhanced and showed excellent esthetic results. The plumping of the IDP could be explained by the unique rheological and highly hydrophilic property of HA, which can bind up to 1000 times more water than its molecular weight. It also increases de novo collagen synthesis. As HA degrade, novel collagen synthesis replaces the HA resulting in long lasting correction. The high level of cross linking alters the ability of water to bind to HA and thereby creates tissue lift, increases cell division and the tubulin concentration, which helps in the proliferation of fibroblasts. HA also acts as biomaterial scaffold for other molecules, such as bone morphogenic protein-2 and platelet-derived growth factor-BB. The insoluble gel portion that persists in the skin and gingiva after injection is the amount of HA that contributes to the clinical effect.[7,8] All the study participants in this study showed uneventful healing with no sign of allergy, infection or any complication during the course of 6 months. Only 3 patients complained of mild pain and irritation at the injection site for first 5–7 days for which analgesic (Diclofenac Na) was advised. Result of the present study is also supported by the finding of similar studies conducted by Becker et al. in 2010,[5] Mansouri et al. in 2013,[9] Lee et al. in 2016,[10] Pi et al. in 2017,[11] Habashneh and Khaleel in 2018,[12] Singh and Vandana in 2018.[13] The pioneering study conducted by Becker et al., where HA injections were repeated up to three times, and patients were followed for 6–25 months, though the follow-ups for all of the patients were not uniform.[5] Besides the present study, Mansouri et al. in 2013 in a longitudinal assessment reported an improvement of results over time, i.e., at 3 months, 10% of sites showed 50% improvement, while at 6 months, about 43% of sites demonstrated improvement in papillary coverage by over 50%, which were statistically significant (P < 0.05).[9] However, in the present study, mostly there was a steady result between 3 and 6 months, while minute relapse had occurred in a few cases. In this study, at 3 months mean reduction of CP-GM, IPW, BTH, and BTW 1.60 mm, 0.88 mm, 1.10 mm, and 0.85 mm and at 6 months 1.08 mm, 0.84 mm, 1.06000 mm, 0.81160 mm, respectively, were found with a single injection of HA filler. Lee et al. found a mean reduction in the BTH and BTW at 6 months, 0.71 mm, 0.32 mm, respectively. However, the mean number of HA applications was 3.42 times.[10] In another study, Lee et al. showed a mean reduction of BTH and BTW of 0.70 mm and 0.30 mm, respectively, at 6 months, comparable with the present study.[14] Bertl et al. showed a mean reduction of BTH from baseline to 3 and 6 months was 0.23 mm and 0.09 mm with a single dose of HA, which is less appreciable than the findings of the present study.[15] Habashneh and Khaleel showed significant reductions in bronchial thermoplasty at 3 and 6 months when compared to baseline (P < 0.001), which correlates with the present study (P < 0.01).[12] Singh and Vandana used different concentrations (1%, 2%, and 5%) of HA gel injection at papillary recession site and found different regenerative potential for different concentrations of HA.[13] Three different patterns of results were grouped as those sites, which showed a continuous improvement, sites with improvement, and rebound at different intervals, and no improvement from baseline to 6 months. While evaluating the sites achieved complete papillary coverage, a variation of results has been found in the literature. Becker et al.[5] reported 21% site (3 out of 14 sites) had 100% papillary regeneration which is lower than the findings of the present study (i.e., 52%), Awartani and Tatakis[16] found 11% sites (2 out of 17 sites) had complete papillary regeneration at 3 months, while at 6 months, 17% sites (3 out of 17 sites) had complete papillary fill which is also lower than the present study. Lee et al. in one study showed 63% sites (36 out of 57 sites) had gained complete papillary regeneration at 6-month intervals[10] and the same author in another study[14] had achieved complete papillary regeneration in 67% sites (29 out of 43 sites), which are higher than the findings of the present study, but the author reported HA was applied for 3–5 times per defect site. These differences in the findings of various studies could be due to the differences in study design, selection criteria of patients, differences in recession sites, dosage and concentration of HA injection and degree of oral hygiene maintenance by the study participants. Through this study, it was also found that sites with complete papillary coverage were maintained for 6 months, but seven sites with partial coverage showed a slight deterioration of clinical results from 3 to 6 months. This could be explained by the fact that partial recession coverage was subjected to plaque and food accumulation at the site, which may negatively affect the treatment outcome. Although 5 sites having partial coverage of recession postoperatively with excellent plaque control by patient showed stable results at 3 and 6 months. Abdelraouf et al.[17] in 2019 revealed a statistically significant higher mean decrease in height and surface area of black triangles using HA at 3 and 6 months when compared with placebo/saline. At 6 months, the HA group showed a statistically significant higher mean satisfaction score than the saline group.
Currently, numerous studies have reported the use of an image analysis method for the evaluation of clinical results, as reported in this study. Sin et al. used imaging tools to analyze clinical photographs to investigate gingival shrinkage at 3 months interval after scaling-root planning.[18] Kerner et al. compare the root coverage rate with the conventional method and image analysis program and validated its usefulness.[19] Ricci emphasized the importance of reproducing the shooting angle for the precise outcome.[20] In the present study, utmost care was taken to maintain a similar distance and angulation to reproduce pre- and post-operative photographs. Similar techniques have been used by Becker et al.[5] and later by Lee et al.,[10] Singh and Vandana.[13]
CONCLUSION
The findings of this study indicate reconstruction of IDP can be achieved and maintained for 6 months by using HA filler. HA filler is a much convenient means to correct the papillary recession in the esthetic zone and considered as a minimally invasive method to overcome the problem of the black triangle. Meticulous plaque control can correlate with the study findings. The use of the image analysis method was validated and found a precise and more appreciable means of evaluating the clinical outcome. Long-term follow-up with more sample size will further establish HA as a minimally invasive technique, which can be a breakthrough for successful papillary reconstruction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:30–43. doi: 10.1902/jop.1999.70.1.30. [DOI] [PubMed] [Google Scholar]
- 2.Kassab MM, Cohen RE. The etiology and prevalence of gingival recession. J Am Dent Assoc. 2003;134:220–5. doi: 10.14219/jada.archive.2003.0137. [DOI] [PubMed] [Google Scholar]
- 3.Hennig MA, Mustafa JM, Mussk JM. Absence of interdental papilla– systematic review of available therapeutic modalities. Stomatos. 2016;22:32–43. [Google Scholar]
- 4.Chambrone L, Chambrone D, Pustiglioni FE, Chambrone LA, Lima LA. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-type defects? J Dent. 2008;36:659–71. doi: 10.1016/j.jdent.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Becker W, Gabitov I, Stepanov M, Kois J, Smidt A, Becker BE. Minimally invasive treatment for papillae deficiencies in the esthetic zone: A pilot study. Clin Implant Dent Relat Res. 2010;12:1–8. doi: 10.1111/j.1708-8208.2009.00247.x. [DOI] [PubMed] [Google Scholar]
- 6.Nordland WP, Tarnow DP. A classification system for loss of papillary height. J Periodontol. 1998;69:1124–6. doi: 10.1902/jop.1998.69.10.1124. [DOI] [PubMed] [Google Scholar]
- 7.Vedamurthy M. Soft tissue augmentation: Use of hyaluronic acid as dermal filler. Indian J Dermatol Venereol Leprol. 2004;70:383–7. [PubMed] [Google Scholar]
- 8.Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (Restylane) in facial cosmetic surgery: Review and technical considerations. Plast Reconstr Surg. 2007;120:41S–54S. doi: 10.1097/01.prs.0000248794.63898.0f. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri SS, Ghasemi M, Salmani Z, Shams N. Clinical application of hyaluronic acid gel for reconstruction of interdental papilla at the esthetic zone. J Islamic Dent Assoc Iran. 2013;25:152–7. [Google Scholar]
- 10.Lee WP, Kim HJ, Yu SJ, Kim BO. Six month clinical evaluation of interdental papilla reconstruction with injectable hyaluronic acid gel using an image analysis system. J Esthet Restor Dent. 2016;28:221–30. doi: 10.1111/jerd.12216. [DOI] [PubMed] [Google Scholar]
- 11.Pi S, Choi YJ, Hwang S, Lee DW, Yook JI, Kim KH, et al. Local injection of hyaluronic acid filler improves open gingival embrasure: Validation through a rat model. J Periodontol. 2017;88:1221–30. doi: 10.1902/jop.2017.170101. [DOI] [PubMed] [Google Scholar]
- 12.Habashneh RA, Khaleel B. Interdental papilla reconstruction using injectable hyaluronic acid (HYADENT BG): A six month prospective clinical study. Clin Oral Implants Res. 2018;29:271. [Google Scholar]
- 13.Singh S, Vandana KL. Use of different concentrations of hyaluronic acid in interdental papillary deficiency treatment: A clinical study. J Indian Soc Periodontol. 2019;23:35–41. doi: 10.4103/jisp.jisp_332_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WP, Seo YS, Kim HJ, Yu SJ, Kim BO. The association between radiographic embrasure morphology and interdental papilla reconstruction using injectable hyaluronic acid gel. J Periodontal Implant Sci. 2016;46:277–87. doi: 10.5051/jpis.2016.46.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertl K, Gotfredsen K, Jensen SS, Bruckmann C, Stavropoulos A. Can hyaluronan injections augment deficient papillae at implant-supported crowns in the anterior maxilla.A randomized controlled clinical trial with 6 months follow-up? Clin Oral Implants Res. 2017;28:1054–61. doi: 10.1111/clr.12917. [DOI] [PubMed] [Google Scholar]
- 16.Awartani FA, Tatakis DN. Interdental papilla loss: Treatment by hyaluronic acid gel injection: A case series. Clin Oral Investig. 2016;20:1775–80. doi: 10.1007/s00784-015-1677-z. [DOI] [PubMed] [Google Scholar]
- 17.Abdelraouf SA, Dahab OA, Elbarbary A, El-Din AM, Mostafa B. Assessment of hyaluronic acid gel injection in the reconstruction of interdental papilla: A randomized clinical trial. Open Access Maced J Med Sci. 2019;7:1834–40. doi: 10.3889/oamjms.2019.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sin YW, Chang HY, Yun WH, Jeong SN, Pi SH, You HK. Association of gingival biotype with the results of scaling and root planing. J Periodontal Implant Sci. 2013;43:283–90. doi: 10.5051/jpis.2013.43.6.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerner S, Etienne D, Malet J, Mora F, Monnet-Corti V, Bouchard P. Root coverage assessment: Validity and reproducibility of an image analysis system. J Clin Periodontol. 2007;34:969–76. doi: 10.1111/j.1600-051X.2007.01137.x. [DOI] [PubMed] [Google Scholar]
- 20.Ricci A. An objective method to measure soft tissue behavior around single-tooth implants.Part 1: Vertical measurements. Eur J Esthet Dent. 2007;2:406–18. [PubMed] [Google Scholar]


