Abstract
Background:
Recent evidence suggests an interconnection between chronic periodontal disease and systemic diseases.
Aim:
The aim of this study is to evaluate the possible association between nonalcoholic fatty liver disease (NAFLD) and inflammatory periodontal disease among north Indian population.
Settings and Design:
Tertiary health care center, cross-sectional case-control observational study.
Materials and Methods:
A total of 40 cases, i.e., patients with NAFLD and 40 healthy volunteers were included over a period of 8 months and their periodontal status was compared. The status of their hepatic health was ascertained by anthropometric, imaging, and biochemical evaluation including ultrasound examination of abdomen and transient elastography.
Statistical Data Analysis:
Paired t-test, multivariate logistic regression analysis using IBM SPSS STATISTICS (version 22.0, Armonk, NY: IBM Corp).
Results:
The study revealed that only 11.9% and 20% of participants had periodontitis, in healthy controls and hepatic disease patients, respectively. A statistically significant difference was observed in clinical parameters of periodontal status, except for malocclusion. Comparative analysis of tumor necrosis factor-α (TNF-α), interleukin-6, C-reactive protein, and cytokeratin-18 revealed differences in mean scores, though statistically nonsignificant. Only aspartate transaminase, number of missing teeth, and bleeding on probing (BOP) were observed with higher odds ratios for hepatic disease patients. Spearman correlation analysis revealed significant positive correlations between TNF-α and BOP, for cases.
Conclusion:
Patients with hepatic disease showed a higher prevalence of periodontal disease, worse oral hygiene and periodontal health status compared to healthy individuals.
Keywords: Bleeding on probing, case control, nonalcoholic fatty liver disease, oral health, periodontal disease, systemic health, tumor necrosis factor
INTRODUCTION
Periodontal disease is an immune inflammatory disease caused by microbial infection and results in the loss of gingiva and of the supporting structures of the teeth.[1,2,3,4,5,6,7,8] The shedding of lipopolysaccharide from periodontal flora stimulates the endothelial cells, monocytes, and macrophages to initiate a proinflammatory response resulting in long-standing sustained increase in many cytokines enhancing inflammation, i.e., interleukins 1β (IL-1β), 6, and tumor necrosis factor (TNF)-α.[3,4,6,9,10,11] A huge body of contemporary epidemiological studies have reported periodontal disease as a risk factor for various overall health conditions, including cardiovascular disease, type 2 diabetes, adverse pregnancy outcomes, and rheumatoid arthritis.[12]
The term nonalcoholic fatty liver disease (NAFLD) encompasses a disease spectrum which includes variable degrees of simple steatosis (nonalcoholic fatty liver disease, NAFLD), nonalcoholic steatohepatitis (NASH) and cirrhosis.[13] The overall prevalence of NAFLD varies from 15% to 40% and 9%–40% in Western countries and in Asian countries, respectively.[14,15,16] NAFLD is an emerging cause of liver disease in our country as well. The prevalence of NAFLD is 9%–32% in the general Indian population, with a higher incidence among specific groups, namely, obese and diabetic patients.[17,18,19,20,21,22,23]
With the rising incidence and prevalence, an enhanced understanding of the diverse aspects of NAFLD is appearing and is alarming in context of its clinical implications.[24,25] Approximately 10%–30% have the potentially advancing type of NAFLD, NASH (NASH/hepatocellular injury and inflammation). Further, 25%–40% of NASH patients may progress to liver fibrosis over time, resulting in cirrhosis in 20%–30%. Such cirrhotic patients are highly susceptible to develop hepatocellular carcinoma (2.6%/year).[26] NASH is considered synonymous to the metabolic syndrome (MS) affecting the liver, i.e., as an associate morbidity to diabetes mellitus (DM) type 2, hyperlipidemia, and hypertension (HTN).[27]
NAFLD has been associated with infections of gastrointestinal tract, such as small intestinal bacterial overgrowth.[28,29] Oral bacteria have also been implicated in the induction of endotoxemia and subsequent hepatic inflammatory responses directly or indirectly through imparting alterations in gut flora.[30] More recently, Porphyromonas gingivalis, most prevalent pathogen was detected in significantly higher frequency in periodontal disease in NAFLD patients than in the non-NAFLD participants (46.7% vs. 21.7%, odds ratio [OR]: 3.16). Moreover, NASH patients showed a higher detection rate of P. gingivalis than that in the non-NAFLD participants (52.0%, OR: 3.91).[31]
Indian NAFLD patients vary from their Western counterparts in diverse aspects as these are less obese and have lower frequency of DM and MS.[10] As available literature regarding the interrelationship among NAFLD, periodontal disease and MS is largely from nonIndian populations. The current investigation was planned to conduct a cross-sectional case-control observational study aimed at investigating the possible association between NAFLD and periodontitis in regional population.
SUBJECT POPULATION AND CATEGORIZATION
Forty patients diagnosed as suffering from NAFLD in the Department of Hepatology, PGIMER, Chandigarh, a tertiary care Centre were included over a period of 6 months (case group) and their periodontal status were compared with 40 normal healthy volunteers (HVs) (control group) selected from the accompanying attendants other than those selected for this study. The study protocol was duly reviewed and approved by the Institutional Ethics Committees of both institutes (Ref no's: PUIEC/2016/18/1-A/20/05 and PGI/IEC/2016/349). All study participants were explained about the need and detailed course of the study and were asked for the voluntary participation in the investigation. An informed consent regarding willingness to participate in study was obtained from the patients in written and signed format.
Inclusion criteria
The nonalcoholic fatty liver disease group (cases)
Nonalcoholic fatty liver disease patients
Showing hepatic steatosis on liver ultrasound and at least 1.5 times increased levels of liver enzymes, i.e., aspartate transaminase (AST) and alanine transaminase (ALT) at least for a duration of >3 months, and where available, liver biopsy confirmed diagnosis of NAFLD
Absence of alcohol intake history or intake limited to <20 g/day
Absence of viral markers (HBsAg and anti-hepatitis C virus), autoimmune markers (antinuclear anti bodies, anti-smooth muscle antibody), anti-liver kidney-microsomal antibody anti-mitochondrial antibody), Kayser–Fleischer rings
Normal ceruloplasmin levels and iron studies of the patients.
Exclusion criteria
Expectant mothers and patients with history of drug intake contributing to fatty liver disease
Patients with history of anti-TNF-a or anti-oxidant drugs in the past 3 months.
Healthy volunteers (controls)
This group included healthy controls without a history of DM and with normal blood pressure, abdominal ultrasound, liver enzyme levels, and fasting plasma glucose.
Study method
Anthropometric, imaging, and biochemical evaluation
All patients with NAFLD underwent a detailed evaluation in the Department of Hepatology of a tertiary care medical center. The evaluation included a detailed medical history keeping inclusion and exclusion criteria in mind. Family history of diabetes, HTN, coronary artery disease, and stroke was also recorded. Body mass index (BMI) and waist-hip ratio for central obesity and abdominal examination for any organomegaly were recorded. After routine hematological, biochemical (including fasting plasma glucose, lipid profile), all patients were subjected to an ultrasound examination of abdomen and transient elastography (fibroscan) for the assessment of hepatic steatosis and fibrosis.[32]
HVs were also assessed in the Department of Hepatology and underwent selective investigations of liver function tests, fasting plasma glucose, and abdominal ultrasound.
Assessment of severity of hepaticdisease
Hepatic steatosis-was diagnosed on abdominal ultrasonography and was graded into mild, moderate, and severe as per Saverymuttu et al.[33] In addition to ultrasound, hepatic steatosis was also graded noninvasively into S0, S1, and S3 based on the controlled attenuation parameter (CAP)-a software available with the transient elastography (fibroscan) machine[34]
Hepatic inflammation – patients with NAFLD were assessed with the help of serum biomarkers of hepatic inflammation and apoptosis. Noninvasive biomarkers included TNF-alpha, IL-6, C-reactive protein (CRP), and (CK)-18[35]
Hepatic fibrosis – was assessed noninvasively by measuring the liver stiffness measurement (LSM) with the help of transient elastography. Patients were divided into NASH and no-NASH based on the LSM (LSM ≥7 Kpa = NASH). In addition to Fibroscan, and histological assessment was made according to NAFLD activity score in patients who underwent liver biopsy[36] and patients were divided into NASH (absent), NASH (borderline), and NASH.
Periodontal examination in patients with nonalcoholic fatty liver disease and healthy volunteers
A single previously calibrated and trained examiner made all measurements for parameters of periodontal status in the Department of Periodontology, Dr. HS Judge Institute of Dental Sciences, Punjab University, Chandigarh, India. The examiner was blinded about the hepatic status of patients. Probing pocket depths (PDs) were measured at standard six sitesper tooth using a calibrated periodontal probe. Gingival recession was recorded as the distance from the cementoenamel junction to the gingival margin. Clinical attachment loss (CAL) and the percentage of bleeding sites in each participant were also noted.
Gingivitis was categorized as the absence of PD (≤3 mm), bleeding on probing (BOP), and clinical signs of gingival inflammation. Chronic periodontitis was defined by the presence of two or more sites with PD (≥4 mm), attachment loss (≥4 mm), and BOP. Periodontal disease was ascertained by radiographic alveolar bone loss. Chronic periodontitis was further classified in to categories based on the severity of involvement: incipient (4–5 mm CAL) and moderate/severe (≥6 mm CAL). Periodontal health was determined when there was an absence of obvious clinical inflammation, BOP, and no periodontal attachment loss or bone loss.[37]
Data analysis
The baseline demographic, anthropologic, and clinical data have been tabulated [Table 1 and Figures 1, 2]. Bilirubin, AST, ALT, debris index simplified (DIS), calculus index simplified, missing teeth, malocclusion, number of sites, BOP, mean PD, mean CAL, PD, oral hygiene index simplified (OHIS) were expressed by mean ranks and standard deviations, and paired t-test was performed [Tables 2 and 3]. The prevalence of periodontitis based on the predefined CAL criteria have been presented by frequency distribution and compared between cases and controls [Table 4]. The periodontitis prevalence was further analyzed and compared within NASH and non-NASH patients in case group [Table 5].
Table 1 a.
Demographic and hepatic status parameters in NAFLD and HVs
| Parameters | n=40 (Study Group) | n= 42 |
|---|---|---|
| Age | 44±10.90 | 30.24±7.30 |
| Sex(Male: Female) | 14:26 | 14:28 |
| AST | 73.10±88.26 | 23.36±8.85 |
| ALT | 70.25±38.18 | 25.09±15.11 |
| TGs | 190±80.8 | - |
| HDL | 41.3±7.4 | - |
| Fibro scan | ||
| LSM | 5.96±1.94 | - |
| CAP | 306.35±56.05 | - |
NAFLD: Non-alcoholic Fatty Liver Disease, HV: Healthy Volunteer, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, TGs: Triglycerides, HDL: high density lipoproteins, LSM: Liver stiffness measurement, CAP: Controlled Attenuation Parameter
Figure 1.
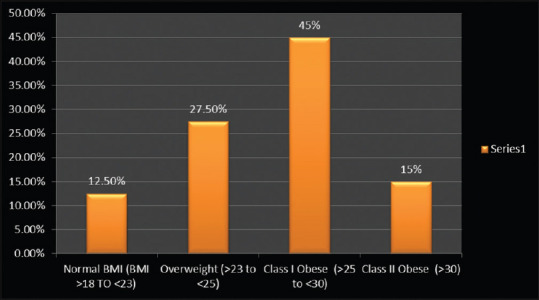
Graph showing distribution of study subjects based on anthropologic parameters in NAFLD case group population. NAFLD: Nonalcoholic fatty liver disease; BMI: Body mass index
Figure 2.
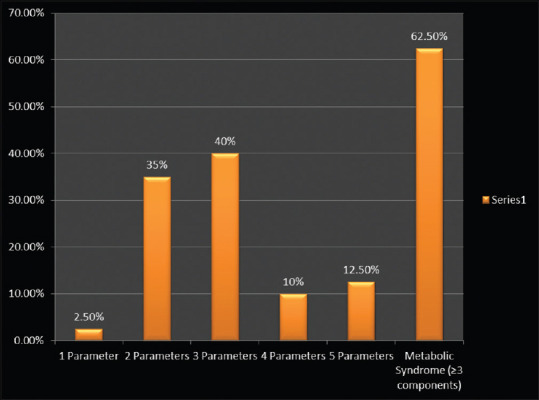
Graph showing distribution of study subjects based on presence of parameters of metabolic syndrome in NAFLD case group population. NAFLD: Nonalcoholic fatty liver disease
Table 2.
Comparison of hepatic health parameters and oral clinical parameters in nonalcoholic fatty liver disease and healthy volunteers
| Clinical measurements | Controls Mean rank | Case NAFLD Mean rank | P (paired t-test) |
|---|---|---|---|
| Bilirubin | 32.98 (n=42) | 49.64 (n=39) | 0.001 |
| AST | 25.95 (n=42) | 57.21 (n=39) | 0.000 |
| ALT | 25.81 (n=42) | 57.36 (n=39) | 0.000 |
| DIS | 50.65 (n=42) | 31.89 (n=40) | 0.000 |
| CIS | 50.61 (n=42) | 31.94 (n=40) | 0.000 |
| Missing teeth | 35.07 (n=42) | 48.25 (n=40) | 0.004 |
| Malocclusion | 41.57 (n=42) | 41.43 (n=40) | 0.974 |
| BOP | 31.00 (n=42) | 52.53 (n=40) | 0.000 |
| Mean PD | 46.14 (n=42) | 36.63 (n=40) | 0.052 |
| Mean CAL | 31.45 (n=42) | 52.05 (n=40) | 0.000 |
| OHIS | 51.07 (n=42) | 30.68 (n=40) | 0.000 |
The P value is considered significant, for P<0.05. NAFLD – Nonalcoholic fatty liver disease; AST – Aspartate aminotransferase; ALT – Alanine aminotransferase; DIS – Debris index simplified; CIS – Calculus index simplified; BOP – Bleeding on probing; PD – Pocket depth; CAL – Clinical attachment loss; OHIS – Oral hygiene index simplified; P – P-value or probability value
Table 3.
Comparison of hepatic health parameters and oral clinical parameters in non-nonalcoholic steatohepatitis and nonalcoholic steatohepatitis groups
| Clinical measurements | Controls Non-NASH Mean rank | Case NASH Mean rank | P (paired t-test) |
|---|---|---|---|
| Bilirubin | 17.79 (n=28) | 24.30 (n=10) | 0.111 |
| AST | 17.09 (n=28) | 26.25 (n=10) | 0.025 |
| ALT | 18.23 (n=28) | 23.05 (n=10) | 0.239 |
| SR | 19.83 (n=29) | 20.50 (n=10) | 0.834 |
| IQR | 17.50 (n=29) | 27.25 (n=10) | 0.019 |
| CAP | 16.74 (n=29) | 29.45 (n=10) | 0.002 |
| TNF-α | 18.52 (n=29) | 24.30 (n=10) | 0.166 |
| IL6 | 19.64 (n=29) | 21.05 (n=10) | 0.735 |
| CRP | 18.79 (n=29) | 23.50 (n=10) | 0.260 |
| CK18 | 20.84 (n=29) | 17.55 (n=10) | 0.431 |
| DIS | 18.69 (n=29) | 23.80 (n=10) | 0.214 |
| CIS | 19.47 (n=29) | 21.55 (n=10) | 0.614 |
| Missing teeth | 20.48 (n=29) | 18.60 (n=10) | 0.631 |
| Mean PD | 18.93 (n=29) | 23.10 (n=10) | 0.288 |
| Mean CAL | 19.50 (n=29) | 21.45 (n=10) | 0.631 |
| BOP | 18.67 (n=29) | 23.85 (n=10) | 0.202 |
| Pocket depth | 18.93 (n=29) | 23.10 (n=10) | 0.288 |
| OHIS | 19.00 (n=29) | 22.90 (n=10) | 0.349 |
The P value is considered significant, for P<0.05. NASH – Nonalcoholic steatohepatitis; AST – Aspartate aminotransferase; ALT – Alanine aminotransferase; SR – Success rate; IQR – Interquartile range; CAP – Controlled attenuation parameter; TNF-α – Tumor necrosis factor-alpha; IL6 – Interleukin-6; CRP – C-reactive protein; CK18 – Cytokeratin-18; PD – Pocket depth; CAL – Clinical attachment loss; BOP – Bleeding on probing; OHIS – Oral hygiene index simplified; P – P-value or probability value
Table 4.
Prevalence of periodontitis (cases & controls)
| No. of sites with CAL > 4mm * group Cross tabulation | ||||
|---|---|---|---|---|
| No. of sites with CAL > 4mm | Group | Total | ||
| NAFLD patients | Control | |||
| NO | % within group | 80.0% | 88.1% | 84.1% |
| YES | % within group | 20.0% | 11.9% | 15.9% |
NAFLD: Non-alcoholic Fatty Liver Disease, CAL: Clinical attachment loss
Table 5.
Prevalence of periodontitis (nash and non-nash patients in cases)
| No. of sites with CAL > 4mm * NASH (based on LSM >=7) * group Cross tabulation | |||||
|---|---|---|---|---|---|
| Group | No. of sites with CAL > 4mm | NASH (based on LSM >=7) | Total | ||
| NO | YES | ||||
| NAFLD patients | NO | % within NASH (based on LSM >=7) | 82.8% | 70.0% | 79.5% |
| YES | % within NASH (based on LSM >=7) | 17.2% | 30.0% | 20.5% | |
NAFLD: Non-alcoholic Fatty Liver Disease, NASH: Non-Alcoholic Steatohepatitis, LSM: Liver stiffness measurement, CAL: Clinical attachment loss
Multivariate logistic regression models were applied and the association between periodontitis and risk indicators was expressed by ORs at 95% confidence interval [Table 6]. A spearman correlation analysis in hepatic status and oral health status parameters has been performed [Figure 3].
Table 6.
Multivariate logistic regression analysis expressing the odds ratio, 95% confidence intervals and P values*
| OR | 95% CI for OR | P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Bilirubin | 0.234 | 0.022 | 2.505 | 0.230 |
| AST | 1.177 | 1.023 | 1.355 | 0.023 |
| ALT | 1.059 | 0.995 | 1.127 | 0.070 |
| DIS | 0.008 | 0.000 | 2.196 | 0.091 |
| CIS | 4.004 | 0.017 | 939.593 | 0.618 |
| Missing teeth | 2.062 | 0.991 | 4.293 | 0.053 |
| Malocclusion | 0.132 | 0.009 | 2.022 | 0.146 |
| Number of sites | 0.884 | 0.571 | 1.369 | 0.580 |
| BOP | 1.199 | 1.021 | 1.407 | 0.027 |
| Mean PD | 0.017 | 0.000 | 1.432 | 0.072 |
| Mean CAL | 3.992 | 0.257 | 62.021 | 0.323 |
| Constant | 10.345 | 0.462 | ||
*The value of P is considered statistically significant. <0.05. AST – Aspartate aminotransferase; ALT – Alanine aminotransferase; CAL – Clinical attachment loss; DIS – Debris Index simplified; CIS – Calculus index simplified; BOP – Bleeding on probing; PD – Pocket depth; OR – Odds ratio; CI – Confidence interval; P – P-value or probability value
Figure 3.
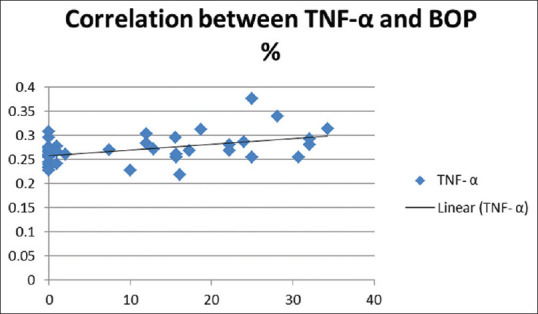
Graph showing correlation between BOP and TNF-α in NAFLD case population. BOP: Bleeding on probing; TNF-α: Tumor necrosis factor-alpha; NAFLD: Nonalcoholic fatty liver disease
RESULTS
Forty NAFLD patients (14 males and 26 females) with a mean age 44 ± 10.90 years were included over an 8-month period. Five patients of the case subject population (12.5%) had normal body weight, 11 (27.5%) were overweight, 18 (45%) had class I obesity, and 6 (15%) had class II obesity [Table 1 and Figure 1]. Case population was analyzed for the presence of salient parameters of MS. 25 NAFLD cases (62.5%) has >3 components of MS [Figure 2]. A control group of 42 HV consisting of 14 males and 28 females with a mean age of 30.24 ± 7.30 were included during the same time span [Table 1]. All enrolled HVs were normotensive, nondiabetic, normal range cholesterol levels and normal level of hepatic enzymes AST and ALT. A comparative analysis of all hepatic health indicators was performed and significant differences were observed in liver enzymes (AST, ALT), bilirubin levels [Table 2]. Case controls were divided into NASH (10) and non-NASH (28) based on the LSM (LSM ≥7 Kpa = NASH. The mean scores for LSM were 8.8 ± 0.99 and 5.01 ± 1.05 for NASH and non-NASH patients, respectively (data not shown).
Table 1 b.
Anthropologic and metabolic status parameters in NAFLD patients
| Parameters | No.(percentage ) of study subjects |
|---|---|
| Anthropometry | |
| Normal BMI (BMI >18 TO <23) | 5 (12.5%) |
| Overweight (>23 to <25) | 11 (27.5%) |
| Class I Obese (>25 to <30) | 18 (45%) |
| Class II Obese (>30) | 6 (15%) |
| Metabolic syndrome | |
| 0 Parameter | 0 |
| 1 Parameter | 1 (2.5%) |
| 2 Parameters | 14 (35%) |
| 3 Parameters | 16 (40%) |
| 4 Parameters | 4 (10%) |
| 5 Parameters | 5 (12.5%) |
| Metabolic Syndrome (≥3 components) | 25 (62.5%) |
| Diabetes Mellitus (DM) | 10 (25%) |
| Hypertension (HTN) | 12 (30%) |
NAFLD: Non-alcoholic Fatty Liver Disease, BMI: Body Mass index,
Comparison of hepatic health parameters in nonalcoholic steatohepatitis and non-nonalcoholic steatohepatitis
The mean CAP scores were 351 ± 33.1 and 291 ± 54.4 for NASH and non-NASH patients, respectively. The mean levels of all systemic biomarkers, namely, TNF-α, IL-6, CRP, CK18 were lower in non-NASH patients then observed in NASH patients. Inflammatory biomarkers, namely, TNF-α, IL-6, CRP, and CK18 revealed differences in mean scores, but no statistically significant differences could be traced in. A statistically significant difference was revealed in median scores of CAP, AST levels for NASH and non-NASH patients [Table 3].
Prevalence of periodontitis in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis cases population
Only 11.9% and 20% of the study participants had periodontitis, with in the healthy controls and patients suffering from hepatic disease, respectively, in total study population [Table 4]. The prevalence of periodontitis was observed to be 17.2% and 30%, respectively, in non-NASH and NASH patients [Table 5].
Comparison of clinical parameters of periodontal health in nonalcoholic fatty liver disease and healthy volunteers
Table 3 shows the mean rank scores of clinical parameters of hepatic health as well as periodontal status for total study population. Statistically significant difference was observed in clinical parameters of periodontal disease, except for malocclusion between HVs and hepatic disease patients (cases) [Table 2].
Comparison of clinical parameters of periodontal health in nonalcoholic steatohepatitis and non-nonalcoholic steatohepatitis case population
Similarly, a statistically significant difference for mean rank scores of AST, interquartile range (IQR) and CAP scores was observed, when patients with NASH and non-NASH status were compared for hepatic health parameters. None of the periodontal health parameters showed any statistically significant difference with in these groups [Table 3].
Association between hepatic disease and clinical parameters of periodontal and hepatic health
Multivariate logistic regression analysis was performed and the association between hepatic disease and study parameters was expressed as ORs. Only three study parameters viz. AST, number of missing teeth and BOP were observed with ORs with statistically significant difference for healthy and hepatic disease subjects [Table 6]. None of the indicator of periodontal status could be ascertained for differentiation of NASH and non-NASH patients based on logistic regression (results not shown).
Co-relation of systemic biomarkers and clinical parameters of periodontal status
Spearman correlation analysis revealed significant positive correlations between TNF-α and BOP, for case population group, i.e., patients with hepatic disease [Figure 3], whereas significant positive correlations were observed between BOP and CAL, PD and OHIS in all study population.(Data not shown).
DISCUSSION
The present study is the first report on the prevalence of periodontitis in NAFLD patients in the Indian population and explores the effect of periodontitis on the severity of NAFLD. The study findings regarding mean BMI and metabolic derangements are in agreement with the previous investigations conducted in India in NAFLD patient populations,[10,21] however these are lower than those reported from the West.[22,23,24] Findings revealed a periodontitis prevalence of 20% in patients of hepatic disease in total and 30% in patients suffering from NASH. The mentioned prevalence is definitely greater than the respective compared groups i.e., healthy controls (11.9%) and non-NASH individuals (17.2%), but the study could not delineate a statistically significant association between periodontal disease and NAFLD, owing to limited set of data.
There was a statistically significant difference for liver enzymes (AST, ALT), bilirubin levels and all clinical parameters of periodontal status evaluated, except for malocclusion between HVs and patients with hepatic disease, which surely points to some bearing of periodontal status on NAFLD status of the individual. Research studies in Japanese university students have reported significantly higher levels of alanine aminotransferase (ALT) in periodontitis patients.[38] The incidence of periodontal disease in healthy japenese females was significantly greater when elevated serum levels of aspartate aminotransferase (AST), ALT and cholinesterase were present.[39,40] In a recent investigation of 7.7 years follow-up, 605 incident NAFLD cases were documented. In participants with CAL <3 mm, incidence of liver disease was slightly higher with <30% of sites affected and moderately higher in participants with ≥30% of sites affected (multivariable-adjusted incidence rate ratio = 1.28, 95% confidence interval [CI], 0.84, 1.95 and 1.60, 95% CI, 1.05–2.43), respectively. Although PD did not reveal a similar dose-response curve.[41]
When hepatic status and periodontal status parameters were compared between NASH and non-NASH patients, a significant difference was noted only in the AST levels, IQR and CAP scores. None of the periodontal status parameters or biochemical mediators differed significantly, though NASH patients had worse scores than non-NASH patients for all indicators of periodontal health, except missing teeth. Inflammatory biomarkers also showed a trend toward higher levels in NASH as compared to non-NASH patients, with only CK-18 showing a reverse trend. A recent large sample investigation reportedan association of periodontitis with hepatic steatosis and significant liver fibrosis (NHANES III cohort). Similar findings have been revealed regarding significant liver fibrosis in a prospective study of largely biopsy-proven NAFLD. In fact, authors confirmed the association with steatosis and demonstrated a gradient of periodontitis with worsening liver injury.[42] This investigation did not take in account of any biochemical markers such as hepatic enzymes or inflammatory serum markers, but similar clinical periodontal findings could not be replicated in our investigation.
AST, number of missing teeth and BOP could discretely differentiate between hepatic health and disease status, based on ORs calculated by multivariate logistic regression. A recent large study population revealed similar observation trends replicated for all periodontal parameters, i.e., adults with steatosis showed a higher percentage of oral sites with BOP, periodontal probing depth ≥4 mm or CAL ≥3 mm. A statistically significant crude OR for steatosis was observed for all markers of periodontitis.[42]
However, in the current investigation, some of the periodontal status parameters did reveal significant association with hepatic status indicators and provided significant insights into association. A significant positive correlation was observed between BOP and serum TNF-α in patients suffering from hepatic disease. TNF-α is an important inflammatory mediator secreted from hepatocytes and is known to perpetuate inflammation, proliferation and apoptosis in chronic liver disease.[43,44] This raises the possibility of this mediator being a connecting link between periodontal and hepatic disease, as it has been previously implicated in many systemic diseases and conditions linked to periodontal disease.[45,46,47,48] Other correlations observed in the study are also much in accordance with previously observed correlations which have been validated with significant body of evidence for example, LSM and CAP scores,[49] IL-6 and CRP in patients with hepatic disease.[50,51,52] Significant positive correlations were observed between BOP and CAL, PD and OHIS in all study population which is again a very old and time tested correlation in multiple cross sectional and longitudinal studies in periodontal disease patients.[53]
There is mounting evidence documenting association between oral and systemic health, however the potential link between periodontitis and NAFLD patients has received scanty attention by now. Few previously published studies have provided conflicting results regarding the association of periodontal disease and NAFLD. Lack of infrastructure in terms of bringing in the hepatological and dental assessment together in a research environment is the major barrier perceived for meager number of such studies. The limited subject population reflects on the same aspects as we did this investigation in collaboration of a tertiary care medical center over a span of 1 year.
Another major challenge in studying associations between oral and systemic health is that diverse definitions of periodontitis, which pose serious differences in the measurement of disease in different studies, questioning the interpretation. Majority of investigations have been placed on diverse population of different ethnicity and variable experimental study settings and design. Hence, there is huge need of well-designed multicentric studies on this specific patient population, i.e., NAFLD patients with chronic periodontal disease to conclusively understand the association as well as causality link between periodontal disease and NAFLD.
CONCLUSION
Patients with hepatic disease showed a higher prevalence of periodontal disease as compared to healthy individuals. The same pattern was observed with respect to hepatic disease severity, revealing a higher prevalence of periodontal disease in patients of more sever hepatic disease. A worse oral hygiene and periodontal health status was observed in patients with hepatic disease compared to healthy individuals. Similar differences between NASH and non-NASH individuals, could not reach the statistical significance owing to the limited study population. Some of the systemic inflammatory biomarkers correlated well with clinical indicators of periodontal status, for example, TNF-α and BOP for case population group, i.e., patients with hepatic disease pointing to the plausible mediating link of periodontal and systemic health. Thus, based on the current observations and within the limitations of investigation, it may be concluded, there appears to be an association between NAFLD and inflammatory periodontal disease, which needs to be ascertained by very well designed, multicentric cross sectional and longitudinal investigations.
Financial support and sponsorship
Financial support sanction S&T/Sanc/07/2015/738-743 (Department of Science and Technology, Chandigarh).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors sincerely acknowledge the financial support from Department of Science and Technology (Chandigarh State Branch) via Sanction no. S&T/Sanc/07/2015/738-743 and analytical support extended for biochemical analysis by the technical staff Mrs. Kiran at Dr. HSJ Institute of dental sciences and hospital, Panjab University, Chandigarh and Mr. Jatinder Raj Sharma at Department of Hepatology, PGIMER, Chandigarh.
REFERENCES
- 1.Van Dyke TE, van Winkelhoff AJ. Infection and inflammatory mechanisms. J Periodontol. 2013;84:1–7. doi: 10.1902/jop.2013.1340018. [DOI] [PubMed] [Google Scholar]
- 2.Grossi SG. Treatment of periodontal disease and control of diabetes: An assessment of evidence and need for future research. Ann Periodontol. 2001;6:138–45. doi: 10.1902/annals.2001.6.1.138. [DOI] [PubMed] [Google Scholar]
- 3.Iacopino AM, Cutler CW. Pathophysiological relationships between periodontitis and systemic disease: Recent concepts involving serum lipids. J Periodontol. 2000;71:1375–84. doi: 10.1902/jop.2000.71.8.1375. [DOI] [PubMed] [Google Scholar]
- 4.Klokkevold PR. Periodontal medicine: Assessment of risk factors for disease. J Calif Dent Assoc. 1999;27:135–42. [PubMed] [Google Scholar]
- 5.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–58. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scannapieco FA. Systemic effects of periodontal diseases. Dent Clin N Am. 2005;49:533–50. doi: 10.1016/j.cden.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Soory M. Chronic periodontitis as a risk marker for systemic diseases with reference to cardiometabolic disorders: Common pathways in their progression. Immunol Immunogenetics Insights. 2010;2:7–21. [Google Scholar]
- 8.Southerland JH, Taylor GW, Offenbacher S. Diabetes and periodontal infection: Making the connection. Clin Diabetes. 2005;23:171–8. [Google Scholar]
- 9.Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol. 2013;84:51–69. doi: 10.1902/jop.2013.134006. [DOI] [PubMed] [Google Scholar]
- 10.Garcia RI, Henshaw M, Krall EA. Relationship between periodontal disease and systemic health. Periodontol 2000. 2001;25:21–36. doi: 10.1034/j.1600-0757.2001.22250103.x. [DOI] [PubMed] [Google Scholar]
- 11.Srinivas SR, Nagarajappa S, Jithendra KD. Periodontal infections as a risk factor for preterm low birth weight deliveries: Speculation or reality? J Dent Sci Res. 2011;2:81–6. [Google Scholar]
- 12.Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP, et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–86. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 13.Vannia E, Bugianesia E, Kotronenb A, Minicisd SD, Järvinenb HY, Baronid GS. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320–30. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43:100–12. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 15.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: A global perspective. Semin Liver Dis. 2008;28:339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 16.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 17.Duseja A. Nonalcoholic fatty liver disease in India a lot done, yet more required. Indian J Gastroenterol. 2010;29:217–25. doi: 10.1007/s12664-010-0069-1. [DOI] [PubMed] [Google Scholar]
- 18.Singh SP, Nayak S, Swain M, Rout N, Mallik RN, Agrawal O, et al. Prevalence of nonalcoholic fatty liver disease in coastal eastern India: A preliminary ultrasonographic survey. Trop Gasteroenterol. 2004;25:76–9. [PubMed] [Google Scholar]
- 19.Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshreshtta P, Pramanik S, et al. Non-alcoholic steatohepatitis in Type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854–8. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 20.Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, et al. Prevalence of non-alcoholic fatty liver disease: Population based study. Ann Hepatol. 2007;6:161–3. [PubMed] [Google Scholar]
- 21.Mohan V, Farooq S, Deepa M, Ravikumar R, Pitchumoni CS. Prevalence of Non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84:84–91. doi: 10.1016/j.diabres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Uchil D, Pipalia D, Chawla M, Patel R, Maniar S, Narayani, et al. Non-alcoholic fatty liver disease (NAFLD)--the hepatic component of metabolic syndrome. J Assoc Physicians India. 2009;57:201–4. [PubMed] [Google Scholar]
- 23.Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, et al. Prevalence of nonalcoholic fatty liver disease in patients with Type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–10. [PubMed] [Google Scholar]
- 24.Kalra S, Vithalani M, Gulati G, Kulkarni CM, Kadam Y, Pallivathukkal J, et al. Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in Type 2 diabetes patients in India (SPRINT) J Assoc Physicians India. 2013;61:448–53. [PubMed] [Google Scholar]
- 25.Dhiman RK, Duseja A. Nonalcoholic fatty liver disease. In: Gupta SB, editor. Medicine Update. New Delhi: Association of Physicians of India; 2005. pp. 469–75. [Google Scholar]
- 26.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: A practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211–8. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WGO Global Guidelines Non-alcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. 2012. [[Last accessed on 2020 Feb 03]]. Available from: https://www.worldgastroenterology.org/guide lines/global-guidelines/nafld-nash/nafld-nash-eng lish .
- 28.Brown AJ. Viral hepatitis and fatty liver disease: How an unwelcome guest makes pâté of the host. Biochem J. 2008;416:15–7. doi: 10.1042/BJ20081916. [DOI] [PubMed] [Google Scholar]
- 29.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–11. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:16. doi: 10.1186/1471-230X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, Sharma A, Duseja A, Das A, Dhiman RK, Chawla YK, et al. A patients with nonalcoholic fatty liver disease (NAFLD) have higher oxidative stress in comparison to chronic viral hepatitis. J Clin Exp Hepatol. 2013;3:12–8. doi: 10.1016/j.jceh.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saverymuttu SH, Joseph AEA, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J. 1986;292:13–5. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasso M, Beaugrand M, Ledinghen VD, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–35. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Pearce SG, Thosani NC, Pan J. Noninvasive biomarkers for the diagnosis of steatohepatitis and advanced fibrosis in NAFLD. Biomark Res. 2013;1:7. doi: 10.1186/2050-7771-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 37.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Furuta M, Ekuni D, Yamamoto T, Irie K, Koyama R, Sanbe T, et al. Relationship between periodontitis and hepatic abnormalities in young adults. Acta Odontol Scand. 2010;68:27–33. doi: 10.3109/00016350903291913. [DOI] [PubMed] [Google Scholar]
- 39.Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between periodontitis and hepatic condition in Japanese women. J Int Acad Periodontol. 2006;8:89–95. [PubMed] [Google Scholar]
- 40.Kudo C, Kessoku T, Kamata Y, Hidaka K, Kurihashi T, Iwasaki T, et al. Relationship between non-alcoholic fatty liver disease and periodontal disease: A review and study protocol on the effect of periodontal treatment on non-alcoholic fatty liver disease. J Transl Sci. 2016;2:340–5. [Google Scholar]
- 41.Akinkugbe AA, Slade GD, Barritt AS, Cole SR, Offenbacher S, Petersmann A, et al. Periodontitis and non-alcoholic fatty liver disease, a population-based cohort investigation in the Study of health in pomerania. J Clin Periodontol. 2017;44:1077–87. doi: 10.1111/jcpe.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alazawi W, Bernabe E, Tai D, Janicki T, Kemos P, Samsuddin S, et al. Periodontitis is associated with significant hepatic fibrosis in patients with non-alcoholic fatty liver disease. PLoS One. 2001;12:e0185902. doi: 10.1371/journal.pone.0185902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mannaa FA, Abdel-Wahhab KG. Physiological potential of cytokines and liver damages. Hepatoma Res. 2016;2:131–43. [Google Scholar]
- 44.Yang YM, Seki E. TNF-α in liver fibrosis. Curr Pathobiol Rep. 2015;3:253–61. doi: 10.1007/s40139-015-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khosravi R, Ka K, Huang T, Khalili S, Nguyen BH, Nicolau B, et al. Tumor necrosis factor-α and interleukin-6: Potential inter organ inflammatory mediators contributing to destructive periodontal disease in obesity or metabolic syndrome. Mediators Inflamm. 2013;2013:728987. doi: 10.1155/2013/728987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang H, Zhang Y, Xiong X, Harville EW, Karmin O, Qian X. Salivary and serum inflammatory mediators among pre-conception women with periodontal disease. BMC Oral Health. 2016;16:131. doi: 10.1186/s12903-016-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddy NR, Madhu Babu DS, Reddy V, Sarath N, Reddy CV, Kumar AK. Estimation of tumor necrosis factor-α in chronic periodontitis and its co-relation with preterm gestation: A clinico biochemical study. J Orofac Sci. 2012;4:108–13. [Google Scholar]
- 48.Farhad SZ, Amini S, Khalilian A, Barekatain M, Mafi M, Barekatain M, et al. The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease. Dent Res J. 2014;11:549–52. [PMC free article] [PubMed] [Google Scholar]
- 49.Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease where do we stand? World J Gastroenterol. 2016;22:7236–51. doi: 10.3748/wjg.v22.i32.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dascălu DN. Markers of systemic inflammation in nonalcoholic fatty liver disease. Acta Medica Transilvanica. 2010;2:245–7. [Google Scholar]
- 51.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:727–35. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borough M, Maghsoudi Z, Khayyatzadeh S, Ghiasvand R, Askari G, Iraj B. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. 2016;15:28. doi: 10.4103/2277-9175.176368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Checchi L, Montevecchi M, Checchi V, Zappulla F. The relationship between bleeding on probing and subgingival deposits.An endoscopical evaluation. Open Dent J. 2009;3:154–60. doi: 10.2174/1874210600903010154. [DOI] [PMC free article] [PubMed] [Google Scholar]


