Abstract
The relationship between arterial hypertension and cognitive decline, two among the conditions with higher prevalence in the elderly population, has gained significant interest, in the scientific community, during the last few years, stemming from the numerous epidemiologic, experimental, and therapeutic evidences suggesting a non-casual correlation between the two conditions. In fact, the brain, for its substantial metabolic and functional complexity, is more susceptible to the harmful effect of high blood pressure than the other target organs. Chronic ischaemic impairment, microvascular damage, and neurodegenerative phenomena are the likely pathophysiologic basis for the correlation between hypertension and cognitive decline. Vascular dementia and Alzheimer’s disease, the two prominent forms of senile dementia, seem to represent the end result of the chronic exposure, during the lifetime, to harmful stimuli, among which the most relevant are the cardiovascular risk factors, at least from an epidemiological perspective. Evidences from interventional studies, although limited, seems to support the concept that to limit the spread of senile dementia, the early optimization of the control of cardiovascular risk factors, first and foremost hypertension, is crucial. The occurrence of a variable degree of mental decline, till overt dementia, in the hypertensive patient, represents the final step of a pathophysiologic process that began many years before. There is, then, the clear opportunity to control the pathophysiologic mechanisms leading to cognitive decline in the hypertensive patient.
Keywords: Hypertension, Cognitive decline, Dementia
Introduction
The progressive aging of the population has led to a profound nosographic change over the last few decades characterized by the progressive expansion of some clinical problems particularly frequent in the geriatric age. Among these, dementia stands out for its clinical and socio-economic relevance, a condition whose prevalence is destined to triple in the world over the next 30 years. These projections are alarming if we consider the severity of the pathology which in the course of a few years from the onset, leads to the complete loss of self-sufficiency, and the scarce efficacy of the therapeutic resources currently available in arresting them or at least in slowing down their progressive process.1 The appearance of dementia does not, however, represent the inevitable fate of those who age, as studies conducted on centenarians clearly indicate. The demonstration that the pathophysiological path that leads to dementia develops over decades opens the door to the possibility of preventive interventions that must necessarily be put in place in the preclinical phase of the disease, through early identification and correction of the main pathophysiological determinants.1
Hypertension, cognitive impairment, and dementia
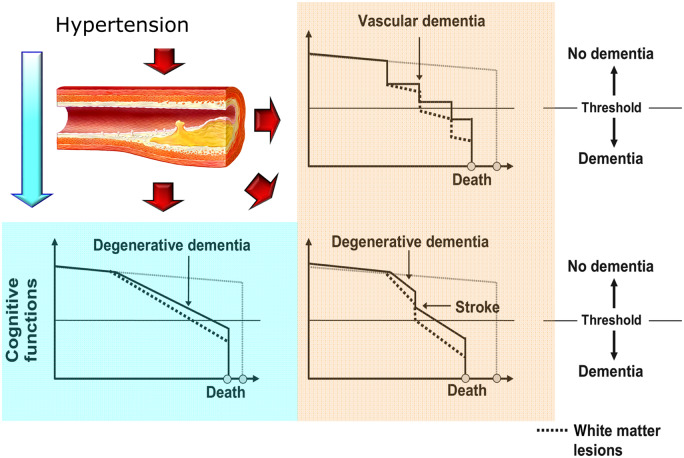
The development of a variable degree of cognitive deficit up to full-blown dementia, both vascular and Alzheimer-type, represents a rather common occurrence in those who during the life have been exposed for years to the harmful action of the various cardiovascular risk factors, such as diabetes and hypertension.2 The Coronary Artery Risk Development in Young Adults (CARDIA) study, for example, showed that cumulative exposure to various cardiovascular risk factors from adolescence is associated with worse cognitive performance over a 25-year observation period, with a prevalent impairment of executive functions and verbal memory.3 More recently, the evidence deriving from the data of the English cohort Insight 46 revealed a significant association between the increase in blood pressure values in young adulthood and greater damage in the brain white matter level and a smaller brain volume in the following decades.4 The hypertensive patient, even without clinical evidence of cerebrovascular disease, has an average cognitive performance lower than the normotension. It is interesting to note that the relationship between blood pressure and cognitive decline is linear and evident already starting from normal-high pressure values. This relationship, analogous to the one that has long been known between blood pressure and cerebrovascular events, demonstrates the existence of a continuum of cognitive impairment in the hypertensive patient that ranges from a modest impairment of the superior cortical functions to full-blown dementia. In a pathophysiological key, hypertension has all the potential to promote the appearance and progression of cognitive impairment (Figure 1).5,6
Figure 1.
Mechanisms of cognitive impairment in arterial hypertension (modified from ref. 5).
In fact, arterial hypertension represents the most important modifiable risk factor for stroke which, in turn, in addition to exacerbating the evolutionary process of Alzheimer's disease, exposes the patient to an increased risk of dementia.5 Furthermore, the hypertensive patient is more exposed to ischaemic cerebrovascular lesions which, although often occurring in an asymptomatic form, can lead to the development of dementia, especially if numerous and bilateral.5 Hypertension is often associated with the finding of white matter lesions, alterations of the cerebral white matter frequently found in patients with cognitive impairment and characterized by areas of demyelination and narrowing of the lumen of small-calibre arteries.5 Finally, it is possible that alterations of the cerebral blood flow, district or diffuse, sustained by the exposure of the cerebral vessels to chronically elevated blood pressure levels, even without being able to determine the appearance of frank ischaemic lesions, could induce neuronal metabolic impairment capable of triggering over time, the neuronal inflammatory and degenerative phenomena that underlie Alzheimer's disease, phenomena in which the accumulation of β-amyloid and other neurodegenerative proteins plays a central role.5–7 After the clinical manifestation of dementia, the pressure levels often tend to decrease gradually, even up to complete normalization, probably due to the patient's reduced perception of environmental hypertensive stimuli and to a possible direct influence of the specific brain lesions on the mechanisms of regulation of the blood pressure.8 Considering the enormous spread of high blood pressure, it is easy to imagine the impact in terms of prevention regarding the cognitive decline that could derive from an adequate control of blood pressure in all hypertensive patients.
Antihypertensive therapy and cognitive decline
Numerous longitudinal studies over the past few years have led to the hypothesis that antihypertensive treatment may represent a valuable tool to prevent the appearance of cognitive deterioration and dementia.9 The evidence derived from clinical studies, indeed, are not defined mainly because of the short duration of the various therapeutic interventions experimented from time to time with respect to the pathophysiological course of the pathology and the lack in many of these studies of a clear diagnostic characterization of dementia or mild cognitive impairment (MCI). The most solid evidence in this regard derives from controlled clinical trials. In the Systolic Hypertension in Europe (Syst-Eur) study, conducted in the elderly with isolated systolic hypertension, a 50% reduction in the risk of dementia was observed after 2 years of treatment with nitrendipine compared to what was observed in patients who received placebo.10 Prolonged follow-up with extension of active treatment also in patients initially randomized to placebo demonstrated a 55% reduction in the risk of dementia at 4 years in patients who had received active treatment immediately, compared to those initially randomized to placebo.11 Similarly, in the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), the perindopril/indapamide combination resulted in a 30% reduction in the risk of dementia compared to placebo in patients with pre-existing cerebrovascular disease.12 More recently, the Study on Cognition and Prognosis in the Elderly (SCOPE) has shown significant efficacy of treatment with the angiotensin II AT1 receptor inhibitor candesartan in preserving cognitive functions in elderly hypertensive patients with initial cognitive deficits,13 while in the Hypertension in the Very Elderly (HYVET) study, conducted in hypertensive patients over the age of 80, a trend was described towards a reduction in new cases of dementia in patients assigned to antihypertensive treatment.14 Quite recently, the results of the Systolic Blood Pressure Intervention Trial Memory and Cognition in Decreased Hypertension (SPRINT MIND) study have provided new and more vigorous support to the hypothesis of possible prevention of dementia through effective treatment of high blood pressure, producing the first convincing demonstration of the effectiveness of antihypertensive therapy in preventing senile cognitive decline.15 The SPRINT study enrolled ∼9400 hypertensive individuals (average age 68 years) at increased cardiovascular risk (but without a history of stroke or diabetes), randomized to a standard antihypertensive treatment (systolic pressure target <140 mmHg) or intensive (pressure target systolic <120 mmHg). The study was stopped early due to the evident superiority of intensive treatment in reducing cardiovascular events.16 The SPRINT MIND study was designed as part of the SPRINT study with a primary outcome represented by the diagnosis of probable dementia and with a composite secondary outcome of probable dementia or MCI. The evaluation of cognitive aspects was prolonged for at least 3 years after the conclusion of the SPRINT study with an average follow-up of 6 years. In patients assigned to intensive treatment (systolic pressure target <120 mmHg), an interesting trend was observed towards a reduction of dementia of all types (7.2 vs. 8.6 cases per 1000 person-years, hazard ratio 0.83 with a confidence interval between 0.67 and 1.04) and a significant reduction in the risk of developing MCI (14.6 vs. 18.3 cases per 1000 person-years, hazard ratio 0.81 with a confidence interval between 0.69 and 0.95) and in the risk of the composite outcome of MCI or probable dementia (20.2 vs. 24.1 cases per 1000 person-years, hazard ratio 0.85 with a confidence interval between 0.84 and 0.97). It does not seem useless to underline how the blood pressure in the SPRINT study was measured in the majority of cases directly by the patient in a context that could substantially replicate the home self-measurement of blood pressure. It is therefore legitimate to hypothesize that in the interpretation of the pressure values reached in the SPRINT study, the absence of a ‘white coat effect’, which could justify differences in the measured pressure values of even 12–15 mmHg compared to the traditional measurement, should be considered, compared to ‘office’ measurement adopted in all other clinical trials (thus bringing the pressure target reached in the intensive arm of the SPRINT study to values not far from the target of 140–130 mmHg suggested for the systolic pressure measured in the clinical setting).
Antihypertensive drugs and pathogenesis of cognitive decline
The main benefit of antihypertensive treatment is related to the reduction of blood pressure. Some interesting evidence, however, seems to suggest the possibility that some classes of antihypertensive drugs present added value in the prevention of cognitive impairment in the hypertensive patient. Calcium antagonists, in particular, have shown a particular protective efficacy against cerebrovascular events and cognitive impairment.10,11 Some experimental evidence suggests the possibility that these alleged neuroprotective and antidegenerative effects may be at least partially mediated by a beneficial effect on calcium homeostasis in the brain and by a scavenging action of oxygen-free radicals.9 Similarly, the protective effect against cognitive functions exerted by drugs active on the renin–angiotensin–aldosterone system—angiotensin-converting enzyme (ACE-I) inhibitors and angiotensin II type 1 (AT1) receptor blockers (ARB)—seems to be at least partially independent of the blood pressure reduction.9 The biological assumptions of the possible neuroprotective efficacy of these drugs are to be found in the existence of an intrinsic brain renin–angiotensin system capable of modulating cognitive processes at various levels, probably through the AT2 and AT4 receptors, and of intervening in the pathogenesis of the neurodegenerative damage through the induction of inflammatory cytokines and free radicals of oxygen, the inhibition of the release of acetylcholine, the accumulation of β-amyloid and the district reduction of cerebral blood flow. The different point of action of ACE-I and ARB on the renin–angiotensin system accounts for some evidence of a possible superiority of ARB compared to ACE-I in improving cognitive performance and in preventing the appearance of both vascular and type Alzheimer's dementia, evidence however, in line with what has already been hypothesized also with regard to stroke prevention.9
The earlier the better
The appearance of a variable degree of decline up to overt dementia in the hypertensive patient represents the final moment of a complex pathophysiological path started many years earlier. It is therefore clear that the opportunity for early intervention should be taken to defuse the pathophysiological mechanisms underlying the development and progression of cognitive decline in the hypertensive patient as soon as possible. The current possibilities of an early clinical diagnosis of a possible cognitive deficit in the hypertensive patient are rather limited due to the relative complexity of adequately sensitive and specific neuropsychological tests which makes them difficult to apply on a large scale. The tools commonly used in clinical practice, such as the Mini Mental State Examination, despite the undeniable advantage of being able to be administered by anyone who has performed a minimum of training, cannot identify the earliest stages of cognitive impairment from hypertension. Modern technology seems to be able to open the doors to a really early diagnosis. A recent study based on an advanced magnetic resonance technique—tractography or diffusion tensor—through which it is possible to reconstruct the white matter fibres for each subject and study the microstructural integrity of the same to obtain a precise profile of the hypertensive damage, it seems to provide interesting perspectives in this regard.17 The study was conducted on a group of hypertensive patients who were compared with normotensive subjects. In hypertensive patients, a deterioration of those nerve fibres that connect brain areas typically involved in attention, emotions, and memory has been found. An important aspect to consider is that all the patients studied did not show clinical signs of dementia and on a conventional neuroimaging radiological examination they were free of signs of damage.
Hypotension and dementia: the downside
In elderly patients, the impact of arterial hypertension on the progression of cognitive decline tends to become progressively less evident while the impact of low blood pressure values is increasingly relevant18,19 (Figure 2). The causes of this association between low blood pressure and cognitive decline are probably to be found in the reduction of cerebral blood flow due to low blood pressure in a vascular bed which, due to age and/or chronic exposure to cardiovascular risk factors, has lost much of its self-regulation capacity.20 Therefore, the evidence of a more rapid cognitive decline in elderly patients with low blood pressure induced by antihypertensive treatment but not in those spontaneous hypotheses is not surprising. It is therefore clear the importance of avoiding excessively low blood pressure in the elderly and of routinely seeking orthostatic hypotension as this condition, very often iatrogenic, is associated with an increased risk of cognitive decline and dementia.18
Figure 2.
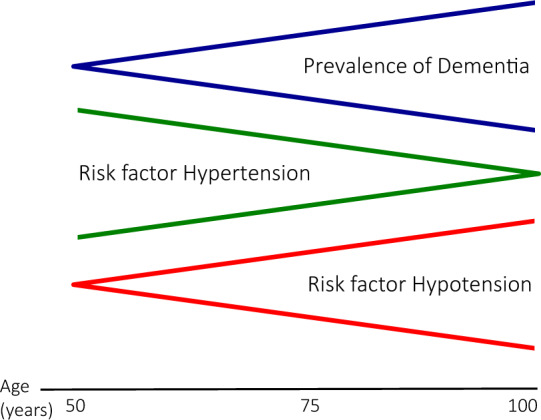
Relationship between hypertension, hypotension and dementia (from ref. 18).
Conclusions
The progressive aging of the population is causing a progressive expansion of those age groups in which hypertension is more frequent and the susceptibility to develop dementia is greater. The socio-economic impact of dementia, which is already very significant, appears destined to assume even greater proportions in the near future. Prevention is more than ever the only truly winning strategy against dementia. The association between hypertension and cognitive decline is certainly not causal. In the meantime, that the pathophysiological mechanisms underlying this relationship are definitively clarified and that the tools for a certain early diagnosis of cerebral organ damage from hypertension are available for large sections of the population, considering the devastating clinical and socio-economic impact of dementia, it has a profound ethical as well as clinical sense to draw from the numerous scientific evidence already available a further incentive to seek, with determination, the optimal blood pressure control in all hypertensive patients.
Conflict of interest: none declared.
References
- 1. Querfurth HW, LaFerla FM.. Alzheimer’s disease. N Engl J Med 2010;362:329–344. [DOI] [PubMed] [Google Scholar]
- 2. Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS.. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer RA, Coker LH, Sidney S.. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Parker TD, Malone IB, Lu K, James S-N, Keshavan A, Murray-Smith H, Wong A, Buchanan SM, Keuss SE, Gordon E, Coath W, Barnes A, Dickson J, Modat M, Thomas D, Crutch SJ, Hardy R, Richards M, Fox NC, Schott JM.. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol 2019;18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Staessen JA, Richart T, BirkenhäGer WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension 2007;49:389–400. [DOI] [PubMed] [Google Scholar]
- 6. de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 2004;3:184–190. [DOI] [PubMed] [Google Scholar]
- 7. Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH.. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skoog I, Nilsson L, Persson G, Lernfelt B, Landahl S, Palmertz B, Andreasson L-A, Odén A, Svanborg A.. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 9. Duron E, Hanon O. Antihypertensive treatments, cognitive decline, and dementia. J Alzheimer’s Dis 2010;20:903–914. [DOI] [PubMed] [Google Scholar]
- 10. Forette F, Seux M-L, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene M-R, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R.. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998;352:1347–1351. [DOI] [PubMed] [Google Scholar]
- 11. ) Forette F, Seux ML, Staessen JA; Systolic Hypertension in Europe Investigators . The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002;162:2046–2052. [DOI] [PubMed] [Google Scholar]
- 12. Tzourio C, Anderson C, Chapman N; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003;163:1069–1075. [DOI] [PubMed] [Google Scholar]
- 13. Saxby BK, Harrington F, Wesnes KA, McKeith IG, Ford GA.. Candesartan and cognitive decline in older patients with hypertension: a substudy of the SCOPE trial. Neurology 2008;70:1858–1866. [DOI] [PubMed] [Google Scholar]
- 14. Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C.. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008;7:683–689. [DOI] [PubMed] [Google Scholar]
- 15. Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh M-K, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT, Wright CB; SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. Erratum in: N Engl J Med 2017;377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carnevale L, D’Angelosante V, Landolfi A, Grillea G, Selvetella G, Storto M, Lembo G, Carnevale D.. Brain MRI fiber-tracking reveals white matter alterations in hypertensive patients without damage at conventional neuroimaging. Cardiovasc Res 2018;114:1536–1546. [DOI] [PubMed] [Google Scholar]
- 18. Messerli FH, Streit S, Grodzicki T.. The oldest old: does hypertension become essential again? Eur Heart J 2018;39:3144–3146. [DOI] [PubMed] [Google Scholar]
- 19. Streit S, Poortvliet RKE, Gussekloo J.. Lower blood pressure during antihypertensive treatment is associated with higher all-cause mortality and accelerated cognitive decline in the oldest-old—data from the Leiden 85-plus Study. Age Ageing 2018;47:545–550. [DOI] [PubMed] [Google Scholar]
- 20. Novak V, Hajjar I.. The relationship between blood pressure and cognitive function. Nat Rev Cardiol 2010;7:686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]