Abstract
Left ventricular non-compaction (LVNC) is defined by the triad: prominent trabecular anatomy, thin compacted layer, and deep inter-trabecular recesses. No person, sick or healthy, demonstrates identical anatomy of the trabeculae; their configuration represents a sort of individual dynamic ‘cardiac fingerprinting’. LVNC can be observed in healthy subjects with normal left ventricular (LV) size and function, in athletes, in pregnant women, as well as in patients with haematological disorders, neuromuscular diseases, and chronic renal failure; it can be acquired and potentially reversible. When LVNC is observed in patients with dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, restrictive cardiomyopathy, or arrhythmogenic cardiomyopathy, the risk exists of misnaming the cardiomyopathy as ‘LVNC cardiomyopathy’ rather than properly describe, i.e. a ‘DCM associated with LVNC’. In rare infantile CMPs (the paradigm is tafazzinopathy or Barth syndrome), the non-compaction (NC) is intrinsically part of the cardiac phenotype. The LVNC is also common in congenital heart disease (CHD) as well as in chromosomal disorders with systemic manifestations. The high prevalence of LVNC in healthy athletes, its possible reversibility or regression, and the increasing detection in healthy subjects suggest a cautious use of the term ‘LVNC cardiomyopathy’, which describes the morphology, but not the functional profile of the cardiac disease. Genetic testing, when positive, usually reflects the genetic causes of an underlying cardiomyopathy rather than that of the NC, which often does not segregate with CMP phenotype in families. Therefore, when associated with LV dilation and dysfunction, hypertrophy, or CHD, the leading diagnosis is cardiomyopathy or CHD followed by the descriptor LVNC.
Keywords: Left ventricular non-compaction (LVNC), Cardiomyopathy, Trabeculae, Genetics
Definition of LVNC
The myocardial ventricular wall consists of a compact layer and a trabecular layer characterized by the alternation of trabeculae and inter-trabecular recesses. The definition of non-compact myocardium implies the absence or reduction of a compact layer of the left ventricular (LV) wall. The three anatomical components are:
The thin compact layer;
The prominent trabeculae that protrude into the endocavitary side of the ventricle; and
The deep inter-trabecular recesses.
The definition left ventricular non-compaction (LVNC) does not include descriptors of the left ventricular function and size. Per se, the non-compacted myocardium describes a structural/anatomical condition of the LV wall.
Function of the trabeculae
Trabeculae work as small, mechanically active, levers during early systolic ejection and increase perfusion of the sub-endocardium by expanding the interface between the endocardium and endoventricular blood. The embryogenic hypothesis of the origin of LVNC postulates that during the early embryogenesis the myocardial compaction arrests in favour of the trabecular myocardium. In the process of cardiac chamber maturation, the trabeculae constitute the primordial state of development of the ventricular myocardial wall. Indeed, in the embryonic myocardium, the trabeculae contribute to the cardiac output, nutrition of trabecular myocytes, and oxygen uptake prior to coronary vascularization.1–3 As the cardiac walls mature, the compact myocardium and the coronary artery system develop concomitantly, the trabeculae undergo progressive remodelling.
LVNC in newborn/infant vs. LVNC in adults
LVNC in newborn with either cardiomyopathy or congenital heart disease (CHD) is likely to be attributable to an arrest in the maturation of the ventricular walls in the context of the underlying cardiac disease. LVNC in an adult in whom it was not present at birth and did not cause clinical manifestation until the time of clinical observation is unlikely to have the same origin as that observed in the newborn.
The embryogenic hypothesis does not explain why in adult age LVNC has been observed as an acquired and potentially reversible trait4 in athletes or in pregnant women or in patients with hypertension, renal, and haematological disorders.5–12
Diagnosis
No person, sick or healthy, demonstrates identical anatomy of the trabeculae. The trabecular anatomy constitutes a true cardiac fingerprint. The criteria for defining LVNC are based exclusively on imaging by two-dimensional transthoracic echocardiography, cardiovascular magnetic resonance, or computed tomography without any mention of LV dysfunction. Imaging provides qualitative descriptors of the LV trabeculae (prominent, pronounced, marked, severe, etc.) and quantitative assessments of ratios between thickness, mass, and volumes of non-compacted and compacted LV layers. Cut-off values of these ratios are obtained by either increasing the trabecular thickness or decreasing the thickness of the compact layer.13,14 In most indexes, the ratios between compacted and NC thickness/volume/mass drive the definition of LVNC.4 Nosological assignment exists in the absence of diagnostic guidelines and consensus criteria.13,14
Cardiac magnetic resonance (CMR) is generally superior to echocardiography in the identification of NC myocardium, with 12 times higher prevalence of LVNC when the assessment is made with CMR respect to echocardiography amongst different cardiac patients cohorts.15 Multidetector computed tomography angiography is useful when cardiovascular CMR is not feasible or when echocardiography and CMR provide discordant data; it adds the advantage of non-invasive investigation of the coronary tree. Nevertheless, the sensitivity and specificity of NC/compaction ratios by echocardiography, cardiovascular magnetic resonance imaging, and computed tomography are difficult to establish because of the absence of referral gold standards.
The diagnosis and the definition of LVNC do not mention LV dysfunction. LV function and haemodynamic can be normal in individuals with LVNC. Tissue Doppler regional deformation seems to distinguish isolated LVNC from dilated cardiomyopathy (DCM),13,16 and 2D speckle-tracking echocardiography seems to detect myocardial dysfunction in children with LVNC and normal LV function by conventional methods.17
LVNC as isolated and non-isolated trait
Isolated LVNC in healthy subjects
Isolated LVNC can be observed in individuals with normal LV systolic and diastolic function, size, and wall thickness. The pathogenesis in otherwise normal hearts is unknown. The long-term follow-up MESA study cohort first showed that LVNC does not impact prognosis (MESA study cohort).18 TASCFORCE study gave similar results showing that long-axis NC/C ratio was negatively correlated with systolic blood pressure and LV mass, but the effect sizes were minimal and without clinical significance.19 In a meta-analysis including 574 patients with LVNC and 677 without LVNC, no hard cardiac events occurred among 158 patients with LVNC, no late gadolinium enhancement (LGE) and preserved LV ejection fraction (LVEF) demonstrating that, when matched for LVEF, individuals with and without LVNC share the same prognosis.20
LVNC in cardiomyopathies
LVNC can occur in hearts with DCM, hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM), or arrhythmogenic cardiomyopathy (ACM). The prominent trabeculae in DCM may represent the effect of a greater need for leverage in early systole and may contribute to the maintenance of the contractile function. The prominent trabeculae in a heart with HCM may instead respond purposely to greater demands of nutrition of the trabecular myocytes and oxygen uptake.1,3 LVNC can be observed in cardiomyopathies showing overlapping phenotypes, such as in mitochondrial DNA-related cardiomyopathies (HCM with potential dilated evolution)4 or associated with LV dilation and dysfunction at onset, such as in the paradigmatic infantile DCM in Barth syndrome.21 In a prospective study including 113 patients with LVNC followed-up for 4-years the adverse outcome was associated with the presence of cardiac symptoms, positive family history of non-ischaemic cardiomyopathy, neuromuscular diseases, prior malignant arrhythmias, and thromboembolic event. The extent of trabeculation alone had no prognostic implications above and beyond the well-established prognostic markers such as LV dilation or LGE.22
LVNC in congenital heart diseases
LVNC is common in CHDs (from patent ductus arteriosus, septal defects, Ebstein’s anomaly, to hypoplastic left heart syndrome)23 as well as in chromosomal disorders (1p36 deletion syndrome; interstitial 1q43–q43del; del(1)(q) syndrome; del5q35; 7p14.3p14.1 deletion, 8p23.1 del syndrome; 18p subtelomeric deletion; 22q11.2 deletion syndrome; 22q11.2 distal deletion syndrome; trisomy 13 and 18; tetrasomy 5q35.2–5q35; Robertsonian translocation 13;14; and mosaics such as 45,X/46XX and 45,X/46,X,i(Y)(p11)).24
LVNC in individuals without cardiac diseases
As anticipated, isolated LVNC may occur in athletes,8,11,12 in pregnant women,10 in patients with haematological disorders,7 neuromuscular disorders,5,25 and chronic renal failure.6 The review and meta-analysis by Ross et al.15 provided the comparative prevalence of LVNC when assessed with 2DTTE or with CMR in different cohorts. Between athletes, the estimated LVNC prevalence was 3.16% in 2DTTE- and 27.29% in CMR-assessed-cohorts, respectively. In non-cardiac cohorts, LVNC prevalence was 2.21% and 36.2% with 2DTTE and CMR, respectively. Furthermore, among the 1480 asymptomatic, free from known cardiovascular diseases, participants to TASCFORCE study a significant proportion (15%) fulfilled CMR-based diagnostic criteria for LVNC.19
LVNC in syndromic diseases
In sporadic or familial syndromes with LVNC, the NC morphology is one of the cardiac traits associated with both monogenic defects and chromosomal anomalies. The former include rare diseases: some of them (i.e. Anderson–Fabry disease and Danon disease)26,27 are well known by cardiologists because their HCM-like phenotype is often the first clinically overt manifestation. The latter are complex syndromes that display several multiorgan defects: chromosomal abnormalities include deletions, translocations, and trisomy or tetrasomy.23
Genetics and LVNC
Current recommendations suggest genetic evaluation for patients with cardiomyopathy and LVNC, or syndromes in which LVNC may recur, or incidentally detected LVNC.4,28 The clinical family screening (including cardio-imaging) should be performed in order to assess whether the trait is sporadic or familial (more family members show LVNC) and whether it segregates with the major cardiac phenotype, either cardiomyopathy or CHD or other, non-cardiac diseases/syndromes. The family screening further deepens with genetic test exploring multiple candidate genes. The possible scenarios are as follows:
The disease-causing mutation segregates with the cardiomyopathy phenotype and also segregates with the LVNC. In this scenario, the coincidence of the cardiomyopathy with the LVNC may imply that the trabecular anatomy is part of the cardiomyopathy itself (the typical example is tafazzinopathy in new borns/children).
The mutation that is associated with the cardiomyopathy in the family does not segregate with the LVNC: asymptomatic family members with normal LV size and function may show LV hypertrabeculation or vice-versa, affected and mutated family members may show the cardiomyopathy but not LVNC. A dual interpretation is possible at the baseline evaluation: (i) the family members demonstrating LVNC and normal LV size and function are younger (offspring) than the proband and affected relatives; the phenotype might manifest later on in the course of their life; (ii) the cause of LVNC is additional to that of the cardiomyopathy (two or more genetic mutations/variants, with one potentially influencing LV trabecular anatomy).
Clinical monitoring of families may demonstrate that the trabecular anatomy can evolve: LVNC may progress or regress. Although a few genes have been proposed in the past as specifically associated with isolated LVNC, there are no confirmations and validations. The genes routinely investigated are usually those that cause cardiomyopathies in which LVNC may be present.25
Turn healthy subjects into sick: the case
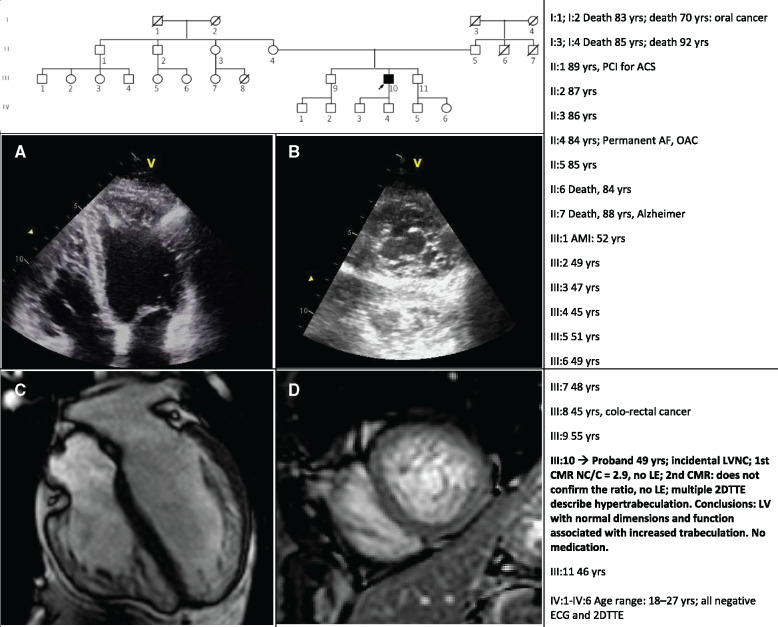
The diagnostic work-up is especially complex when healthy individuals with prominent trabeculae, up to LVNC are sent to the genetic path. The genetic test is often asked to compensate for the clinical uncertainty. The example described in the next paragraph demonstrates the devastating effect of the diagnosis of ‘LVNC cardiomyopathy’ in a healthy individual and in his family. The 49-year-old male, agonistic dance sport athlete, was addressed to our attention after the incidental detection of LVNC during a sport suitability evaluation. This led from 2DTTE to a 1st CMR that confirmed NC/C = 2.9 and 1 year later, to a second CMR that did not report LVNC. A further 2DTTE confirmed hypertrabeculation, normal LV size and function. A large panel of genes tested negative. Clinical family screening (1st grade relatives: in parents, sibs, and offspring) gave normal results in the absence of family history of cardiac diseases or sudden cardiac death (Figure 1).
Figure 1.
The figure shows the family pedigree; the table summarizes the clinical history of the proband and his relatives: there is no evidence of cardiac disease in the family. Images show the 2DTTE (Panels A and B) and the CMR (Panels C and D) views of the proband’s heart. During 3 years of follow-up, no signs of dilatation and left ventricular dysfunction were observed. Repeated 24 h-ECG Holters have not documented any significant arrhythmias. The proband, who drastically reduced his physical activity, is asymptomatic. 2DTTE, two-dimensional transthoracic echocardiography; ACS, acute coronary syndrome; AF, atrial fibrillation; AMI, acute myocardial infarction; CMR, cardiac magnetic resonance; LE, late enhancement; LV, left ventricle; OAC, oral anticoagulant; PCI, primary coronary intervention.
Why are there no novel guidelines: since 2006 (AHA) and 2008 (ESC) the LVNC problem is still not solved
The scientific societies probably look at the LVNC with caution: is LVNC a CMP? More than 10 years ago the formulation of guidelines would have certainly had non-sufficient evidence. Today, in 2020, before writing new nosology or new definitions or diagnostic criteria, the evidences from hundreds of imaging studies in patients with cardiac diseases, in cohorts of healthy controls and athletes and in patients with non-cardiac diseases should be considered. The conclusions of a recent review and meta-analysis contain an essential warning: ‘Left ventricular non-compaction in adult populations is a poorly defined entity which is likely to be both physiological adaptation and pathological disease. There is a higher prevalence with CMR imaging, which identify LVNC changes more readily. The clinical significance of these findings remains unclear; however, there is significant potential for overdiagnosis, overtreatment, and unnecessary follow-up’. We fully agree with the conclusions of this study, as we have already anticipated in the past.4,24,28
Conclusions
The LVNC can be present at birth in association with syndromic or non-syndromic CHD or rare cardiomyopathies. However, in adults the LVNC is an acquired trait, potentially reversible, recurring in cardiac diseases (cardiomyopathies and CHD), in neurologic, renal and haematologic diseases, in hypertension and during pregnancy or in athletes. As such, the misleading term ‘LVNC cardiomyopathy’ should be abandoned in favour of DCM or HCM or ACM or RCM with LV hypertrabeculation or LVNC. Healthy individuals incidentally diagnosed with increased trabeculation should not be labelled as affected by a cardiomyopathy when their LV size and function are normal.
Conflict of interest: none declared.
References
- 1. Liu J, Bressan M, Hassel D, Huisken J, Staudt D, Kikuchi K, Poss KD, Mikawa T, Stainier DYR.. A dual role for ErbB2 signaling in cardiac trabeculation. Development 2010;137:3867–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minot CS. The embryological basis of pathology. Science 1901;13:481–498. [DOI] [PubMed] [Google Scholar]
- 3. Rychter Z, Ostadal B.. Mechanism of the development of coronary arteries in chick embryo. Folia Morphol 1971;19:113. [PubMed] [Google Scholar]
- 4. Arbustini E, Favalli V, Narula N, Serio A, Grasso M.. Left ventricular noncompaction: a distinct genetic cardiomyopathy? J Am Coll Cardiol 2016;68:949–966. [DOI] [PubMed] [Google Scholar]
- 5. Hofer M, Stöllberger C, Finsterer J.. Acquired noncompaction associated with myopathy. Int J Cardiol 2007;121:296–297. [DOI] [PubMed] [Google Scholar]
- 6. Markovic N, Dimkovic N, Damjanovic T, Loncar G, Dimkovic S.. Isolated ventricular noncompaction in patients with chronic renal failure. Clin Nephrol 2008;70:72–76. [DOI] [PubMed] [Google Scholar]
- 7. Piga A, Longo F, Musallam KM, Veltri A, Ferroni F, Chiribiri A, Bonamini R.. Left ventricular noncompaction in patients with β‐thalassemia: uncovering a previously unrecognized abnormality. Am J Hematol 2012;87:1079–1083. [DOI] [PubMed] [Google Scholar]
- 8. Gati S, Chandra N, Bennett RL, Reed M, Kervio G, Panoulas VF, Ghani S, Sheikh N, Zaidi A, Wilson M, Papadakis M, Carré F, Sharma S.. Increased left ventricular trabeculation in highly trained athletes: do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013;99:401–408. [DOI] [PubMed] [Google Scholar]
- 9. Gati S, Papadakis M, Van Niekerk N, Reed M, Yeghen T, Sharma S.. Increased left ventricular trabeculation in individuals with sickle cell anaemia: physiology or pathology? Int J Cardiol 2013;168:1658–1660. [DOI] [PubMed] [Google Scholar]
- 10. Gati S, Papadakis M, Papamichael ND, Zaidi A, Sheikh N, Reed M, Sharma R, Thilaganathan B, Sharma S.. Reversible de novo left ventricular trabeculations in pregnant women: implications for the diagnosis of left ventricular non-compaction in low risk populations. Circulation 2014;130:475–483. [DOI] [PubMed] [Google Scholar]
- 11. D'Ascenzi F, Pelliccia A, Natali BM, Bonifazi M, Mondillo S.. Exercise-induced left-ventricular hypertrabeculation in athlete's heart. Int J Cardiol 2015;181:320–322. [DOI] [PubMed] [Google Scholar]
- 12. Caselli S, Jost CHA, Jenni R, Pelliccia A.. Left ventricular noncompaction diagnosis and management relevant to pre-participation screening of athletes. Am J Cardiol 2015;116:801–808. [DOI] [PubMed] [Google Scholar]
- 13. Jenni R, Oechslin EN, van der Loo B.. Isolated ventricular non-compaction of the myocardium in adults. Heart 2007;93:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ulusoy RE, Kucukarslan N, Kirilmaz A, Demiralp E.. Noncompaction of ventricular myocardium involving both ventricles. Eur J Echocardiogr 2006;7:457–460. [DOI] [PubMed] [Google Scholar]
- 15. Ross SB, Jones K, Blanch B, Puranik R, McGeechan K, Barratt A, Semsarian C.. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur Heart J 2020;41:1428–1436. [DOI] [PubMed] [Google Scholar]
- 16. Niemann M, Liu D, Hu K, Cikes M, Beer M, Herrmann S, Gaudron PD, Hillenbrand H, Voelker W, Ertl G, Weidemann F.. Echocardiographic quantification of regional deformation helps to distinguish isolated left ventricular non‐compaction from dilated cardiomyopathy. Eur J Heart Fail 2012;14:155–161. [DOI] [PubMed] [Google Scholar]
- 17. Ari ME, Cetin II, Kocabas A, Ekici F, Ceylan O, Surucu M.. Decreased deformation in asymptomatic children with isolated left ventricular non-compaction and normal ejection fraction. Pediatr Cardiol 2016;37:201–207. [DOI] [PubMed] [Google Scholar]
- 18. Zemrak F, Ahlman MA, Captur G, Mohiddin SA, Kawel-Boehm N, Prince MR, Moon JC, Hundley WG, Lima JAC, Bluemke DA, Petersen SE.. The relationship of left ventricular trabeculation to ventricular function and structure over a 9.5-year follow-up: the MESA study. J Am Coll Cardiol 2014;64:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weir-McCall JR, Yeap PM, Papagiorcopulo C, Fitzgerald K, Gandy SJ, Lambert M, Belch JJF, Cavin I, Littleford R, Macfarlane JA, Matthew SZ, Nicholas RS, Struthers AD, Sullivan F, Waugh SA, White RD, Houston JG.. Left ventricular noncompaction: anatomical phenotype or distinct cardiomyopathy? J Am Coll Cardiol 2016;68:2157–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grigoratos C, Barison A, Ivanov A, Andreini D, Amzulescu M-S, Mazurkiewicz L, De Luca A, Grzybowski J, Masci PG, Marczak M, Heitner JF, Schwitter J, Gerber BL, Emdin M, Aquaro GD.. Meta-analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular noncompaction. JACC Cardiovasc Imaging 2019;12:2141–2151. [DOI] [PubMed] [Google Scholar]
- 21. Ferreira C, Thompson R, Vernon H. Barth syndrome. 2014 Oct 9. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, eds. GeneReviews® [Internet]. Seattle (WA: ): University of Washington, Seattle; 1993. –2016. http://www.ncbi.nlm.nih.gov/books/NBK247162/PubMed PMID: 25299040. [Google Scholar]
- 22. Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, Schwitter J, Mushtaq S, Vovas G, Sormani P, Aquaro GD, Monney P, Segurini C, Guglielmo M, Conte E, Fusini L, Dello Russo A, Lombardi M, Gripari P, Baggiano A, Fiorentini C, Lombardi F, Bartorelli AL, Pepi M, Masci PG.. Long-term prognostic value of cardiac magnetic resonance in left ventricle noncompaction: a prospective multicenter study. J Am Coll Cardiol 2016;68:2166–2181. [DOI] [PubMed] [Google Scholar]
- 23. Stähli BE, Gebhard C, Biaggi P, Klaassen S, Valsangiacomo Buechel E, Attenhofer Jost CH, Jenni R, Tanner FC, Greutmann M.. Left ventricular non‐compaction: prevalence in congenital heart disease. Int J Cardiol 2013;167:2477–2481. [DOI] [PubMed] [Google Scholar]
- 24. Arbustini E, Toro D, Giuliani A, Narula L, Favalli N, V. Left ventricular noncompaction In: Camm AJL, Thomas F., Serruys PW, Maurer G, eds. The ESC Textbook of Cardiovascular Medicine. 3rd ed: Oxford University Press; 2018. [Google Scholar]
- 25. Arbustini E, Di Toro A, Giuliani L, Favalli V, Narula N, Grasso M.. Cardiac phenotypes in hereditary muscle disorders: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2485–2506. [DOI] [PubMed] [Google Scholar]
- 26. Kozor R, Callaghan F, Tchan M, Hamilton-Craig C, Figtree GA, Grieve SM.. A disproportionate contribution of papillary muscles and trabeculations to total left ventricular mass makes choice of cardiovascular magnetic resonance analysis technique critical in Fabry disease. J Cardiovasc Magn Reson 2015;17:22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Der Starre P, Deuse T, Pritts C, Brun C, Vogel H, Oyer P.. Late profound muscle weakness following heart transplantation due to Danon disease. Muscle Nerve 2013;47:135–137. [DOI] [PubMed] [Google Scholar]
- 28. Arbustini E, Weidemann F, Hall JL.. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol 2014;64:1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]