Abstract
Background and Aims
The gut microbiota plays an important role in the metabolization and modulation of several types of drugs. With this study we aimed to review the literature relating to microbial drug metabolism of medication prescribed in inflammatory bowel disease [IBD] practice.
Methods
A systematic literature search was performed in Embase and PubMed from inception to October 2019. The search was conducted with predefined MeSH/Emtree and text terms. All studies regarding drug metabolism by microbiota of medication prescribed in IBD practice were eligible. A total of 1018 records were encountered and 89 articles were selected for full text reading.
Results
Intestinal bacterial metabolism or modulation is of influence in four specific drugs used in IBD (mesalazines, methotrexate, glucocorticoids and thioguanine). The gut microbiota cleaves the azo-bond of sulfasalazine, balsalazide and olsalazine and releases the active moiety 5-aminosalicylic acid. It has an impact on the metabolization and potentially on the response of methotrexate therapy. In particular, thioguanine can be converted by intestinal bacteria into the pharmacologically active 6-thioguanine nucleotides without the requirement of host metabolism. Glucocorticoid compounds can be prone to bacterial degradation.
Conclusion
The human intestinal microbiota can have a major impact on drug metabolism and efficacy of medication prescribed in IBD practice. A better understanding of these interactions between microbiota and drugs is needed and should be an integral part of the drug development pathway of new IBD medication.
Keywords: Inflammatory bowel disease, drug metabolism, microbiota
1. Introduction
The microbiome refers to the collection of genomes from all the microorganisms in the environment. Microbiota, on the other hand, usually refers to specific microorganisms [e.g. bacteria, viruses and fungi] that are found within a specific environment, leading to localized differences in the microbiota of each person, depending on where in the body the microbiota is collected from. The human gut microbiome is a complex, dense and diverse microbial community. It has an estimated composition of more than five million unique genes and more than 100 trillion cells.1 Despite this diversity, the gut microbes are mainly distributed in four bacterial phyla, namely Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria.2 The gut microbiota plays an important role in many aspects of human health, including metabolic, immune and neurobehavioural traits, but is also an important drug target.3
The gut microbiota can activate, inactivate or make a drug toxic. It can affect drug metabolism directly through biotransformation, which is the conversion of organic compounds into other chemical forms or metabolites and can be aided by microorganisms.4 The most common mechanisms of gut microbiota drug metabolism are hydrolytic and reductive reactions. In addition, many other chemical reactions including acetylation, deamination, dehydroxylation, decarboxylation, demetylation, deconjugation and proteolyse have been reported.5 Besides biotransforming medication, the gut microbiota can control the efficacy of medication indirectly by altering the host metabolism and producing metabolites that compete with the drug receptor.5
The importance of the interaction between microbiota and medication was observed in 1993 when soriduvine was introduced to the Japanese market as a drug for the treatment of varicella-zoster virus infection. Within 40 days after introduction 18 people died after they were co-administrated with soriduvine next to oral 5-fluouracil, an anti-cancer drug. Later it was discovered that soriduvine was transformed by gut microbiota into (E)-5-(2-bromovinyl)uracil, which inhibits the metabolism of 5-fluorouracil leading to toxic levels of this particular drug.6 This drug–drug interaction underlined the importance of studying drug metabolism by the gut microbiota.
Besides these possible toxic effects, the gut microbiota can also be used to activate or enhance the efficacy of medication, i.e transforming pro-drugs to active drugs. One example of this mechanism is demonstrated in lactulose therapy. This drug is hydrolysed by intestinal bacteria to form acetic and lactic acid, which lower the pH in the gastrointestinal tract. Due to the lower pH ammonia and other amines become protonated and tend to be excreted in the faeces, leading to the laxative effect of lactulose and its use as a treatment for hyperammonia and hepatic encephalopathy.7,8
In recent years extensive research has been conducted regarding the role of the microbiome in the pathogenesis of inflammatory bowel disease [IBD] and the therapeutic potential of gut microbiota in treating IBD, including faecal microbiota transplantation.2 Less attention has been paid to the role of gut microbiota in the metabolization and modulation of several drugs given for IBD. In this review we aim to describe the role of microbial drug metabolism in IBD and thus the potential for targeted IBD therapy.
2. Methods
A systematic electronic literature search was performed by using the Embase and PubMed electronic databases to collect publications before October 2019. The search was performed using a combination of the following MeSH/Emtree and text terms with asterisks[*] where applicable: ‘Inflammatory bowel disease, Crohn’s disease, ulcerative colitis, gastrointestinal microbiome, microflora, metagenome, microbiota, bacteria, pharmacokinetics, biotransformation, activation, inactivation, metabolism, prodrugs, conversion, toxicity, efficacy and drug microbiome interaction’. The whole search was combined with generic and branded names for medication given for IBD [Supplementary data 1]. The search was not restricted exclusively to human subjects to gather all available studies with regard to this topic. The reference lists of identified papers were checked to find additional relevant studies missed during the original search. Any original full article or conference abstract written in Dutch or English was eligible and there was no restriction in terms of publication date. Non-original articles, case reports, duplicates and articles written in a language other than Dutch or English were excluded from this review.
After the search, the collected literature was screened on title and abstract by the first and last author [F.C. and N.dB.] for eligibility for full text evaluation. Disagreements regarding study eligibility was resolved by consensus between the first and last author [F.C. and N.d.B.]. The same process was used for the full text screening. Studies were included if they reported outcomes on drug metabolism by microbiota even if this metabolism was observed in subjects without IBD but with medication regularly prescribed in IBD practice. When studies reported the use of coating compounds for delayed release, such as polysaccharide, they were not considered a true microbiota-activated delivery system and were therefore excluded. Furthermore studies that described the effect of medication on the microbiome itself were not eligible. Studies regarding microbial drug metabolization of experimental IBD medication or studies of probiotics were excluded as well.
2.1. Results
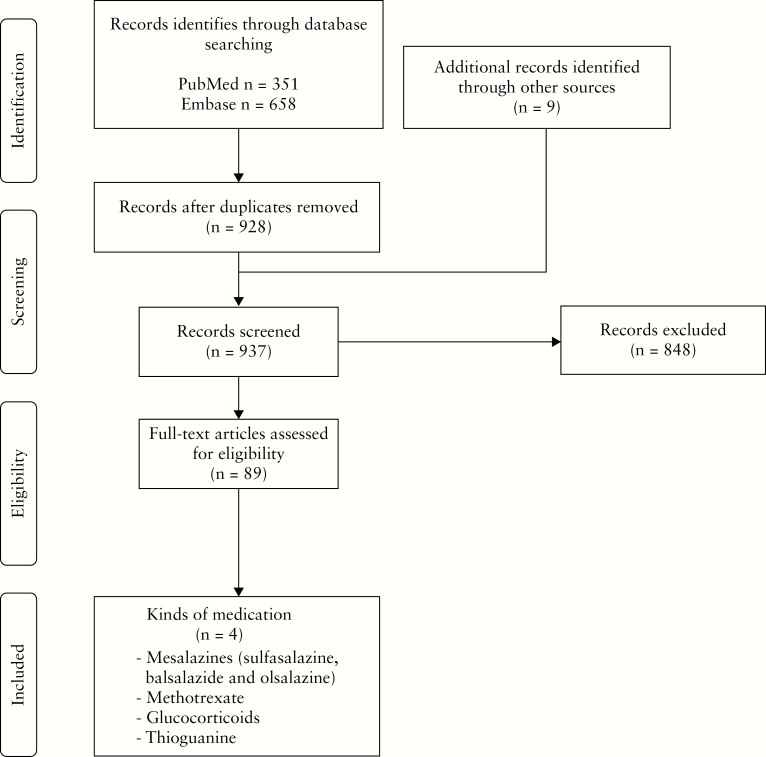
The search strategy yielded 1018 articles which were screened for eligibility. After removing duplicates 937 articles were screened on title and abstract. The 89 selected full text articles described four specific drugs used to treat patients with IBD. The selection process is depicted in Figure 1. A consensus for eligibility was reached between the two independent reviewers in all selection stages.
Figure 1.
Flowchart of study selection.
3. Microbial drug metabolism in IBD
3.1. Sulfasalazine
Sulfasalazine, discovered in the 1940s, is a drug consisting of an anti-inflammatory moiety [salicylic acid] linked to the antimicrobial drug [sulfanomide].9 Sulfasalazine was made by linking sulfapyridine [a sulfanomide molecule] to 5-aminosalicylic acid [5-ASA] by diazo-coupling. Sulfasalazine was initially developed for the treatment of inflammatory conditions that were believed to be of bacterial origin, but it was later found to be beneficial for the treatment of ulcerative colitis [UC].9
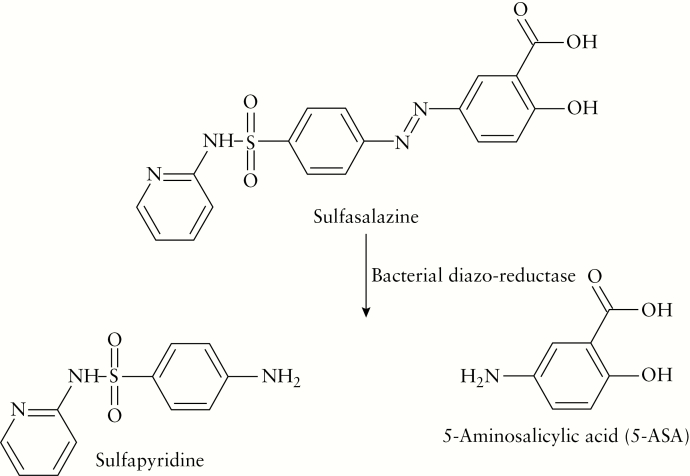
Sulfasalazine has limited absorption in the upper intestine, but in the colon a reduction of the diazo-bond occurs with the aid of diazo-reductase enzymes produced by the intestinal microbiota in the colon. After this cleavage, 5-ASA and sulfapyridine become available for systemic absorption; the former is a topical active drug while the latter is not [Figure 2].8,10 5-ASA is effective in UC as it induces anti-inflammatory effects by inhibiting pro-inflammatory mediators, and sulfapyridine acts primarily as an inactive carrier molecule.11 The pharmacological actions of 5-ASA are not fully understood but studies have shown that its anti-inflammatory action occurs by targeting peroxisome proliferator-activated receptor-γ, as well as modulating multiple cellular metabolism activities.12
Figure 2.
Cleavage of sulfasalazine by bacterial diazo-reductase.
This cleavage of the diazo-bond by gut flora was first described in rats by Peppercorn and Goldman in 1972.13 They demonstrated that antibiotic-treated or germ-free rats on sulfasalazine had unchanged sulfasalazine in their caecum and faeces, in contrast to the excreta of normal rats which did not contain unchanged sulfasalazine. When germ-free rats were infected with four specific bacteria normally found in the gastrointestinal tract, sulfasalazine was metabolized as in normal rats and no unchanged sulfasalazine was detected in their excreta. This cleavage of the diazo-bond was also detected when bacterial strains, representative of those found in the intestinal tract of humans, were cultured in the presence of sulfasalazine.13 In addition, Schröder and Campbell showed in a pharmacokinetic study in healthy human volunteers a very small amount of renally excreted parent drug and no faecal excretion.14 These findings indicate that intestinal bacteria are essential for activating this drug and also in part explain why sulfasalazine appears to be more effective in UC than in Crohn’s disease [CD]. The latter can be localized in the whole gastrointestinal tract and is not limited to the colonic region, which is consistent with the drug being more effective when the intestinal inflammation is in a region where bacterial breakdown is more likely to occur.10 Various species of bacteria are involved in the secretion of diazo-reductase in the human large intestine, but the largest amount is produced by the anaerobic genus Clostridium.15 Multiple in vitro studies have confirmed that the genus Clostridium produces the highest amount of diazo-reductase but also showed that bacteria from all the main bacterial phyla are able to metabolize sulfasalazine.13,15,16
The intestinal metabolism of sulfasalazine can be increased by co-administration with probiotics. An in vitro study showed that after anaerobic incubation of rat colon contents with sulfasalazine or sulfasalazine with probiotics [freeze-dried cultures of Lactobacillus acidophilus, Bifidobacterium lactus and Lactobacillus rhamonus] a higher concentration of 5-ASA and sulfapyridine was recovered in the latter.17 This suggests that these probiotic species possess diazo-reductase activity.17
Interestingly, the intestinal metabolism of sulfasalazine can be hampered by co-administration with cholestyramine, a bile acid sequestrant which can be used to treat bile acid diarrhoea in patients with [extensive] ileal CD or after a surgical ileocaecal resection. Rats treated with this combination had a higher faecal extraction of intact sulfasalazine compared to controls, suggesting a cholestyramine-induced inhibition of intestinal sulfasalazine metabolism.18 There are many possible explanations for this finding, but the most plausible is the occurrence of a direct cholestyramine–sulfasalazine interaction which leads to a less accessible sulfasalazine diazo-bond for bacterial diazo-reductase.18 The results of the study by Pieniaszek and Bates suggest that co-administration of cholestyramine and sulfasalazine could cause a significant reduction in the absorption and metabolism of the latter, resulting in a diminished efficacy of sulfasalazine due to an incomplete bacterial metabolism.18 Gastrointestinal transit time is also important for complete bacterial metabolism of sulfasalazine, as patients with medication-induced diarrhoea had an increased 72-h faecal recovery of unsplit sulfasalazine from 0.5% to 45.4%.19 The importance of contact duration with microbiota for sulfasalazine metabolization was confirmed by an in vitro study which, after incubation in human faecal contents, demonstrated a gradual decrease of sulfasalazine and an increase of 5-ASA with time. In the absence of faecal material, sulfasalazine remained stable during the experiment.20
Although sulfasalazine is an effective and low-cost treatment for UC, it has lost popularity as up to 30% of patients experience unwanted effects such as nausea, skin rash or anorexia, which seem to be related to the circulating sulfapyridine.21 To reduce these side effects some studies have used sulfasalazine retention enemas leading to a significant endoscopic and histological improvement compared to placebo without the occurrence of known side effects, even in patients who had previously experienced sulfasalazine hypersensitivity.22 It was demonstrated that after rectal admission of sulfasalazine patients had reduced plasma levels of sulfapyridine, suggesting that this may be one reason for the lowered frequency of undesired effects.22,23 Although plasma 5-ASA levels were not measured after rectal admission of sulfasalazine, a reduction of these levels seems unlikely to hamper the efficacy because the mode of action of 5-ASA seems to be topically rather than systemically.24
3.2. Different diazo-bonded mesalazine pro-drugs
When administered orally, 5-ASA undergoes rapid and almost complete systemic absorption in the small intestine, whereas it is known that 5-ASA works locally in the more distal intestinal mucosa. Preferably, the release of 5-ASA from a drug formulation takes place at the site of inflammation, i.e. distal ileum or colon, because the effectiveness of the medication is related more to drug mucosal concentration than to blood concentration.24,25
To overcome the problem of the toxic carrier moiety in sulfasalazine and the rapid systemic absorption of 5-ASA, other diazo-bonded mesalazine pro-drugs have been developed.
One of these pro-drugs is olsalazine, which consists of two molecules of 5-ASA linked by a diazo-bond between their amino groups. The complex is poorly absorbed in the upper gastrointestinal tract but in the large intestine the diazo-bond is cleaved by anaerobic and aerobic bacteria leading to the release of two 5-ASA molecules for every mole of olsalazine.26 In patients with an ileostomy almost all of the olsalazine is recovered in the ileostomy fluid and 5-ASA is not detected in the urine, suggesting that this pro-drug indeed passes through the small intestine with minimal absorption and without cleavage of the diazo-bond.26 These findings are supported by multiple studies which showed that the concentration of diazo-bonded 5-ASA in faeces was less than 5% of an ingested dose in nearly all cases, which suggests an almost complete colonic diazo-reduction.19,27 The extent of olsalazine metabolism is also dependent on the transit time; in patients with an accelerated gastrointestinal transit time the 72-h faecal recovery of olsalazine increased to 50%.19,27 The importance of the duration of contact between olsalazine and microbiota has also been demonstrated in an in vitro study which observed a decrease in olsalazine with time and an increase in 5-ASA.20
Balsalazide is another diazo-bonded pro-drug, which was developed in 1983 and approved in 1997 in Europe for the treatment of UC. Balsalazide consists of 4-aminobenzoyl-beta-alanine [4-ABA] diazo-linked to 5-ASA. This carrier is designed to be less toxic than sulfapyridine, due to minimal absorption of this pro-moiety after diazo-reduction in the colon, while maintaining the poor absorbability of the pro-drug in the upper gastrointestinal tract.28 Reduction of the diazo-bond by bacterial diazo-reductase results in the release of equimolar amounts of 5-ASA and 4-ABA. After oral administration of balsalazide there is almost complete colonic diazo-reduction, with less than 1% of the parent drug being excreted renally or in faeces. Approximately 25% of its metabolites are systemically absorbed and approximately 15% of these systemically absorbed metabolites consists of 4-ABA, although plasma concentrations of 4-ABA are below the level of detection.29 As previously demonstrated in sulfasalazine and olsalazine, the duration of contact with microbiota is also important for the extent of bacterial metabolism of balsalazide.20
In contrast to the previously described 5-ASA pro-drug diazo-reduction, some studies have proposed a more complex mechanism. They suggest a more rapid azo-reduction mechanism for sulfasalazine compared to the other pro-drugs of 5-ASA, as a lower percentage of unchanged sulfasalazine is recovered in the faeces compared to olsalazine and balsalazide.20,27,30 This is in line with an in vitro faecal microbial model which observed a faster rate of sulfasalazine metabolization.20 By contrast, Ryan et al. studied the substrate specificity of three azoreductase-encoding genes from Pseudomonas aeruginosa and found, depending on the gene, a higher binding specificity for balsalazide or olsalazine compared to sulfasalazine.31 These findings highlight a more complex mechanism of 5-ASA pro-drug diazo-reduction and suggest that bacterial metabolism of 5-ASA pro-drugs may not only be dependent on the presence of the diazo-bond but also on enzyme substrate specificity of the molecule surrounding this chemical bond.20
After diazo-reduction of the parent drug, 5-ASA is released in the colon and can be acetylated by N-acetyltransferase [NAT] enzymes in, among others, the metabolite N-acetyl-5-ASA [Ac-5-ASA].25 Whether the effective moiety is 5-ASA or Ac-5-ASA remains unclear, although a systematic review of the efficacy of Ac-5-ASA concluded that two out of three placebo controlled trials demonstrated that this metabolite is inactive.29 This acetylation into Ac-5-ASA is mainly mediated by mucosal enzymes as incubation of colonic biopsies with 5-ASA leads to almost complete acetylation after 10 min.32 However, after incubation of 5-ASA in faecal samples a slower and limited acetylation was witnessed after 24 h, also suggesting that acetylation can be mediated by faecal bacterial processes.32 These findings suggest that microbiota, besides parent pro-drug reduction and the release of the active moiety, can also inactivate 5-ASA by acetylation to a certain extent. In addition, colonic inflammation may also affect the efficacy of 5-ASA therapy; in a mouse model acute colonic inflammation diminished the capacity for 5-ASA metabolism by impairing the expression and function of one of the NAT enzymes.33
Although other diazo-bonded oral pro-drugs with alternative carrier molecules have been formulated in an attempt to reduce side-effects, at present mostly drugs that combine a 5-ASA pro-drug with pH-dependent and/or time-dependent release formulations are used to treat UC.34
3.3. Methotrexate
Methotrexate [MTX] was introduced in the late 1940s as an anti-neoplastic therapy for the treatment of acute leukaemia in children and later as treatment for solid organ cancers.35 In the late 1950s, low-dose MTX was established as an anti-inflammatory therapy for rheumatoid arthritis [RA], psoriatic arthritis and psoriasis, and in 1987 the first results of intramuscular MTX therapy in patients with refractory IBD were published.36,37 MTX is now a well-known immunomodulator that can induce and maintain remission in patients with CD and is often combined with biologicals.38,39 Inside the cell MTX is polyglutamated [PG] by folylpolyglutamate synthase [FPGS] to MTX-PG. Intracellular MTX and MTX-PG act as competitive inhibitors of dihydrofolic reductase [DHFR], which ultimately leads to a decrease in compounds involved in DNA and RNA synthesis. MTX-PG can also lead to an anti-inflammatory effect by the inhibition of 5-aminoimidazole-4-carboxamide ribonucleotide [AICAR] transformylase, which causes accumulated adenosine to leave the cell and bind receptors on surrounding cells.40
One of the reasons to investigate the role of microbiota in the metabolization of MTX was the observation that administration of the antibiotic neomycin prior to MTX administration increased mortality in mice. The authors concluded that a possible explanation for this increased lethality was a decrease in intestinal flora that normally metabolizes MTX into a non-toxic form.41 These findings were supported by another animal study in which they compared the amount of radioactivity in faeces between normal mice and germ-free mice, both treated with intraperitoneal injected radiolabelled MTX. They detected a higher amount of radioactivity in germ-free mice as compared to normal mice, indicating that bacteria of the intestinal tract probably play a prominent role in the degradation of MTX and may be responsible for the changed MTX toxicity observed during antibiotic treatment in mice.41 Another study in mice showed in vitro that the caecal contents from mice can cleave MTX to APA [2,4-diaminomethylpteroic acid], the major intestinal metabolite. This cleavage reaction is largely prevented by pretreatment of mice with antibiotics before excision of their caecum.42
As previously mentioned, in humans, intracellular MTX is converted into MTX-PG, which is poorly transported in and out of cells. MTX-PG can be exported after removal of the polyglutamate tail either by glutamate carboxypeptidase II or folate hydrolase but this removal reduces its efficacy as an inhibitor of DHFR.40 This removal of glutamate entities can also be performed by a carboxypeptidase G2 [CPG2] produced by strains of Pseudomonas and other orthologous enzymes in bacterial species.43,44 These enzymes give bacterial species the ability to metabolize and inactivate MTX in vitro, thereby altering its efficacy. In addition to removal of glutamate via carboxypeptidase, at least two bacterial species are able to metabolize MTX to MTX-PG by adding glutamate to MTX via FPGS-like enzymes.45 It is unlikely that removal of glutamate entities from MTX via bacterial carboxypeptidase leads to a decreased efficacy of oral MTX, because it would not affect post-absorption formation of MTX-PG. However, switching from oral to parenteral administration of MTX leads to a significant increase in [very] long-chain MTX-PGs, which are known to be more potent inhibitors.46 This suggests that the oral route may inhibit formation of the more potent MTX-PG, possibly due to conversion of MTX by bacterial carboxypeptidase. However, parenterally administrated MTX can, just as with orally administrated MTX, come into contact with the intestinal microbiota due to biliary secretion and could also be prone to bacterial metabolism.47 This interaction between parenterally administrated MTX and microbiota was confirmed in mice colonized with stool from an MTX-naïve RA patient. After either intraperitoneal injection of MTX or oral administration of MTX, the same effect was seen in the microbiota composition, namely a decrease in the phylum Bacteroidetes and an increase in two other phyla, Proteobacteria and Firmicutes.47 These results confirm that, in small laboratory animals, regardless of the administration route, MTX comes into contact with the intestinal microbiota and could alter its composition; however, to really elucidate the influence of bacterial metabolism on orally and parenteral administrated MTX more [human] studies are needed.
One study has investigated the role of the microbiota on determining response to oral MTX in patients with RA. The authors generated predictive models using microbiota data and were able to differentiate between good and poor responders.48 This suggests that the gut microbiota may contribute to interindividual variations in pharmacokinetics and clinical outcome in patients treated with oral MTX.
3.4. Glucocorticoids
Since the 1950s the first generation of glucocorticoids, i.e. prednisolone, methylprednisolone and hydrocortisone, have been effectively used to induce remission in IBD patients.49 They are well absorbed in the upper intestine and only a small fraction is delivered to the inflamed ileum or colon.50 Due to this systemic absorption, long-term administration of glucocorticoids can cause serious systemic side effect such as oedema, osteoporosis, hypertension or diabetes. Therefore, the administration of oral or intravenous first-generation glucocorticoids is generally reserved for the treatment of severely active disease and not for long-term maintenance therapy.50 To reduce the systemic side effects, second-generation glucocorticoids have been developed, e.g. budesonide and beclomethasone diproprionate [BDP], which are designed to release corticosteroids in the ileum or proximal colon, and the budesonide Multi Matrix System [MMX], which was constructed to release corticosteroids in the entire colon.51 This local release is mainly achieved by a pH-dependent coating and gives the advantage of high topical delivery of the active compound and a reduction in systemic side-effects due to, among other things, first-pass metabolism in the liver. Despite these advantages there is no increased effectiveness compared to first-generation systemically acting glucocorticoids.52
Due to the local release of glucocorticoids in the colon there is a direct contact with microbiota in the colon, and studies have demonstrated that the intestinal microbiota plays a role in metabolization of glucocorticoids.53,54
To determine the in vitro colonic bacterial metabolism of various types of glucocorticoids, the amount of each glucocorticoid was measured with high-performance liquid chromatography [HPLC] at different time intervals after incubation in caecal contents of rats.53 Their study showed a different susceptibility toward the caecal metabolism depending on the type of glucocorticoid. The concentration of cortisone decreased most drastically and was not measurable after 4 h of incubation, while the concentration of fluocinolone acetonide was unchanged.53 The metabolic stability of glucocorticoids appears to be dependent on their chemical structures, i.e. glucocorticoids with a 1,2 single bond, such as hydrocortisone and cortisone, are very susceptible to caecal metabolism, while glucocorticoids with substituents on the 6 and/or 9 position, such as fluocinolone acetonide and betamethasone, are more resistant, in vitro.53 The duration of contact between the intestinal microbiota and various glucocorticoids is also of influence on the metabolism of these drugs. When incubating different types of glucocorticoids in colonic contents of rats, respectively 22%, 35%, 53% and 92% of cortisone, hydrocortisone, prednisolone and dexamethasone remained after 3 h. These values decreased to 0%, 8%, 28% and 85% after 7 h of incubation.55 This variable degradation in time depending on the type of glucocorticoids was also observed in another in vitro study.53 In addition, in human colonic fluid or human faecal slurry, glucocorticoids were prone to colonic bacterial degradation.54 This bacterial degradation occurred to varying degrees depending on the kind of glucocorticoid: prednisolone and BDP degraded significantly faster than budesonide.54 This metabolism by the colonic microflora could affect therapeutic availability at the target site, although in vivo studies for this are lacking.
Additional studies are needed to elucidate the influence of the route of administration on bacterial degradation of glucocorticoids, although the enterohepatic circulation is also known to be a factor in the metabolism of glucocorticoids, so in theory also parenterally administrated glucocorticoids could be prone to bacterial metabolism.56
3.5. Thiopurines
Thioguanine [TG] and the more known conventional thiopurines azathioprine [AZA] and mercaptopurine [MP] are immunosuppressive drugs initially that were developed as chemotherapeutic agents for the treatment of acute lymphoblastic leukaemia.57 Due to their immunosuppressive potential thiopurines were, in the late 1950s and early 1960s, slowly introduced for the prevention of organ transplant rejection and the management of chronic idiopathic inflammatory diseases such as RA and IBD.58,59 Today, thiopurines are important immunomodulating agents to maintain remission in patients with IBD, but with a relatively slow therapeutic action.60 TG, AZA and MP are intracellularly metabolized into the same therapeutically effective end-metabolites, the phosphorylated 6-thioguanine nucleotides [6-TGNs]. Their mode of action is partly explained by the incorporation of 6-TGN in DNA during replication.60 However, the main immunosuppressive mode of action is due to competition with the endogenous GTP in binding of intracellular Ras-related C3 botulinum toxin substrate 1 [RAC1], inducing apoptosis and impairment of the capacity of T lymphocytes to form complexes with antigen-presenting cells.61
The metabolism of the ‘conventional’ thiopurines, MP and its pro-drug AZA, is complex and requires multiple enzymatic steps.62 Although excessive conversion to 6-TGN can cause unwanted leukopaenia, the toxicity of conventional thiopurines is mainly associated with the formation of potentially hepatotoxic 6-methylmercaptopurine [6-MMP] metabolites.63 TG on the other hand has a much less complicated metabolism. After oral absorption, TG is rapidly transported into the cell where hypoxanthine phosphoribosyl transferase [HPRT] directly converts TG into the pharmacological active 6-TGN. The competing thiopurine S-methyl transferase [TPMT] and xanthine oxidase [XO] yield 6-methylthioguanine [6-MTG] and 6-thiouric acid [6-TUA] respectively; the generation of 6-MMP is avoided in this pathway.62
In contrast to these well-established pathways requiring systemic conversion, Movva et al. demonstrated that the gut microbiota can metabolize TG, and to a far lesser extent MP, to the therapeutically effective 6-TGN.64 They cultured Escherichia coli DH5α in a medium containing TG or MP and after lysing the bacteria the intracellularly produced 6-TGN were measured according to the method of Dervieux et al.64 They discovered that Escherichia coli DH5α is able to convert TG and to a minimal extent MP into 6-TGN, underlined by a three-fold higher 6-TGN production with TG incubation in comparison to MP.64
This bacterial metabolism of TG into 6-TGN was also observed in vitro by using Escherichia coli, Enterococcus faecalis and Bacteroides thetaiotaomicron, which are representative gut bacteria from the bacterial phyla Bacteroidetes, Firmicutes and Proteobacteria. After incubation of bacterial pellets with TG, the 6-TGN were detected, whereas incubation with MP led to minimal 6-TGN detection. This finding of TG metabolism by bacteria belonging to different phyla is in line with the knowledge that the critical TG metabolizing enzyme HPRT is present in many forms of life. This suggests that all intestinal bacteria are able to make this conversion and implies that individual variations in the microbiota composition of the host may not make a large difference in the ability to locally metabolize TG, although this has not yet been investigated.
In vivo it was shown that dextran sulphate sodium [DSS]-induced colitis in HPRT-deficient mice improved after treatment with oral TG and that 6-TGN metabolites were detected in their faecal slurries. This suggests a local metabolization of TG due to bacterial metabolism, as HPRT is the host enzyme required for the conversion of TG in 6-TGN and an HPRT-deficient murine host is unable to make this conversion. Consistent with the inability of the HPRT-deficient murine host to convert TG to 6-TGN, there was no reduction in leukocyte counts in either mesenteric lymph nodes [MLNs] or peripheral blood compartments.65 Furthermore, intrarectal treatment with TG in Winnie mice rapidly led to significant improvement of the colitis in the regions of the colon with direct contact to the administrated TG. This was not the case in mice treated with intrarectal MP.
Thus, local delivery of TG could lead to a local conversion into 6-TGN by microbial HPRT, permitting a more rapid therapeutic action with potential avoidance of unwanted systemic adverse effects.65 Following these findings, controlled-release oral formulations of TG are being developed and a few patients have been treated with daily TG enemas or suppositories with a promising treatment response and with low systemic levels of 6-TGN.66
4. Discussion
The human intestinal microbiota can have a major impact on drug metabolism and this drug–microbiota interaction could alter the toxicity and efficacy of medication given for the treatment of IBD. The gut microbiota can cleave the azo-bond of sulfasalazine, balsalazide and olsalazine and release the active moiety 5-ASA, it has an impact on the metabolization of MTX, it can convert TG into the pharmacologically active 6-TGN without the requirement of host metabolism, and glucocorticoids can be prone to bacterial degradation. Moreover, individual patient responses to IBD medication may [partly] be explained by the effect of the host microbiota on the metabolism of these drugs. A better understanding of microbiota and medication interactions is needed and should be an integral part of the drug development pathway of new IBD medication.
Multiple new therapeutic compounds with oral delivery systems are now being developed for IBD treatment. Ideally these oral medications have minimal systemic side effects and are potent and effective at the site of inflammation. Oral therapies with pharmacodynamic effects in the colon can accomplish this, but their efficacy depends on whether adequate drug concentrations are delivered to the site of inflammation. Different approaches have been previously used for site-specific delivery to the colon with minimized proximal gastrointestinal absorption of medication such as the use of time-controlled release systems or by the use of a pH-sensitive coating.67 The latter approach has been optimized in recent years and is currently being used to develop ileo-colonic-targeted zero-order release tablets of budesonide with promising in vitro results and to produce oral infliximab for the local treatment of IBD in the ileo-colonic region.68,69
The intestinal microbiota can also be used for a colon-specific drug delivery system and seems to be the most site-specific approach due to the abrupt rise in the density of colonic microbiota and associated enzymatic activities in the lower gastrointestinal tract. There is a pro-drug approach, where an inactive derivate of a drug molecule requires bacterial metabolic processes in the colon to release the active drug moiety from the drug carrier, which is the case in sulfasalazine.70 Several studies have also investigated the possibility of a pro-drug of glucocorticoids, leading to a decrease of side effects and an increase of therapeutic concentrations at the target site.71,72 Azo polymers or polysaccharides can also be used as coating material over drug cores, which can be broken down by the gut microbiota and release the entrapped drug in the colon.73,74 Combined dual activating approaches are also being developed to overcome the limitations of a single approach, for example by a novel coating which combined microbiota-triggered and pH-dependent systems used to design, among others, a once-daily 1600-mg tablet-based mesalazine preparation.75,76 Newer approaches for colon-targeted drug delivered are still being developed, such as a pressure-controlled drug delivery system, which uses the difference in peristalsis in the gut, charge-based systems, ligand/receptor-mediated drug delivery systems, nanoparticulate systems or osmotically controlled drug delivery methods.77
This site-specific drug delivery can be used to design more stable and effective therapeutics with a reduction of drug-related systemic side effects. However, when applying colon-specific drug delivery there is direct contact between microbiota in the gut and the locally released drug, leading to a possible drug–microbiota interaction. This interaction could alter the efficacy and toxicity of medication which, as described before, is the case in vitro with bacterial degradation of glucocorticoids. Although this has not yet been studied in vivo, one can imagine that this metabolism by colonic microflora could affect therapeutic availability at the target site with colonic-specific delivery of glucocorticoids.53,54 Given the development of new IBD medications that are locally released in the colon, such as oral infliximab, it is important that these possible interactions between microbiota and drugs are an integral part of the drug development pathway. Inter- and intra-individual changes in microbial diversity also need to be taken into consideration during drug development, because the composition of the human microbiome is not static and this could also impact the efficacy and toxicity of medication over time.78,79
Considering the effect of the microbiota on drug metabolism, there is also the potential to alter the microbiota to enhance the therapeutic efficacy or decrease the toxicity of IBD medication. Antibiotics are the most obvious category of drugs to modify the gut microbiota, and after administration significant changes in the microbiota composition are witnessed, but faecal microbiota transplantation is also a way to modify the composition.80 The extent to which probiotics influence the host intestinal microbiota is not yet clear but it has been demonstrated that co-administration of colon-targeted probiotics ameliorates the efficacy of sulfasalazine in rodent models of colitis.81 However, before speculating on modulating the gut microbiota as a potential target to enhance therapeutic efficacy, there needs to be an improved understanding of the precise interaction between microbes and drugs and how an alteration of the microbiota composition effects this interaction.
The human intestinal microbiota can have a major impact on drug metabolism and a better understanding of all these interactions between microbiota and IBD medication should be an integral part of the drug development path. This contribution of the gut microbiota can be used as a precision medicine approach to design more stable and effective therapeutics while reducing drug-related side effects. Moreover, modulating the microbiota, e.g. by faecal microbiota transplantation, co-administration of antibiotics or probiotics, could also be a potential target to enhance the therapeutic efficacy of IBD medication.
Supplementary Material
Acknowledgments
There was no writing assistance required for this study. This paper, including related data and figures, has not been previously published and is not under consideration elsewhere.
Funding
No funding was required for the performance and writing of this study.
Conflict of Interest
F.C. and H.B. have nothing to declare. N.d.B. has served as a speaker for AbbVie and MSD and has served as consultant and principal investigator for TEVA Pharma BV and Takeda. He has received a [unrestricted] research grant from Dr Falk, TEVA Pharma BV, MLDS and Takeda.
Author Contributions
F.C. performed the systematic literature search. F.C. and N.d.B. performed the data extraction. F.C. drafted the first version of the manuscript. N.d.B. and H.B. critically revised the manuscript. All authors approved the final version of the article, including the authorship list.
References
- 1. Qin J, Li R, Raes J, et al.; MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggeletopoulou I, Konstantakis C, Assimakopoulos SF, Triantos C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb Pathog 2019;137:103774. [DOI] [PubMed] [Google Scholar]
- 3. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sousa T, Paterson R, Moore V, Carlsson A, Abrahamsson B, Basit AW. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm 2008;363:1–25. [DOI] [PubMed] [Google Scholar]
- 5. Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res 2017;179:204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okuda H, Ogura K, Kato A, Takubo H, Watabe T. A possible mechanism of eighteen patient deaths caused by interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. J Pharmacol Exp Ther 1998;287:791–9. [PubMed] [Google Scholar]
- 7. Elkington SG, Floch MH, Conn HO. Lactulose in the treatment of chronic portal-systemic encephalopathy. N Eng J Med 1969;281:408–12. [DOI] [PubMed] [Google Scholar]
- 8. Peppercorn MA Drug–bacteria interaction. Drug Metab Drug Interact 1976:75–88. [Google Scholar]
- 9. Bachrach WH Sulfasalazine: I. An historical perspective. Am J Gastroenterol 1988;83:487–96. [PubMed] [Google Scholar]
- 10. Peppercorn MA, Goldman P. Distribution studies of salicylazosulfapyridine and its metabolites. Gastroenterology 1973;64:240–5. [PubMed] [Google Scholar]
- 11. Hayllar J, Bjarnason I. Sulphasalazine in ulcerative colitis: in memoriam? Gut 1991;32:462–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lichtenstein GR, Kamm MA. Review article: 5-aminosalicylate formulations for the treatment of ulcerative colitis–methods of comparing release rates and delivery of 5-aminosalicylate to the colonic mucosa. Aliment Pharmacol Ther 2008;28:663–73. [DOI] [PubMed] [Google Scholar]
- 13. Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther 1972;181:555–62. [PubMed] [Google Scholar]
- 14. Schröder H, Campbell DE. Absorption, metabolism, and excretion of salicylazosulfapyridine in man. Clin Pharmacol Ther 1972;13:539–51. [DOI] [PubMed] [Google Scholar]
- 15. Rafii F, Cerniglia CE. Reduction of azo dyes and nitroaromatic compounds by bacterial enzymes from the human intestinal tract. Environ Health Perspect 1995;103[Suppl 5]:17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Azad Khan AK, Guthrie G, Johnston HH, Truelove SC, Williamson DH. Tissue and bacterial splitting of sulphasalazine. Clin Sci (Lond) 1983;64:349–54. [DOI] [PubMed] [Google Scholar]
- 17. Lee HJ, Zhang H, Orlovich DA, Fawcett JP. The influence of probiotic treatment on sulfasalazine metabolism in rat. Xenobiotica 2012;42:791–7. [DOI] [PubMed] [Google Scholar]
- 18. Pieniaszek HJ Jr, Bates TR. Cholestyramine-induced inhibition of salicylazosulfapyridine (sulfasalazine) metabolism by rat intestinal microflora. J Pharmacol Exp Ther 1976;198:240–5. [PubMed] [Google Scholar]
- 19. Rijk MC, van Hogezand RA, van Schaik A, van Tongeren JH. Disposition of 5-aminosalicylic acid from 5-aminosalicylic acid-delivering drugs during accelerated intestinal transit in healthy volunteers. Scand J Gastroenterol 1989;24:1179–85. [DOI] [PubMed] [Google Scholar]
- 20. Sousa T, Yadav V, Zann V, Borde A, Abrahamsson B, Basit AW. On the colonic bacterial metabolism of azo-bonded prodrugsof 5-aminosalicylic acid. J Pharm Sci 2014;103:3171–5. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen OH Sulfasalazine intolerance. A retrospective survey of the reasons for discontinuing treatment with sulfasalazine in patients with chronic inflammatory bowel disease. Scand J Gastroenterol 1982;17:389–93. [DOI] [PubMed] [Google Scholar]
- 22. Palmer KR, Goepel JR, Holdsworth CD. Sulphasalazine retention enemas in ulcerative colitis: a double-blind trial. Br Med J (Clin Res Ed) 1981;282:1571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allgayer H, Kruis W, Eisenburg J, Paumgartner G. Comparative pharmacokinetics of sulphasalazine and sulphapyridine after rectal and oral administration to patients with ulcerative colitis. Eur J Clin Pharmacol 1984;26:275–7. [DOI] [PubMed] [Google Scholar]
- 24. Klotz U Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid. Clin Pharmacokinet 1985;10:285–302. [DOI] [PubMed] [Google Scholar]
- 25. De Vos M, Verdievel H, Schoonjans R, Praet M, Bogaert M, Barbier F. Concentrations of 5-ASA and Ac-5-ASA in human ileocolonic biopsy homogenates after oral 5-ASA preparations. Gut 1992;33:1338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandberg-Gertzén H, Ryde M, Järnerot G. Absorption and excretion of a single 1-g dose of azodisal sodium in subjects with ileostomy. Scand J Gastroenterol 1983;18:107–11. [DOI] [PubMed] [Google Scholar]
- 27. Wadworth AN, Fitton A. Olsalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in inflammatory bowel disease. Drugs 1991;41:647–64. [DOI] [PubMed] [Google Scholar]
- 28. Green JRB The treatment of ulcerative colitis with balsalazide sodium. InflammoPharmacology 1993;2:289–95. [Google Scholar]
- 29. Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther 2003;17:29–42. [DOI] [PubMed] [Google Scholar]
- 30. Chan RP, Pope DJ, Gilbert AP, Sacra PJ, Baron JH, Lennard-Jones JE. Studies of two novel sulfasalazine analogs, ipsalazide and balsalazide. Dig Dis Sci 1983;28:609–15. [DOI] [PubMed] [Google Scholar]
- 31. Ryan A, Wang CJ, Laurieri N, Westwood I, Sim E. Reaction mechanism of azoreductases suggests convergent evolution with quinone oxidoreductases. Protein Cell 2010;1:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allgayer H, Ahnfelt NO, Kruis W, et al. Colonic N-acetylation of 5-aminosalicylic acid in inflammatory bowel disease. Gastroenterology 1989;97:38–41. [DOI] [PubMed] [Google Scholar]
- 33. Ramírez-Alcántara V, Montrose MH. Acute murine colitis reduces colonic 5-aminosalicylic acid metabolism by regulation of N-acetyltransferase-2. Am J Physiol Gastrointest Liver Physiol 2014;306:G1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ye B, van Langenberg DR. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J Gastrointest Pharmacol Ther 2015;6:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farber S Chemotherapy in the treatment of leukemia and Wilms’ tumor. JAMA 1966;198:826–36. [PubMed] [Google Scholar]
- 36. Benedek TG Methotrexate: from its introduction to non-oncologic therapeutics to anti-TNF-α. Clin Exp Rheumatol 2010;28:S3–8. [PubMed] [Google Scholar]
- 37. Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med 1989;110:353–6. [DOI] [PubMed] [Google Scholar]
- 38. Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med 2000;342:1627–32. [DOI] [PubMed] [Google Scholar]
- 39. Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med 1995;332:292–7. [DOI] [PubMed] [Google Scholar]
- 40. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis 2007;65:168–73. [PubMed] [Google Scholar]
- 41. Zaharko DS, Bruckner H, Oliverio VT. Antibiotics alter methotrexate metabolism and excretion. Science 1969;166:887–8. [DOI] [PubMed] [Google Scholar]
- 42. Valerino DM, Johns DG, Zaharko DS, Oliverio VT. Studies of the metabolism of methotrexate by intestinal flora. I. Identification and study of biological properties of the metabolite 4-amino-4-deoxy-N 10 -methylpteroic acid. Biochem Pharmacol 1972;21:821–31. [DOI] [PubMed] [Google Scholar]
- 43. Levy CC, Goldman P. The enzymatic hydrolysis of methotrexate and folic acid. J Biol Chem 1967;242:2933–8. [PubMed] [Google Scholar]
- 44. Webb M Inactivation of analogues of folic acid by certain non-exacting bacteria. Biochim Biophys Acta 1955;17:212–25. [DOI] [PubMed] [Google Scholar]
- 45. Nayak RR, O’Loughlin C, Fischbach M, Turnbaugh PJ. Methotrexate is an antibacterial drug metabolized by human gut bacteria [abstract]. Arthritis Rheumatol 2016;68[suppl 10]. https://acrabstracts.org/abstract/methotrexate-is-an-antibacterial-drug-metabolized-by-human-gut-bacteria/ Accessed August 3, 2020. [Google Scholar]
- 46. Dervieux T, Zablocki R, Kremer J. Red blood cell methotrexate polyglutamates emerge as a function of dosage intensity and route of administration during pulse methotrexate therapy in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:2337–45. [DOI] [PubMed] [Google Scholar]
- 47. Nayak RR, Alexander M, Stapleton-Grey K, et al. Perturbation of the human gut microbiome by a non-antibiotic drug contributes to the resolution of autoimmune disease. bioRxiv 2019:600155. [Google Scholar]
- 48. Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 2015;21:895–905. [DOI] [PubMed] [Google Scholar]
- 49. Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2008;2008:CD006792-CD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Domènech E Inflammatory bowel disease: current therapeutic options. Digestion 2006;73[Suppl 1]:67–76. [DOI] [PubMed] [Google Scholar]
- 51. Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014;63:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Cassan C, Fiorino G, Danese S. Second-generation corticosteroids for the treatment of Crohn’s disease and ulcerative colitis: more effective and less side effects? Dig Dis 2012;30:368–75. [DOI] [PubMed] [Google Scholar]
- 53. Kong H, Lee Y, Kim H, et al. Susceptibility of glucocorticoids to colonic metabolism and pharmacologic intervention in the metabolism: implication for therapeutic activity of colon-specific glucocorticoid 21-sulfate sodium at the target site. J Pharm Pharmacol 2012;64:128–38. [DOI] [PubMed] [Google Scholar]
- 54. Yadav V, Gaisford S, Merchant HA, Basit AW. Colonic bacterial metabolism of corticosteroids. Int J Pharm 2013;457:268–74. [DOI] [PubMed] [Google Scholar]
- 55. Kim IH, Kong HS, Choi BI, et al. Synthesis and in vitro properties of dexamethasone 21-sulfate sodium as a colon-specific prodrug of dexamethasone. Drug Dev Ind Pharm 2006;32:389–97. [DOI] [PubMed] [Google Scholar]
- 56. Hyde PM, Williams RH. Absorption and metabolism of hydrocortisone-4-C14. J Biol Chem 1957;227:1063–81. [PubMed] [Google Scholar]
- 57. Elion GB The purine path to chemotherapy. Science 1989;244:41–7. [DOI] [PubMed] [Google Scholar]
- 58. Zweiman B Immunosuppressive effects of specific classes of agents with special reference to organ transplantation. Immunosuppression by thiopurines. Transplant Proc 1973;5:1197–201. [PubMed] [Google Scholar]
- 59. Brooke BN, Hoffmann DC, Swarbrick ET. Azathioprine for Crohn’s disease. Lancet 1969;2:612–4. [DOI] [PubMed] [Google Scholar]
- 60. de Boer NKH, Peyrin-Biroulet L, Jharap B, et al. Thiopurines in inflammatory bowel disease: new findings and perspectives. J Crohns Colitis 2018;12:610–20. [DOI] [PubMed] [Google Scholar]
- 61. Seinen ML, van Nieuw Amerongen GP, de Boer NK, van Bodegraven AA. Rac attack: modulation of the small GTPase Rac in inflammatory bowel disease and thiopurine therapy. Mol Diagn Ther 2016;20:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Derijks LJ, Gilissen LP, Hooymans PM, Hommes DW. Review article: thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther 2006;24:715–29. [DOI] [PubMed] [Google Scholar]
- 63. de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol 2007;4:686–94. [DOI] [PubMed] [Google Scholar]
- 64. Movva R, Lobb M, Ó Cuív P, Florin THJ, Duley JA, Oancea I. Microbial metabolism of thiopurines: a method to measure thioguanine nucleotides. J Microbiol Methods 2016;128:102–7. [DOI] [PubMed] [Google Scholar]
- 65. Oancea I, Das ID, De Carcer DA, et al. Colonic microbiota can promote rapid improvement of murine colitis by thioguanine independently of t-lymphocytes and host metabolism. Gastroenterology 2016;150:S196–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Florin THJ, Wright JD, Jambhrunkar SD, Henman MG, Popat A. A well-tolerated and rapidly acting thiopurine for IBD? Drug Discov Today 2019;24:37–41. [DOI] [PubMed] [Google Scholar]
- 67. Gazzaniga A, Iamartino P, Maffione G, Sangalli ME. Oral delayed-release system for colonic specific delivery. Int J Pharm 1994;108:77–83. [Google Scholar]
- 68. Gareb B, Dijkstra G, Kosterink JGW, Frijlink HW. Development of novel zero-order release budesonide tablets for the treatment of ileo-colonic inflammatory bowel disease and comparison with formulations currently used in clinical practice. Int J Pharm 2019;554:366–75. [DOI] [PubMed] [Google Scholar]
- 69. Gareb B, Posthumus S, Beugeling M, et al. Towards the oral treatment of ileo-colonic inflammatory bowel disease with infliximab tablets: development and validation of the production process. Pharmaceutics 2019;11:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Philip AK, Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J 2010;25:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim I, Kong H, Lee Y, et al. Dexamethasone 21-sulfate improves the therapeutic properties of dexamethasone against experimental rat colitis by specifically delivering the steroid to the large intestine. Pharm Res 2009;26:415–21. [DOI] [PubMed] [Google Scholar]
- 72. Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci 2003;6:33–66. [PubMed] [Google Scholar]
- 73. Van den Mooter G, Samyn C, Kinget R. In vivo evaluation of a colon-specific drug delivery system: an absorption study of theophylline from capsules coated with azo polymers in rats. Pharm Res 1995;12:244–7. [DOI] [PubMed] [Google Scholar]
- 74. Ashford M, Fell J, Attwood D, Sharma H, Woodhead P. An evaluation of pectin as a carrier for drug targeting to the colon. J Control Release 1993;26:213–20. [Google Scholar]
- 75. Ibekwe VC, Khela MK, Evans DF, Basit AW. A new concept in colonic drug targeting: a combined pH-responsive and bacterially-triggered drug delivery technology. Aliment Pharmacol Ther 2008;28:911–6. [DOI] [PubMed] [Google Scholar]
- 76. D’Haens GR, Sandborn WJ, Zou G, et al. Randomised non-inferiority trial: 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther 2017;46:292–302. [DOI] [PubMed] [Google Scholar]
- 77. Javdan B, Lopez JG, Chankhamjon P, et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell 2020;181:1661–1679.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scarpellini E, Ianiro G, Attili F, Bassanelli C, De Santis A, Gasbarrini A. The human gut microbiota and virome: potential therapeutic implications. Dig Liver Dis 2015;47:1007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Voigt RM, Forsyth CB, Green SJ, et al. Circadian disorganization alters intestinal microbiota. PLoS One 2014;9:e97500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010;156:3216–23. [DOI] [PubMed] [Google Scholar]
- 81. Prudhviraj G, Vaidya Y, Singh SK, et al. Effect of co-administration of probiotics with polysaccharide based colon targeted delivery systems to optimize site specific drug release. Eur J Pharm Biopharm 2015;97:164–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.